Abstract
The neuroendocrine and autonomic nervous systems are known regulators of brain–immune interaction. However, the functional significance of this interaction under stress is not fully understood. We investigated the effect of a stress paradigm by applying electric foot shock followed by three reminders, on behavior, immune parameters, and lymphoma tumor growth. Male C3H mice were divided into two groups: Group 1—exposed to electric foot shock followed by three reminders, and Group 2—untreated (controls). Sets of mice underwent the elevated plus maze, staircase, and hot plate tests. After foot shock, natural killer (NK) cell activity, and lymphocyte proliferation were measured. In addition, sets of mice were either vaccinated twice with B-cell lymphoma 38C-13 immunoglobulin for determination of anti-idiotype (Id) antibodies in sera, or inoculated with tumor cells and monitored for tumor development and survival time. Mice exposed to electric foot shock followed by the three reminders had higher NK cell activity, levels of anti-Id antibodies, and a higher proliferation rate of splenocytes in response to mitogens, than the control mice. The exposed mice also showed attenuated tumor growth. Thus, the stress paradigm inhibited tumor development and lead to some immune changes that were not accompanied by behavioral changes.
Introduction
Stress is a constellation of events, initiated by a stimulus (stressor) that precipitates a reaction in the brain (stress perception) which, in turn, activates physiologic systems in the body (stress response). The physiologic stress response results in the release of neurotransmitters and hormones that serve as the brain's messengers to the body (Dhabhar Citation2000). The consequences of the physiological response are generally adaptive in the short-term, but can be damaging when stress is long-lasting (Dhabhar Citation2009). Stress has long been suspected of playing a role in the etiology of many diseases. Numerous studies have shown that stress can be immunosuppressive. Glucocorticoid stress hormones are also widely regarded as being immunosuppressive and are used clinically as anti-inflammatory agents (Webster Marketon and Glaser Citation2008). At the same time stress and stress hormones could be also beneficial in preparing the immune system for dealing with potential immunological challenges (Dhabhar Citation2000, Citation2009).
A wide variety of stressors may alter different aspects of the immune response (Maier et al. Citation1994). Acute stress was associated with increased skin allograft survival time, increased retention of viruses in tissues, decreased numbers of circulating lymphocytes, and depressed in vitro mitogen responses. Furthermore, stress decreases natural killer (NK) cell cytotoxicity, a mechanism for the nonspecific killing of tumor cells (Moynihan et al. Citation1990; Li et al. Citation2005). The effects of stress on humoral (antibody-mediated) immunity are less uniform. In rats, for instance, different stressors have diverse effects on antibody responses. Moreover, after immunization there seemed to be a critical period in which electrical foot-shock reduced the immune cell response (Zalcman et al. Citation1988; Moynihan et al. Citation1990). The antibody response was markedly suppressed in mice restrained before antigen injection, but not in mice where the stress was applied after immunization (Okimura and Nigo Citation1986).
In animal models, a great deal of evidence has demonstrated the effects of behavioral stress on tumorigenesis and the biological mechanisms involved (Reiche et al. Citation2004; Antoni et al. Citation2006). Recent animal studies showed deleterious effects of stress on cancer development, and suggested a mediatory role for the sympathetic nervous system (Ben-Eliyahu et al. Citation2007). Experimental stressors were also found to increase the pathogenesis of various virally-mediated tumors in animal models (Reiche et al. Citation2004; Antoni et al. Citation2006). However, other studies showed converse results (Dhabhar et al. Citation2010; Hasen et al. Citation2010). Generally, when measuring the effects of stress on cancer, it is important to consider the duration of the stress application, type of stressor, and type of tumor (Trainor et al. Citation2009).
In addition to the physiological response, stress may also cause a behavioral reaction. Using a model of post-traumatic stress (Pynoos et al. Citation1996; Benaroya-Milshtein et al. Citation2004), we previously showed that mice exhibited an extended freezing time (freezing is defined as the lack of all movement apart from that necessary for respiration) during the first reminder of an aversive stimulus, and a significant increase in circulating corticosterone levels after the stress paradigm (achieved by electric shock with reminders) (Benaroya-Milshtein et al. Citation2004): both these parameters are accepted measures of fear and anxiety. As alterations in the stress-response system are believed to trigger many anxiety-related disorders (Weninger et al. Citation1999), in this study we applied anxiety-like behavior tests (Simiand et al. Citation1984; Hogg Citation1996; Weizman et al. Citation2001) to the same model. We also evaluated immunological parameters. In order to evaluate the effect of the stress paradigm on cancer progression, we used the experimental model of murine 38C-13 B-cell lymphoma (Kaminski et al. Citation1987; Haimovich et al. Citation1999; Benaroya-Milshtein et al. Citation2007).
Our hypothesis was that stress manipulation would increase the levels of anxiety-like parameters and induce some behavioral and immunological changes, as well as alterations in tumor progression. According to published literature, it is not yet clear whether stress manipulation enhances or suppresses immunity, and whether tumor growth is inhibited or enhanced under stress conditions. Thus, our study aimed to further elucidate these issues.
Materials and methods
Animals and rearing conditions
Ten-week-old C3H/eB male mice were obtained from the animal facility of Tel Aviv University, Israel. The C3H/eB mouse strain was selected because this inbred strain is the origin of the carcinogen-induced 38C-13 B-cell tumor that expresses immunoglobulin (IgM) on the cell surface. Thus, we used this strain for an experimental tumor model that included vaccination against the tumor-specific IgM-idiotype (Id) of 38C-13 cells (Benaroya-Milshtein et al. Citation2007). Experiments were conducted under permit number M 02-152, authorized by the Animal Care Committee of the Sackler Faculty of Medicine, Tel Aviv University. The minimal number of animals was used, and all efforts were made to minimize their suffering. Throughout the experiment, the animals were maintained on a 12 h light: 12 h dark lighting schedule (lights on at 06:00 h) in a room thermostatically maintained at 22 ± 1°C. Food and water were available ad libitum. Mice were housed, 10 per cage, in standard plastic cages (31 × 22 × 15 cm); the housing conditions conformed with the European Economic Community directive 86/609.
Experimental design
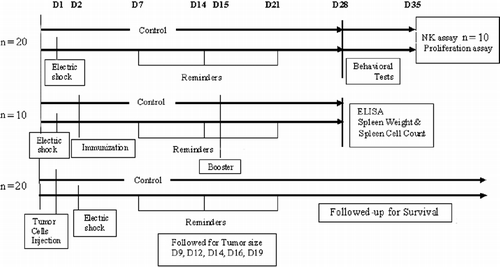
A total of 50 mice were used. At the age of 10 weeks, 25 mice were assigned to the stressed group and 25 to the control group. Mice were weighed before and after the stress paradigm, but there was no difference in body weight gain between the stressed and control groups. Different sets of mice were used for each of the following experiments: (1) determination of behavioral parameters (n = 20), NK-cell-mediated cytotoxicity (n = 10), and lymphocyte proliferation (n = 20); (2) determination of anti-Id antibodies in serum, spleen weight and spleen cell counts (n = 10); and (3) tumor cell challenge (n = 20). The timeline () illustrates the sequence of events.
Stress paradigm
We employed a modified time-dependent sensitization model of repeated exposures to situational reminders of a prior exposure to an aversive stimulus (Pynoos et al. Citation1996; Benaroya-Milshtein et al. Citation2004). Foot shock was produced by a Gemini Avoidance System (San Diego Instruments, San Diego, CA, USA). The experiment was conducted between 09:00 and 12:00 h in the same room in which the mice were housed. The mice were placed in a dual-compartment apparatus. After a 10 s (s) adaptation period, a guillotine door was opened and a bright light switched on in the compartment where the mouse was placed. The door remained open until the mouse entered the dark compartment. The door was then closed, and the mouse received a series of foot shocks of 1 mA intensity for 10 s (12 pulses per minute, duration of pulse 2 ms). Shocks were delivered to the chamber floor via a 150-V source. The situational reminder, performed once a week for 3 weeks, necessitated placing a mouse in the lit chamber with the gate closed to prevent it from entering the shock compartment. Due to the high tumorigenicity of the 38C-13 tumor (most mice died within 25 days after inoculation) we shortened the stress model from five to three reminders. Our previous study showed that three reminders led to a significant increase in circulating corticosterone level, an accepted measure of physiological stress (Pynoos et al. Citation1996; Benaroya-Milshtein et al. Citation2004).
Cell lines
For this study, we used 38C-13 cells (produced in the laboratory of N.H.), a carcinogen-induced B-cell tumor that expresses IgM on the cell surface (Bergman and Haimovich Citation1977; Haimovich et al. Citation1999). Cells were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM 2-mercaptoethanol. One set of mice (from each of the study and control groups) was injected subcutaneously (right side of back) with tumor cells (1 × 105 38C-13) 24 h before electric shock induction in the stress paradigm.
Immunization
The 38C-13 IgM protein (produced in the laboratory of N.H.) was coupled to keyhole limpet hemocyanin (KLH) using 0.1% glutaraldehyde (Maloney et al. Citation1985). Ten-week-old mice were injected i.p with Id-KLH (25 μg/mouse) emulsified in complete Freund's adjuvant (Difco Laboratories Inc., Detroit, MI, USA) at a total volume of 0.2 ml, and received a booster dose 2 weeks later with the same conjugate in incomplete Freund's adjuvant (Difco Laboratories Inc). The stressed mice were inoculated with 38C-13 IgM 24 h after electric shock induction in the stress paradigm, and boosted 24 h after the second reminder.
Behavioral tests
At age 14 weeks (1 week after the last reminder in the stress paradigm) the study and control mice were subjected to the following behavioral tests, in random order: elevated plus maze (Hogg Citation1996; Holmes et al. Citation2002; Benaroya-Milshtein et al. Citation2004), staircase (Simiand et al. Citation1984; Weizman et al. Citation2001), and hot plate (Pick et al. Citation1991). The behavioral experiments were conducted between 09:00 and 12:00 h in the same room in which the mice were housed. Each behavioral test was performed on a different day. Scoring of the tests was performed during the testing period.
Elevated plus maze
The elevated plus maze was constructed according to the description of Holmes et al. (Citation2002). The elevated plus maze was made from polyvinylchloride and built in a “plus” form, comprising two opposing closed black arms (34 × 7.5 × 17.5 cm) and two opposing open white arms (34 × 7.5 × 1 cm). The center of the four arms comprised a square area, 7.5 × 7.5 cm. The maze was elevated 50 cm above ground level; to prevent mice from falling off the apparatus, a small lip (1 cm) encompassed the edge of the open arms. In the current study, each mouse was placed separately in the center of the maze, facing an open arm, and allowed to freely explore the apparatus for 5 min. Parameters measured during the testing period included the total number of arm entries, the total closed-arm entries, the percentage of open-arm entries (open/total × 100), and the percentage time spent in open arms of the maze (time in open arms/session duration × 100). Closed-arm entries are an accepted index of motor function, and the open-arm measures are accepted indices of anxiety-like behavior (Hogg Citation1996; Holmes et al. Citation2002; Benaroya-Milshtein et al. Citation2004). A four-paw entry criterion was used for arm entries. The number of fecal boli was also recorded. After 5 min the mouse was removed, and the apparatus was cleaned with an alcohol sponge to eliminate residual odors.
Staircase
The staircase was constructed according to the description of Simiand et al. (Citation1984). The staircase was made from polyvinylchloride and consisted of five identical steps (height 2.5 cm × width 10 cm × depth 7.5 cm). The height of the walls was constant (12.5 cm above the stairs) along the entire length of the staircase. In this study, each mouse was placed individually onto the staircase. A step was considered as climbed only if the mouse placed all four paws on the stair. The number of stairs climbed is an accepted locomotor and exploratory index. The number of steps descended was not counted. Rearing was recorded when the mouse rose on its hind legs either on the step, or against the wall, to sniff the air. Rearing is considered an anxiety and exploratory index. Scoring of the test was performed during the testing period. After 3 min the mouse was removed, and the staircase was cleaned with an alcohol sponge to eliminate residual odors (Simiand et al. Citation1984; Weizman et al. Citation2001).
Hot plate
Mice were tested with the hot plate analgesia meter Model 35D (IITC Inc., Woodland Hills, CA, USA; Pick et al. Citation1991) to determine the nociceptive threshold. This device comprises a metal plate (40 × 35 cm) on which a plastic cylinder is placed. In our study, the analgesia meter was set to a plate temperature of 52 ± 0.5°C. The latency (in s) from the time the mouse was placed on the hot plate surface until it licked its back paw, or robustly jerked its back paw, or jumped out was recorded during the testing period (Schreiber and Pick Citation2006).
Physiological tests
Cytotoxicity assay
For this assay, a set of 15-week-old mice (half of which had been exposed to the stress paradigm) was killed by cervical dislocation without anesthesia. NK cell activity was assessed by a standard chromium (Cr) release assay that measures cell anti-tumor cytotoxicity. To prepare the effector cells, spleens were gently teased apart to obtain cell suspensions, and erythrocytes were removed by hypo-osmolar lysis with ACK lysing buffer. The spleen cells were then washed three times, resuspended in RPMI medium supplemented with 10% fetal calf serum, and distributed into 96-well V-bottom microplates at different concentrations according to the desired four effector-to-target-cell ratios (100:1, 50:1, 25:1, 12.5:1). YAC-1 target cells were labeled by incubation for 1 h with 200 μCi of 51Cr (NEN). The labeled cells were washed three times, resuspended in medium, and added to the microplate wells at 1 × 104 cells/well. Plates were centrifuged for 5 min at 100g and incubated for 4 h at 37°C in a humidified incubator containing 5% CO2. After incubation, the plates were centrifuged for 5 min at 300g, and the radioactivity in the supernatants was determined with a gamma counter (Model 1185; Tracor Analytic, Elk Grove. Village, IL, USA). Spontaneous 51Cr release was evaluated by incubation of target cells without effector cells. Maximal releasable radioactivity was evaluated by incubation of target cells with 1% Triton X-100. Samples were run in triplicate, and the percentage of lysis was calculated using the formula: (Experimental counts per minute (CPM) − spontaneous CPM)/(maximal releasable CPM − spontaneous CPM) × 100 (Zalcman et al. Citation1991).
Lymphocyte proliferation
Lymphocyte proliferation was determined in response to concanavalin A (Con A) and lipopolysaccharide (LPS) at age 15 weeks. Spleen cells, prepared as aforementioned, were cultured at 2 × 105 cells/well in flat-bottom microtiter plates in 0.2 ml medium containing no mitogen, 5 μg Con A/ml (Sigma-Aldrich, St Louis, MO, USA), or 50 μg LPS/ml (Sigma-Aldrich). The cultures were incubated in a humidified 37°C incubator in an atmosphere of 5% CO2. After 24 h incubation, the cultures were pulsed with 1 μ Ci/well (10 μl) of [3H]thymidine and incubated for an additional 24 h. Cells were harvested using a cell harvester, and the incorporated radioactivity was determined by liquid scintillation counting. The results were expressed as mean CPM of triplicate cultures. The proliferation index (PI) represents the ratio of CPM of lymphocytes incubated with mitogen divided by CPM of lymphocytes incubated without mitogen (Hollander Citation1985; Esquifino et al. Citation1996).
Enzyme-linked immunosorbent assay
Levels of anti-Id antibodies in sera of immunized mice were determined by enzyme-linked immunosorbent assay (ELISA). Blood was drawn from the retro-orbital sinus of the mice; serum was separated by centrifugation (1560g. for 15 min). IgM-Id protein at a concentration of 2 μg/ml was adsorbed to 96-well ELISA plates for 18 h at 4°C. Plates were then washed, blocked with 1% bovine serum albumin (BSA) for 1 h at 37°C, and rewashed. Serial dilutions of sera were then added to the plates for 2 h at room temperature. The plates were washed and horseradish-peroxidase-conjugated rabbit anti-mouse IgG (Jackson Immuno Research, West Grove, PA, USA) was added for 1 h at room temperature. The plates were then washed again and developed for 15 min with 1,2-orthophenylenediamine. Mouse serum containing a known amount of anti-Id antibodies (0.1 mg/ml) was used as a standard in all antibody titrations (a quality control for sensitivity, which was 1 ng/ml). Sera from two nonimmunized mice served as a quality control for specificity. The coefficient of variation in the assay was 12%. Absorbance at 450 nm was measured by an ELISA plate reader (SPECTRAmax 340, Molecular Devices, Sunnyvale, CA, USA).
Spleen weight and spleen cell counts of immunized mice
A set of 15-week-old mice (50% of which had been exposed to the stress paradigm) was killed by cervical dislocation, spleens were harvested and immediately weighed. Spleens were gently teased apart to obtain cell suspensions, and cells counted under a light microscope. Spleens were gently teased apart to obtain cell suspensions, and cells were counted under a light microscope.
Tumor development and survival time
Mice were challenged subcutaneously with 1 × 105 38C-13 tumor cells and followed-up for tumor growth and survival time. Tumor size was measured by calipers 3 times a week. Tumor area was calculated by multiplying horizontal diameter by vertical diameter (Benaroya-Milshtein et al. Citation2007). For determination of survival time, mice were allowed to die from the disease (this procedure was approved by the Animal Care Committee at the time of experiments). Nevertheless, mice that were in distress were euthanized.
Statistical analysis
All results were calculated as mean ± SEM values and analyzed with the statistical package for the social sciences (SPSS) version 14 for Windows (SPSS Inc., Chicago, IL, USA). Unpaired Student's t-tests were used to compare behavioral parameters in the stressed and control mice. The lymphocyte proliferation results were evaluated by one-way ANOVA. When significant group effects were detected, the least squares difference test indicated significant post-hoc differences between individual groups. Differences between groups in NK cell activity, ELISA results, spleen weight, spleen cells, and tumor size were analyzed by ANOVA with repeated measures. Significance was set at the P < 0.05 level.
Results
Effect of stress paradigm on behavior
Analysis of the behavioral battery results at age 14 weeks yielded no significant differences between the stressed and control mice ().
Table I. Results of behavioral tests in stressed and control mice.
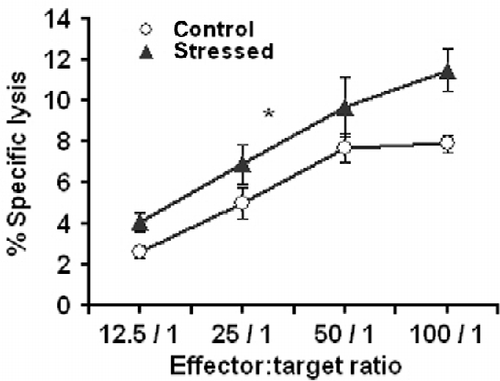
NK cell activity in stressed mice
shows the dose-response curve for splenic NK cell activity in mice killed at age 15 weeks after stress conditioning, and in control mice. ANOVA with repeated measures yielded a significantly higher NK cytotoxicity in the stressed mice than the controls (F1,8 = 5.39, P < 0.05).
Figure 2. NK-cell-mediated cytotoxic activity. Effect of stress conditioning on spleen NK-cell-mediated cytotoxicity assessed by the Cr release assay in 15-week-old mice. In this assay, target tumor cells were labeled intracellularly with 51Cr and then incubated with NK cells. Lysis of the target cells by the effector NK cells released intracellular 51Cr which was measured. The amount of 51Cr released correlates directly with the number of target cells lysed by the NK cells. The x-axis represents different effector cell concentrations expressed as effector: Target cell ratios (100: 1, 50: 1, 25: 1 and 12.5: 1). The y-axis represents the percentage of lysis, calculated as follows: 100 × (experimental CPM–spontaneous CPM)/(maximal releasable CPM–spontaneous CPM). *Denotes significant differences between the groups; P < 0.05 (ANOVA with repeated measures). Values are expressed as mean ± SEM (n = 5 per group).
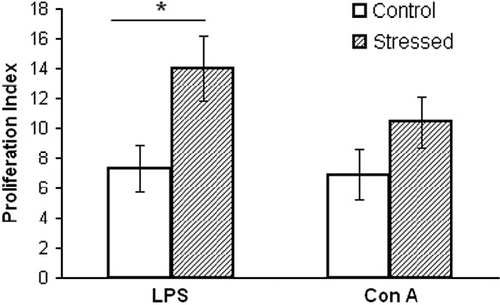
Lymphocyte proliferation in stressed mice
In the absence of mitogens, the proliferation of spleen lymphocytes at age 15 weeks was similar in the stress and control groups. However, as depicted in , the presence of mitogens resulted in significant differences among the mice in the two groups (F3,36 = 3.38, P < 0.05). The PI of the total lymphocyte population in the presence of LPS was significantly (P < 0.05) higher in the stressed mice.
Figure 3. Spleen lymphocyte response to the mitogens, LPS, and Con A, was tested in 15-week-old mice after stress conditioning, and controls. The PI was calculated in each mouse as the ratio of lymphocytes incubated with and without mitogen. The stressed mice demonstrated a greater lymphocyte response. *Denotes significant differences between the groups; P < 0.05, (ANOVA followed by post-hoc LSD). Values are expressed as mean ± SEM (n = 10 per group).
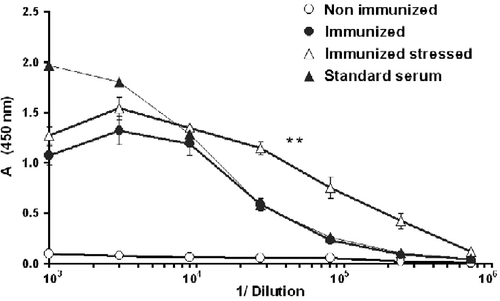
Levels of anti-Id antibodies
To study anti-Id antibody levels, sera were collected 2 weeks after the second immunization. Mouse serum containing a known amount of anti-Id antibodies (0.1 mg/ml) was used as a standard in all antibody titrations. ANOVA with repeated measures yielded significantly higher levels of anti-Id antibodies in the stressed than in the control mice (F1,8 = 17, P < 0.01; ).
Figure 4. Ten-week-old mice were immunized twice with 38C-13 IgM, conjugated to KLH. Sera were collected 2 weeks after the second immunization and assessed for anti-idiotype (Id) antibody levels by ELISA. Mouse serum, containing a known amount of anti-Id antibodies (0.1 mg/ml) was used as a standard. Two mice that were neither immunized nor stressed served as controls. Absorbance (A) at 450 nm was measured by an ELISA reader. Stress conditioning increased antibody levels in IgM-Id-immunized mice. **Denote significant differences between the groups; P < 0.01 (ANOVA with repeated measures; n = 5). Values are expressed as mean ± SEM.
Spleen weight and cell count of immunized mice
The spleens of stressed-immunized, or untreated mice killed at age 15 weeks were weighed and spleen cells counted. The stressed mice had slightly, but not significantly, higher spleen weights (274 ± 26 vs. 256 ± 18 mg). Total white blood cells (WBC) of stressed-immunized mice was higher (658 ± 155 × 106 vs. 357 ± 42 × 106), tending to significance (P = 0.062). No difference was found in total RBC counts (423 ± 183 × 106 vs. 425 ± 90 × 106).
Tumor development and survival
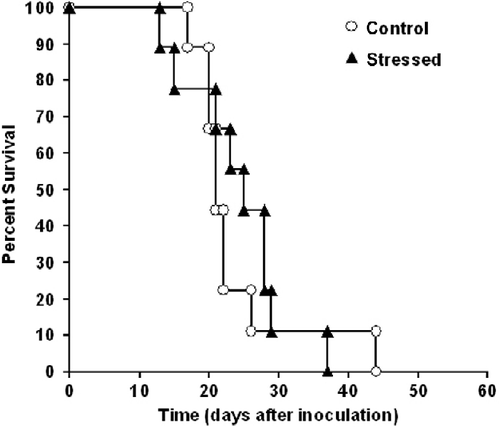
At the age of 10 weeks, sets of mice were inoculated subcutaneously with 1 × 105 38C-13 lymphoma cells and monitored for tumor growth and survival. The next day (as illustrated in ) the stressed mice received an electric shock, followed by reminders once a week for 3 weeks. The results revealed a significant difference in the size of the primary subcutaneous tumor between the two groups, with smaller tumors in the stressed mice (F1,16 = 5.66, P < 0.05; ). Although the point of 50% survival was day 21 for control mice and day 25 for stressed mice, no significant difference was found in overall survival time between the groups. Survival time denotes the time from inoculation of the tumor cells until the death of each mouse ().
Figure 5. Ten-week-old mice were inoculated subcutaneously with 1 × 105 38C-13 B-cells. The next day, 50% of the mice underwent electric shock, followed by three reminders. Stress conditioning decreased primary subcutaneous tumor growth. **Denotes significant differences between groups; P < 0.05 (ANOVA with repeated measures; n = 9). Values are expressed as mean ± SEM.
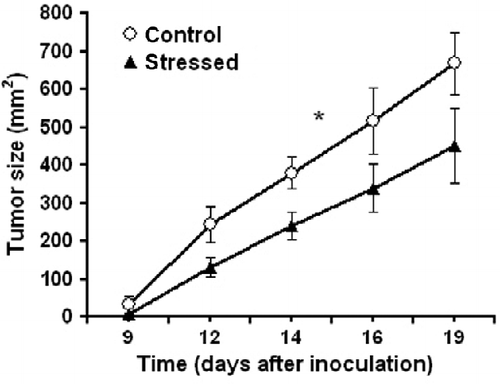
Figure 6. No significant difference was found in survival time between stressed and control mice. Ten-week-old mice were inoculated subcutaneously with 1 × 105 38C-13 B-cells. The next day, 50% of the mice underwent electrical foot-shocks (followed by three reminders). Mice were monitored for survival time (log rank; n = 9 in each group).
Discussion
No differences in behavioral measures were found between stressed and control mice, even though stress was associated with prominent physiological and cellular changes. Specifically, the stress paradigm led to an increased immune reactivity and attenuated tumor development. The immune changes were manifested by higher NK cell cytolytic activity, levels of anti-Id antibodies in immunized mice, and a higher PI of splenocytes in the presence of LPS.
In our previous study using this paradigm, the results showed an increase in corticosterone production (serum concentration: 117 ± 20 ng/ml in stressed mice vs. 23.5 ± 1 ng/ml in the controls) (Benaroya-Milshtein et al. Citation2004), a known measure of stress. Nevertheless, in the current study, we did not observe fear and anxiety-like behaviors on the elevated plus maze test. This finding concurs with earlier reports that some stressors induce fear potentiation in the elevated plus maze whereas other stressors, such as foot shock, do not (Steenbergen et al. Citation1991; Falter et al. Citation1992; Grahn et al. Citation1995; Korte and De Boer Citation2003).
Besides the various types of stressors, this discrepancy may be explained by differences in the intensity and duration of the stress in relation to changes in hypothalamic–pituitary–adrenal axis (HPA) activity, e.g. in animal models, social defeat, forced swimming, and restraint were all associated with higher plasma corticosterone stress responses (300–600 ng/ml) (Heinrichs et al. Citation1992, Citation1994; Koob et al. Citation1993; Koolhaas et al. Citation1997) than foot shock (100–200 ng/ml) (Roozendaal et al. Citation1991; Korte et al. Citation1992; Benaroya-Milshtein et al. Citation2004). The interval between the stress application and the elevated plus maze test may be of relevance. Ruis et al. (Citation1999) in his model reported a significantly enhanced anxiety state in the elevated plus maze immediately and 24 h after social defeat (day 0), but not 2 days later (Ruis et al. Citation1999).
Handling may alter behavior, HPA axis, opioid, and dopaminergic systems (Gariepy et al. Citation2002). As a consequence of the procedure, it could be contended that the stressed mice were handled more frequently than the controls. However, as the mice were handled briefly before and after the procedure, it is unlikely that this issue could be considered a confounding factor.
Our important findings included the enhancement of NK cell activity, humoral immune response, and LPS-induced lymphocyte proliferation by the stress paradigm. Psychological and physical stressors can substantially alter the distribution pattern of immune cells in the body, especially of B and NK cells. Studies have also shown that glucocorticoid and catecholamine hormones induce rapid and significant changes in leukocyte distribution (Dhabhar Citation2009). Pharmacological tests have shown that prednisolone induces retention of circulating lymphocytes within the bone marrow, spleen, and some lymph nodes (Dhabhar et al. Citation1995; Engler et al. Citation2004). However, endogenous hormones in physiological concentrations could have immune-enhancing effects (Dhabhar Citation2009). Our previous findings of higher corticosterone levels within the normal physiological range may explain the increased levels of WBC in the spleen, and a higher humoral immune response in lymph nodes.
The humoral immune response reflects a well-controlled multistep homeostatic response to antigen challenge (Stanojevic et al. Citation2003): The antibodies are responsible for neutralizing and clearing the antigen, and play a major role in the destruction of tumor cells. Studies of Id-vaccinated mice showed that anti-Id antibodies were involved in the destruction of B-cell lymphoma (Campbell et al. Citation1988; George et al. Citation1988). NK cells are a subpopulation of leukocytes involved in innate immunity, and are known to recognize and kill a variety of tumors and virally-infected cells (Lu et al. Citation1998; Ben-Eliyahu et al. Citation1999). Therefore, their enhanced activity under stress conditions has important implications. In our previous paper, (Benaroya-Milshtein et al. Citation2004) we could not show a significant effect of stress on NK cell activity, most probably due to technical obstacles (we used different effector-to-target cell ratios in each experiment). In the same mouse strain, it was shown that individually-housed, tumor-injected mice had higher NK activity compared to a control group on day 21 after tumor cell injection (Hoffman-Goetz et al. Citation1992). The NK cells are known to be activated via neuroendocrine pathways, thus acute stress may be sufficient to stimulate the increase of NK cell activity (Sharify et al. Citation2007).
Several studies have demonstrated a similar effect of stress on these immunological parameters (Dhabhar and Viswanathan Citation2005; Sharify et al. Citation2007). However, other studies noted converse effects. Specifically, inescapable and uncontrollable stress, applied as a series of intermittent shocks, was found to suppress immune function, especially NK cell activity (Shavit et al. Citation1984; Laudenslager et al. Citation1988; Zalcman et al. Citation1991). These variations could be explained by the overall duration of the stress session.
The mice were placed in our paradigm for only 10 s. Other investigators employed extended periods occasionally up to 90 min, or repeated the session over a few days (Rowse et al. Citation1995; Li et al. Citation2005). We measured the NK cell response 5 weeks after the initial shock and 2 weeks after the last reminder; others measured the response immediately after the stress procedure or within the next few days. We have previously shown that the freezing response to reminders disappeared 2 weeks after the initial shock (Benaroya-Milshtein et al. Citation2004). The nature of the stress, its duration, and the interval between the stress and immune measurements are extremely important. During the first weeks a decrease was noted in lymphocyte cytotoxicity and mitogen responsiveness, whereas later a significant increase in the same immune functions may occur. This is a rebound overshoot indicating the importance of timing the stress relative to the measurement (Borysenko and Borysenko Citation1982). Thus, an acute stress response may induce biphasic changes in blood leukocyte numbers. At onset of stress, or during mild acute stress, or exercise, catecholamine hormones induce the leukocytes to enter the blood vessels, the effect being most prominent for NK cells and granulocytes. As the stress response continues, activation of the HPA axis releases glucocorticoid hormones that induce leukocytes to emerge from the blood and enter the body organs, including lymph nodes and spleen, in preparation for immune challenges (Dhabhar Citation2009).
It is of interest that Stanojevic et al. (Citation2003) demonstrated that direct exposure to an electric shock during primary immunization with BSA significantly suppressed specific anti-BSA antibody production, but shock-witnessing procedures enhanced the specific secondary humoral immune response. The shock-witnessing model involved placing the animals in the apparatus for electric shock delivery and exposing them to pheromones and vocalization from the stressed animals throughout the 5 days of testing (Stanojevic et al. Citation2003). These experiments suggest that contrary to severe stress, mild stress may be immunoenhancing and not immunosuppressive. The release of endogenous opioid peptides may partially mediate the enhancing and suppressive effects of stress on the immune system (Stanojevic et al. Citation2003). Similarly, endogenous opioids play a role in the enhancement of the secondary antibody response observed after moderate exercise (Kapasi et al. Citation2001). Opioids could have contradictory effects as different doses of opioid peptides, such as met-enkephalin (Met-Enk), are involved. It was shown that lower doses of Met-Enk potentiated, but higher doses suppressed numerous immunological functions. It is also possible that other opioid peptides and receptors are activated during severe and mild stress (Stanojevic et al. Citation2003).
The majority of studies in the field of psychoneuro-immunology have focused on the immunosuppressive effects of stress. Nevertheless, several studies revealed that under certain conditions, stress could be immunoenhancing. Generally, acute stress is immunoenhancing and chronic stress is immunosuppressive. The stress-induced enhancement of immune function may be an adaptive response to prepare the organism for potential immunologic challenges (e.g. a wound, or infection inflicted by an attacker) for which stress perception by the brain, and subsequent stress hormone and neurotransmitter release, may serve as an early warning (Dhabhar Citation2000). In addition to their well-known immunosuppressive effects, glucocorticoids also possess immunomodulating and immunoenhancing effects. Overall, pharmacological concentrations of glucocorticoids exert immunosuppressive effects, whereas under different conditions, physiological concentrations may exert immunomodulatory, immunoenhancing, or immunosuppressive effects (Jefferies Citation1991; Wilckens Citation1995; Wilckens and De Rijk Citation1997).
In addition to the immune changes, the exposed mice in our paradigm also demonstrated attenuated tumor growth, but there were no differences from controls regarding survival time. Immune mechanisms are possibly the underlying cause of the impact of stress on tumor growth. Short-term stress induces leukocyte trafficking to the skin and sentinel lymph nodes accompanied by an increase in innate and adaptive cutaneous immunity (Dhabhar et al. Citation2010). In our study, tumor cells were injected subcutaneously at a site accessible to redistributed immune cells. Specifically, stressed mice manifested an increased immunologic reactivity in response to Id-vaccination. Furthermore, we have demonstrated that the stress paradigm enhances NK cell activity. These two immune components are enhanced in stressed mice and may contribute to their tumor resistance.
Stress attenuation of tumor growth was confirmed by previous studies (Pradhan and Ray Citation1974; Ray and Pradhan Citation1974; Bhattacharyya and Pradhan Citation1979; Riley Citation1981; Dhabhar Citation2009). In mice maintained in a low-stress environment rather than in a conventional animal room, transplanted tumors showed slower growth (Riley Citation1981). However, other studies reported a converse effect (Amkraut and Solomon Citation1972; Ben-Eliyahu et al. Citation1999, Citation2007). Justice (Citation1985) reviewed the effects of stress on cancer in laboratory animals, and indicated the importance of time of stress application and type of tumor. He suggested that nonviral tumor growth was inhibited by ongoing exposure to stressors, but were stimulated by the rebound following stress termination. Although the immune response of nonviral tumors is less clear than that of viral tumors, the effects on both types are consistent with mediation by biphasic changes of sympathetic and parasympathetic activity (Justice Citation1985).
Interestingly, when the present findings are combined with our previous results, we can show that the effects of the stress paradigm on immune parameters are analogous with the effects of environmental enrichment (Benaroya-Milshtein et al. Citation2004, Citation2007). Thus, enriched conditions may be viewed as a form of repeated mild stress. Indeed, both environmental enrichment and acute stress are forms of behavioral interventions that lead to endogenous immune-enhancement, at least in some immune parameters. These findings merit further investigation: (1) To broaden the understanding of immune changes after behavioral manipulations; (2) to reveal the mediators between behavioral manipulations and immune changes; and (3) to attempt demonstration of a direct relationship between immune changes and tumor attenuation. By investigating the stress anti-tumor mechanisms, we hope to obtain a better understanding of cancer progression and therapy.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amkraut A, Solomon GF. 1972. Stress and murine sarcoma virus (Moloney)-induced tumors. Cancer Res. 32:1428–1433.
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. 2006. The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nat Rev Cancer. 6:240–248.
- Benaroya-Milshtein N, Hollander N, Apter A, Kukulansky T, Raz N, Wilf A, Yaniv I, Pick CG. 2004. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur J Neurosci. 20:1341–1347.
- Benaroya-Milshtein N, Apter A, Yaniv I, Kukulansky T, Raz N, Haberman Y, Halpert H, Pick CG, Hollander N. 2007. Environmental enrichment augments the efficacy of idiotype vaccination for B-cell lymphoma. J Immunother. 30:517–522.
- Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. 1999. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 80:880–888.
- Ben-Eliyahu S, Page GG, Schleifer SJ. 2007. Stress, NK cells, and cancer: Still a promissory note. Brain Behav Immun. 21:881–887.
- Bergman Y, Haimovich J. 1977. Characterization of a carcinogen-induced murine B lymphocyte cell line of C3H/eB origin. Eur J Immunol. 7:413–417.
- Bhattacharyya AK, Pradhan SN. 1979. Effects of stress on DMBA-induced tumor growth, plasma corticosterone and brain biogenic amines in rats. Res Commun Chem Pathol Pharmacol. 23:107–116.
- Borysenko M, Borysenko J. 1982. Stress, behavior, and immunity: Animal models and mediating mechanisms. Gen Hosp Psychiatry. 4:59–67.
- Campbell MJ, Esserman L, Levy R. 1988. Immunotherapy of established murine B cell lymphoma: Combination of idiotype immunization and cyclophosphamide. J Immunol. 141:3227–3233.
- Dhabhar FS. 2000. Acute stress enhances while chronic stress suppresses skin immunity: The role of stress hormones and leukocyte trafficking. Ann N Y Acad Sci. 917:876–893.
- Dhabhar FS. 2009. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation. 16:300–317.
- Dhabhar FS, Viswanathan K. 2005. Short-term stress experienced at time of immunization induces a long-lasting increase in immunologic memory. Am J Physiol Regul Integr Comp Physiol. 289:R738–R744.
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. 1995. Effects of stress on immune cell distribution: Dynamics and hormonal mechanisms. J Immunol. 154:5511–5527.
- Dhabhar FS, Saul AN, Daugherty C, Holmes TH, Bouley DM, Oberyszyn TM. 2010. Short-term stress enhances cellular immunity and increases early resistance to squamous cell carcinoma. Brain Behav Immun. 24:127–137.
- Engler H, Bailey MT, Engler A, Sheridan JF. 2004. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol. 148:106–115.
- Esquifino AI, Selgas L, Arce A, Maggiore VD, Cardinali DP. 1996. Twenty-four-hour rhythms in immune responses in rat submaxillary lymph nodes and spleen: Effect of cyclosporine. Brain Behav Immun. 10:92–102.
- Falter U, Gower AJ, Gobert J. 1992. Resistance of baseline activity in the elevated plus-maze to exogenous influences. Behav Pharmacol. 3:123–128.
- Gariepy JL, Rodriguiz RM, Jones BC. 2002. Handling, genetic and housing effects on the mouse stress system, dopamine function, and behavior. Pharmacol Biochem Behav. 73:7–17.
- George AJ, Folkard SG, Hamblin TJ, Stevenson FK. 1988. Idiotypic vaccination as a treatment for a B cell lymphoma. J Immunol. 141:2168–2174.
- Grahn RE, Kalman BA, Brennan FX, Watkins LR, Maier SF. 1995. The elevated plus-maze is not sensitive to the effect of stressor controllability in rats. Pharmacol Biochem Behav. 52:565–570.
- Haimovich J, Kukulansky T, Weissman B, Hollander N. 1999. Rejection of tumors of the B cell lineage by idiotype-vaccinated mice. Cancer Immunol Immunother. 47:330–336.
- Hasen NS, O'Leary KA, Auger AP, Schuler LA. 2010. Social isolation reduces mammary development, tumor incidence, and expression of epigenetic regulators in wild-type and p53-heterozygotic mice. Cancer Prev Res (Phila PA). 3:620–629.
- Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. 1992. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 581:190–197.
- Heinrichs SC, Menzaghi F, Pich EM, Baldwin HA, Rassnick S, Britton KT, Koob GF. 1994. Anti-stress action of a corticotropin-releasing factor antagonist on behavioral reactivity to stressors of varying type and intensity. Neuropsychopharmacology. 11:179–186.
- Hoffman-Goetz L, MacNeil B, Arumugam Y. 1992. Effect of differential housing in mice on natural killer cell activity, tumor growth, and plasma corticosterone. Proc Soc Exp Biol Med. 199:337–344.
- Hogg S. 1996. A review of the validity and variability of the elevated plus-maze as an. Pharmacol Biochem Behav. 54:21–30.
- Hollander N. 1985. Antibodies to nonpolymorphic determinants of the Thy-1 molecule inhibit T cell proliferation. J Immunol. 134:2916–2921.
- Holmes A, Yang RJ, Crawley JN. 2002. Evaluation of an anxiety-related phenotype in galanin overexpressing. J Mol Neurosci. 18:151–165.
- Jefferies WM. 1991. Cortisol and immunity. Med Hypotheses. 34:198–208.
- Justice A. 1985. Review of the effects of stress on cancer in laboratory animals: Importance of time of stress application and type of tumor. Psychol Bull. 98:108–138.
- Kaminski MS, Kitamura K, Maloney DG, Levy R. 1987. Idiotype vaccination against murine B cell lymphoma: Inhibition of tumor immunity by free idiotype protein. J Immunol. 138:1289–1296.
- Kapasi ZF, Catlin PA, Beck J, Roehling T, Smith K. 2001. The role of endogenous opioids in moderate exercise training-induced enhancement of the secondary antibody response in mice. Phys Ther. 81:1801–1809.
- Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. 1993. The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Found Symp. 172:277–289 discussion 290–275.
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. 1997. Social stress in rats and mice. Acta Physiol Scand Suppl. 640:69–72.
- Korte SM, De Boer SF. 2003. A robust animal model of state anxiety: Fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 463:163–175.
- Korte SM, Buwalda B, Bouws GA, Koolhaas JM, Maes FW, Bohus B. 1992. Conditioned neuroendocrine and cardiovascular stress responsiveness accompanying behavioral passivity and activity in aged and in young rats. Physiol Behav. 51:815–822.
- Laudenslager ML, Fleshner M, Hofstadter P, Held PE, Simons L, Maier SF. 1988. Suppression of specific antibody production by inescapable shock: Stability under varying conditions. Brain Behav Immun. 2:92–101.
- Li Q, Liang Z, Nakadai A, Kawada T. 2005. Effect of electric foot shock and psychological stress on activities of murine splenic natural killer and lymphokine-activated killer cells, cytotoxic T lymphocytes, natural killer receptors and mRNA transcripts for granzymes and perforin. Stress. 8:107–116.
- Lu ZW, Song C, Ravindran AV, Merali Z, Anisman H. 1998. Influence of a psychogenic and a neurogenic stressor on several indices of immune functioning in different strains. Brain Behav Immun. 12:7–22.
- Maier SF, Watkins LR, Fleshner M. 1994. Psychoneuroimmunology: The interface between behavior, brain, and immunity. Am Psychol. 49:1004–1017.
- Maloney DG, Kaminski MS, Burowski D, Haimovich J, Levy R. 1985. Monoclonal anti-idiotype antibodies against the murine B cell lymphoma 38C13: Characterization and use as probes for the biology of the tumor in vivo and in vitro. Hybridoma. 4:191–209.
- Moynihan JA, Ader R, Grota LJ, Schachtman TR, Cohen N. 1990. The effects of stress on the development of immunological memory following low-dose antigen priming in mice. Brain Behav Immun. 4:1–12.
- Okimura T, Nigo Y. 1986. Stress and immune responses. I. Suppression of T cell function in restraint-stressed mice. Jpn J Pharmacol. 40:505–511.
- Pick CG, Cheng J, Paul D, Pasternak GW. 1991. Genetic influences in opioid analgesic sensitivity in mice. Brain Res. 566:295–298.
- Pradhan SN, Ray P. 1974. Effects of stress on growth of transplanted and 7,12-dimethylbenz(alpha)anthracene-induced tumors and their modification by psychotropic drugs. J Natl Cancer Inst. 53:1241–1245.
- Pynoos RS, Ritzmann RF, Steinberg AM, Goenjian A, Prisecaru I. 1996. A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biol Psychiatry. 39:129–134.
- Ray P, Pradhan SN. 1974. Growth of transplanted and induced tumors in rats under a schedule of punished behavior. J Natl Cancer Inst. 52:575–577.
- Reiche EM, Nunes SO, Morimoto HK. 2004. Stress, depression, the immune system, and cancer. Lancet Oncol. 5:617–625.
- Riley V. 1981. Psychoneuroendocrine influences on immunocompetence and neoplasia. Science. 212:1100–1109.
- Roozendaal B, Koolhaas JM, Bohus B. 1991. Attenuated cardiovascular, neuroendocrine, and behavioral responses after a single footshock in central amygdaloid lesioned male rats. Physiol Behav. 50:771–775.
- Rowse GJ, Weinberg J, Emerman JT. 1995. Role of natural killer cells in psychosocial stressor-induced changes in mouse mammary tumor growth. Cancer Res. 55:617–622.
- Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. 1999. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 24:285–300.
- Schreiber S, Pick CG. 2006. From selective to highly selective SSRIs: A comparison of the antinociceptive properties of fluoxetine, fluvoxamine, citalopram and escitalopram. Eur Neuropsychopharmacol. 16:464–468.
- Sharify A, Mahmoudi M, Izad MH, Hosseini MJ, Sharify M. 2007. Effect of acute pain on splenic NK cell activity, lymphocyte proliferation and cytokine production activities. Immunopharmacol Immunotoxicol. 29:465–476.
- Shavit Y, Lewis JW, Terman GW, Gale RP, Liebeskind JC. 1984. Opioid peptides mediate the suppressive effect of stress on natural killer cell cytotoxicity. Science. 223:188–190.
- Simiand J, Keane PE, Morre M. 1984. The staircase test in mice: A simple and efficient procedure for primary. Psychopharmacology. 84:48–53.
- Stanojevic S, Dimitrijevic M, Kovacevic-Jovanovic V, Miletic T, Vujic V, Radulovic J. 2003. Stress applied during primary immunization affects the secondary humoral immune response in the rat: Involvement of opioid peptides. Stress. 6:247–258.
- Steenbergen HL, Farabollini F, Heinsbroek RP, Van de Poll NE. 1991. Sex-dependent effects of aversive stimulation on holeboard and elevated plus-maze behavior. Behav Brain Res. 43:159–165.
- Trainor BC, Sweeney C, Cardiff R. 2009. Isolating the effects of social interactions on cancer biology. Cancer Prev Res (Phila PA). 2:843–846.
- Webster Marketon JI, Glaser R. 2008. Stress hormones and immune function. Cell Immunol. 252:16–26.
- Weizman R, Paz L, Peter Y, Toren P, Pick CG. 2001. Behavioral effects of agents active at the gamma-aminobutyric acid. Brain Res. 901:137–142.
- Weninger SC, Dunn AJ, Muglia LJ, Dikkes P, Miczek KA, Swiergiel AH, Berridge CW, Majzoub JA. 1999. Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc Natl Acad Sci USA. 96:8283–8288.
- Wilckens T. 1995. Glucocorticoids and immune function: Physiological relevance and pathogenic potential of hormonal dysfunction. Trends Pharmacol Sci. 16:193–197.
- Wilckens T, De Rijk R. 1997. Glucocorticoids and immune function: Unknown dimensions and new frontiers. Immunol Today. 18:418–424.
- Zalcman S, Minkiewicz-Janda A, Richter M, Anisman H. 1988. Critical periods associated with stressor effects on antibody titers and on the plaque-forming cell response to sheep red blood cells. Brain Behav Immun. 2:254–266.
- Zalcman S, Irwin J, Anisman H. 1991. Stressor-induced alterations of natural killer cell activity and central catecholamines in mice. Pharmacol Biochem Behav. 39:361–366.