Abstract
Gestational stress may have lasting effects on the physical and neurocognitive development of offspring. The mechanisms that may underlie these effects are of interest. Progesterone and its 5α-reduced metabolites, dihydroprogesterone and 5α-pregnan-3α-ol-20-one (3α,5α-THP), maintain pregnancy, have neurotrophic effects, and can enhance cognitive performance. We hypothesized that some of the deleterious effects of gestational stress on the cognitive performance of offspring may be related to progestogen formation. Pregnant rat dams were exposed to restraint under a bright light (thrice daily for 45 min) on gestational days 17–21 or were minimally handled controls. Dams that were exposed to restraint had lower circulating levels of 3α,5α-THP and significantly greater concentrations of corticosterone at the time of birth than did control dams. Male and female offspring, that were gestationally stressed or not, were cross-fostered to non-manipulated dams. Between postnatal days 28–30, offspring were assessed for object recognition, a prefrontal cortex (PFC)-dependent cognitive task. Restraint-exposed offspring performed more poorly in the object recognition task than did control offspring, irrespective of sex. As well, progesterone turnover to its 5α-reduced metabolites in the medial PFC (but not the diencephalon) was significantly reduced among restraint-exposed, compared to control, offspring. Progesterone turnover, and levels of 3α,5α-THP, positively correlated with performance in the object recognition task. Thus, restraint stress in late pregnancy impaired cognitive development and dysregulated progestogen formation in brain.
Introduction
Pregnant women may be exposed to a variety of environmental, physical, and/or psychological stressors, and it is important to understand the implications of these experiences on offspring. Prospective and retrospective studies suggest that adverse life events during pregnancy, including physical abuse, infection, risk taking, poverty, self-reported anxiety, and/or poor coping skills are associated with low birth weight and/or preterm delivery (Knipschild et al. Citation1981; Done et al. Citation1991; Mutale et al. Citation1991; Steer et al. Citation1992; Hedegaard et al. Citation1993; Wadhwa et al. Citation1993; Orr et al. Citation1996; Zambrana et al. Citation1999; Facchinetti et al. Citation2008; Glynn et al. Citation2008; Pearce et al. Citation2008; Rodrigues and Barros Citation2008; Rodrigues et al. Citation2008). While low birth weight and preterm delivery are major determinants of infant morbidity (McCormick Citation1985; Newton Citation1986; Hakulinen et al. Citation1988; van Zeben-van der Aa et al. Citation1989), it is also important to understand the implications of prenatal stress exposure on surviving infants. Children born under these circumstances are at risk for neurological and behavioral aberrations (Hadders-Algra et al. Citation1988; Holst et al. Citation1989), as well as impairment of cognitive skills (Brouwers et al. Citation2001; Weinstock Citation2008).
Many physical challenges could directly influence the gestating fetus; however, negative birth outcomes associated with psychological stressors imply that endogenous factors may also be important. Irrespective of the type of stress experienced, nearly all stressors elicit a ubiquitous, hypothalamic–pituitary–adrenal (HPA) axis response that is generally characterized by production of corticotropin-releasing hormone (CRH) in the hypothalamus, which promotes adrenocorticotropic hormone (ACTH) secretion from the pituitary into circulation. ACTH activates the production of glucocorticoid (primarily cortisol in people and corticosterone in most rodents) from the adrenals. Glucocorticoids can then feed back through the circulation to act on the hypothalamus and pituitary to downregulate CRH and ACTH production. During human pregnancy, inappropriate fetal exposure to endogenous HPA factors may be harmful. Gestational stress activates the maternal HPA axis and can increase placental CRH (Petraglia et al. Citation1996). In rodents, gestational stress can result in aberrant HPA axis response in adulthood, characterized by greater and more prolonged elevations of plasma ACTH and/or corticosterone (Weinstock Citation2001; Fan et al. Citation2009; Louvart et al. Citation2009). Furthermore, rats that are exposed to gestational stress can have adrenal hypertrophy, which may be the result of chronic overstimulation of the adrenal gland by ACTH (Ward et al. Citation2000; Fan et al. Citation2009). Thus, endogenous stress factors may contribute to aberrations that are associated with prenatal stress.
One common mechanism that is associated with both the response to stress and maintenance of pregnancy is formation of the neuroactive progesterone metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP, allopregnanolone). Both female and male rats demonstrate rapid increases in the central production of 3α,5α-THP in response to forced swim, foot shock, CO2 inhalation, or social stress (Purdy et al. Citation1991; Barbaccia et al. Citation2001; Frye et al. Citation2007a). This rapid response occurs independently from peripheral glands, indicating that these increases in 3α,5α-THP are due to neurosteroid formation in neural cells (Frye Citation2001; Nguyen et al. Citation2004). In the brain, cholesterol precursor can easily diffuse through cell membranes and is readily converted to pregnenolone via sequential actions of p450 side chain cleavage enzyme and steroid acute regulatory protein. Once formed, pregnenolone serves as a precursor to progesterone (P) which can be 5α-reduced to form dihydroprogesterone (DHP). DHP can be further reduced by the 3α-hydroxysteroid dehydrogenase enzyme to form 3α,5α-THP. Regulation of 3α,5α-THP formation may be critical given that 3α,5α-THP levels rise throughout pregnancy in women and in rats and decline coincident with parturition (Concas et al. Citation1998; Gilbert-Evans et al. Citation2005). Notably, the stress response is observed to be attenuated at the end of pregnancy when 3α,5α-THP levels are most elevated (Neumann et al. Citation1998; Brunton and Russell Citation2008; Paris and Frye Citation2008). As such, 3α,5α-THP may play an important role in mediating stress effects during pregnancy, which can have implications for offspring development.
The present investigation examined the effects of a physical/psychological stressor, late in gestation (gestational days 17–21), on the cognitive and neuroendocrine profile of male and female rat offspring, with a focus on the medial prefrontal cortex (mPFC). Prefrontal cortex (PFC) may be an important region for stress-related cognitive deficits. Exposure to unpredictable stressors on gestational days 15–20 reduces dendritic spine density in the dorsal anterior cingulate and orbitofrontal cortical regions of male and female offspring (Murmu et al. Citation2006). As well, maternal restraint, or corticosterone injection, on gestational days 14–21 upregulates the expression of regenerative factors in response to a PFC insult among juvenile offspring (Jutapakdeegul et al. Citation2010). Yet, the cognitive effects of gestational stress on the PFC have been studied less than those on the hippocampus. Stressors administered late in gestation may have particularly salient teratogenic effects, given that the expression of placental 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which inactivates maternal corticosterone prior to fetal exposure, is reduced (Seckl Citation1997). Glucocorticoid exposure at this time can have deleterious effects on offspring (Frye and Orecki Citation2002a,Citationb; Setiawan et al. Citation2007; Kapoor et al. Citation2008, Citation2009). As such, dams were exposed to minimal handling (daily weighing to monitor weight throughout gestation), with or without exposure to thrice daily restraint stress for 45 min on gestational days 17–21. Given that pup-rearing strategies can lead to changes in the HPA function of offspring (Fish et al. Citation2004; Meaney and Szyf Citation2005), all pups in the present study were cross-fostered to non-perturbed dams and were assessed for cognitive performance on an object recognition task when juvenile (between 28 and 30 days of age). Following testing, the mPFC and diencephalon (a control region that is important for the stress response but is not expected to be involved in object recognition) were assessed for concentrations of progestogens (P, DHP, 3α,5α-THP) and estradiol (E2). In order to assess prenatal hormone exposure, circulating corticosterone, progestogens, and E2 were also assessed in plasma taken from dams following parturition. We anticipated that juvenile offspring that were prenatally exposed to restraint would have poorer performance on the cognitive task and show dysregulation of the progestogen milieu in brain compared to control offspring. Additionally, we anticipated greater circulatory corticosterone and/or dysregulated progestogen formation among restraint-exposed dams, compared to control dams, following parturition.
Methods
Subjects and housing
These methods were pre-approved by the Institutional Animal Care and Use Committee at The University at Albany-SUNY and were conducted in accordance with ethical guidelines defined by the National Institutes of Health (NIH Publication No. 85-23). Female, timed-pregnant, Long Evans rats (N = 16), that were approximately 55 days old, were obtained from Taconic Farms (Germantown, NY, USA) and weighed daily. Dams were randomly assigned to be exposed to restraint stress (thrice daily for 45 min; n = 8) or to not be further manipulated (n = 8) from gestational days 17 to 21. Given that differences in maternal care can alter subsequent HPA function among offspring (Fish et al. Citation2004; Meaney and Szyf Citation2005), it was important to equate restraint-exposed and control pups in their experience of early-life rearing by dams. To achieve this, a cross-fostering strategy was employed. Following parturition, the number of pups per litter was noted and litters were culled such that all dams had three to four pups represented in the study. Specifically, eight dams (four control and four restrained) were left with two male and two female pups, four dams (two control and two restrained) were left with two male pups and one female pup, and four dams (two control and two restrained) were left with one male pup and two female pups. Pups (28 males and 28 females) were removed from control and restrained dams and cross-fostered to non-manipulated dams in our colony in the Laboratory Animal Care Facility at The University at Albany-SUNY, Life Sciences Research Building (Albany, NY, USA). With cross-fostered pups, and their own litters to care for, each non-manipulated dam had 16 pups to rear. Cross-fostered pups (identified by an ear notch) were reared in the homecages of the non-manipulated dams until weaning at 21 days of age (one gestationally stressed male died prior to weaning). Once weaned, juvenile offspring were housed in same-sex groups, six per cage (with the exceptions of one male cage that housed only three rats and one female cage that housed only four rats), in polycarbonate cages (45 × 24 × 21 cm) in a temperature-controlled room (21 ± 1°C) in our colony. Rats were maintained on a 12/12 h reversed-light cycle (lights off at 08:00 h) with continuous access to Purina Rat Chow and tap water. Offspring were weighed and tested between 28 and 30 days of age.
Restraint paradigm
Dams were placed in a plexiglass restrainer for 45 min, three times a day under a 60-W light from gestational days 17 to 21. We have previously demonstrated this, and similar restraint stress regimen in rodents, to increase HPA response in dams and pups and to alter later 3α,5α-THP-dependent behaviors among offspring, including seizure threshold (Frye and Bayon Citation1999b), reproductive behavior (Frye and Orecki Citation2002a,Citationb), and affective behavior (Walf and Frye Citation2005, Citation2007).
Object recognition
The object recognition task, used herein, assesses memory consolidation and primarily relies on PFC functioning and, to a lesser extent, hippocampal functioning (Ennaceur et al. Citation1997; Broadbent et al. Citation2004). This task was used as modified from previously published methods (Ennaceur and Delacour Citation1988; Frye and Lacey Citation2001 Luine et al. Citation2003) and as described (Walf et al. Citation2006; Paris and Frye Citation2008). During training, rats were placed in a white open field (76 × 57 × 35 cm) in a brightly lit testing room. Rats were allowed 3 min to explore the open field which contained two identical, spheric objects in adjacent corners (plastic toys in the shape of oranges). After training, rats were placed in a dark, sound-dampened room for 4 h. Prior to testing, one of the spheric objects was replaced with a cone-shaped object (a blue plastic toy in the shape of a buoy). Following the 4-h interval, rats were returned to the open field and were allowed to explore for 3 min. Although rats did not show a bias toward exploring objects on the right versus the left side of the open field, placement of the novel object was counterbalanced across treatment groups and testing sessions to eliminate any possible effects of side preference. A greater percentage of time spent exploring the novel object, as a function of the total amount of time spent exploring both objects during testing [duration spent with novel object/(duration spent with novel object+duration spent with familiar object) × 100], is considered an index of enhanced cognitive performance in this task and was used for statistical analyses ().
Table I. Depiction of the amount of time spent exploring objects in the object recognition task among juvenile rats (28–30 days of age) exposed to minimal daily handling (control) or thrice daily episodes of 45-min restraint (restraint) on gestational days 17–21.
Radioimmunassay
Dam plasma collection
For dams, plasma was collected 30 min following parturition of pups and was stored at − 20°C for later assessment of corticosterone, E2, P, DHP, and 3α,5α-THP.
Offspring tissue collection
For offspring, whole brains were collected immediately following behavioral testing between 28 and 30 days of age. Brains were rapidly frozen on dry ice and stored at − 80°C for later assessment of E2, P, DHP, and 3α,5α-THP.
Tissue preparation
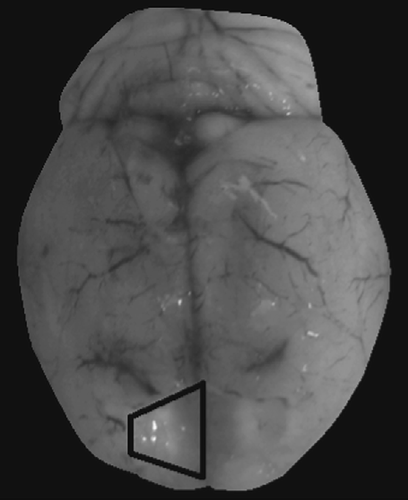
At the time of radioimmunoassay, plasma and brains were slowly thawed on ice, and mPFC was grossly dissected from brains as modified from previous methods (Dunnett et al. Citation1987). Briefly, the mPFC (including aspects of the anterior cingulate and posterior PFC) was extracted in a section that was rostral to the frontal pole, caudal and ventral to the genu of the corpus callosum, and lateral to the minor forceps of the corpus callosum (). As well, diencephalon was grossly dissected as a control region as previously described (Frye et al. Citation2007a). Concentrations of central steroids were assessed, modified from previously reported methods, as described below (Frye et al. Citation1998; Choi and Dallman Citation1999; Frye and Bayon Citation1999a).
Radioactive probes
[3H] E2 (NET-317: specific activity = 51.3 Ci/mmol), [3H] P (NET-208: specific activity = 47.5 Ci/mmol), and [3H] 3α,5α-THP (used for DHP and 3α,5α-THP, NET-1047: specific activity = 65.0 Ci/mmol) were purchased from Perkin-Elmer (Boston, MA, USA).
Extraction of steroids from brain tissues
In serum, corticosterone was extracted by heating at 60°C for 30 min (Choi and Dallman Citation1999). E2, P, DHP, and 3α,5α-THP were extracted with ether following incubation with water and 800 cpms of [3H] steroid (Frye and Bayon Citation1999a,Citationb). Test tubes containing steroid and ether were snap frozen twice and evaporated to dryness. Dried down tubes were reconstituted with phosphate assay buffer to the original serum volume.
In cortical tissues, E2, P, DHP, and 3α,5α-THP were extracted following homogenization with a glass/glass homogenizer in 50% MeOH, 1% acetic acid. Tissues were centrifuged at 3,000 × g, and the supernatant was chromatographed on Sep-Pak C18 cartridges (Waters Corp., Milford, MA, USA). Steroids were eluted with increasing concentrations of MeOH (50% MeOH followed by 100% MeOH) and solvents were removed using a speed drier. Samples were reconstituted in 500 μl assay buffer.
Setup and incubation of radioimmunoassays
The range of the standard curves was 0–4 ng for corticosterone, 0–1000 pg for E2, and 0–8000 pg for P, DHP, and 3α,5α-THP. Standards were added to assay buffer followed by the addition of the appropriate antibody (described below) and [3H] steroid. Total assay volumes were 800 μl for E2 and P, 950 μl for DHP, and 1250 μl for 3α,5α-THP. All assays were incubated overnight at 4°C except for corticosterone which was incubated for 60 min at room temperature.
Antibodies
The corticosterone antibody (#B3-163, Endocrine Sciences), which typically binds 40–60% of [3H] corticosterone was used in a 1:20,000 dilution. This corticosterone antibody has little cross-reactivity with deoxycorticosterone (4%) but negligible ( < 1%) cross-reactivity with other steroid hormones, including cortisol, aldosterone, and P (McCormick et al. Citation2005). The E2 antibody (E#244, Dr G.D. Niswender, Colorado State University, Fort Collins, CO), which generally binds between 40 and 60% of [3H] E2, was used in a 1:40,000 dilution. This E2 antibody has negligible ( < 1%) cross-reactivity with other steroid hormones, including estrone, 17α-estradiol, P, and 17-hydroxyprogesterone (England et al. Citation1974). The P antibody (P#337 from Dr G.D. Niswender, Colorado State University) used in a 1:30,000 dilution typically binds between 30 and 50% of [3H] P. This P antibody has very low levels ( < 4%) of cross-reactivity with DHP and 3α,5α-THP (Niswender Citation1973). The DHP (X-947) and 3α,5α-THP antibodies (#921412-5, purchased from Dr Robert Purdy, Veterans Medical Affairs, La Jolla, CA) were used in a 1:5000 dilution and typically bind between 40 and 60% of [3H] 3α,5α-THP. The DHP antibody cross-reacts with 3α,5α-THP (100%), 5α-pregnan-3,20-dione (50%), 4-pregnen-3α-ol-20-one (50%), and P (17%; Purdy et al. Citation1990). The 3α,5α-THP antibody cross-reacts with 3α-hydroxypregn-4en-20-one (84%) and DHP (11%), and its β-isomer (7%), P (6%), and pregnenolone ( < 2%; Purdy et al. Citation1990; Finn and Gee Citation1994).
Termination of binding
Separation of bound and free steroid was accomplished by the rapid addition of dextran-coated charcoal. Following incubation with charcoal, samples were centrifuged at 3000 × g and the supernatant was pipetted into a glass scintillation vial with 5-ml of scintillation cocktail. Sample tube concentrations were calculated using the logit-log method of Rodbard and Hutt (Citation1974), interpolation of the standards and correction for recovery were done with AssayZap. The inter-assay reliability coefficients were corticosterone 4.1 ± 0.2%, E2 = 6.4 ± 0.2%, P = 9.9 ± 0.3%, DHP = 9.5 ± 0.2%, and 3α,5α-THP = 10.2 ± 0.2%. The intra-assay reliability coefficients were corticosterone = 6.6 ± 0.2%, E2 = 8.2 ± 0.2%, P = 11.0 ± 0.3%, DHP = 9.4 ± 0.3%, and 3α,5α-THP = 10.6 ± 0.3%.
Analyses
Progestogen data were analyzed as raw data and as a ratio of progesterone to its 5α-reduced metabolites [(DHP+3α,5α-THP)/P] per previous methods (Kellogg and Frye Citation1999). Behavioral and endocrine data were analyzed using two-way analyses of variance (sex × dam condition) and were followed up with Fisher's protected least significant differences post hoc tests to determine group differences. Simple linear regressions were performed to assess the amount of variance in object recognition performance that could be explained by concentrations of each steroid measured in dam and offspring tissues. An α level of p < 0.05 was used to determine statistical significance in all analyses.
Results
Gestational stress alters litter size and corticosterone concentrations among dams
Among dams, those exposed to gestational restraint had significantly smaller litters (8 ± 2 pups) compared to those that were minimally handled (12 ± 1 pup) [F(1,14) = 4.69, p < 0.05]. Moreover, circulatory corticosterone was significantly higher [F(1,14) = 5.16, p < 0.05] among those exposed to restraint, compared to those exposed to minimal handling, during GD 17–21 (). Circulatory concentrations of neither E2, P, DHP, 3α,5α-THP nor turnover of P to its 5α-reduced metabolites were significantly different ().
Figure 2. Dams exposed to thrice daily restraint stress on gestational days (GD) 17–21 (restraint; n = 8) had significantly greater concentrations of corticosterone in plasma 30 min following parturition than did dams that were minimally handled on GD 17–21 (control; n = 8). * indicates significant main effect, p < 0.05.
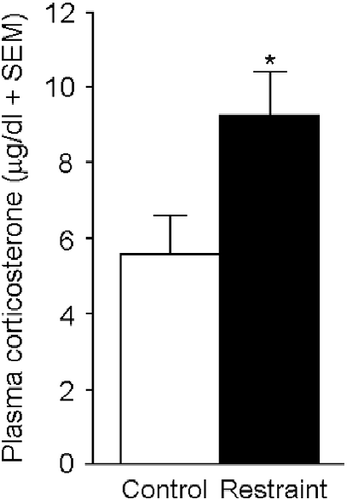
Table II. Depiction of circulatory concentrations of estradiol, progesterone (P), DHP, 3α,5α-THP, and P turnover to its 5α-reduced metabolites among dams exposed to minimal daily handling (control) or thrice daily episodes of 45-min restraint stress (restraint) on gestational days 17–21.
Gestational stress, sex, and dam 3α,5α-THP levels influence P metabolite formation in adolescent offspring
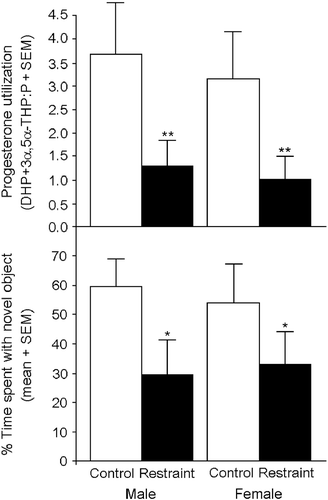
Gestational stress significantly altered P metabolite formation in adolescent offspring. Irrespective of sex, progesterone turnover to its 5α-reduced metabolites in the mPFC was significantly lower among offspring that were exposed to gestational restraint compared to control offspring [F(1,51) = 9.76, p < 0.05] (, top).
Figure 3. Juvenile offspring (28–30 days of age; n = 13–14/group) exposed to gestational restraint (45 min thrice daily on prenatal days 17–21; closed bars) have significantly reduced progesterone turnover to its 5α-reduced metabolites in mPFC than do control offspring (open bars) as indicated by the ratio of DHP and 3α,5α-THP to the pro-hormone, progesterone (P; top). As well, gestationally stressed offspring (closed bars) spend significantly less time investigating a novel object in an object recognition task compared to control offspring (open bars) that were exposed to minimal handling (bottom). * indicates significant main effect for restraint-exposed offspring to perform worse on object recognition compared to control offspring. ** indicates significant main effect for restraint-exposed offspring to have reduced progesterone utilization compared to control offspring, p < 0.05.
As well, the sex of the offspring contributed to the formation of P metabolites in response to gestational stress exposure. Medial PFC DHP concentrations were higher among male, compared to female, offspring and significantly interacted with gestational restraint, such that restraint-exposed males had a greater decrease in DHP than did restraint-exposed females [F(1,51) = 4.13, p < 0.05] ().
Table III. Depiction of concentrations of estradiol, progesterone, DHP, and 3α,5α-THP in the mPFC and the diencephalon of juvenile rats (28–30 days of age) exposed to minimal daily handling (control) or thrice daily episodes of 45-min restraint (restraint) on gestational days 17–21.
Notably, 3α,5α-THP plasma levels of dams, but not other steroids examined, were associated with P metabolite formation in the mPFC of adolescent offspring. Circulating 3α,5α-THP of dams positively correlated with cortical concentrations of DHP [β = 0.11, t(53) = 2.39, p < 0.05] and 3α,5α-THP [β = 0.34, t(53) = 2.03, p < 0.05] of adolescent offspring and accounted for a significant amount of variance within these measures [offspring cortical DHP: R2 = 0.10, F(1,54) = 5.69, p < 0.05; offspring cortical 3α,5α-THP: R2 = 0.07, F(1,54) = 4.10, p < 0.05].
Irrespective of gestational stress, concentrations of E2 were significantly greater in the mPFC [F(1,51) = 14.47, p < 0.05] and diencephalon [F(1,51) = 17.15, p < 0.05] of females compared to males (). No other differences were observed in diencephalon steroid concentrations between groups.
Gestational stress and dam 3α,5α-THP levels influence cognitive phenotype of adolescent offspring
This report presents the first instance wherein we have conducted the object recognition task in juvenile rats. Compared to adult rats that have been observed to investigate objects for 4.7 ± 1.0 s during the training phase and 4.1 ± 0.8 s during the testing phase of the task (Walf et al. Citation2006), we found that juvenile rats spend less time investigating objects during the training (3.1 ± 0.5 s) or testing (1.8 ± 0.6 s) phases. Despite reduced exploration time among juvenile rats, we are readily able to measure the amount of time spent investigating the objects utilizing “ANY-maze” animal tracking software (Stoelting Co., Wood Dale, IL, USA). Notably, the mean difference in the amount of time that treatment groups spent investigating the novel object in the present investigation was 0.6 s (1.3 ± 0.3 s with the novel object among control juveniles versus 0.7 ± 0.3 s with the novel object among gestationally restrained juveniles) which is commensurate with that of past investigations that reported mean differences of 0.8 s (Walf et al. Citation2006).
Gestational stress significantly altered cognitive performance of male and female offspring [F(1,51) = 4.77, p < 0.05]. Irrespective of sex, control offspring spent a significantly greater percentage of time with the novel object than did restraint-exposed offspring (, bottom). In the mPFC, P turnover to its 5α-reduced metabolites (DHP+3α,5α-THP:P ratio; β = 4.49, t(53) = 4.53, p < 0.05) or 3α,5α-THP (β = 5.04, t(53) = 6.16, p < 0.05), but not P or DHP concentrations alone, positively correlated with performance on the object recognition task. Either measure accounted for a significant amount of variance in task performance (P utilization: R2 = 0.08, F(1,54) = 4.81, p < 0.05; 3α,5α-THP: R2 = 0.08, F(1,54) = 4.38, p < 0.05).
In addition to gestational stress exposure, plasma levels of 3α,5α-THP, but not other steroids examined, in dams significantly influenced cognitive performance of adolescent offspring. Dam circulatory 3α,5α-THP positively correlated with offspring performance in the object recognition task (β = 7.97, t(53) = 2.65, p < 0.05) and accounted for a significant proportion of variance in their performance (R2 = 0.12, F(1,54) = 7.00, p < 0.05). At the time of testing, gestational stress did not significantly alter mean subject weight (male = 102 ± 2 g, female = 103 ± 2 g) compared to minimal handling (male = 104 ± 2 g, female = 99 ± 3 g).
Discussion
The present study supported the hypothesis that exposure to a physical/psychological stressor on gestational days 17–21 would alter the formation of progesterone's metabolites and the cognitive performance of offspring. Compared to minimal handling, exposure to gestational stress was associated with significantly increased corticosterone in circulation of parturient dams, concomitant with poorer cognitive performance, and reduced turnover of P to its 5α-reduced metabolites among adolescent offspring. These data support the findings of prior investigations and extend them to elucidate the importance of progestogen formation during gestation.
Harmful effects of gestational stress on cognition may involve actions of maternal glucocorticoids on fetal development. In later gestation, expression of the 11β-HSD2 enzyme (which rapidly metabolizes maternal corticosterone to an inert substance prior to fetal exposure) is reduced. In vivo studies demonstrate that inhibiting 11β-HSD, which increases early exposure of the gestating fetus to glucocorticoids, permanently programs offspring anxiety behavior concomitant with increased glucocorticoid receptor mRNA expression in amygdala (Welberg et al. Citation2000; Seckl and Walker Citation2004). As well, when placental 11β-HSD2 expression is dampened, (as is the case among rats bred for high levels of anxiety) hippocampal neurogenesis is reduced among gestationally stressed offspring (Lucassen et al. Citation2009). Lifelong effects of early stress may partly depend on the timing of exposure during development. In adult rats, a history of stress exposure (up to the first three weeks of life) is associated with greater circulating corticosterone in response to subsequent stressors (Frisone et al. Citation2002; McCormick et al. Citation2002). This effect is not observed when stressors are administered later in development, around the time of puberty (McCormick et al. Citation2005; Toledo-Rodriguez and Sandi Citation2007). These data lend support to a “programming” model wherein the HPA response is perturbed when stressors are administered during prenatal and/or perinatal development. In the present study, we observed that restraint-exposed dams had higher circulating corticosterone levels compared to control dams at the time of parturition, suggesting that gestationally stressed offspring in the present study were exposed to greater levels of maternal glucocorticoid. Notably, maternal concentrations of corticosterone did not predict the neuroendocrine or behavioral profile of offspring; however, litter size was reduced among stressed, compared to control, dams. While differences were not observed among offspring body weights at the time of testing, litter weights prior to culling may have demonstrated differences in response to gestational stress that would be indicative of programing effects on offspring growth trajectory. Future investigations should assess litter birth weights, prior to cross-fostering, as well as circulating corticosterone and/or ACTH among offspring in response to a subsequent stressor.
The mechanisms that underlie stress-induced changes in neuroendocrine milieu and cognitive performance of offspring may involve the formation of progestogens. In later stages of development, P's metabolite, 3α,5α-THP, plays an important role in neural development, maintenance of neural health, and can be neuroprotective (Djebaili et al. Citation2004; Griffin et al. Citation2004; He et al. Citation2004a,Citationb; Mellon et al. Citation2004; Rhodes et al. Citation2004; Ahmad et al. Citation2005; Wang et al. Citation2007, Citation2008). In studies of adult rats, natural or exogenous 3α,5α-THP enhancement improves performance in the object recognition or object placement tasks (Walf et al. Citation2006; Frye et al. Citation2007b; Paris and Frye Citation2008). Here, we find that higher levels of circulating maternal 3α,5α-THP at parturition predicted not only higher DHP and 3α,5α-THP concentrations in the mPFC of offspring later in life but also better cognitive performance on the object recognition task. These data are striking in light of recent findings that reduced P, during the course of gestation, may be associated with re-activation of placental corticosterone, increasing fetal glucocorticoid exposure in utero (Mark et al. Citation2009). Together, these data suggest that progestogen formation may play an important role in regulating fetal glucocorticoid exposure, and that exposure to maternal 3α,5α-THP may influence later progestogen milieu and, consequently, cognitive performance. Notably, sex differences have been observed in past investigations among adult gestationally stressed offspring (Bowman et al. Citation2004), but were not observed in the present pre-pubertal sample, suggesting a later interaction between stress and activational hormone effects. Future investigations, utilizing pharmacological enhancement and/or blockade of pregnane neurosteroid formation, should focus on the mPFC as a target for cognitive performance, both in juvenile and adult offspring.
The findings of the current investigation demonstrate that exposure to gestational stress in late pregnancy can impair pre-pubertal cognitive performance of offspring in a rat model. Circulating maternal 3α,5α-THP predicted the formation of 5α-reduced P metabolites in the mPFC of offspring concomitant with performance in the object recognition task. In particular, these data elucidate the need for assessment of 5α-reduction and/or 3α-hydroxylation among gestationally stressed offspring given that perturbation of enzymatic processes could account for observed effects. Future investigations should aim to assess the activity, as well as the mRNA and the protein expression, of 5α-reductase and/or 3α-HSD in response to restraint or other environmental stressors.
Acknowledgements
This work was supported in part by grants from the National Science Foundation (IBN9896263, IBN0316083, IBN0957148) and the National Institute of Mental Health (MH06769801). Technical assistance provided by Kassandra Edinger is greatly appreciated.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahmad I, Lope-Piedrafita S, Bi X, Hicks C, Yao Y, Yu C, Chaitkin E, Howison CM, Weberg L, Trouard TP, Erickson RP. 2005. Allopregnanolone treatment, both as a single injection or repetitively, delays demyelination and enhances survival of Niemann-Pick C mice. J Neurosci Res. 82:811–821.
- Barbaccia ML, Serra M, Purdy RH, Biggio G. 2001. Stress and neuroactive steroids. Int Rev Neurobiol. 46:243–272.
- Bowman RE, MacLusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN. 2004. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 145:3778–3787.
- Broadbent N, Squire L, Clark R. 2004. Spatial memory, recognition memory, and the hippocampus. Neuroscience. 101:14515–14520.
- Brouwers P, van Engelen M, Lalonde F, Perez L, de Haan E, Wolters P, Martin A. 2001. Abnormally increased semantic priming in children with symptomatic HIV-1 disease: Evidence for impaired development of semantics?. J Int Neuropsychol Soc. 7:491–501.
- Brunton PJ, Russell JA. 2008. Attenuated hypothalamo–pituitary–adrenal axis responses to immune challenge during pregnancy: The neurosteroid opioid connection. J Physiol. 586:369–375.
- Choi S, Dallman MF. 1999. Hypothalamic obesity: Multiple routes mediated by loss of function in medial cell groups. Endocrinology. 140:4081–4088.
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. 1998. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U S A. 95:13284–13289.
- Djebaili M, Hoffman SW, Stein DG. 2004. 3α,5α-THP and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 123:349–359.
- Done DJ, Johnstone EC, Frith CD, Golding J, Shepherd PM, Crow TJ. 1991. Complications of pregnancy and delivery in relation to psychosis in adult life: Data from the British perinatal mortality survey sample. BMJ. 302:1576–1580.
- Dunnett SB, Ryan CN, Levin PD, Reynolds M, Bunch ST. 1987. Functional consequences of embryonic neocortex transplanted to rats with prefrontal cortex lesions. Behav Neurosci. 101:489–503.
- England BG, Niswender GD, Midgley ARJr. 1974. Radioimmunoassay of estradiol-17β without chromatography. J Clin Endocrinol Metab. 38:42–50.
- Ennaceur A, Delacour J. 1988. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 31:47–59.
- Ennaceur A, Neave N, Aggleton JP. 1997. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 113:509–519.
- Facchinetti F, Dante G, Venturini P, Paganelli S, Volpe A. 2008. 17α-hydroxy-progesterone effects on cervical proinflammatory agents in women at risk for preterm delivery. Am J Perinatol. 25:503–506.
- Fan JM, Chen XQ, Jin H, Du JZ. 2009. Gestational hypoxia alone or combined with restraint sensitizes the hypothalamic–pituitary–adrenal axis and induces anxiety-like behavior in adult male rat offspring. Neuroscience. 159:1363–1373.
- Finn DA, Gee KW. 1994. The estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroid. J Pharmacol Exp Ther. 271:164–170.
- Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. 2004. Epigenetic programming of stress responses through variations in maternal care. Ann N Y Acad Sci. 1036:167–180.
- Frisone DF, Frye CA, Zimmerberg B. 2002. Social isolation stress during the third week of life has age-dependent effects on spatial learning in rats. Behav Brain Res. 128:153–160.
- Frye CA. 2001. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Rev. 37:201–222.
- Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THP and 3α-Diol. J Neuroendocrinol. 1999a; 11:839–847.
- Frye CA, Bayon LE. Prenatal stress reduces the effectiveness of the neurosteroid 3α,5α-THP to block kainic-acid-induced seizures. Dev Psychobiol. 1999b; 34:227–234.
- Frye CA, Lacey EH. 2001. Progestins influence performance on cognitive tasks independent of changes in affective behavior. Psychobiology. 28:550–563.
- Frye CA, Orecki ZA. Prenatal stress produces deficits in socio-sexual behavior of cycling, but not hormone-primed, Long-Evans rats. Pharmacol Biochem Behav. 2002a; 73:53–60.
- Frye CA, Orecki ZA. Prenatal stress alters reproductive responses of rats in behavioral estrus and paced mating of hormone-primed rats. Horm Behav. 2002b; 42:472–483.
- Frye CA, Bayon LE, Pursnani NK, Purdy RH. 1998. The neurosteroids, progesterone and 3α,5α-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 808:72–83.
- Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5α-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007a; 133:663–674.
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007b; 88:208–216.
- Gilbert-Evans SE, Ross LE, Sellers EM, Purdy RH, Romach MK. 2005. 3α-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecol Endocrinol. 21:268–279.
- Glynn LM, Schetter CD, Hobel CJ, Sandman CA. 2008. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychol. 27:43–51.
- Griffin LD, Gong W, Verot L, Mellon SH. 2004. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 10:704–711.
- Hadders-Algra M, Huisjes HJ, Touwen BC. 1988. Perinatal correlates of major and minor neurological dysfunction at school age: A multivariate analysis. Dev Med Child Neurol. 30:472–481.
- Hakulinen A, Heinonen K, Jokela V, Launiala K. 1988. Prematurity-associated morbidity during the first two years of life. A population-based study. Acta Paediatr Scand. 77:340–348.
- Hedegaard M, Henriksen TB, Sabroe S, Secher NJ. 1993. Psychological distress in pregnancy and preterm delivery. BMJ. 307:234–239.
- He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and 3α,5α-THP reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004a; 189:404–412.
- He J, Hoffman SW, Stein DG. 3α,5α-THP, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004b; 22:19–31.
- Holst K, Andersen E, Philip J, Henningsen I. 1989. Antenatal and perinatal conditions correlated to handicap among 4-year-old children. Am J Perinatol. 6:258–267.
- Jutapakdeegul N, Afadlal S, Polaboon N, Phansuwan-Pujito P, Govitrapong P. 2010. Repeated restraint stress and corticosterone injections during late pregnancy alter GAP-43 expression in the hippocampus and prefrontal cortex of rat pups. Int J Dev Neurosci. 28:83–90.
- Kapoor A, Leen J, Matthews SG. 2008. Molecular regulation of the hypothalamic–pituitary–adrenal axis in adult male guinea pigs after prenatal stress at different stages of gestation. J Physiol. 586:4317–4326.
- Kapoor A, Kostaki A, Janus C, Matthews SG. 2009. The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behav Brain Res. 197:144–149.
- Kellogg CK, Frye CA. 1999. Endogenous levels of 5α-reduced progestins and androgens in fetal vs. adult rat brains. Brain Res Dev Brain Res. 115:17–24.
- Knipschild P, Meijer H, Sallé H. 1981. Aircraft noise and birth weight. Int Arch Occup Environ Health. 48:131–136.
- Louvart H, Maccari S, Vaiva G, Darnaudéry M. 2009. Prenatal stress exacerbates the impact of an aversive procedure on the corticosterone response to stress in female rats. Psychoneuroendocrinology. 34:786–790.
- Lucassen PJ, Bosch OJ, Jousma E, Krömer SA, Andrew R, Seckl JR, Neumann ID. 2009. Prenatal stress reduces postnatal neurogenesis in rats selectively bred for high, but not low, anxiety: Possible key role of placental 11β-hydroxysteroid dehydrogenase type 2. Eur J Neurosci. 29:97–103.
- Luine VN, Jacome LF, Maclusky NJ. 2003. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 144:2836–2844.
- Mark PJ, Augustus S, Lewis JL, Hewitt DP, Waddell BJ. 2009. Changes in the placental glucocorticoid barrier during rat pregnancy: Impact on placental corticosterone levels and regulation by progesterone. Biol Reprod. 80:1209–1215.
- McCormick MC. 1985. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 312:82–90.
- McCormick CM, Kehoe P, Mallinson K, Cecchi L, Frye CA. 2002. Neonatal isolation alters stress hormone and mesolimbic dopamine release in juvenile rats. Pharmacol Biochem Behav. 73:77–85.
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE. 2005. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm Behav. 48:64–74.
- Meaney MJ, Szyf M. 2005. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 7:103–123.
- Mellon S, Gong W, Griffin LD. 2004. Niemann pick type C disease as a model for defects in neurosteroidogenesis. Endocr Res. 30:727–735.
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. 2006. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 24:1477–1487.
- Mutale T, Creed F, Maresh M, Hunt L. 1991. Life events and low birthweight–analysis by infants preterm and small for gestational age. Br J Obstet Gynaecol. 98:166–172.
- Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ. 1998. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol. 508:289–300.
- Newton ER. 1986. Antepartum care in multiple gestation. Semin Perinatol. 10:19–29.
- Nguyen PN, Ross Young I, Walker DW, Hirst JJ. 2004. Allopregnanolone in the brain and blood after disruption of the hypothalamic–pituitary–adrenal axis in fetal sheep. J Endocrinol. 182:81–88.
- Niswender GD. 1973. Influence of the site of conjugation on the specificity of antibodies to progesterone. Steroids. 22:413–424.
- Orr ST, James SA, Miller CA, Barakat B, Daikoku N, Pupkin M, Engstrom K, Huggins G. 1996. Psychosocial stressors and low birthweight in an urban population. Am J Prev Med. 12:459–466.
- Paris JJ, Frye CA. 2008. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 136:105–115.
- Pearce BD, Garvin SE, Grove J, Bonney EA, Dudley DJ, Schendel DE, Thorsen P. 2008. Serum macrophage migration inhibitory factor in the prediction of preterm delivery. Am J Obstet Gynecol. 199:46e1–46e6.
- Petraglia F, Florio P, Nappi C, Genazzani AR. 1996. Peptide signaling in human placenta and membranes: Autocrine, paracrine, and endocrine mechanisms. Endocr Rev. 17:156–186.
- Purdy RH, Moore PHJr., Rao PN, Hagino N, Yamaguchi T, Schmidt P, Rubinow DR, Morrow AL, Paul SM. 1990. Radioimmunoassay of 3α-hydroxy-5α-pregnan-20-one in rat and human plasma. Steroids. 55:290–296.
- Purdy RH, Morrow AL, Moore PHJr., Paul SM. 1991. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 88:4553–4557.
- Rhodes ME, McCormick CM, Frye CA. 2004. 3α,5α-THP mediates progestins' effects to protect against adrenalectomy-induced cell death in the dentate gyrus of female and male rats. Pharmacol Biochem Behav. 78:505–512.
- Rodbard D, Hutt DM. 1974. Statistical analysis of radioimmunoassay and immunoradiometric assays: A generalized, weighted iterative, least squares method for logistic curve fitting. In: International Atomic Energy Agency. editors. Symposium on Radioimmunoassay and Related Procedures in Medicine. New York: Uniput209–233.
- Rodrigues T, Barros H. 2008. Maternal unemployment: An indicator of spontaneous preterm delivery risk. Eur J Epidemiol. 23:689–693.
- Rodrigues T, Rocha L, Barros H. 2008. Physical abuse during pregnancy and preterm delivery. Am J Obstet Gynecol. 198:171e1–171e6.
- Seckl JR. 1997. Glucocorticoids, feto-placental 11β-hydroxysteroid dehydrogenase type 2, and the early life origins of adult disease. Steroids. 62:89–94.
- Seckl JR, Walker BR. 2004. 11β-hydroxysteroid dehydrogenase type 1 as a modulator of glucocorticoid action: From metabolism to memory. Trends Endocrinol Metab. 15:418–424.
- Setiawan E, Jackson MF, MacDonald JF, Matthews SG. 2007. Effects of repeated prenatal glucocorticoid exposure on long-term potentiation in the juvenile guinea-pig hippocampus. J Physiol. 581:1033–1042.
- Steer RA, Scholl TO, Hediger ML, Fischer RL. 1992. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 45:1093–1099.
- Toledo-Rodriguez M, Sandi C. 2007. Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plast. 2007:71203.
- Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. 1993. The association between prenatal stress and infant birth weight and gestational age at birth: A prospective investigation. Am J Obstet Gynecol. 169:858–865.
- Walf AA, Frye CA. 2005. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic–pituitary–adrenal axis activity. Neuropsychopharmacology. 30:1288–1301.
- Walf AA, Frye CA. 2007. Estradiol decreases anxiety behavior and enhances inhibitory avoidance and gestational stress produces opposite effects. Stress. 10:251–260.
- Walf AA, Rhodes ME, Frye CA. 2006. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurbiol Learn Mem. 86:35–46.
- Wang JM, Irwin RW, Liu L, Chen S, Brinton RD. 2007. Regeneration in a degenerating brain: Potential of allopregnanolone as a neuroregenerative agent. Curr Alzheimer Res. 4:510–517.
- Wang JM, Liu L, Irwin RW, Chen S, Brinton RD. 2008. Regenerative potential of allopregnanolone. Brain Res Rev. 57:398–409.
- Ward HE, Johnson EA, Salm AK, Birkle DL. 2000. Effects of prenatal stress on defensive withdrawal behavior and corticotropin releasing factor systems in rat brain. Physiol Behav. 70:359–366.
- Weinstock M. 2001. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 65:427–451.
- Weinstock M. 2008. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 32:1073–1086.
- Welberg LA, Seckl JR, Holmes MC. 2000. Inhibition of 11β-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 12:1047–1054.
- Zambrana RE, Dunkel-Schetter C, Collins NL, Scrimshaw SC. 1999. Mediators of ethnic-associated differences in infant birth weight. J Urban Health. 76:102–116.
- van Zeben-van der Aa TM, Verloove-Vanhorick SP, Brand R, Ruys JH. 1989. Morbidity of very low birthweight infants at corrected age of two years in a geographically defined population. Report from Project on Preterm and Small for gestational age infants in The Netherlands. Lancet. 1:253–255.