Abstract
Mechanisms underlying the relationship between exercise and mood are not well understood. This study sought to investigate the role of pro- and anti-inflammatory cytokines and autonomic balance in determining the impact of exercise withdrawal on negative mood. Healthy men and women who regularly exercised (N = 26, mean age = 25.5 years, SD = 4.5 years) were randomised to exercise withdrawal or exercise maintenance for 2 weeks. Protocol adherence was monitored using accelerometers. Inflammatory markers from plasma (interleukin-6, IL-6; tumour necrosis factor-alpha; interleukin-10; and interleukin-1 receptor antagonist), heart-rate variability (HRV) and measures of mood (General Health Questionnaire-28 (GHQ) and the Profile of Mood States (POMS)) were assessed at study entry and at 2-week follow-up. Exercise withdrawal resulted in significant increases in negative mood over time on both the GHQ (p = 0.028) and the POMS (p = 0.005). Following the intervention, IL-6 concentration was lower in the exercise withdrawal than exercise maintenance condition (p = 0.05). No intervention effects were observed for other cytokines or HRV. The mood changes were significantly related to changes in IL-6 concentration (β = − 0.50, p = 0.011), indicating that reduction in IL-6 was related to increased negative mood. Our results are consistent with positive effects of exercise on mental health, but further research on inflammatory pathways is warranted.
Introduction
Exercise has an important role in the promotion of mental well-being (Steptoe Citation2006). In recent years, several large-scale epidemiological studies have supported an association between lower depressive symptomatology and higher physical activity levels (Kritz-Silverstein et al. Citation2001; van Gool et al. Citation2003). A recent meta-analysis of 13 prospective cohort studies found physical activity to be associated with a lower incidence of depression (OR = 0.78; 95% CI = 0.71–0.86); this relationship was particularly prevalent in women (OR = 0.69, 95% CI = 0.60–0.79; Hamer and Chida Citation2008). This association remained significant even after controlling for sociodemographic and behavioural factors. However, the mechanisms underlying the relationship are not well understood.
One biological pathway implicates inflammatory cytokines. Patients with symptoms of depression have been reported to exhibit disturbances of several aspects of immune functioning and this association is related to the severity of the mood disturbance (Raison et al. Citation2006). A meta-analysis of 136 studies indicated that depression is positively associated with elevations in circulating peripheral blood levels of C-reactive protein (CRP), interleukin-1 (IL-1), interleukin-6 (IL-6) and interleukin-1 receptor antagonist (IL-1ra; Howren et al. Citation2009). Interestingly, these cytokines impact the neurochemical pathways associated with depression and it has been hypothesised that low-grade inflammation and negative mood represent features of sickness behaviour (Dantzer and Kelley Citation2007).
Exercise is known to have a profound and unique effect on the production of cytokines. The characteristic pro-inflammatory cytokines, tumour necrosis factor-alpha (TNF-α) and interleukin-1beta do not increase with exercise as would be typical of an infection. Instead, the first cytokine usually present in the circulation is IL-6, which increases in an exponential fashion and decreases again after exercise stops (Petersen and Pedersen Citation2005). Many cell types may contribute to the release of IL-6, but the dominant source is thought to be exercising muscle (Shephard Citation2002). IL-6 has been classified as both a pro- and anti-inflammatory cytokine (Tilg et al. Citation1997). Increases in circulating IL-6 are observed after exercise without muscle damage and this in turn reduces pro-inflammatory cytokines via the stimulation of the anti-inflammatory cytokines IL-1ra and interleukin-10 (IL-10; Petersen and Pedersen Citation2005). It has been suggested that in the case of exercise, IL-6 exerts anti-inflammatory effects (Pedersen and Febbraio Citation2008). This mechanism may explain why lower baseline levels of pro-inflammatory cytokines are present in those people who are most physically active (Abramson and Vaccarino Citation2002). Although there is growing evidence in support of the anti-inflammatory effects of regular exercise, few studies have investigated this mechanism experimentally in relation to the anti-depressive effects of exercise.
A related body of research has studied autonomic nervous-system regulation of immune function; and one key aspect is the control of cytokine response to inflammatory stimuli (Tracey Citation2002). Parasympathetic nervous-system activity has been shown to respond in real time to inflammation via the vagus nerve (Pavlov and Tracey Citation2004). Heart-rate variability (HRV) can be used as a measure of parasympathetic nervous-system activity changes through measurement of beat-to-beat variations in R–R interval length (Berntson et al. Citation1997); a recent review by Thayer (Citation2009) suggested a cholinergic anti-inflammatory pathway accounting for the inverse relationship between HRV and inflammation. Physical inactivity is associated with reduced parasympathetic nervous-system function and increased sympathetic activity (Buchheit et al. Citation2005), while exercise training results in increased parasympathetic and decreased sympathetic activity (Dixon et al. Citation1992).
Many regular exercisers find enforced inactivity as stressful and increased negative mood is common (Berlin et al. Citation2006). Only one study to date has investigated the relationship between inflammatory factors, parasympathetic activity, mood and exercise withdrawal (Kop et al. Citation2008). Lower HRV [indexed by the low- to high-frequency (LF/HF) ratio] at baseline predicted the onset of negative mood after exercise withdrawal (Weinstein et al. Citation2007). However, although exercise withdrawal increased negative mood, the inflammatory markers IL-6, CRP, fibrinogen and soluble intracellular adhesion molecule-1 (sICAM-1) did not change during the intervention (Kop et al. Citation2008). In the present study, we utilise an exercise withdrawal paradigm but assess a wider range of circulating cytokines (IL-6, IL-1ra, IL-10 and TNF-α) potentially relevant to the effects of exercise on mood.
The aim of the study is to examine the association between mood changes after exercise withdrawal and pro- and anti-inflammatory cytokines. We hypothesised that 2-week exercise withdrawal would result in increased negative mood and reduced HRV, and that these mood changes would be associated with increases in inflammatory cytokines.
Methods
Participants
The study recruited 26 participants (mean age = 25.5 years, SD = 4.5 years, 50% females). Inclusion criteria were: (1) physically active, defined as aerobic exercise lasting a minimum of 30 min at least three times per week, for the previous 6 months; (2) aged 18–39 years; (3) non-smoking; and (4) fluent in the English language. Exclusion criteria were: (1) any history of cardiovascular, immune or respiratory disease; (2) taking regular prescribed medications except for oral contraceptives; (3) currently having a diagnosis or under treatment for a psychiatric or psychological disorder; or (4) currently experiencing cold or influenza symptoms. First appointments for women were scheduled to coincide with the beginning of their new menstrual cycle to control for the possible confounding effect of gonadal steroids on mood and cytokine levels (Sanders et al. Citation1983). Written informed consent was obtained for all participants and ethical approval was obtained from the University College London Hospital Ethics Committee.
Design
This randomised controlled study had a 2-week follow-up period which was deemed appropriate since previous work demonstrated robust mood changes during this period of exercise withdrawal (Berlin et al. Citation2006). Participants were randomly allocated to one of two conditions using a computer-generated random number table. Participants were assigned to either the control condition (i.e. exercise maintenance, n = 13) or the intervention condition (i.e. exercise withdrawal, n = 13). The main investigator (L. P.) was blinded to the treatment allocation until the completion of the study. Blinding was made possible by the use of an external researcher (M. H.) who provided a sealed, opaque envelope containing condition assignment and written instructions to each participant.
Participants were required to abstain from exercise for the 12 h prior to the laboratory assessment. All baseline and follow-up assessments took place between 08:30 and 12:00 h. Baseline assessments included: anthropometrics (weight, height and waist circumference), personal information (date of birth, ethnicity, smoking habit, alcohol usage, medical history, current use of medications and cold/influenza symptoms), measures of mood, physical activity levels and cardiac autonomic activity. Blood was taken in a non-fasting state after a 15-min rest period to allow familiarisation to the laboratory environment and to minimise the effects of stress on the inflammatory markers under study. Following the blood sample, a submaximal fitness test was performed. After randomisation, all participants were fitted with an ambulatory accelerometer to measure protocol adherence. Follow-up appointments included mood and cardiac autonomic assessments and a second blood sample.
Blood collection and assays for inflammatory markers
To assess inflammatory activity, a 20-ml blood sample was drawn into EDTA coated tubes by venipuncture from the forearm at each of the study appointments. Blood was centrifuged for 10 min at 1246g. Plasma was divided into aliquots and frozen at − 80°C until analysis at a later date. IL-6 assays were performed using commercially available high-sensitivity ELISA kits (Quanitkine HS Human IL-6; RandD Systems, Minneapolis, MN, USA). TNF-α, IL-10 and IL-1ra were analysed in duplicate using fluorescent-labelled capture antibody beads from a commercially available kit (Milliplex Human Cytokine/Chemokine Immunoassay Kit, #MPXHCYTO-60K-03; Millipore Corporation, Billerica, MA, USA); concentrations were determined using a Luminex flow cytometer (Bio-Plex; Bio-Rad, Hercules, CA, USA). For IL-6, the limit of detection of the IL-6 assay was 0.039 pg/ml, the mean intra-assay CV was 3.60% and the mean inter-assay CV was 3.61%. For TNF-α, IL-10 and IL-1ra, the limit of detection was 0.2, 0.3 and 2.3 pg/ml, respectively. The mean intra-assay CV was 10.5% for TNF-α, 9.1% for IL-10 and 8.0% for IL-1ra. The mean inter-assay CV was 10.1% for TNF-α, 9.1% for IL-10 and 8.4% for IL-1ra. These mean intra- and inter-assay CVs were within the acceptable range published by Milliplex; samples whose CVs were out of the acceptable range were re-analysed.
Assessment of negative mood and physical activity levels
Mood was assessed using the Profile of Mood States (POMS; McNair et al. Citation1971). This is a factor-analytically derived questionnaire, measuring six different moods: tension–anxiety, depression–dejection, anger–hostility, vigour–activity, fatigue–inertia and confusion–bewilderment. Each of the 36 items was scored as 0 = not at all to 4 = extremely. The total mood disturbance score on the POMS was calculated by summing the scores (with vigour weighted negatively), with higher scores indicative of greater negative affective state. In the present sample, the POMS demonstrated adequate internal reliability (Cronbach's α = 0.78). The General Health Questionnaire-28 was also administered (Goldberg and Hillier Citation1979). This is a brief, standardised measure designed to detect psychological distress among community dwelling individuals. Responses were marked on a Likert scale of 0–1–2–3 and summed to compute a score for each subscale and a total score. In the present sample, the GHQ-28 demonstrated good internal reliability (Cronbach's α = 0.86).
Self-reported physical activity was assessed using the International Physical Activity Questionnaire (IPAQ; Booth Citation2000). This asks respondents to provide details about the average amount of time spent doing vigorous activities, moderate activities, walking and sitting per day, over the previous week. Summary scores of the number of hours of vigorous and moderate activity per week were derived.
Submaximal cardio-respiratory fitness testing using a cycle ergometer (Model 864, Monark, Sweden) was conducted in accordance with the Astrand protocol (Astrand and Rodahl Citation1986). Participants were required to cycle for a period of 12–16 min, with incremental workloads at 4-min intervals. Workloads started at 60 W and increased until 120 or 180 W depending on the individual's verbal rating of perceived exertion (RPE; Borg Citation1982). Participants were asked to stop when they reached an RPE of 16 or 17 out of 20. Estimated peak oxygen consumption in ml/kg/min was computed.
Cardiac autonomic assessment
Cardiac autonomic function was monitored using an Actiheart device (Cambridge Neurotechnology Ltd., Cambridge, Cambridgeshire, UK), which is a valid and reliable heart rate and movement sensor (Brage et al. Citation2005). Data were analysed using Actiheart Software version 2.132. Actiheart recordings were averaged over a 5-min period while participants were in a seated position. Prior to HRV analyses, the data were cleaned using the Actiheart software, to remove any abnormal beat-to-beat intervals. Two indices of cardiac autonomic function were examined, namely the root mean square successive difference of R–R intervals (rMSSD; in milliseconds) and the LF/HF ratio in the frequency domain (Berntson et al. Citation1997).
Resting blood pressure was measured using an automated measuring device (Digital Blood Pressure Monitor model UA767+; A and D Medical, Tokyo, Japan). Two consecutive recordings were taken with participants in a seated position, separated by a 2-min interval.
Ambulatory monitoring of protocol adherence
Accelerometers are devices that measure body movements in terms of acceleration, which can then be used to estimate the intensity of physical activity over time. Most accelerometers are piezoelectric sensors that detect acceleration in one to three orthogonal planes (anteroposterior, mediolateral and vertical; Chen and Basset Citation2005). The ActiGraph GT1M (ActiGraph, LLC, Pensacola, FL, USA) was used to measure physical activity levels during this study. This has been previously validated as a measure of whole body movements during free-living physical activity (Patterson et al. Citation1993). The hip position was adopted in this research, and care was taken for proper placement of the ActiGraph on each participant. Activity counts were summed over 60 s epochs and recorded continuously during waking hours. Data were analysed using the MAH/UFFE Analyser version 1.9.0.3. Intensity band boundaries were selected in accordance with the recommendations from Matthews (Citation2005) and are displayed in . The first and last days of data were excluded from analysis, as were any days on which the ActiGraph had been worn for fewer than 10 h. These parameters were chosen to obtain accurate physical activity measurement and to minimise selection bias (Ward et al. Citation2005).
Table I. Intensity band boundaries for accelerometer calibration (min/day).
Statistical analyses
Statistical analyses were conducted using SPSS version 16.0 software (SPSS, Inc., Chicago, IL, USA). The dataset was screened for outliers and normality of distribution, using the Kolmogorov–Smirnov test and by examining kurtosis and skewness z scores. Cytokine and HRV data were log transformed prior to analysis. The demographic and baseline characteristics of the two groups were compared using t-tests. To compare the responses of exercise withdrawal and exercise maintenance groups, repeated measures analysis of variance was used, with group (exercise withdrawal vs. exercise maintenance) as the between-person factor and time (pre- vs. post-intervention assessment) as the within-person factor. Significant interaction effects were examined post hoc by comparing pre- to post-intervention change scores with independent t-tests (or non-parametric Mann–Whitney tests as appropriate). Associations between inflammatory markers and mood and HRV were examined, with age and sex as covariates, using hierarchical regression analyses and Spearman ρ correlations as appropriate.
A total of 28 participants were recruited to take part in this study, 15 were randomised to the exercise withdrawal group and 13 to the exercise maintenance group. One participant in the exercise withdrawal group withdrew from the study. A further participant in the exercise withdrawal group was excluded from all analyses after falling ill during the study interval. In total, this reduced the exercise withdrawal group to a total of 13 participants. Several participants (n = 5) had missing rMSSD data due to technical failure and missing data from questionnaires (n = 2).
Results
Sample characteristics
provides baseline characteristics of the sample. T-tests demonstrated that the groups were comparable with respect to all baseline characteristics prior to study entry, including demographic characteristics, BMI, activity levels, estimated cardiovascular fitness, mood and inflammatory markers. The groups were also comparable with respect to sex (t(24) = − 0.38, p = 0.71). There were no differences between males and females at baseline on the POMS (t(23) = − 0.24, p = 0.81) or on the GHQ (t(24) = − 1.82, p = 0.81).
Table II. Characteristics of the sample at baseline.
A repeated measures analysis of variance was used to test whether weight changed significantly over the study interval for either group. Results showed no main effect of time (F = 0.10, ns) and no main effect of group (F = 0.10, ns), indicating that weight change was not a confounding variable in this study.
Ambulatory ActiGraph data largely confirmed compliance to the exercise withdrawal protocol with average daily counts per minute being significantly lower in exercise withdrawn participants as compared with the exercise maintenance group (U = 47.00, p = 0.05). Exercise withdrawn participants also performed significantly lower levels of very vigorous activity (U = 27.00, p = 0.003) than exercise maintenance participants. There was no significant difference in lower intensity boundaries including light (t(24) = 0.63, p = 0.53), moderate (t(24) = 0.08, p = 0.94) and vigorous (t(24) = − 0.82, p = 0.42) activity.
Changes in negative mood during exercise withdrawal
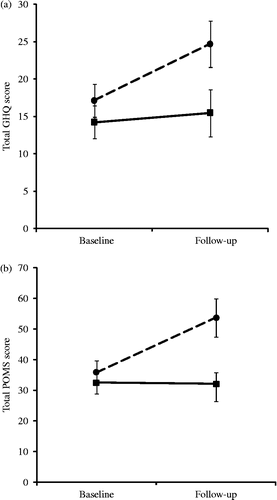
Analysis of the GHQ scores showed a main effect of time (F(1,24) = 10.51, p = 0.003), coupled with a significant group by time interaction (F(1,24) = 5.44, p = 0.028). There was no difference at baseline, but the exercise withdrawal group showed a larger increase than the exercise maintenance participants in GHQ score over the intervention period (means = 7.54 and 1.23, SEs = 2.23 and 1.53, respectively, p = 0.030). This interaction is illustrated in CitationFigure 1a.
The analysis of POMS scores identified main effects of time (F(1,22) = 9.15, p = 0.006) and group (F(1,22) = 5.10, p = 0.034), together with a significant group by time interaction (F(1,22) = 9.78, p = 0.005). There was no difference at baseline, but the exercise withdrawal group showed a larger increase than the exercise maintenance participants in POMS score over the intervention period (means = 18.73 and − 0.31, SEs = 5.71 and 2.86, respectively, p = 0.009). This interaction is characterised in .
Figure 1. (a) Mean GHQ score at baseline and 2-week follow-up assessments in exercise withdrawal (dashed line; n = 13) and exercise maintenance (solid line; n = 13) conditions. Vertical lines represent SE of the means. Significant group difference at follow-up, and with time in the withdrawal group (independent t-tests, p < 0.05). (b) Mean POMS score at baseline and 2-week follow-up assessments in exercise withdrawal (dashed line; n = 13) and exercise maintenance (solid line; n = 13) conditions. Vertical lines represent SE of the means. Significant group difference at follow-up, and with time in the withdrawal group (independent t-tests, p < 0.01).
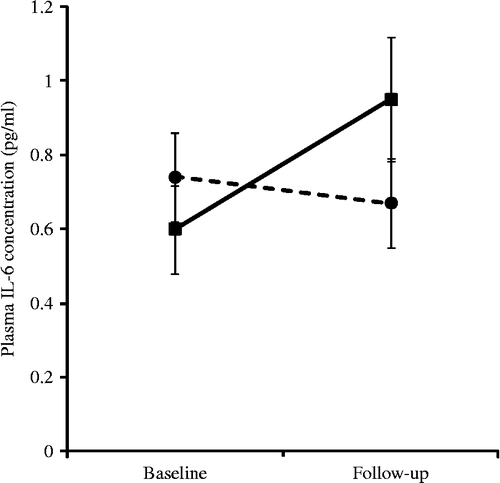
Effects of exercise withdrawal on inflammatory markers and HRV
Analysis of IL-6 showed a group by time interaction that approached a significant difference (F(1,24) = 3.89, p = 0.060). This is illustrated in using non-transformed data. It can be seen that the exercise maintenance group displayed a small increase in IL-6 concentration over the study period (ΔIL-6: mean = − 0.07, SE = 0.06), while levels fell marginally in the exercise withdrawal group (ΔIL-6: mean = 0.09, SE = 0.05). Post-hoc analysis of change scores found a borderline significant effect of group (t(24) = − 2.03, p = 0.05). There were no effects of exercise withdrawal on any of the other cytokines. Similarly, there were no significant changes on either of the HRV indices; for example, the LF/HF ratio averaged 2.04 ( ± 1.03) and 3.71 ( ± 2.72) in the exercise withdrawal and exercise maintenance groups, respectively, following the intervention period.
Relationship between changes in negative mood, IL-6 and cardiac autonomic activity
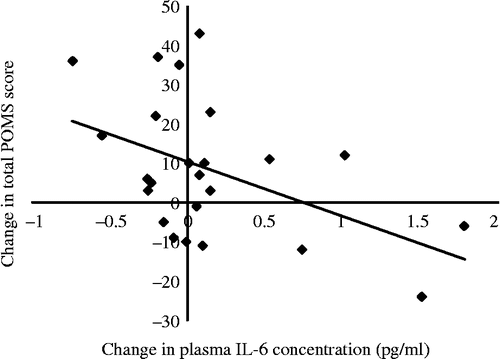
Since total POMS score appeared to be the most sensitive indicator of mood change in response to exercise withdrawal, this measure was used to examine the association with change in IL-6 concentration for the entire sample. Using total POMS change as the dependent variable, hierarchical regression analyses were conducted, controlling for age, sex and baseline POMS score (see ). The final model demonstrates that baseline POMS score, age and gender accounted for 23.0% of the variance, while change in IL-6 accounted for an additional 22.5% of the total POMS change (ΔR2 = 0.23). The relationship was negative, so increases in mood disturbance were associated with decreases in IL-6 (t(23) = − 2.72, p = 0.011; see ). The final model also showed a significant independent relationship between total POMS change and sex (t(23) = − 2.18, p = 0.040), since women (ΔPOMS mean = 14.54, SE = 4.84) reported larger increases in negative mood than men (ΔPOMS mean = 1.18, SE = 4.57). No associations were observed between mood change and the other cytokines.
Table III. Summary of hierarchical regression analysis for variables predicting total POMS change.
Figure 3. Correlation between change in mood disturbance and 2-week change in plasma IL-6 concentration (r = − 0.49, n = 24, p = 0.016). Note that an increase in POMS score reflects an increase in mood disturbance at follow-up.
Cross-sectional correlations of follow-up data were performed using Spearman's ρ (two-tailed) correlations; they found no significant relationships between any of the cytokine, cardiac sympathetic or mood variables. Associations between baseline HRV and mood change were also examined, though no significant relationships were found. The relationship between changes in mood and HRV and between cytokine responses and HRV was analysed using partial correlation analyses, adjusting for age, gender and fitness. However, no significant correlations were found between changes in mood or cytokines and either of the measures of HRV: rMSSD and LF/HF ratio.
Changes in mood, IL-6 and cardiac autonomic activity in relation to fitness
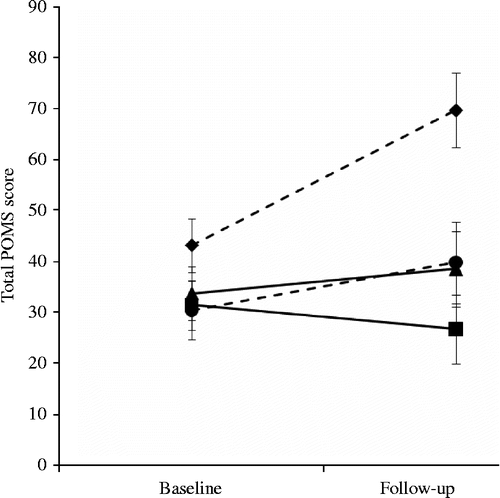
The sample was stratified around the median split to produce groups of low and high fitness, before being divided further based on condition allocation of exercise maintenance or exercise withdrawal. These groups were then compared in a 4 × 2 repeated measures analysis of variance in which exercise dependence/group stratification was the four-level between subjects factor, and POMS score over the two study visits was included as the two-level within subjects factor. Results show a main effect of time (F(1,20) = 10.19, p = 0.005) and group (F(1,20) = 4.46, p = 0.015), together with a significant group by time interaction (F(1,20) = 5.75, p = 0.005). There was no difference at baseline, but the exercise withdrawal/high-fitness group (mean = 26.5, SE = 4.92) showed a larger increase than exercise maintenance high- and low-fitness groups (means = 4.83 and − 4.71, SEs = 2.40 and 4.38, p = 0.057 and 0.003, respectively) in POMS score over the intervention period. The exercise withdrawal/high-fitness group also showed a larger increase than exercise withdrawal/low fitness (mean = 9.40, SE = 10.15, p = 0.201) in POMS score over the intervention period, but this was not significant. This interaction is characterised in . No significant effects were observed for fitness and exercise withdrawal on any of the cytokines or HRV.
Figure 4. Mean POMS score at baseline and 2-week follow-up assessments in exercise withdrawal/low fitness (dashed line, circle; n = 5), exercise withdrawal/high fitness (dashed line, diamond; n = 6), exercise maintenance/low fitness (solid line, square; n = 7) and exercise maintenance/high fitness (solid line, triangle; n = 6). Vertical lines represent SE of the means. The exercise withdrawal/high-fitness group POMS score was greater at follow-up than the exercise maintenance high and low-fitness groups (independent t-tests, p < 0.005).
Discussion
The main aim of the present study was to induce a negative mood state through 2 weeks of exercise cessation in habitual exercisers and to examine if this effect was mediated through cytokines. Exercise withdrawal was monitored using accelerometry with results showing exercise withdrawn participants performed significantly less overall activity (counts per minute) and very vigorous activity than the exercise maintenance group. No differences were observed in lower intensity boundaries, but since the validity of intensity boundary cut-points has been criticised recently (Strath et al. Citation2003; Matthews Citation2005); overall measures are thought to be more accurate indicators, so we can assume the intervention was successful. We demonstrated that 2 weeks exercise withdrawal can induce robust increases in negative mood state, though there were negligible changes in cytokines and HRV. Sex was found to be an independent predictor of change in mood, with females showing greater increase in negative mood than men. The reason for this is unclear, but fluctuations in mood due to menstrual cycle stage may be a contributing factor. We observed a modest reduction in IL-6 concentration in participants withdrawn from exercise, which was associated with an increase in negative mood state. Contrary to our hypothesis, the rise in negative mood following exercise withdrawal was not associated with increased IL-6 levels. IL-6 is regarded by some authorities as an anti-inflammatory cytokine in the case of exercise, with increases in IL-6 in regular exercisers being associated with decreases in pro-inflammatory cytokines (Petersen and Pedersen Citation2005). Assays performed on other anti-inflammatory markers Il-1ra and IL-10, and the pro-inflammatory marker TNF-α, did not corroborate the IL-6 findings. Interestingly, exercise maintenance participants increased in IL-6 as opposed to remaining stable as we expected. These findings might suggest an acute carry-over effect of muscle-derived IL-6 from a recent bout of physical activity (Petersen and Pedersen Citation2005), or alternatively that participants in the control group increased the intensity of their exercise regime rather than maintaining their habitual levels during the 2-week study interval (perhaps as a consequence of being monitored by wearing the ActiGraph). However, this is speculative since we did not collect any objective physical activity data prior to study entry. Indeed, the relationship observed between mood changes and the decrease in IL-6 concentration may be non-causal and instead reflect a reduction in muscle-derived IL-6.
Kop et al. (Citation2008) also examined mood and inflammatory responses to a 2-week exercise withdrawal intervention, measuring several inflammatory markers including IL-6, CRP, fibrinogen and sICAM-1. Although the authors reported increased negative mood with exercise withdrawal, there were no associations with inflammatory markers or autonomic nervous-system function. The results reported here regarding the effects of exercise withdrawal on mood are consistent with the findings of Kop et al. (Citation2008). Exercise withdrawal consistently predicted greater negative mood compared to exercise maintenance participants.
An association between negative mood and various inflammatory markers has been found in previous studies (Maier and Watkins Citation1998; Segerstrom and Miller Citation2004; Kop and Gottdiener Citation2005). In addition, the effect has been observed after administration of a low dose of endotoxin, which acts to increase the levels of circulating pro-inflammatory cytokines. For example, Wright et al. (Citation2005) found significant increases in negative mood and circulating IL-6 in healthy male participants after injection of Salmonella typhi vaccine compared with those injected with a placebo. Additionally, animal data suggest that the pro-inflammatory (TNF-α) response to stress in IL-6 deficient mice is heightened in comparison with controls (Chida et al. Citation2004). The non-significant results from the TNF-α data in the current study may be partially explained by the fact that we observed small changes within a healthy sample.
The homeostatic role of the autonomic nervous-system over the inflammatory response has been well documented (Tracey Citation2002), showing an inflammatory reflex whereby the nervous-system regulates cytokine production in real time via the vagus nerve. In the present study, no associations were found between exercise withdrawal and HRV, or HRV and IL-6 concentration. In addition, HRV was not associated with any measures of negative mood. These findings are consistent with a recent study that showed no effects of exercise withdrawal on measures of HRV (Goedhart et al. Citation2008). However, an earlier study reported that reduced parasympathetic nervous-system activity as measured by HRV (including the LF/HF ratio) was predictive of negative mood following exercise withdrawal in a slightly older sample than the one used in the current study (mean age = 31.3 years, SD = 7.5 years; Weinstein et al. Citation2007). Supposing that exercise withdrawal does indeed affect autonomic nervous-system activity, more sensitive markers might be necessary to better assess the changes in sympathovagal balance, such as 24-h ambulatory assessments of HRV. In general, findings relating mood disorders with HRV are not completely consistent (Rottenberg Citation2007), and in some population studies, findings may be driven by anti-depressant medication (Licht et al. Citation2008).
It is probable that the relationship between exercise and negative mood is mediated by pathways other than the hypothesised autonomic and inflammatory mechanisms studied here. For example, decreased fitness has been previously implicated (Berlin et al. Citation2006); or quite plausibly, the relationship may be explained by decreased pleasure associated with withdrawing from an enjoyable activity.
Study limitations
Several limitations should be considered. The exercise withdrawal paradigm (specifically, the duration and intensity of the activities that participants were permitted to maintain) may not have been powerful enough to establish inter-relationships between mood, inflammatory markers and cardiac autonomic functioning in healthy participants (Kop et al. Citation2008). Indeed, the ActiGraph data showed that moderate and vigorous intensity activity levels were not significantly different between groups. This could be an important factor given that some studies have shown that it may be exercise intensity and not total time of exercise that predicts changes in serum cytokines (Balducci et al. Citation2010). The reason for this finding is not clear, though as mentioned previously the validity of using cut-points has been brought under scrutiny; self-report diaries could be useful in future research to help document the exercise withdrawal process. In addition, more sensitive immunoassays and provocation procedures (lipopolysaccharide stimulation of lymphocytes) may be needed to identify significant changes in inflammatory cytokine responses in healthy participants. The use of the Luminex platform for IL-10, IL-1ra and TNF-α and ELISA for IL-6 may partly explain the disparate findings. Given the naturalistic design of the study, we were unable to control all of the potential confounding variables; the association between mood changes and IL-6 concentration might be explained by a third unmeasured variable. No objective data were collected regarding diet or perceived distress associated with abstaining from a pleasurable activity. While we tried to minimise menstrual cycle effects in females by timing the intervention with the start of the new menstrual cycle, no objective data regarding menstrual cycle length were gathered. In addition, the temporal association between changes in mood and IL-6 concentration could not be determined in the current study since this would have required additional assessments mid-way through the protocol.
In conclusion, this study found evidence supporting the relationship between exercise withdrawal and negative mood. Negligible changes were found in inflammatory markers and HRV in response to exercise withdrawal. However, changes in mood were associated with changes in IL-6 concentration, though there was no evidence of a causal relationship. To test the causal mechanisms underlying the exercise–mood dynamic, studies employing longitudinal designs with greater withdrawal periods are required, so as to delineate the cause–effect relationship between inflammatory, autonomic nervous-system and mood indices.
Funding sources: L. P. is funded by a British Heart Foundation, UK, PhD scholarship. M. H. and A. S. are funded by the British Heart Foundation, UK (Grant RG/05/006).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abramson JL, Vaccarino V. 2002. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 162:1286–1292.
- Astrand P-O, Rodahl K. 1986. Textbook of work physiology: Physiological bases of exercise. Singapore: McGraw Hill Book Co.
- Balducci S, Zanuso S, Nicolucci A, Fernando F, Cavallo S, Cardelli P, Fallucca S, Alessi E, Letizia C, Jimenez A, Fallucca F, Pugliese G. 2010. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 20:608–617.
- Berlin A, Kop W, Deuster P. 2006. Depressive mood symptoms and fatigue following exercise withdrawal: The potential role of decreased fitness. Psychosom Med. 68:224–230.
- Berntson G, Bigger J, Eckberg D, Grossman P, Kaufman P, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. 1997. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 34:623–648.
- Booth M. 2000. Assessment of physical activity: An international perspective. Res Q Exerc Sport. 71 Suppl: S114–S120.
- Borg G. 1982. Psychological basis of perceived exertion. Med Sci Sports Exerc. 14:377–381.
- Brage S, Brage N, Franks P, Ekelund U, Warehams N. 2005. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr. 59:561–570.
- Buchheit M, Simon C, Charloux A, Doutreleau S, Piquard F, Brandenberger G. 2005. Heart rate variability and intensity of habitual physical activity in middle-aged persons. Med Sci Sports Exerc. 37:1530–1534.
- Chen K, Bassett D. 2005. The technology of accelerometry-based activity monitors: Current and future. Med Sci Sports Exerc. 37 Suppl: S490–S500.
- Chida Y, Sudo N, Motomura Y, Kubo C. 2004. Electric foot-shock stress drives TNF-alpha production in the liver of IL-6 deficient mice. Neuroimmunomodulation. 11:419–424.
- Dantzer R, Kelley KW. 2007. Twenty years of research on cytokine-induced sickness behaviour. Brain Behav Immun. 21:153–160.
- Dixon EM, Kamath MV, McCartney N, Fallen EL. 1992. Neural regulation of heart rate variability in endurance athletes and sedentary controls. Cardiovasc Res. 26:713–719.
- Goedhart AD, de Vries M, Kreft J, Bakker FC, de Geus EJC. 2008. No effect of training state on ambulatory measures of cardiac autonomic control. J Psychophysiol. 22:130–140.
- Goldberg D, Hillier V. 1979. A scaled version of the General Health Questionnaire. Psychol Med. 9:139–145.
- Hamer M, Chida Y. 2008. Exercise and depression: A meta-analysis and critical review. In: Hansson W, Olsson E. editors. New perspectives on women and depression. New York: Nova Science Publishers. p 245–256.
- Howren MB, Lamkin DM, Suls J. 2009. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 7:71–86.
- Kop W, Gottdiener J. 2005. The role of immune system parameters in the relationship between depression and coronary artery disease. Psychosom Med. 67:537–541.
- Kop W, Weinstein A, Deuster P, Whittaker K, Tracy R. 2008. Inflammatory markers and negative mood symptoms following exercise withdrawal. Brain Behav Immun. 22:1190–1196.
- Kritz-Silverstein D, Barrett-Connor E, Corbeau C. 2001. Cross-sectional and prospective study of exercise and depressed mood in the elderly. Am J Epidemiol. 153:596–603.
- Licht CM, de Geus EJ, Zitman FG, Hoogendijk WJ, van Dyck R, Penninx BW. 2008. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA). Arch Gen Psychiatry. 65:1358–1367.
- Maier SF, Watkins LR. 1998. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 105:83–107.
- Matthews C. 2005. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 37 Suppl: S512–S522.
- McNair D, Lorr M, Droppleman L. 1971. Manual for the profile of mood states. San Diego, CA: Educational and Industrial Testing Service.
- Patterson S, Krantz D, Montgomery L, Deuster P, Hedges S, Nebel L. 1993. Automated physical activity monitoring: Validation and comparison with physiological and self-report measures. Psychophysiology. 30:296–305.
- Pavlov VA, Tracey KJ. 2004. Neural regulators of innate immune responses and inflammation. Cell Mol Life Sci. 61:2322–2331.
- Pedersen BK, Febbraio MA. 2008. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol Rev. 88:379–406.
- Petersen AM, Pedersen BK. 2005. The anti-inflammatory effect of exercise. J Appl Physiol. 98:1154–1162.
- Raison CL, Capuron L, Miller AH. 2006. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 27:24–31.
- Rottenberg J. 2007. Cardiac vagal control in depression: A critical analysis. Bio Psychol. 74:200–211.
- Sanders D, Warner P, Backstrom T, Bancroft J. 1983. Mood, sexuality, hormones and the menstrual cycle. Cycle. I. Changes in mood and physical state: Descriptions of subjects and method. Psychosom Med. 45:487–501.
- Sergerstrom S, Miller G. 2004. Psychological stress and the human immune system: A meta-analytic study of 30-years inquiry. Psychol Bull. 130:601–630.
- Shephard RJ. 2002. Cytokine responses to physical activity, with particular reference to IL-6: Sources, actions, and clinical implications. Crit Rev Immunol. 22:165–182.
- Steptoe A. 2006. Depression and physical activity. In: Steptoe A. editors. Depression and physical illness. Oxford: Oxford University Press348–368 Chapter 16.
- Strath SJ, Bassett DR, Swartz AM. 2003. Comparison of MTI accelerometer cut-points for predicting time spent in physical activity. Int J Sports Med. 24:298–303.
- Thayer JF. 2009. Vagal tone and the inflammatory reflex. Cleve Clin J Med. 76 Suppl 2: S23–S26.
- Tilg H, Dinarello CA, Mier JW. 1997. IL-6 and APPs: Anti-inflammatory and immunosuppressive mediators. Immunol Today. 18:428–432.
- Tracey KJ. 2002. The inflammatory reflex. Nature. 420:853–859.
- van Gool CH, Kempen GI, Pennix BW, Deeg DJ, Beekman AT, van Eijk JT. 2003. Relationship between changes in depressive symptoms and unhealthy lifestyles in late middle aged and older persons: Results from the Longtitudinal Aging Study Amsterdam. Age Ageing. 32:81–87.
- Ward D, Evenson K, Vaughn A, Brown Rodgers A, Troiano R. 2005. Accelerometer use in physical activity: Best practices and research recommendations. Med Sci Sports Exerc. 37 suppl: S582–S588.
- Weinstein AA, Deuster PA, Kop WJ. 2007. Heart rate variability as a predictor of negative mood symptoms induced by exercise withdrawal. Med Sci Sports Exerc. 39:735–741.
- Wright C, Strike P, Brydon L, Steptoe A. 2005. Acute inflammation and negative mood: Mediation by cytokine activation. Brain Behav Immun. 19:345–350.