Abstract
Acute stress is increasingly recognized as a precipitant of acute myocardial infarction (AMI). However, the role of chronic stress in developing AMI is less clear. We have developed a method to measure cortisol in hair, which allows longitudinal assessment of cortisol levels prior to an acute event. We aimed to evaluate the hypothesis that chronic stress, as assessed by hair cortisol content, is associated with the development of AMI. A prospective case–control study included 56 patients admitted to hospital with AMI and 56 control patients, admitted to internal medicine wards for other indications. An enzyme immunoassay technique was used to measure cortisol in the most proximal 3 cm of hair, considered to represent the most recent 3 months of exposure. Median hair cortisol contents (range) were 295.3 (105.4–809.3)ng/g in AMI patients and 224.9 (76.58–949.9)ng/g in controls (p = 0.006, Mann–Whitney U-test). After controlling for other risk factors for AMI using multiple logistic regression, log-transformed hair cortisol content remained the strongest predictor (OR 17.4, 95% CI 2.15–140.5; p = 0.007). We demonstrated elevated hair cortisol concentrations in patients with AMI. This suggests that chronic stress, as assessed by increased hair cortisol in the 3 months prior to the event, may be a contributing factor for AMI.
Introduction
The association between acute stress and cardiovascular morbidity and mortality is well established (Mittleman et al. Citation1993, Citation1995; Leor et al. Citation1996; Albert et al. Citation2000; Strike et al. Citation2006; Brotman et al. Citation2007; Chida and Steptoe Citation2010). Acute stressors have also been linked to sustained cardiovascular responses. A 2005 study found that systolic blood pressure of an American population was increased during the 2 months following the World Trade Center attacks on September 11, 2001, compared to the two previous months (Gerin et al. Citation2005). With regard to chronic stress, most research indicate a similar link to cardiovascular disease (CVD). Chronic psychosocial stressors, including job strain, marital and financial stress, have been linked to increased risk for developing CVD including acute myocardial infarction (AMI; Rosengren et al. Citation2004; Kornitzer et al. Citation2006; Aboa-Eboulé et al. Citation2007). For example, studies have shown that increased systolic blood pressure and greater risk of the metabolic syndrome are linked with chronic job stress (Kario et al. Citation2002; Chandola et al. Citation2006). However, other studies have failed to show such association (Hlatky et al. Citation1995; Lee et al. Citation2002; Eaker et al. Citation2004). To date, studies on the effects of chronic stress on cardiovascular events have used psychosocial questionnaires to collect data on chronic stress (Dimsdale Citation2008). While the association between psychosocial stress and CVD is fairly well established, as seen by the many reports of the Whitehall II studies, it is important to note that the assessment of past stress at the time of an event is subject to significant recall bias, as the acute event may prompt the patient to strive harder to identify previous stressors (Dimsdale Citation2008). Moreover, the limited availability of objective and reliable biological markers for the measurement of chronic stress has been a critical hurdle in evaluating the role of chronic stress and the risk for CVDs even in the large prospective studies.
Allostasis refers to the body's ability to adapt and respond to changes in the internal and external environment using a variety of mechanisms, such as the hypothalamic–pituitary–adrenal (HPA)-axis. These changes can take different forms, but often present as some form of stress. Physical and emotional stress both activate several neuroendocrine systems, the most important being the HPA-axis that regulates the production and secretion of glucocorticoids (especially cortisol) from the adrenal cortex (Brotman et al. Citation2007). Cortisol is considered to be a “stress hormone” and as such, its secretion is increased during times of stress. Steptoe et al. (Citation2004) showed that higher salivary cortisol levels in the morning, as well as greater cortisol output averaged over the day, were positively associated in men who were overcommitted to their jobs. This study also found an association with overcommitment and increased systolic blood pressure, leading the researchers to suggest that perhaps cortisol is one of the links between chronic job stress and CVD risk (Steptoe et al. Citation2004). Additionally, two recent studies conducted by CitationReynolds et al. (2010a,b) suggest that greater serum level in the morning and greater plasma cortisol levels are associated with increased CVD, as well as cognitive decline, in an elderly population. Interestingly, higher levels of serum cortisol have been observed in patients presenting with AMI, compared to those in controls (Adair and Kasahara Citation1980; Zouaghi et al. Citation1985).
Traditionally, cortisol has been measured in serum, urine, and saliva. All of these matrices measure cortisol levels in the last few hours to days and, therefore, do not reflect the stress response over prolonged periods of time. Recently, the validity of measuring cortisol in hair as a biomarker of chronic stress has been documented. Lipophilic compounds circulating in the blood, including cortisol, can primarily become incorporated into hair following diffusion from capillaries nourishing the hair into the growing hair follicle. As the hair emerges from the scalp, the compound remains trapped inside the inner hair shaft (Henderson Citation1993; Cone Citation1996; Pragst and Balikova Citation2006). Other mechanisms of incorporation may include sebum and sweat secretions, as well as external contamination after formation of the hair strand (Henderson Citation1993; Cone Citation1996).
While the exact extent of time during which cortisol remains stable along the hair shaft is unknown, it is agreed that cortisol is stable in hair for at least up to 6 months (corresponding to the proximal 6 cm of a human hair sample; Davenport et al. Citation2006; Van Uum et al. Citation2008; Kirschbaum et al. Citation2009). Indeed, a recent study demonstrated the presence of cortisol in the hair of ancient Peruvian mummies dating back to as early as AD 550–1000 (Webb et al. Citation2010), indicating stability over many years. Hair grows at an average of approximately 1 cm per month (0.35 mm per day; Hayashi et al. Citation1991; D'Amico et al. Citation2001), hence hair analysis can accurately reflect long-term endogenous production of cortisol. For example, cortisol measurements from the most proximal 3 cm of hair represent the most recent 3 months of exposure (comparable to the assessment of blood glucose levels by using hemoglobin A1C). It is important to note that, as the hair follicle is embedded approximately 3 mm beneath the scalp, cortisol (or any compound) measured in hair does not reflect immediate or recent exposure (i.e. previous few days; Pragst and Balikova Citation2006).
This technique has provided for the first time a reliable method for the measurement of cortisol exposure over prolonged time and a potential biomarker of chronic stress (Davenport et al. Citation2006, Citation2008; Kalra et al. Citation2007; Sauvé et al. Citation2007; Yamada et al. Citation2007; Van Uum et al. Citation2008; Kirschbaum et al. Citation2009; Thomson et al. Citation2010). Several recent reports have demonstrated an association between high levels of hair cortisol and chronic stress in both animal models and in humans (Davenport et al. Citation2006, Citation2008; Kalra et al. Citation2007; Sauvé et al. Citation2007; Yamada et al. Citation2007; Van Uum et al. Citation2008; Kirschbaum et al. Citation2009; Thomson et al. Citation2010). Importantly, measurements in hair also allow longitudinal assessment of cortisol levels prior to an acute event. The objective of the present study was to test the hypothesis that chronic stress, as assessed by hair cortisol levels, is associated with the development of AMI. For this study, we define the term “chronic” as a 3-month period of time.
Patients and methods
We included male patients above the age of 18 years who were admitted to the Intensive Cardiac Care Unit at the Meir Medical Center with either ST-elevation or non-ST elevation AMI (corresponding to the S-T segment on an electrocardiogram). AMI was defined as the combination of elevated circulating levels of cardiac biomarkers (troponin or creatine kinase and its MB isoenzyme) with typical chest pain lasting for at least 30 min, or typical ECG changes (new Q waves and/or ST and T-wave changes). The control group consisted of male patients admitted to an internal medicine ward for reasons other than acute coronary syndrome or stroke ().
Table I. Diagnosis of the control patients at discharge.
Patients were excluded if they had any of the following: age below 18 years, glucocorticosteroid treatment within the last 12 months, diagnosis of Cushing's or Addison's disease, dyed hair, morbid obesity [defined as body mass index (BMI)>35], or inability to sign an informed consent form. Patients for whom a hair sample of at least 3 cm from vertex posterior could not be obtained were excluded for technical reasons. Since AMI is largely more prevalent in men than in women, and considering the hormonal differences between genders, our study focused solely on male patients.
Hypertension was defined as repeated systolic or diastolic blood pressure measurements ≥ 140 and/or ≥ 90 mmHg, respectively, and/or chronic treatment with anti-hypertensive medications. Diabetes mellitus was defined based on self-report by the patient combined with either documentation in his medical records or regular treatment with oral hypoglycemic agents or insulin. Blood samples for lipid measurements were drawn after a minimum 12-h fast. The biochemical analysis of blood lipids was performed on fresh samples in a core laboratory facility with the use of a BM/Hitachi917 automated analyzer (Boehringer, Mannheim, Germany).
Dyslipidemia was defined as high-density lipoprotein (HDL) cholesterol levels below 40 mg/dl and/or as low-density lipoprotein (LDL) cholesterol above the target level according to the NCEP-3 recommendations, or if in chronic treatment, lipid lowering drugs were given. Prior CVD was defined as either a history of hospital admission due to acute coronary artery occlusion, percutaneous coronary interventions, coronary artery bypass grafting, heart failure, cerebro-vascular accident or peripheral vascular disease prior to the index admission. Family history of CVD was defined as the occurrence of CVD in a male first-degree relative under the age of 55 years or in a female first-degree relative under the age of 65 years. BMI was calculated as the weight in kilograms divided by the square of the height in meters.
Hair sampling
Hair samples for the measurement of hair cortisol were obtained from the posterior vertex during the first 2–3 days of admission. The hair samples were taken in accordance with standard protocols previously established by the Motherisk Laboratory (Kalra et al. Citation2007; Sauvé et al. Citation2007; Yamada et al. Citation2007; Van Uum et al. Citation2008; Thomson et al. Citation2010). Briefly, hair samples were taken from the vertex posterior of the head and as close to the scalp as possible. Using a low-tack tape, the hair samples were attached to a collection page with the scalp end of the sample indicated with an arrow. Hair samples were stored at room temperature and sent through the mail in a sealed envelope.
Quantification of hair cortisol
Once received, hair samples were measured and the two most proximal 1.5-cm sections were cut. The hair sections were placed in a glass vial and weighed, a minimum of 10 mg hair was required for each sample. The hair was finely chopped with surgical scissors and 1 ml of methanol was added. The vial was incubated on a shaker at 100 RPM for 16 h at 50°C. Methanol was then removed to a glass test tube where it was dried under a stream of nitrogen gas at 50°C. The remaining white residue was reconstituted with 250 μl of phosphate buffered saline to increase the concentration four fold. The sample was vortexed and 50 μl phosphate buffered saline was added to the plate wells in duplicate. The analysis was done using a commercially available salivary enzyme immunoassay kit from Alpco Diagnostics (Salem, NH, USA). Both a positive and a negative control were used to ensure accuracy and specificity of measurement. A negative control (containing the buffer only) was used to assess any non-specific binding and this value was subtracted from all other values prior to interpretation. The intra- and inter-day coefficients of variation were determined using a standard sample of hair measured over several weeks and was 3.8% (n = 5) and 8% (n = 6), respectively. The limit of detection of the enzyme immunoassay kit was 1.14 ng/ml (Alpco Diagnostics).
Statistical analysis
The AMI and control groups were compared on a large number of characteristics using either a student unpaired t-test or Mann–Whitney U-test based on the distribution of the data for continuous variables, and Fisher's exact test for dichotomous variables. This was followed by multiple logistic regression to identify factors that significantly predict AMI. We included in the logistic regression all determinants proven to affect the risk for AMI, including age, LDL and HDL cholesterol levels, BMI, smoking status, previous MI, as well as log-transformed hair cortisol values. This analysis was conducted using GraphPad Prism version 5.00 (GraphPad Software, Inc., La Jolla, CA, USA) and SigmaStat version 3.11 (Systat Software, Inc., Richmond, CA, USA).
Ethical considerations
The study was approved by the research ethics committee at Meir Medical Center and written informed consent was obtained from each participant. The study was registered in the Clinical Trials system (ID: NCT00682487).
Results
The 120 consecutive male patients recruited to the study were divided equally between the AMI and the control group and underwent hair sampling. The mean ± SD age of all patients was 61 ± 10.9 years. None of the study's participants had any hospital admission during the 3 months prior to the index admission. Among the control patients, 31 (51.7%) were hospitalized for chest pain not found to be due to an acute coronary syndrome, and 9 (15%) for other cardiac conditions (including heart failure and atrial fibrillation that were not related to acute ischemia). The remaining patients were hospitalized for non-cardiac conditions, mainly infectious diseases ().
One control and three case patients did not provide sufficient quantity of hair to proceed with the analysis, and, therefore, hair analysis was conducted in only 59 controls and 57 AMI patients. One control patient was excluded because of hair cortisol levels beyond the detection limit of the kit and additional three individuals (two controls, one case) with cortisol content higher than 1500 ng/g were excluded from this analysis on the basis of possible external contamination.
Of the 56 patients in the AMI group, 30 (54%) patients presented with non-ST elevation and 26 (46%) with ST elevation myocardial infarction. Furthermore, according to the ECG and echocardiographic findings, 24 (42%) patients had inferior, 20 (36%) had anterior, and 2 (3.6%) had lateral wall AMI. In the other 10 (18%) patients, the location of MI could not be determined. There were no cases of death during the index admission in either the AMI or control groups.
The final analysis included 56 subjects in each group (). The patients admitted with AMI had significantly lower age and HDL, and significantly higher LDL and BMI than the control group. The two groups did not differ in total cholesterol, rates of current or former cigarette smoking, diabetes mellitus, hypertension, prior CVD including coronary artery disease (CAD) or family history of CVD ().
Table II. Patient characteristics of the AMI case and control groups.
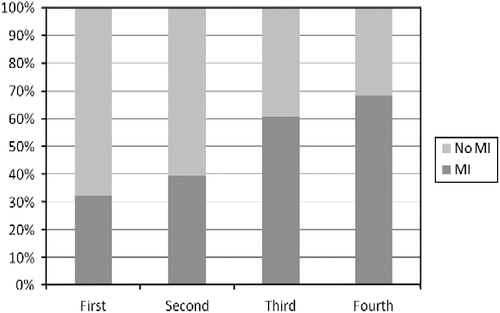
Hair cortisol concentrations [median (range)] in the proximal 3-cm segments of the AMI patients [295.3 (105.4–809.3 ng/g)] were significantly higher compared to those of controls [224.9 (76.6–949.9 ng/g)] using a Mann–Whitney U-test (p = 0.006). Within the AMI patients, there was no difference in hair cortisol levels between the first 1.5-cm and the second 1.5-cm samples (p = 0.48, Mann–Whitney U-test). When we divided the entire study population into quartiles according to the hair cortisol concentrations, the occurrence of acute MI (in %) increased with hair cortisol concentration, escalating from 32 to 68% from the first (lowest hair cortisol levels) to fourth quartiles (highest hair cortisol levels; ; p < 0.01, Fisher's exact test).
Figure 1. The proportions of case (acute MI) and control (no acute MI) patients per quartile of hair cortisol concentration (x-axis; ng of cortisol/g of hair) are displayed in percentage (y-axis). Patients were divided into quartiles according to increasing hair cortisol content. This resulted in four quartiles of 28 participants each. The range of cortisol for each quartile is as follows: first (76.6–195.6 ng/g), second (197.0–252.9 ng/g), third (254.4–358.3 ng/g), and fourth (359.6–949.9 ng/g; p < 0.01, Fisher's exact test).
We further conducted a multivariate analysis using multivariate logistic regression. The model included determinants proven to affect the risk for AMI, including age, LDL and HDL cholesterol, BMI, smoking status, previous MI, and log-transformed hair cortisol. Only hair cortisol concentrations (OR 17.4, 95% CI 2.15–140.45; p = 0.007) correlated with AMI.
Discussion
While the role of acute stress in the development of AMI is well established, data regarding the causative role of chronic stress have been less clear. As such information is based on retrospective assessment of stress over several months prior to the acute cardiac event, it is subject to recall and reporting bias.
Hair cortisol content has emerged as a promising biological marker of chronic systemic exposure to this corticosteroid hormone (Kalra et al. Citation2007; Sauvé et al. Citation2007; Yamada et al. Citation2007; Van Uum et al. Citation2008; Kirschbaum et al. Citation2009; Thomson et al. Citation2010). Unlike saliva, blood, and urine cortisol measurements, the hair technique provides a novel method for the quantification of longitudinal accumulation of cortisol over time and, therefore, a possible biological marker for chronic stress. Indeed, several groups have recently shown that hair cortisol levels are higher in patients with Cushing's syndrome (Thomson et al. Citation2010) and in chronic stress secondary to pain (Van Uum et al. Citation2008), mechanical ventilation (Yamada et al. Citation2007), and emotional strain (Kalra et al. Citation2007), as well as in an animal model in which rhesus monkeys were exposed to relocation stress (Davenport et al. Citation2008).
The present study documents the first use of this novel test in evaluating the role of chronic stress in the etiology of AMI. We demonstrated higher hair cortisol levels in the AMI patients compared to those in consecutive patients hospitalized to an internal medicine ward for other causes. The latter were chosen to comprise the control group, since hair cortisol levels are not influenced by the stress associated with the index admission itself or in the previous few days (Pragst and Balikova Citation2006), and due to their risk factor profile that resembles the AMI patients. While the prevalence of diabetes, hypertension, smoking and family history of CAD did not differ significantly between the AMI and control group, LDL-cholesterol levels and BMI were higher, and HDL cholesterol and age were lower in the AMI group. After controlling for the known risk factors for AMI by multiple logistic regressions, hair cortisol content emerged as the strongest predictor of AMI. This highlights the potential of hair cortisol as a biological marker of chronic stress and increased cardiovascular risk. Our findings are in line with the results of the recent study by CitationReynolds et al. (2010a), who found an association between elevated fasting plasma cortisol levels and risk for ischemic heart disease. Together with the Caerphilly study (Smith et al. Citation2005), these observations indicate that cortisol levels are elevated before the clinical manifestation of coronary heart disease, supporting the hypothesis that activation of the HPA-axis may be an important pathway via which increased stress results in increased risk for CAD.
Importantly, as hair grows on an average of 1 cm per month (Hayashi et al. Citation1991; D'Amico et al. Citation2001), our measurements in 3 cm of hair provide information over a period of about 3 months prior to the hospital admission. Furthermore, we did not find any difference in hair cortisol levels between the first and second 1.5-cm samples, which supports the notion that hair cortisol levels are a marker of chronic rather than acute stress.
Possible mechanisms
The pathophysiological mechanisms underlying the relationship between the increased risk for CAD and chronic stress are probably multi-factorial and can be divided into behavioral and physiological mechanisms. Various psychosocial conditions causing increased chronic stress were found to be associated with a higher frequency of adverse health behaviors such as poor diet, smoking, or lack of adherence to medical therapy (Eaker et al. Citation1993; Rozanski et al. Citation1999; Kornitzer et al. Citation2006). Chronic stress, assessed by measures of subjective stress, cortisol levels in plasma, serum, saliva, and/or urine or β1-adrenoceptor activation, has been demonstrated to involve an excessive activation of the HPA-axis and of the sympathetic nervous system (Rozanski et al. Citation1999; Brotman et al. Citation2007). The activation of these two pathways has been shown to be associated with accelerated atherosclerosis (Troxler et al. Citation1977; Kaplan et al. Citation1983, Citation1987; Rozanski et al. Citation1999; Brotman et al. Citation2007), increased endothelial dysfunction (Pettersson et al. Citation1990; Skantze et al. Citation1998; Hizume et al. Citation2006), platelet activation (Markovitz Citation1998; Rozanski et al. Citation1999; Brotman et al. Citation2007), accentuated inflammation of the arterial wall (Sajadieh et al. Citation2004; Cohn and Colucci Citation2006; Nijm et al. Citation2007), and subsequently the formation of thrombosis (Troxler et al. Citation1977; Kaplan et al. Citation1983, Citation1987; Rozanski et al. Citation1999; Brotman et al. Citation2007).
Increased levels of cortisol in the saliva and urine have been associated with the development of the metabolic syndrome (Brunner et al. Citation2002) including obesity, hypertension, dyslipidemia, and dysglycemia, which are all associated with increased cardiovascular risk. Although we found hair cortisol levels to be an independent predictor for AMI after controlling for the conventional risk factors of CVD, it is still reasonable that these abnormalities may contribute to the increased cardiovascular risk associated with chronic stress.
Except for age, LDL, HDL, and BMI, all other known risk factors of atherosclerosis, including smoking, diabetes mellitus, and hypertension, were not different between the AMI and the control group. These similar risk factor profiles suggest that an additional factor (such as chronic stress) may be required to induce an acute event.
Clinical implications
Our results may have several implications for both clinical management and research. The high impact of chronic stress, as measured by hair cortisol levels, on the evolution of AMI supports previous reports in which chronic stress is associated with increased cardiovascular risk (Rozanski et al. Citation1999; Rosengren et al. Citation2004; Kornitzer et al. Citation2006; Aboa-Eboulé et al. Citation2007). Since chronic psychosocial stress may be subjected to clinical modification, its contribution to the underlying development of coronary disease might potentially be reduced by interventions designed to modify it. Evidence to support this hypothesis has been limited by lack of adequate clinical studies. One of the possible explanations may be the absence of a reliable model for quantitative assessment of chronic stress. As hair grows on average 1 cm per month, this novel method may serve as a sensitive biomarker for changes in stress levels secondary to interventions.
Furthermore, the measurement of high levels of hair cortisol may help in identifying patients at high risk for future cardiovascular events. It is possible that such patients may benefit more from an intensive assessment and treatment of the existing conventional cardiovascular risk factors and from lifestyle modifications, such as smoking cessation and physical exercise. Finally, the finding that hair cortisol content has emerged as the strongest predictor of AMI should highlight the possibly tremendous role of chronic stress, which is often overlooked by physicians, as a cardiovascular risk factor.
Limitations
Several limitations of this study warrant consideration. Our analysis pertains only to males. Due to the obvious hormonal differences between men and women, and since atherosclerosis may be differentially affected based on sex, our results should not be applied to females.
Only 112 patients, divided equally between the AMI and control group, were included in the study. The finding that despite a relatively small sample size, hair cortisol levels were significantly higher in the AMI group than in the controls after controlling for confounders, provides further support to the importance of chronic stress as a risk factor for AMI. This will have to be corroborated in larger studies.
In addition, there are limitations to the measurements of cortisol in hair. Obviously, it can only be done in patients with sufficient length of hair and is potentially subject to contamination by cortisol-containing creams. Furthermore, hair cortisol cannot be used to study dynamic impacts of, for example, morning cortisol awakening response. While the average hair growth in males is 1 cm per month (0.35 mm per day; Hayashi et al. Citation1991; D'Amico et al. Citation2001), it may be affected by environmental, ethnic, and nutritional factors. Nevertheless, these effects were reported to be mild (Hayashi et al. Citation1991; D'Amico et al. Citation2001; Loussouarn Citation2001) and do not alter the presumption that hair cortisol measurements reflect the accumulation of the hormone over a long period of time.
An acute MI may be preceded by escalating cardiac ischemia in the days to weeks before the MI, and, thus, may have caused stress. Therefore, we cannot exclude that such ischemia-induced stress caused elevation of hair cortisol content, rather the reverse (stress causing ischemia). However, within the AMI patients, there was no difference in hair cortisol levels between the first 1.5-cm and the second 1.5-cm samples. These findings support the notion that hair cortisol levels are a marker of chronic rather than acute stress and cannot be explained solely by several weeks of preceding anginal symptoms. Furthermore, most of our control patients presented to the hospital with a syndrome that included chest pain. Their discharge diagnoses indicate the presence of disease that also may have caused stress during the weeks before admission.
Our study did not include a behavioral stress scale questionnaire for the assessment of psychosocial stress. We have previously shown that hair cortisol levels correlate positively and significantly with measures of perceived stress (Kalra et al. Citation2007; Van Uum et al. Citation2008). However, it would be important for future studies to include the use of such a questionnaire to determine if a similar correlation exists within this patient group. While we consider that biological quantification of stress using hair cortisol measurements should be superior to retrospective assessment using stress scales (since the latter is subjected to recall and reporting bias), further information is needed. As our results suggest that the AMI patients have greater stress than controls (as measured by hair cortisol content), which may have contributed to their prognosis, it would be valuable to understand whether this stress was of a physical or emotional nature. Future work will need to address this issue using a perceived stress questionnaire.
While the current study indicates that the overall degree of chronic stress, as assessed by hair cortisol levels, was higher in the MI group than in the control group, this study does not address the question whether, within the group of patients at high risk for an MI, the degree of stress would be associated with an increased risk to actually develop an MI. We suggest that this should be addressed in a separate study that compares hair cortisol levels in patients with chronic CAD who did not develop an MI with hair cortisol levels in patients with similar established risk factors who did develop an MI. Moreover, a new study would also need to include women.
Other potential factors that may affect hair cortisol content include sleep deprivation and disruptions of circadian rhythm. As cortisol is secreted on a diurnal rhythm, with higher levels in the morning and lower levels in the afternoon and evening, a disrupted sleep pattern can upset the normal functioning of this rhythm (Van Cauter et al. Citation2007), and may cause excessive cortisol secretion. Research describes the association with sleep loss and the increased risk of obesity and diabetes, and cortisol has been suggested as a pathophysiological intermediary factor (Knutston et al. Citation2007; Van Cauter et al. Citation2008). We did not measure sleep patterns of the subjects, and the effect of sleep disruption on hair cortisol content is currently not known. Furthermore, depressive illness is associated with elevated cortisol production and increased risk of coronary heart disease (Van der Kooy et al. Citation2007), and is, therefore, also a potential confounding factor. The role of these various factors will also need to be addressed in future studies.
In conclusion, hair cortisol concentrations were found to be elevated in the 3 months prior to the event in patients admitted with AMI than in controls. This indicates that similar to acute stress, chronic stress may be a significant contributing factor for AMI, and that chronic elevation in cortisol levels may contribute to its development. Hair cortisol measurements can be used to identify patients at high risk for AMI who may benefit from strategies targeted to manage chronic stress, and as an impetus for more aggressive treatment of other modifiable risk factors.
Acknowledgment
Dr. D Pereg and Miss R Gow share first (leading) authorship. We would like to acknowledge Physicians' Services Incorporated Foundation for supporting this study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aboa-Eboulé C, Brisson C, Maunsell E, Mâsse B, Bourbonnais R, Vézina M, Milot A, Théroux P, Dagenais GR. 2007. Job strain and risk of acute recurrent coronary heart disease events. J Am Med Assoc. 298:1652–1660.
- Adair R, Kasahara M. 1980. Serum cortisol response to acute myocardial infarction in the aged. J Am Geriatr Soc. 28:472–474.
- Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. 2000. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 343:1355–1361.
- Brotman D, Golden SH, Wittstein IS. 2007. The cardiovascular toll of stress. Lancet. 370:1089–1098.
- Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, Shipley MJ, Kumari M, Andrew R, Seckl JR, Papadopoulos A, Checkley S, Rumley A, Lowe GD, Stansfeld SA, Marmot MG. 2002. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: Nested case-control study. Circulation. 106:2659–2665.
- Chandola T, Brunner E, Marmot M. 2006. Chronic stress at work and the metabolic syndrome: Prospective study. Br Med J. 332 7540: 521–525.
- Chida Y, Steptoe A. 2010. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension. 55 4: 1026–1032.
- Cohn JN, Colucci W. 2006. Cardiovascular effects of aldosterone and post-acute myocardial infarction pathophysiology. Am J Cardiol. 97:4F–12F.
- Cone EJ. 1996. Mechanisms of drug incorporation into hair. Ther Drug Monit. 18 4: 438–443.
- D'Amico D, Vaccaro M, Guarneri F, Borgia F, Cannavo S, Guarneri B. 2001. Phototrichogram using videomicroscopy: A useful technique in the evaluation of scalp hair. Eur J Dermatol. 11:17–20.
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. 2006. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol. 147:255–261.
- Davenport MD, Lutz CK, Tiefenbacher S, Novak MA, Meyer JS. 2008. A rhesus monkey model of self-injury: Effects of relocation stress on behavior and neuroendocrine function. Biol Psychiatry. 63:990–996.
- Dimsdale JE. 2008. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 51:1237–1246.
- Eaker ED, Chesebro JH, Sacks FM, Wenger NK, Whisnant JP, Winston M. 1993. Cardiovascular disease in women. Circulation. 88:1999–2009.
- Eaker ED, Sullivan LM, Kelly-Hayes M, D'Agostino RBSr, Benjamin EJ. 2004. Does job strain increase the risk for coronary heart disease or death in men and women? The Framingham offspring study. Am J Epidemiol. 159:950–958.
- Gerin W, Chaplin W, Schwartz JE, Holland J, Alter R, Wheeler R, Duong D, Pickering TG. 2005. Sustained blood pressure increase after an acute stressor: The effects of the 11 September 2001 attack on the New York City World Trade Center. J Hypertens. 23 2: 279–284.
- Hayashi S, Miyamoto I, Takeda K. 1991. Measurement of human hair growth by optical microscopy and image analysis. Br J Dermatol. 125:123–129.
- Henderson GL. 1993. Mechanisms of dug incorporation into hair. Forensic Sci Int. 63:19–29.
- Hizume T, Morikawa K, Takaki A, Abe K, Sunagawa K, Amano M, Kaibuchi K, Kubo C, Shimokawa H. 2006. Sustained elevation of serum cortisol level causes sensitization of coronary vasoconstricting responses in pigs in vivo: A possible link between stress and coronary vasospasm. Circ Res. 99:767–775.
- Hlatky MA, Lam LC, Lee KL, Clapp-Channing NE, Williams RB, Pryor DB, Califf RM, Mark DB. 1995. Job strain and the prevalence and outcome of coronary artery disease. Circulation. 92:327–333.
- Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. 2007. The relationship between stress and hair cortisol in healthy pregnant women. Clin Invest Med. 30:E103–E107.
- Kaplan JR, Manuck SB, Adams MR, Weingand KW, Clarkson TB. 1987. Inhibition of coronary atherosclerosis by propranolol in behaviorally predisposed monkeys fed an atherogenic diet. Circulation. 76:1364–1372.
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM, Miller EW. 1983. Social stress and atherosclerosis in normocholesterolemic monkeys. Science. 220:733–735.
- Kario K, James GD, Marion R, Ahmed M, Pickering TG. 2002. The influence of work- and home-related stress on the levels and diurnal variation of ambulatory blood pressure and neurohumoral factors in employed women. Hypertens Res. 25 4: 499–506.
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. 2009. Hair as a retrospective calendar of cortisol production – increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 34:32–37.
- Knutston K, Spiegel K, Penev P, Van Cauter E. 2007. The metabolic consequences of sleep deprivation. Sleep Med Rev. 11:163–178.
- Kornitzer M, deSmet P, Sans S, Dramaix M, Boulenguez C, DeBacker G, Ferrario M, Houtman I, Isacsson SO, Ostergren PO, Peres I, Pelfrene E, Romon M, Rosengren A, Cesana G, Wilhelmsen L. 2006. Job stress and major coronary events: Results from the job stress, absenteeism and coronary heart disease in Europe study. Eur J Cardiovasc Prev Rehabil. 13:695–704.
- Lee S, Colditz G, Berkman L, Kawachi I. 2002. A prospective study of job strain and coronary heart disease in US women. Int J Epidemiol. 31:1147–1153.
- Leor J, Poole WK, Kloner RA. 1996. Sudden cardiac death triggered by an earthquake. N Engl J Med. 334:413–419.
- Loussouarn G. 2001. African hair growth parameters. Br J Dermatol. 145:294–297.
- Markovitz JH. 1998. Hostility is associated with increased platelet activation in coronary heart disease. Psychosom Med. 60:586–591.
- Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. 1993. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of myocardial infarction onset study investigators. N Engl J Med. 329:1677–1683.
- Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, Friedman R, Benson H, Muller JE. 1995. Triggering of acute myocardial infarction onset by episodes of anger. Determinants of myocardial infarction onset study investigators. Circulation. 92:1720–1725.
- Nijm J, Kristenson M, Olsson AG, Jonasson L. 2007. Impaired cortisol response to acute stressors in patients with coronary disease. Implications for inflammatory activity. J Intern Med. 262:375–384.
- Pettersson K, Bejne B, Björk H, Strawn WB, Bondjers G. 1990. Experimental sympathetic activation causes endothelial injury in the rabbit thoracic aorta via beta 1-adrenoceptor activation. Circ Res. 67:1027–1034.
- Pragst F, Balikova MA. 2006. State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta. 370:17–49.
- Reynolds RM, Labad J, Strachan MW, Braun A, Fowkes FG, Lee AJ, Frier BM, Seckl JR, Walker BR, Price JF. Elevated fasting plasma cortisol is associated with ischemic heart disease and its risk factors in people with type 2 diabetes: The Edinburgh type 2 diabetes study. J Clin Endocrinol Metab. 2010a; 95 4: 1602–1608.
- Reynolds RM, Strachan MW, Labad J, Lee AJ, Frier BM, Fowkes FG, Mitchell R, Seckl JR, Deary IJ, Walker BR, Price JF. Morning cortisol levels and cognitive abilities in people with type 2 diabetes: The Edinburgh type 2 diabetes study. Diabet Care. 2010b; 33:714–720.
- Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S. 2004. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): Case-control study. Lancet. 364:953–962.
- Rozanski A, Blumenthal JA, Kaplan J. 1999. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 99:2192–2217.
- Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. 2004. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 25:363–370.
- Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SH. 2007. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. 30:E183–E191.
- Skantze HB, Kaplan J, Pettersson K, Manuck S, Blomqvist N, Kyes R, Williams K, Bondjers G. 1998. Psychosocial stress causes endothelial injury in cynomolgus monkeys via beta1-adrenoceptor activation. Atherosclerosis. 136:153–161.
- Smith GD, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, Elwood P. 2005. Cortisol, testosterone, and coronary heart disease – prospective evidence from the caerphilly study. Circulation. 112:332–340.
- Steptoe A, Siegrist J, Kirschbaum C, Marmot M. 2004. Effort-reward imbalance, overcommitment, and measures of cortisol and blood pressure over the working day. Psychosomatic Med. 66:323–329.
- Strike PC, Perkins-Porras L, Whitehead DL, McEwan J, Steptoe A. 2006. Triggering of acute coronary syndromes by physical exertion and anger: Clinical and sociodemographic characteristics. Heart. 92:1035–1040.
- Thomson S, Koren G, Fraser LA, Rieder M, Friedman TC, Van Uum S. 2010. Hair analysis provides a historical record of cortisol levels in Cushing's syndrome. Exp Clin Endocrinol Diabet. 118 2: 133–138.
- Troxler RG, Sprague EA, Albanese RA, Fuchs R, Thompson AJ. 1977. The association of elevated plasma cortisol and early atherosclerosis as demonstrated by coronary angiography. Atherosclerosis. 26:151–162.
- Van Cauter E, Holmback U, Knutson K, Leproult R, Miller A, Nedeltcheva A, Pannain S, Penev P, Tasali E, Spiegel K. 2007. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 67 Suppl 1: 2–9.
- Van Cauter E, Spiegel K, Tasali E, Leproult R. 2008. Metabolic consequences of sleep and sleep loss. Sleep Med. 9 Suppl 1: S23–S28.
- Van der Kooy K, van Hout H, Marwiik H, Marten H, Stehouwer C, Beekman A. 2007. Depression and the risk for cardiovascular diseases: Systemic review and meta analysis. Int J Geriatr Psychiatry. 22 7: 613–626.
- Van Uum SH, Sauvé B, Fraser LA, Morley-Forster P, Paul TL, Koren G. 2008. Elevated content of cortisol in hair of patients with severe chronic pain: A novel biomarker for stress. Stress. 11:483–488.
- Webb E, Thomson S, Nelson A, White C, Koren G, Rieder M, Van Uum S. 2010. Assessing individual systemic stress through cortisol analysis of archaeological hair. J Archaeol Sci. 37:807–812.
- Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, Newman C, Koren G. 2007. Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology. 92:42–49.
- Zouaghi H, Savu L, Guerot C, Gryman R, Coulon A, Nunez EA. 1985. Total and unbound cortisol-, progesterone-, oestrone- and transcortin-binding activities in sera from patients with myocardial infarction: Evidence for differential responses of good and bad prognostic cases. Eur J Clin Invest. 15:365–370.
