Abstract
Despite research regarding emotional processing, it is still unclear whether fear-evoking stimuli are processed when they are irrelevant and when attention is oriented elsewhere. In this study, 63 healthy university students with high fear from snakes or spiders participated in two different experiments. In an emotional modification of the spatial cueing task, 31 subjects (5 males) were asked to detect a target letter while ignoring a neutral or fear-related distracting picture. The distribution of attention was independently manipulated by a spatial cue that preceded the appearance of the picture and the target letter. In an emotional modification of the cognitive load paradigm, 32 subjects (4 males) were asked to discriminate between two target letters, while ignoring a central neutral or fear-related picture, and additional 1, 3, or 5 distracting letters that created a varied attentional load. Fear-related pictures interfered with the performance of highly fearful participants, even when the pictures were presented outside the focus of attention and when the task taxed attentional resources. We suggest that highly fearful individuals process fear-related information automatically, either inattentively or with prioritized attention capture over competing items, leading to deteriorated cognitive performance. Different results were shown in healthy individuals while processing negative—but not phobic—pictures, suggesting that emotional processing depends on the fear value of the stimulus for a specific observer.
Introduction
From an evolutionary perspective, stimuli that signal danger or threat should get higher processing priority compared with competing stimuli. Different people can perceive the same stimulus as threatening or harmless, leading to different behavioral influences. An extreme example for such individual differences comes from people with phobia from a specific object such as a snake or a spider (i.e. the phobic stimulus). Exposure of individuals with specific phobia to their phobic stimulus leads to a rapid, exaggerated, and persistent fear response that is dominated by the sympathetic system and includes elevation of autonomic measures, such as blood pressure and heart rate, and release of epinephrine and norepinephrine (Curtis et al. Citation1978; American Psychiatric Association Citation1994; Globisch et al. Citation1999). It has been suggested that stimuli that evoke fear are processed in an automatic manner, do not require attention or consciousness, and attract attention to their location (Seligman Citation1971; Öhman Citation1992, Citation2002; Öhman and Soares Citation1993, Citation1994, Citation1998; Mogg and Bradley Citation1999; Öhman and Mineka Citation2001; Öhman et al. Citation2001).
There is evidence that individuals with specific phobias process stimuli related to their phobias in a prioritized manner. Individuals with phobia were slower to name a word's color when the word described their phobic stimulus, compared with neutral words, in an emotional Stroop-like task (Lavy et al. Citation1993; Amir et al. Citation1996; Williams et al. Citation1996; van den Hout et al. Citation1997). In addition, studies that used a visual search paradigm found that phobic participants showed facilitated reaction to their phobic stimulus (Öhman et al. Citation2001; Miltner et al. Citation2004). However, in most of these studies, the fear-related stimuli were both relevant to the experimental task and presented inside the focus of attention. Thus, it is not clear whether phobia-related stimuli are preferentially processed when they are outside attentional focus, or when their processing is irrelevant to a task being performed. Evidence for inattentive processing of phobia-related stimuli comes from a functional magnetic resonance imaging (fMRI) study that examined the neural correlates of direct vs. indirect processing of phobic material, by manipulating orienting of attention to phobia-related pictures (Straube et al. Citation2006). Individuals with phobia manifested increased activation bilaterally in the amygdala, even when attention was not focused on the phobic picture. The authors suggest that the amygdala is involved in automatic processing of the phobic material that does not depend on attention.
In the current study, individuals fearful of snakes or spiders were asked to ignore fear-relevant pictures (phobia stimuli) while performing an effortful discrimination task. Processing of the picture's content was always irrelevant for task performance. We manipulated attention independently in order to examine whether processing of phobia-related distracters occurs even when attention is directed elsewhere (Experiment 1), and when the experimental task exploits attentional resources (Experiment 2). Unlike previous studies that often used spatial locations to manipulate attention, we specifically manipulated attentional resources in Experiment 2 and not visual–spatial factors. The picture appeared at fixation, between letters that were relevant for task performance, and hence, it was easily perceived. However, the attentional manipulation created situations in which there was not always enough attention to process the picture. Based on the existing findings in individuals with specific phobia, we hypothesized that fear-related stimuli would interfere with performance, even though the stimuli were task irrelevant, and even when they were presented outside the attentional focus or in the absence of attentional resources. Specifically, we hypothesized that we would find deteriorated performance in trials presenting fear-related stimuli, compared with trials depicting neutral stimuli, regardless of whether these stimuli were presented within or outside of attentional focus, and regardless of the size of the cognitive load.
Materials and methods
Participants
Experiment 1
In a prior study (CitationAlyagon 2010), 534 undergraduate students from Ben-Gurion University of the Negev filled in questionnaires that measured fear of snakes and spiders (modified from Klorman et al. Citation1974). The questionnaires were translated to Hebrew and back-translated to English by two of the co-authors (U.A. and O.K.) who are bilingual and native speakers of Hebrew and English, respectively. In the fear questionnaires, 31 undergraduate students were recruited to the current experiment according to their z-score. The study was approved by the ethics committee of Ben-Gurion University of the Negev.
The participants were divided into two groups according to their z-score in the questionnaire; participants who scored above the mean were considered “highly fearful” and those who scored below the mean were considered the “low-fearful” group. The “highly fearful” group was comprised of 21 participants (3 male and 18 female). Of the 21 subjects, 12 were fearful of snakes, and 9 were fearful of spiders. Their questionnaire scores ranged between 0.8 and 2.73 SDs above the average (mean = 1.74). Fear stimuli consisted of either snakes or spiders, according to the specific fear reported by the participant. The “low-fearful” group was comprised of 10 participants (2 male and 8 female). Nine of them participated in a control experiment with pictures of snakes, and one in a control experiment containing pictures of spiders. Their questionnaire scores ranged between 1.1 and 1.76 SDs below the average (mean = − 1.4).
Experiment 2
In this experiment, 32 undergraduate students participated. They were recruited from the same pool of students as in Experiment 1. Different subjects participated in the two experiments. Two participants from the highly fearful group and one from the low-fearful group were excluded due to more than 20% mistakes. Hence, the highly fearful group included 14 participants (2 male and 12 female). Their questionnaire scores ranged between 1.8 and 2.75 SDs above the average (mean = 2.15 SDs). Nine of them were fearful of snakes, and five were fearful of spiders. The low-fearful group included 15 participants (2 male, 13 female). Their questionnaire scores ranged between − 1.13 and − 1.57 SDs below the average (mean = − 1.34 SDs). Of the low-fearful control group, 13 were exposed to pictures of snakes and the other two low-fearful participants to pictures of spiders. The mean age of the highly fearful group was 23.14 years with SD of 1.17, and of the low-fearful group, 23.67 years with SD of 0.9.
Stimuli and procedure
Experiment 1: Emotional variation of the spatial cueing paradigm (Posner Citation1980)
The display consisted of three rectangles, 7.5° wide and 6° high, one at the center of the screen and two plotted with their centers 7.5° to the right or left of the middle of the screen. The cue was a brightening of one of the peripheral rectangles. The target was a 1° letter, O or Q, which was presented inside one of the peripheral rectangles. The distractor was a 6.5° high and 5° wide colored picture, which appeared in the center of one of the peripheral rectangles. For the experiment, 160 pictures were chosen—30 pictures of real snakes, 30 pictures of real spiders, and 100 neutral pictures. The neutral pictures were chosen from the International Affective Picture System (IAPS; CitationLang et al. 2001), according to a validation experiment described elsewhere (Okon-Singer et al. Citation2007). The snake and spider pictures were chosen from the IAPS and the Internet.
The participants sat in a dimly lit and quiet room, facing the computer screen at eye level. They were asked to fixate the center of the screen and to refrain from eye movement. Fixation was monitored by the experimenter in a different room using a camera directed at the participant's eyes. illustrates the order of an experimental trial. Each trial began with a central fixation point for 500 ms. Two hundred milliseconds after the disappearance of the fixation point, one of the peripheral squares (with equal probability) brightened for 100 ms. After a variable stimulus onset asynchrony (SOA) of 140 or 160 ms following the cue onset, a target letter appeared in one of the peripheral rectangles. Half of the trials were target-only no-picture trials. In the other half of the trials, simultaneously with the letter onset in one peripheral rectangle, a picture was presented in the other peripheral rectangle for 100 ms. The picture had an equal probability of being fear related or neutral.
Figure 1. An example of a valid trial in Experiment 1. A fixation cross appears for 500 ms, followed by a 200 ms blank interval. After the interval, one of the peripheral rectangles flashes for 100 ms. Following a variable SOA of 140 or 160 ms, a target letter appears in the cued peripheral rectangle. Simultaneously with the target's appearance, a distracting picture appears in the other peripheral rectangle for 100 ms (ms, milliseconds; SOA, stimulus onset asynchrony).
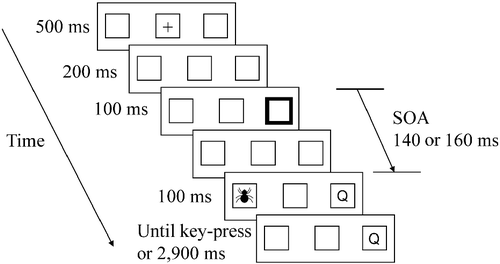
The participants were asked to ignore the picture and respond to the letter as quickly as possible by pressing the “/” and “z” keys using their index fingers. The picture was completely task irrelevant and its content did not correlate with the target letter. Stickers of “O” and “Q” were put on the “/” and “z” keys, respectively. Validity was defined with respect to the location of the target letter used in the discrimination task. In valid trials, the target letter appeared at a previously cued location and the picture appeared at an uncued location (outside attentional focus). In invalid trials, the target letter was presented at an uncued location and the picture was presented at the previously cued location (inside attentional focus). Seventy-five percent of the trials in the practice and experimental blocks were valid. The order of the trials in the practice and experimental blocks was randomly chosen for each participant. As the cue contained both reflexive and intentional elements, it was assumed to attract attention to its location in a very efficient manner. We used a discrimination task and a short interval between cue and target presentations in order to avoid inhibition at the cued location, known as inhibition of return (IOR; e.g. Lupianez et al. Citation2001; Berger et al. Citation2005).
The experiment contained a practice block of 30 trials, followed by two experimental blocks of 240 trials each. One experimental block was a mixed block containing an equal number of neutral and fear-related pictures. The other experimental block was a neutral block including only neutral pictures. The order of the neutral and mixed blocks was counterbalanced between the participants. The neutral block was included in order to examine effects of anxiety. Thus, reaction time (RT) to neutral stimuli in the mixed block, during which fear stimuli could appear, was compared to RT in the neutral block, which included only “safety” signals. As we did not find any effects of anxiety, we will not discuss these results here. RT was measured in milliseconds from the target onset until the participant's key press. The target remained visible until the participant responded or for an additional 2900 ms after the picture disappeared. The next trial began 1500 ms after the participant's response. In case of a mistake or a response before the target's appearance, the words “mistake” or “too soon” appeared in the middle rectangle for 1000 ms, and the next trial began after 1500 ms. The participants were debriefed after the experiment was completed and the rationale of the experiment was explained.
Experiment 2: Emotional variation of a cognitive load paradigm (Lavie Citation1995)
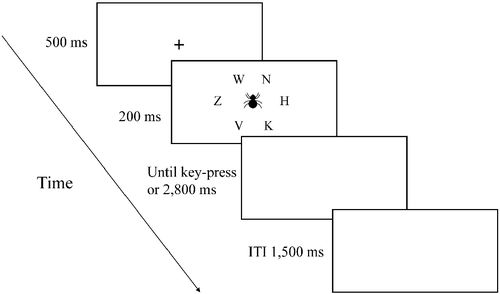
Stimuli were displayed on a 15-inch screen. The display consisted of a target letter that appeared alone or with an additional one, three, or five distracting letters. The task created low- or high-attentional load conditions, depending on the number of distracting letters. Hence, there were four set sizes (1, 2, 4, or 6 letters) each of which occurred in 25% of the trials. Based on Lavie's (Citation1995) study, the target letter was either X or N and the distracting letters were K, H, V, Z, or W. The target letter and the distracting letters appeared randomly in six possible locations that created an imaginary circle at the center of the screen. The distracting pictures were similar to those used in Experiment 1.
The experiment started with a practice block of 40 trials, followed by five experimental blocks of 96 trials each. In both the practice and experimental blocks, half of the trials did not contain any picture, and in half of the trials, simultaneously with the letters, a picture was presented in the middle of the screen. Half of the picture trials presented a neutral picture, and half presented a fear-related picture. The order of the trials in the practice and experimental blocks was randomly chosen by the computer for each participant.
Each trial began with a central fixation point for 500 ms. Immediately after the fixation point disappeared, a target letter appeared for 200 ms alone or with the distracting letters, as described above. The participants were asked to respond to the target letter and ignore the picture, which was irrelevant to task performance, as quickly and accurately as possible by pressing the key that corresponded to the target letter (i.e. X or N on the keyboard) using the index finger of their left and right hands, respectively. RT was measured in milliseconds from target onset until the participant's key press. The screen remained blank until the participant responded up to 3000 ms. In case of a mistake or a response before the target's appearance, the words “mistake” or “too soon” appeared for 1000 ms (). After the experiment was completed, the participants were debriefed and the rationale of the experiment was explained.
Figure 2. An example of a trial in Experiment 2. A fixation cross appears for 500 ms. After the fixation, a target letter appears alone, or with additional one, three, or five distracting letters for 200 ms. Simultaneously with the letters' appearance, a distracting picture appears in the center of the screen for 200 ms in half of the trials (ITI, inter-trial interval; ms, milliseconds).
Statistical analysis
Experiment 1
Data from trials in which the participants responded before the appearance of the target (less than 0.5%), and trials in which RT was faster than 100 ms or slower than 3000 ms (less than 0.5%) were excluded from the analysis. The overall mean error percentage (EP) was 3.49 and 3.31 for the highly fearful and low-fearful group, respectively. EP analysis did not show a main effect of group or any interactions with it, and there was no sign of speed–accuracy tradeoff in any of the effects concerning the group. Hence, we are reporting only the analyses of the RT data.
For each participant, mean RTs of correct responses from the mixed block were subjected to a three-way repeated measures analysis of variance (ANOVA), with trial type (fear related, neutral, no picture) and cue validity (valid, invalid) as within-subjects factor and group (highly fearful, low fearful) as a between-subjects factor.
Experiment 2
Data from trials in which RT was faster than 100 ms or slower than 3000 ms (less than 0.1%) were excluded from the analysis. The overall mean EP was 9.3 and 10.6 for the highly and low-fearful groups, respectively. For each participant, mean RTs of correct responses as well as error data were subjected to a three-way repeated measures ANOVA, with trial type (fear related, neutral, no picture) and set size (1, 2, 4, 6 letters) as within-subjects factor and group (highly fearful, low fearful) as a between-subjects factor.
Results
Experiment 1
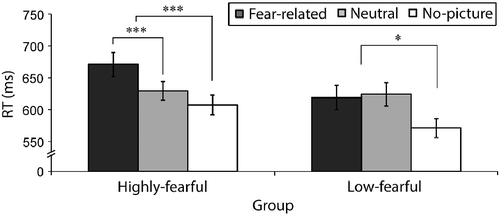
Two main effects were significant: cue validity [F(1,29) = 63.5, p < 0.001, η2 = 0.68] due to faster RTs in valid compared to invalid trials, and trial type [F(2,58) = 13.7, p < 0.001, η2 = 0.32]. Although there was no main effect of group, there was a significant interaction between trial type and group [F(2,58) = 4.4, p < 0.01, η2 = 0.13]. To explore this interaction further, we analyzed the simple effect of trial type in each of the groups, by decomposing it into two orthogonal contrasts: a contrast between fear-related and neutral trials, aimed at testing the hypothesis regarding interference of fear-related pictures; and a contrast between picture and no-picture trials, in order to examine the effect of picture appearance. As expected, in the highly fearful group, RTs were slower in fear-related compared to neutral trials [F(1,20) = 17.3, p < 0.001, η2 = 0.46] and in picture trials compared to no-picture trials [F(1,20) = 26.14, p < 0.001, η2 = 0.57]. In the low-fearful group, as expected, there was no difference between fear-related and neutral trials [F < 1, ns], but, similar to the fearful group, RTs were slower in picture trials compared to no-picture trials [F(1,29) = 6.19, p < 0.05, η2 = 0.41] (). Thus, in both groups, pictures interfered with performance compared with no-picture trials, suggesting that the picture manipulation affected performance. However, the picture content was relevant only for the fearful group. Thus, fear related, but not neutral pictures interfered with performance in highly fearful participants, and had no influence on performance in the low-fearful group.
Figure 3. Mean reaction as a function of group and trial type in Experiment 1. The data are collapsed across valid and invalid trials. In the highly fearful group (N = 21), RTs in fear-related picture trials were slower than in neutral picture trials. In the low-fearful group (N = 10), there was no difference in RT between fear-related and neutral pictures. In addition, RTs were slower in picture trials compared with “no-picture” trials in both groups. The error bars depict the SE in each condition; *p < 0.05, **p < 0.001 (ms, milliseconds; RT, reaction time).
The interaction between trial type and group was not modulated by validity [F < 1, ns; see RT in valid and invalid trials in . Despite the absence of a significant interaction, we wanted to test our hypothesis that the fear-relevant stimulus affected performance more in the fearful participants than in the non-fearful participants, regardless of the allocation of attention. For that, we examined the interaction between the group (highly fearful, low fearful) and picture valence (fear related, neutral) in valid trials and invalid trials separately. As expected, this interaction was significant in both trial types {[F(1,29) = 4.25, p < 0.04, η2 = 0.12] and [F(1,29) = 6.16, p < 0.01, η2 = 0.17], for valid and invalid trials, respectively}. This interaction seemed larger in the invalid trials, however the difference between valid and invalid trials was not significant. We further examined the simple effect of picture valence in each group. As expected, fear-related pictures interfered with performance in the highly fearful group, whether the picture was in the focus of attention [F(1,20) = 9.88, p < 0.01, η2 = 0.33] or not [F(1,20) = 10.8, p < 0.01, η2 = 0.35], while in the low-fearful group there was no difference between fear-related and neutral pictures in valid and invalid conditions [both Fs < 1, ns].
Table I. Mean reaction times (in milliseconds) and SDs (in brackets, in milliseconds) as a function of group, trial type, and validity in Experiment 1.
Experiment 2
RT data analysis
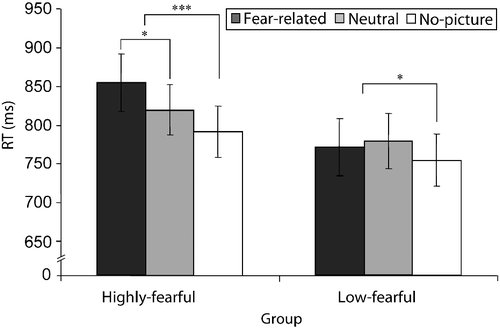
Two main effects were significant: trial type [F(2,54) = 13.63, p < 0.001, η2 = 0.34], and set size [F(3,81) = 197.6, p < 0.001, η2 = 0.88] due to longer RTs for larger set sizes. More importantly, the interaction between trial type and group was significant [F(2,54) = 5.39, p < 0.001, η2 = 0.16]. To examine this interaction, we analyzed the simple effect of trial type in the highly and low-fearful groups separately, by decomposing it into two orthogonal contrasts: a contrast between fear-related and neutral trials, and a contrast between picture trials (neutral and fear related) and no-picture trials. As hypothesized, in the highly fearful group RTs were slower in fear-related vs. neutral trials [F(1,13) = 7.76, p < 0.05, η2 = 0.37] and in picture vs. no-picture trials [F(1,13) = 22.35, p < 0.001, η2 = 0.63]. In the low-fearful group, there was no difference between fear-related and neutral trials [F < 1, ns], and RTs were slower in the picture compared with no-picture trials [F(1,14) = 8.74, p < 0.05, η2 = 0.38] (). Hence, in the highly fearful group, fear-related pictures interfered with performance, while these pictures had no influence on the low-fearful group. In addition, pictures interfered with performance in both groups, showing that participants did not ignore the pictures in general. Since, there was no main effect of group [F(1,27) = 1.2, p = 0.28, η2 = 0.04], the differences between the groups did not result from general differences in RTs.
Figure 4. Mean RTs in Experiment 2 as a function of group and trial type. Phobia-related pictures interfered with performance in the highly fearful group (N = 14), resulting in slower RTs in fear-related vs. neutral trials. There were no differences in RT between phobia-related and neutral pictures in the low-fearful group (N = 15). In addition, RTs were slower in picture vs. no-picture trials in both groups, showing that participants perceived the pictures. The error bars depict the SE in each condition; *p < 0.05, ***p < 0.01 (ms, milliseconds; RT, reaction time).
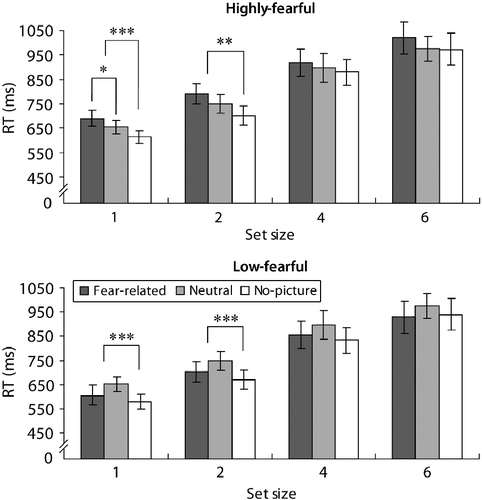
There was no three-way interaction between trial type, group, and set size [F < 1, ns]. Hence, the interaction between trial type and group was not modulated by attention load. illustrates the size of the effect in each group, in the different set sizes. Although the differences in RTs reached significance only in set sizes of one or two letters, a similar trend was shown in the other conditions and there were no interactions between set size and trial type in each of the groups.
Figure 5. Mean RTs in Experiment 2 as a function of group, trial type, and cognitive load. The appearance of phobia-related pictures resulted in slower RTs compared to neutral pictures in the highly fearful group (N = 14), in the low-load condition. There were no differences in RTs between phobia-related and neutral pictures in the low-fearful group (N = 15), in any of the load conditions (all F < 1, ns). In addition, RTs were slower in picture vs. no-picture trials in both groups in low-load conditions. Although the differences in RTs reached significance only in low-load conditions, a similar trend was shown in the other conditions and there were no interactions between load and trial type in each of the groups. The error bars depict the SE in each condition; *p < 0.05, **p < 0.01, ***p < 0.001 (ms, milliseconds; RT, reaction time).
EP data analysis
Two main effects were significant: set size [F(3,81) = 96.1, p < 0.01, η2 = 0.78] due to more mistakes in larger sized sets, and trial type [F(2,54) = 3.8, p < 0.0, η2 = 0.12] due to no differences between fear-related and neutral trials [F < 1, ns], and more mistakes in neutral vs. no-picture trials [F(1,27) = 8.15, p < 0.01, η2 = 0.24]. No higher effects or interactions reached significance. In particular, the interaction between group and trial type was not significant [F(2,54) = 3.8, p = 0.25, η2 = 0.04]. Moreover, there was no evidence of speed–accuracy tradeoff.
Discussion
The current study manipulated spatial attention using peripheral cues, and executive attention using a varied cognitive load, while participants were instructed to ignore distracting fear-related pictures. Both manipulations resulted in similar findings, i.e., fear-related information interfered with task performance even though its processing was completely irrelevant. This interference was not modulated by spatial allocation of attention (Experiment 1) or by significant exploitation of attentional resources (Experiment 2). Hence, our findings suggest prioritized processing of phobia-related material by fearful individuals, even when attention is not directed to these stimuli.
Our findings are in agreement with previous studies that showed interference of phobia-related stimuli in an emotional Stroop task (Amir et al. Citation1996, Citation2002; Williams et al. Citation1996; Becker et al. Citation2001) and a visual search task (Öhman et al. Citation2001; Miltner et al. Citation2004). In addition, our results fit with those of Elsesser et al. (Citation2006), who showed an interference effect of phobia-related pictures presented simultaneously with a target word, similar to the pictures in our study. Finally, our results are in line with the findings of Lavy et al. (Citation1993) who used a modified flanker task to examine the effect of treatment on the behavior of phobic subjects. Participants with spider phobia were asked to respond to a central picture while ignoring two identical flanking pictures. Untreated phobic participants showed faster RTs to phobia-related central pictures, and no deteriorated performance when phobia-related distracting flanker pictures were presented. The opposite pattern was found in a post-treatment group. Interestingly, our findings with fearful participants are similar to those found in the post-treatment group (i.e. deteriorated performance following presentation of fear-related pictures). It is possible that following treatment, subjects with diagnosed phobia reach a behavioral pattern that is similar to individuals with less severe symptoms, as in the fearful participants in our study. In addition, the similar results point to the behavioral abnormality of fearful subjects, even if they do not meet clinical criteria for phobia, and suggest that these individuals may benefit from efficient treatment.
Importantly, our study adds to these studies in three major aspects: (a) Previous studies did not differentiate between task-relevance and attentional deployment. For example, in the study of Lavy et al. (Citation1993), when the central picture depicted fear-related material, it was both task-relevant and inside attentional focus; and when the flanker side pictures presented fear-related material, they were task irrelevant as well as outside attentional focus. In contrast, we manipulated attentional resources independently from task relevance, and thus our study allowed for specific examination of attentional processes. The finding that there was a difference between fear-related and neutral pictures even when attention was oriented to another location (Experiment 1) or when attention was taxed by the task (Experiment 2) supports the view that processing of fear-related information does not depend on initial orienting of attention toward this information. (b) The design of Experiment 1 allowed us to examine different components related to the orienting of spatial attention. Our findings suggest that two different mechanisms are involved in the processing of fear-related stimuli in highly fearful individuals: privileged capture of attention as well as slower disengagement. (c) We showed interference of fear-related material even when the participants were engaged in a difficult discrimination task and the distracting pictures were briefly presented for 100 ms, as opposed to easier tasks and longer presentations in previous studies.
Posner and Cohen (Citation1984) suggested that orienting of attention involves the three following stages: disengaging attention from its current location, moving attention to a new target's location, and engaging attention at the new location. With regard to emotional stimuli, several studies suggested that emotional stimuli attract attention faster at the initial engagement phase, due to their evolutionary significance (Mogg and Bradley Citation1999; Bradley et al. Citation2000), while other authors argued that these stimuli hold attention once detected, resulting in later disengagement (Fox et al. Citation2001, Citation2002; Koster et al. Citation2004). Our findings suggest that both of these processes take place in highly fearful individuals. The finding of slower reaction to fear-related pictures in invalid trials suggests slower disengagement from these stimuli by highly fearful individuals. Furthermore, the finding of slower reaction to fear-evoking pictures in valid trials suggests privileged attentional capture by these pictures. A recent study has used an emotional variation of the spatial cueing task in individuals with high- or low-trait anxiety (Pérez-Dueñas et al. Citation2009). The high-trait anxiety group, as opposed to the low-trait anxiety group, did not show the typical IOR effect for fear-related targets, although they showed IOR to neutral and positive targets. The authors suggested that for anxious individuals, threat-related stimuli can capture attention, even when they are presented at a cognitively hindered location. Similarly, in our study, fear-related pictures appeared in hindered locations in the valid trials. Thus, in these trials, capture of attention might have taken place only for the highly fearful subjects, resulting in a difference between fear-related and neutral pictures in this group, but not in the low-fearful group. As suggested by Pérez-Dueñas et al. (Citation2009), privileged capture of attention in highly anxious or fearful subjects may be revealed only when the stimuli are presented in hindered locations, as was done in their design as well as in ours.
In the current study, fear-related pictures interfered with performance when presented outside attentional focus or when attentional resources were challenged. As participants showed a significant validity effect in all the experimental conditions in Experiment 1, we can conclude that their attention was indeed oriented to the cued location. Similarly, the load conditions in Experiment 2 had an influence on the RTs and EP, and hence we can conclude that attentional resources were indeed exploited. The interference effect may be due to inattentive processing of the fear-related pictures, which results in slower processing of the target letter. Alternatively, it may be due to a difficulty of fearful individuals to ignore the irrelevant fear-related pictures and to inhibit their processing (Kindt and Brosschot Citation1997). The latter suggestion is in line with the concept of automatic detection (Shiffrin and Schneider Citation1977), suggesting that a controlled search for targets is disturbed when a distracter that is detected automatically is presented simultaneously. The distracter causes an automatic attention response, draws attention to its location, and disturbs the processing of the target. Automatic attention response to specific stimuli is a learned process achieved with training. In this context, fearful participants may have learned during their lifetime to automatically detect fear-related information.
The pictures used in the current study were highly negative and arousing for the fearful participants compared with neutral pictures. Therefore, it is impossible to disentangle the effects of arousal and emotional valence on performance. It is possible that the interference produced by negative pictures was, at least in part, due to their high arousal value. This latter possibility is in line with the findings of Schimmack (Citation2005), who asked participants to perform a difficult task while ignoring emotional pictures that varied in their arousal level, emotional valence, and evolutionary significance. The results were in favor of the arousal theory, as both aversive pictures and attractive pictures of opposite-sex models produced the strongest interference effects.
The current findings are important considering our previous findings with non-fearful individuals (Okon-Singer et al. Citation2007). We used extremely negative pictures taken from the IAPS, covering a wide variety of topics, in experiments similar to the current ones. We found that although the pictures were processed, even though their processing was task irrelevant, negative pictures interfered with performance only when presented inside attentional focus or when enough attentional resources were available for their processing. In contrast, the phobia-related pictures used in the present study interfered with performance of fearful participants, even in the absence of attention. Differences between fearful and non-fearful individuals are highly relevant considering that the majority of laboratory experiments are conducted with non-fearful participants. The participants are usually undergraduate students, similar to the participants in our previous study. The findings of these studies may not be relevant to other populations, or for highly threatening stimuli, especially since studies often use stimuli that are not too threatening, such as words or schematic figures. The current findings propose that processing of aversive stimuli depends on a ‘threat threshold’; negative stimuli that are not extremely threatening require attentional resources in order to be processed, while highly threatening stimuli are processed without attention or they receive the necessary attentional resources prior to competing stimuli.
In summary, we showed that fear-related stimuli interfered with the performance of participants who are highly fearful of snakes or spiders, even when attention was directed elsewhere or when attentional resources were exploited. These findings suggest that highly threatening information is processed automatically, either inattentively or with prioritized attention capture over competing items. Prioritized processing of phobia-related stimuli may be due to dedicated neural circuitry, involving cortical and sub-cortical areas, as was shown in several fMRI studies. Participants with phobia showed increased activation in the amygdala, the insula, the prefrontal cortex, the uncus, and the cingulate gyrus, when they were presented with phobia-related information (Birbaumer et al. Citation1998; Dilsger et al. Citation2003; Straube et al. Citation2004a,Citationb, Citation2005; Britton et al. Citation2009; Caseras et al. Citation2010). Importantly, activation in the amygdala was found even in a sub-conscious presentation of phobic stimuli (Carlsson et al. Citation2004), and when attention was not engaged in the phobic items (Straube et al. Citation2006). Our findings fit the suggestion of Straube et al. (Citation2006) that the amygdala is involved in automatic processing of phobic material that does not depend on attention, while other regions are involved in direct threat evaluation and require sufficient attentional resources. Several studies suggested that a subcortical pathway involving the superior colliculus and pulvinar provides the route through which unconscious threatening stimuli access the amygdala, allowing unconscious and inattentive processing of highly threatening stimuli (Morris et al. Citation1998, Citation1999, Citation2001; Linke et al. Citation1999; Vuilleumier et al. Citation2001, Citation2003; Öhman Citation2002). It is possible that the amygdala serves as a mechanism for fast detection of highly threatening stimuli, even in a brief inattentive appearance, such as in the current study.
Acknowledgments
We wish to thank all the participants who kindly took part in this study, and Prof. Judy Auerbach for help in translating the questionnaires.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alyagon U. 2010. Influence of Fear and Anxiety on Human Cognition, Thesis dissertation. Department of Psychology. Ben-Gurion University of the Negev, Beer-Sheva, Israel.
- American Psychiatric Association. 1994. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Press.
- Amir N, McNally R, Riemann BC, Burns J, Lorenz M, Mullen JT. 1996. Suppression of the emotional Stroop effect by increased anxiety in patients with social phobia. Behav Res Ther. 34:945–948.
- Amir N, Freshman M, Foa E. 2002. Enhanced Stroop interference for threat in social phobia. J Anxiety Disord. 16:1–9.
- Becker ES, Rinck M, Margraf J, Roth WT. 2001. The emotional Stroop effect in anxiety disorders: General emotionality or disorder specificity?. J Anxiety Disord. 15:147–159.
- Berger A, Henik A, Rafal R. 2005. Competition between endogenous and exogenous orienting of visual attention. J Exp Psychol Gen. 134:207–221.
- Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Schneider F, Weiss U, Flor H. 1998. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 9:1223–1226.
- Bradley BP, Mogg K, Millar NH. 2000. Covert and overt orienting of attention to emotional faces in anxiety. Cogn Emot. 14:789–808.
- Britton JC, Gold AL, Deckersbach T, Rauch SL. 2009. Functional MRI study of specific animal phobia using an event-related emotional counting stroop paradigm. Depress Anxiety. 26:796–805.
- Carlsson K, Petersson KM, Lundqvist D, Karlsson A, Ingvar M, Öhman A. 2004. Fear and the amygdala: Manipulation of awareness generates differential cerebral responses to phobic and fear-relevant (but nonfeared) stimuli. Emotion. 4:340–353.
- Caseras X, Giampietro V, Lamas A, Brammer M, Vilarroya O, Carmona S, Rovira M, Torrubia R, Mataix-Cols D. 2010. The functional neuroanatomy of blood–injection–injury phobia: A comparison with spider phobics and healthy controls. Psychol Med. 40:125–134.
- Curtis GC, Nesse R, Buxton M, Lippman D. 1978. Anxiety and plasma cortisol at the crest of the circadian cycle: Reappraisal of a classical hypothesis. Psychosom Med. 40:368–378.
- Dilger S, Straube T, Mentzel HJ, Fitzek C, Reichenbach JR, Hecht H, Krieschel S, Gutberlet I, Miltner WH. 2003. Brain activation to phobia-related pictures in spider phobic humans: An event-related functional magnetic resonance imaging study. Neurosci Lett. 348:29–32.
- Elsesser K, Heuschen I, Pundt I, Sartory G. 2006. Attentional bias and evoked heart-rate response in specific phobia. Cogn Emot. 20:1092–1107.
- Fox E, Russo R, Bowles R, Dutton K. 2001. Do threatening stimuli draw or hold visual attention in subclinical anxiety?. J Exp Psychol Gen. 130:681–700.
- Fox E, Russo R, Dutton K. 2002. Attentional bias for threat: Evidence for delayed disengagement from emotional faces. Cogn Emot. 16:355–379.
- Globisch J, Hamm AO, Esteves F, Öhman A. 1999. Fear appears fast: Temporal course of startle reflex potentiation in animal fearful subjects. Psychophysiology. 36:66–75.
- Kindt M, Brosschot JF. 1997. Phobia-related cognitive bias for pictorial and linguistic stimuli. J Abnorm Psychol. 106:644–648.
- Klorman R, Hastings JE, Weerts TC, Melamed BG, Lang PJ. 1974. Psychometric description of some specific-fear questionnaires. Behav Ther. 5:401–409.
- Koster EHW, Crombez G, Verschuere B, Houwer JD. 2004. Selective attention to threat in the dot probe paradigm: Differentiating vigilance and difficulty to disengage. Behav Res Ther. 42:1183–1192.
- Lang PJ, Bradley MM, Cuthbert BN. 2001. International Affective Picture System (IAPS): Instruction manual and affective ratings, Technical report A-5. The Center for Research in Psychophysiology. University of Florida, Gainesville, FL.
- Lavie N. 1995. Perceptual load as a necessary condition for selective attention. J Exp Psychol Hum Percept Perform. 21:451–468.
- Lavy E, van den Hout M, Arntz A. 1993. Attentional bias and spider phobia: Conceptual and clinical issues. Behav Res Ther. 31:17–24.
- Linke R, Lima ADD, Schwegler H, Pape HC. 1999. Direct synaptic connections of axons from superior colliculus with identified thalamo-amygdaloid projection neurons in the rat: Possible substrates of a subcortical visual pathway to the amygdala. J Comp Neurol. 403:158–170.
- Lupianez J, Milliken B, Solano C, Weaver B, Tipper SP. 2001. On the strategic modulation of the time course of facilitation and inhibition of return. Q J Exp Psychol A. 54:753–773.
- Miltner WHR, Krieschel S, Hecht H, Trippe R, Weiss T. 2004. Eye movements and behavioral responses to threatening and nonthreatening stimuli during visual search in phobic and nonphobic subjects. Emotion. 4:323–339.
- Mogg K, Bradley BP. 1999. Some methodological issues in assessing attentional biases for threatening faces in anxiety: A replication study using a modified version of the probe detection task. Behav Res Ther. 37:595–604.
- Morris JS, Öhman A, Dolan RJ. 1998. Conscious and unconscious emotional learning in the human amygdala. Nature. 393:467–470.
- Morris JS, DeGelder B, Weiskrantz L, Dolan RJ. 2001. Differential extrageniculate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain. 124:1241–1252.
- Morris JS, Öhman A, Dolan RJ. 1999. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci USA. 96:1680–1685.
- Öhman A. 1992. Orienting and attention: Preferred preattentive processing of potential phobic stimuli. In: Campbell BA, Richardson R, Haynes H. editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Hillsdale, NJ: Erlbaum. p 263–295.
- Öhman A. 2002. Automaticity and the amygdala: Nonconscious responses to emotional faces. Curr Dir Psychol Sci. 11:62–66.
- Öhman A, Mineka S. 2001. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol Rev. 108:483–522.
- Öhman A, Soares JF. 1993. On the automatic nature of phobic fear: Conditioned electrodermal responses to masked fear-relevant stimuli. J Abnorm Psychol. 102:121–132.
- Öhman A, Soares JF. 1994. “Unconscious anxiety”: Phobic responses to masked stimuli. J Abnorm Psychol. 103:231–240.
- Öhman A, Soares JF. 1998. Emotional conditioning to masked stimuli: Expectancies for aversive outcomes following nonrecognized fear-relevant stimuli. J Exp Psychol Gen. 127:69–82.
- Öhman A, Flykt A, Esteves F. 2001. Emotion drives attention: Detecting the snake in the grass. J Exp Psychol Gen. 130:466–478.
- Okon-Singer H, Tzelgov J, Henik A. 2007. Distinguishing between automaticity and attention in the processing of emotionally significant stimuli. Emotion. 7:147–157.
- Pérez-Dueñas C, Acosta A, Lupianez J. 2009. Attentional capture and trait anxiety: Evidence from inhibition of return. J Anxiety Disord. 23:782–790.
- Posner MI. 1980. Orienting of attention. Q J Exp Psychol. 32:3–25.
- Posner M, Cohen Y. 1984. Components of visual orienting. In: Bouma H, Bouwhuis D. editors. Attention and performance X. London: Lawrence Erlbaum Associates Ltd. p 531–556.
- Schimmack U. 2005. Attentional interference effects of emotional pictures: Threat, negativity, or arousal?. Emotion. 5:55–66.
- Seligman ME. 1971. Phobias and preparedness. Behav Ther. 2:307–320.
- Shiffrin RM, Schneider W. 1977. Controlled and automatic human information-processing: II. Perceptual learning, automatic attending, and a general theory. Psychol Rev. 84:127–190.
- Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WHR. Effect of task conditions on brain responses to threatening faces in social phobics: An event-related functional magnetic resonance imaging study. Biol Psychiatry. 2004a; 56:921–930.
- Straube T, Mentzel HJ, Glauer M, Miltner WHR. Brain activation to phobia-related words in phobic subjects. Neurosci Lett. 2004b; 372:204–208.
- Straube T, Mentzel HJ, Miltner WHR. 2005. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 52:163–168.
- Straube T, Mentzel HJ, Miltner WHR. 2006. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry. 59:162–170.
- van den Hout M, Tenney N, Huygens K, de Jong P. 1997. Preconscious processing bias in specific phobia. Behav Res Ther. 35:29–34.
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. 2001. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 30:829–841.
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. 2003. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat Neurosci. 6:624–631.
- Williams JMG, Mathews A, MacLeod C. 1996. The emotional Stroop task and psychopathology. Psychol Bull. 120:3–24.