Abstract
In this study, effects of weaning on behavioral and physiological stress parameters in young horses (foals) were determined. Foals were weaned either simultaneously without the presence of adult horses (group A, n = 6), or in the presence of two adult females familiar but unrelated to the foals (group B, n = 5), or weaned consecutively by removing two mother horses per day (group C, n = 6). Behavior, locomotion, salivary cortisol concentration, beat-to-beat (RR) interval, heart rate variability (HRV) and weight were determined. Group A foals lost weight for 2 days (mean ± SEM) − 8.3 ± 1.6 kg, p < 0.05. Weaning was followed by increased vocalization which was least pronounced in foals of group B (p < 0.05). Locomotion was most pronounced on weaning day in foals of group A and lowest in group B (p < 0.05). Weaning increased salivary cortisol concentration on the day of weaning in groups A and B and for 2 days in group C (p < 0.05). The RR interval decreased most pronouncedly in group A foals (p < 0.05). There were no consistent changes in HRV. Based on cortisol release and behavior, weaning is associated with stress but this was least pronounced in foals weaned in the presence of two familiar but unrelated adult female horses.
Introduction
In mammals living under natural conditions, weaning usually is a gradual process. The mother by decreasing the benefit and increasing the cost of suckling gradually induces the young to reduce and finally to stop suckling (Martin Citation1984; Hickey et al. Citation2003). Natural weaning is supposed to cause minimal separation stress for the mother and its young (Napolitano et al. Citation2008). Under natural conditions, a horse mare (female horse) will wean her foal (young horse < 1 year of age) shortly before the next foaling at a time when the young is approximately 1 year old. In contrast, in most domestic animals the offspring is weaned artificially and before the time of natural weaning in the respective species. The age at artificial weaning varies from immediately after birth in dairy cattle, a few days in intensive sheep farms (Napolitano et al. Citation2008), 3–5 weeks in pigs (Hameister et al. Citation2010), to approximately 6 months in beef cattle (Price et al. Citation2003; Enriquez et al. Citation2010) and 4–7 months in horses (Houpt et al. Citation1984; Heleski et al. Citation2002; Waran et al. Citation2008). Artificial weaning has been associated with distress behavior, increased release of stress hormones, and altered immune function in the young (Lefcourt and Elsaesser Citation1995; Hickey et al. Citation2003; Price et al. Citation2003; Haley et al. Citation2005; Enriquez et al. Citation2010; Hameister et al. Citation2010).
In horses, weaning has been described as one of the most stressful events in the animal's life (Fraser et al. Citation1975). Until the point of weaning, the mare has provided its foal not only with nourishment but also with emotional security (Hoffman et al. Citation1995). As in cattle (Price et al. Citation2003), the degree of stress can be reduced by favorable environmental conditions at weaning. Traditionally, weaning of horses is performed as an abrupt separation of foals from the mares (Waran et al. Citation2008). In contrast, with gradual weaning, the mare and foal are first separated temporarily. On subsequent days, the time of separation is extended until the mare is not returned to the foal. Gradual weaning has been suggested to result in fewer behavioral responses of the foals than abrupt weaning (CitationMcCall et al. 1985, 1987), but conflicting data have been reported with some authors failing to find an advantage of separating mares and foals transiently before final weaning (Moons et al. Citation2005). In addition, diet (McCall et al. Citation1987; Hoffman et al. Citation1995; Holland et al. Citation1996; Nicol et al. Citation2005) and environment of the foal (Hoffman et al. Citation1995; Holland et al. Citation1996; Heleski et al. Citation2002; Nicol et al. Citation2005) may influence the response to weaning. Differences between studies may at least in part be caused by different horse breeds studied, with race horses being potentially more excitable and impulsive than ponies or other breeds.
Adverse or stressful stimuli initiate both a hypothalamo–pituitary–adrenocortical response and an adrenomedullary and sympathetic nervous system response. Through hypothalamic corticotropin releasing factor and adrenocorticotropin from the anterior pituitary, cortisol release from the adrenal cortex is increased. During short-term stress, cortisol may improve fitness by energy mobilization (Raynaert et al. Citation1976) and changes in behavior (Korte et al. Citation1993). Cortisol concentration is an accepted parameter for stress analysis in animals (Prunier et al. Citation2005). Because cortisol rapidly diffuses into saliva, salivary cortisol concentrations reliably mirror changes in cortisol concentrations in blood plasma (CitationSchmidt et al. 2009). The most immediate stress response is an increase in adrenomedullary and sympathetic nervous activity. An acute stress such as 1-s hot-iron branding elicits an immediate but extremely short-lasting release of epinephrine and increase in heart rate (Lay et al. Citation1992). Besides heart rate, heart rate variability (HRV) is used as an indicator for the response of the autonomic nervous system to stress. HRV, i.e., short-term fluctuations in heart rate, reflects the oscillatory antagonistic influence of the sympathetic and parasympathetic branches of the autonomic nervous system on the sinus node of the heart. In general, an increase in HRV reflects a shift toward more sympathetic dominance, whereas reduced values indicate a shift toward parasympathetic dominance (Von Borell et al. Citation2007).
Vocalization and locomotion have been suggested as objective measures of behavioral distress in foals. Foals standing still and not vocalizing are considered to be less anxious than foals moving and vocalizing frequently (Houpt and Hintz Citation1983; McCall et al. Citation1985; Hoffman et al. Citation1995). The frequency of defecation has been shown to be positively related to both heart rate and the degree of anxiety in adult horses (Momozawa et al. Citation2003).
The aim of this study was to determine the effects of three different weaning protocols on behavioral and physiological parameters in foals and to compare these weaning systems with regard to animal stress. Our hypothesis was that the stress of weaning is reduced when foals are kept with familiar but unrelated adult horses after separation from their mother.
Material and methods
Animals
A total of 17 Warmblood foals of the Brandenburg State Stud in Neustadt (Dosse), Germany, were available for the study. The foals were born at the stud and kept as a group together with their mothers (mares) under identical conditions from 5 to 10 days after birth. In order to exclude potential differences between male and female foals in response to weaning, all foals included in the study were female. The management protocol for all foals concerning handling and feeding was the same. During the last 5 months before weaning, all mares and foals had been together on pasture night and day. In the morning, the three experimental groups were brought into separate, straw-bedded group stables from 06:00 to 10:00 h and received concentrates and hay. Concentrates for the foals were given in a separate area of each stable not accessible to the mares. At the time of the experiment, foals were used to being handled for grooming and routine procedures such as hoof care, deworming, and physical examination for general health. All foals were made familiar to the procedures of saliva sampling, heart rate recordings, and locomotion analysis by pedometers during the 5 days before the experiment.
Experimental design
The foals were weaned with three different methods in September and October. Foals of group A (n = 6) were weaned simultaneously; all mares were removed from the group at the same time and the foals were left together in their familiar group stable. Foals of group B (n = 5) were weaned as for the foals in group A, but two mares not related to the foals and present in the group since birth of the foals stayed with the foals after weaning and throughout the experimental period. Foals of group C (n = 6) were weaned consecutively by removing two mares per day on three successive days (). In this group, the day when its mother was removed was defined as day 0 for the respective foal. At weaning, ages of the foals in groups A, B, and C were 175 ± 16 days ( ± SD; range 160–200 days), 166 ± 18 days (136–183), and 179 ± 8 days (167–187). Age at weaning did not differ significantly between groups (F = 1.0, df = 16, p = 0.39). At weaning of their foals, four out of six mares in group A, all mares in group B and three out of six mares in group C were pregnant again. Out of the two unrelated mares staying with the foals of group B, one was pregnant and one non-pregnant.
Figure 1. (a) Time chart of experimental procedures and observations. (b) Consecutive weaning of foals in group C and alignment of weaning days for data evaluation. Abbreviations: RR, heart beat interval; exam, examination; C, progressive removal of two mares each day as shown.
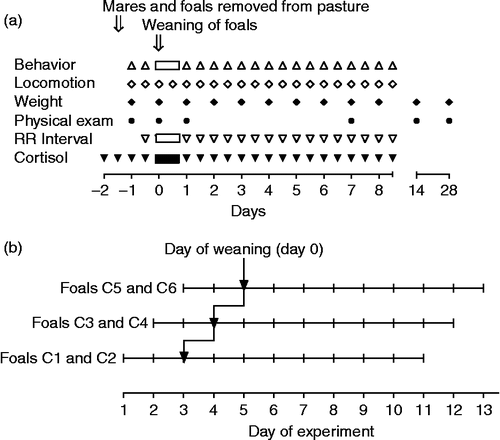
One day before weaning, mares and foals of each group as well as the two unrelated mares of group B were brought into their familiar group stables. The stables were not air-conditioned and no artificial light regime was used. On the days of weaning, the mares which had been removed from the respective groups were taken to a distant pasture out of the foals' visual and auditory range (800 m distance with a row of buildings between the foals' stables and the mares' pasture).
The study was approved by the competent authority for animal experimentation in Brandenburg State, Germany (Landesamt für Ländliche Entwicklung, Landwirtschaft und Flurneuordnung, license number 23-2347-A-5-2-2009).
Measurements
Physical examination
A physical examination of the foals was carried out on the day before weaning, on the day of weaning but before removal of mares, and on days 1, 7, 14, and 28 after weaning. This included assessment of respiratory rate (obtained by auscultation of the lungs), mucosal membranes, capillary refill time, and auscultation of the lungs and abdomen. Weight of the foals was measured daily from 1 day before to 8 days after weaning and also at 2, 4, and 8 weeks after weaning using a tape bearing a centimeter and a kilogram scale validated for determination of weight in horses (Ellis and Hollands Citation1998). The measuring tape was pressed firmly against the skin around the thorax of the foal during measurement.
Behavior
Behavior of the foals was observed for 2 h in the morning (09:00–11:00 h) and afternoon (16:00–18:00 h) from 1 day before to 8 days after weaning. On the actual weaning day, the foals were observed for four consecutive hours, starting when the foals were separated from their mares. The frequency of whinnying (vocalization), defecation, and feeding was recorded for each foal during the observation periods. Frequency of vocalization and defecation was calculated as the number of events recorded per 2 h of observation. Feeding behavior was scored from 1 to 4 (1 represents no interest in hay, pasture, or straw, 2 represents interest in hay, pasture, or straw nearly absent, i.e. feeding less than 1 min in 15 min, 3 represents reduced interest in hay, pasture, or straw, i.e. feeding between 1 and 10 min per 15 min, 4 represents constant or nearly constant eating when hay, straw, or pasture was available, i.e. more than 10 min per 15 min). The foals were checked daily for overt stereotypies from the day before to 8 days after weaning.
Locomotion
Locomotion activity and lying time of the foals were recorded with activity, lying, and temperature (ALT) pedometers (Ingenieurbüro Holz, Falkenhagen, Germany) as described previously (Rose-Meierhöfer et al. Citation2010). Recordings of 3 h each were made in the morning (09:00–12:00 h) and afternoon (16:00–19:00 h) from the day before to 8 days after weaning. On the day of weaning, locomotion activity and lying time were measured continuously from 1 h before to 8 h after weaning. To facilitate comparisons with the 3-h recordings on the days before and after weaning, the recording periods from 0 to 180 min and 180 to 360 min after weaning were chosen for further analysis. The pedometers count and store times of locomotion, standing, and lying for 15-min intervals consecutively, and data storage capacity exceeds the maximal recording time of 8 h used in our study. The pedometers were fixed on a tendon boot placed on one hind leg of the foal. The hind leg was chosen to prevent interaction with front leg activities such as pawing and rearing. After each 3-h recording time, data from the pedometers were transferred to a PC by radio transmission and stored in an MS access database until analysis.
Cortisol
For analysis of salivary cortisol concentration, saliva was collected with a cotton-based swab (Salivette, Sarstedt, Nümbrecht-Rommelsdorf, Germany). The Salivette was grasped with an arterial clamp, inserted at the angle of the lips into the mouth of the foal and placed gently onto the tongue of the horse for 1 min and afterwards returned into a polypropylene tube. After centrifugation for 10 min at 1000g, at least 1 ml of saliva was aspirated from each sample and frozen at − 20°C until analysis. The sampling procedure was well accepted by all foals and conducted by a single person without restraining the foals. Saliva samples were obtained from 2 days before to 8 days after weaning at half-hour intervals in the morning (07:30 and 8:00 h), at noon (12:00 and 12:30 h), and in the evening (18:30 and 19:00 h). On the day of weaning, saliva was collected at 60 and 30 min before weaning, immediately after weaning and 15 and 30 min thereafter, followed by half-hour intervals until 480 min after weaning.
Salivary cortisol concentration was determined with a direct enzyme immunoassay without extraction validated for equine saliva (Palme and Möstl Citation1997; CitationSchmidt et al. 2009). The antiserum cross-reacts with cortisone and several corticosterone metabolites, and the measured values have to be interpreted as cortisol immunoreactivity. The intra-assay coefficient of variation was 5.0%, the inter-assay variation was 6.7% and the minimal detectable concentration was 0.3 pg per well.
RR interval and HRV
Cardiac beat-to-beat (RR) interval was recorded as described (Schmidt et al. Citation2010a) with a portable recording system (S 810i and 800i, Polar, Kempele, Finland) attached to a girth around the thorax of the horse. The positive electrode was positioned on the right side of the withers (highest point of the dorsal spinal processes of the 3rd through 11th thoracic vertebrae) and the negative electrode on the left side next to the heart base. A second girth with a pocket for the recorder was fixed upon the chest belt. At the end of each recording period and on the weaning day, due to limited storage capacity, once during the recordings, data from the recorders were retrieved via infrared transmission. RR intervals were recorded from the afternoon of the day before to 8 days after weaning over a period of 3 h in the morning (9:00–12:00 h) and afternoon (16:00–19:00 h). On the weaning day, recordings were made from 1 h before to 8 h after separating the mares from their foals.
HRV was analyzed with the Kubios HRV software (Biomedical Signal Analysis Group, Department of Applied Physics, University of Kuopio, Kuopio, Finland). To remove trend components, data were de-trended and, in addition, an artefact correction was made following established procedures (Tarvainen et al. Citation2002; Schmidt et al. Citation2010a). The RR interval was recorded and the HRV variables standard deviation of RR interval (SDRR) and root mean square of successive RR differences (RMSSD) were calculated. The means for both HRV variables were determined for subsequent periods of 30 min each. The RMSSD are determined by calculating the difference between consecutive RR intervals before squaring and summing them, the values are then averaged and square roots obtained. The RMSSD are used to estimate high frequency RR variations that represent mainly vagal regulatory activity (Von Borell et al. Citation2007). The RR interval, SDRR, and RMSSD are expressed in milliseconds (ms).
Statistical analysis
The statistical analysis was carried out with the Predictive Analysis Software (PASW) statistics package (version 17.0, SPSS, Chicago, IL, USA). Data for all continuous variables were normally distributed (Kolmogorov–Smirnov test). For all parametric data, changes over time and differences between groups (weaning protocols) were analyzed using a general linear model (GLM) for repeated measures procedure with group as between-subject factor and univariate tests of within-subject effects. For analysis of categorical variables (scored feeding behavior) non-parametric tests were used. Comparisons between groups were made by the Kruskal Wallis H-test. For comparisons over time, the Friedman test was used. A p-value < 0.05 was considered significant. All data given are means ± SEM. Statistical evaluation of variables before and directly after weaning are summarized in supplementary .
Table I. Significant differences (p-values; F-values; degrees of freedom, df) for salivary cortisol concentrations, cardiac RR interval, and HRV variables, SDRR, and RMSSD, on different experimental days.
Results
Physical examination
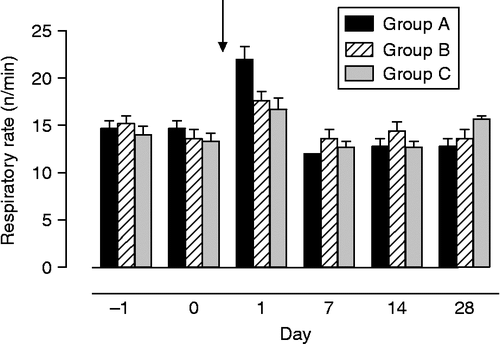
Respiratory rate in all groups of foals changed significantly over time (F = 18.5, df = 5, p < 0.001). On the day after weaning (day 1), respiratory rate was higher than that before weaning and higher than that the days thereafter (). Interactions group × time were significant (F = 3.4, df = 10, p < 0.01) and on day 1, respiratory rate was higher in foals of group A than in foals of groups B and C. No abnormalities or differences between groups were found for the other clinical parameters determined such as mucosal membranes, capillary refill time, and auscultation of the lungs and abdomen (data not shown).
Figure 2. Respiratory rate in foals subjected to different weaning protocols (group A: simultaneous weaning without unrelated adult mares, n = 6; group B: simultaneous weaning in the presence of two adult mares unrelated to the foals, n = 5; group C: consecutive weaning without unrelated adult mares) from 1 day before to 4 weeks after weaning, n = 6. Arrow indicates time of weaning (GLM for repeated measures: differences between times p < 0.001; interactions time × group p < 0.01). Data are mean ± SEM.
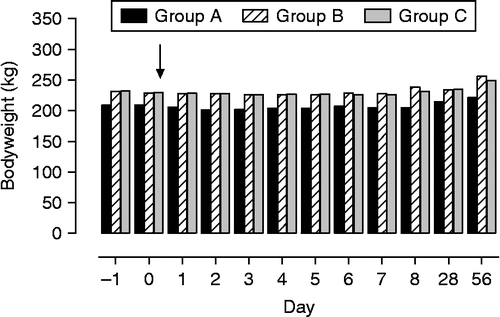
In foals weaned simultaneously and without unrelated mares present (group A), a significant weight loss from the day of weaning until 2 days thereafter occurred (weight loss in foals of groups A, B, and C, − 8.3 ± 1.6, − 0.8 ± 1.5, and − 1.8 ± 1.6 kg, respectively). Although the body weights of foals in group B (weaning with two unrelated mares) exceeded weaning values after 14 days by 10.0 ± 1.5 kg, only a slight weight gain had occurred in foals of groups A (simultaneous weaning, 0.4 ± 1.3 kg) and C (consecutive weaning, 1.5 ± 1.4 kg). Weight changes over time versus the day of weaning were significant (F = 24.1, df = 10, p < 0.001) as well as differences between groups (F = 4.7, df = 2, p < 0.05) and interactions group × time (F = 3.0, df = 20, p < 0.001). Also actual body weight of foals differed significantly over time (F = 23.1, df = 10, p < 0.001) and between groups (F = 3.8, df = 2, p < 0.05) with significant interactions group × time (F = 2.9, df = 20, p < 0.001; ).
Figure 3. Body weight development of foals subjected to different weaning protocols (group A: simultaneous weaning without unrelated adult mares, n = 6; group B: simultaneous weaning in the presence of two adult mares unrelated to the foals, n = 5; group C: consecutive weaning without unrelated adult mares) from 1 day before to 8 weeks after weaning, n = 6. Arrow indicates time of weaning (GLM for repeated measures: differences between times p < 0.001, differences between groups p < 0.05; interactions time × group p < 0.001). Data are mean ± SEM.
Behavior
Weaning was followed by a marked increase in vocalization in foals weaned without unrelated older mares (groups A and C) and increased slightly in foals staying together with two unrelated mares after weaning (group B). Changes in vocalization events over time were significant for all groups (F = 35.7, df = 19; p < 0.001) but frequency of vocalization differed between groups (F = 14.6, df = 2, p < 0.001; interactions group × time F = 8.4, df = 38, p < 0.001). In the first 2 h after removal of mares, vocalization was most frequent in foals of group A and least frequent in those of group B. Values had returned to near baseline by day 2 after weaning ().
Figure 4. (a) Vocalization, (b) defecation, and (c) feeding behavior in foals subjected to different weaning protocols (group A: simultaneous weaning without unrelated adult mares, n = 6; group B: simultaneous weaning in the presence of two adult mares unrelated to the foals, n = 5; group C: consecutive weaning without unrelated adult mares) from 1 day before to 8 days after weaning, n = 6. Arrow indicates time of weaning; GLM for repeated measures: vocalization, differences between times p < 0.001, differences between groups p < 0.001, interactions time × group p < 0.001; defecation, differences over time p < 0.001; interactions time × group p < 0.001; feeding score: Friedman test, differences between times p < 0.001; Kruskal Wallis H-test, *significant differences between groups p < 0.05. Data are mean ± SEM.
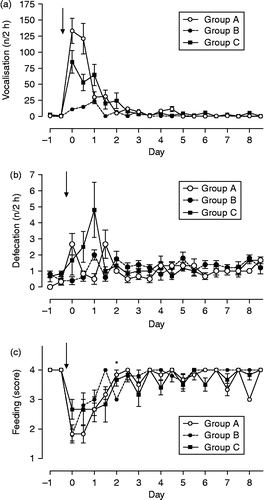
The frequency of defecation differed significantly over time (F = 3.6, df = 19, p < 0.001) but not between groups, whereas interactions group × time were significant (F = 3.5, df = 38, p < 0.001; ). All foals were eating nearly continuously during both observation periods on the day before weaning. The time spent on feed intake was significantly reduced in response to weaning in foals of all three groups (Friedman test: χ2 163.8, df = 19, p < 0.001). Significant differences in eating behavior between groups existed only for the morning of day 2 after weaning with lowest feed intake in group B foals (Kruskal Wallis H test χ2 7.82, df = 2, p < 0.05; ). At no time were stereotypies observed in any of the foals.
Locomotion
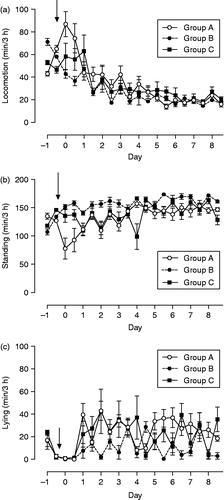
Locomotion activity in response to weaning determined by ALT pedometers changed significantly over time (F = 27.2, df = 19, p < 0.001) with an increase in groups A and C but not in group B (interactions group × time F = 2.9, df = 38, p < 0.001), whereas overall differences between groups were not significant. Locomotion activity decreased on day 1 in foals of groups A and B and on day 2 in group C foals (). The time foals were standing without moving changed over time (F = 6.75, df = 19, p < 0.001), and was longer for group B foals than for foals of groups A and C (differences between groups F = 27.2, df = 2, p < 0.001; interactions group × time F = 2.4, df = 38, p < 0.001; ). The time foals spent lying changed over time (F = 3.9, df = 19, p < 0.001) and foals spent only little time lying on the day of weaning, day 0. From day 2 onwards, foals of all groups were again lying regularly (), but the time was less in foals of group B compared with those of groups A and C (differences between groups F = 8.9, df = 2, p < 0.01; interactions group × time F = 2.1, df = 38, p < 0.001).
Figure 5. (a) Locomotion activity, (b) standing, and (c) lying time determined by pedometers in foals subjected to different weaning protocols (group A: simultaneous weaning without unrelated adult mares, n = 6; group B: simultaneous weaning in the presence of two adult mares unrelated to the foals, n = 5; group C: consecutive weaning without unrelated adult mares) from 1 day before to 8 days after weaning, n = 6. Arrow indicates time of weaning (GLM for repeated measures: locomotion, differences between times p < 0.001, interactions time × group p < 0.001, standing, differences between times p < 0.001, differences between groups p < 0.001, interactions time × group p < 0.001; lying, differences between times differences between groups p < 0.01, interactions time × group p < 0.001). Data are mean ± SEM.
Cortisol
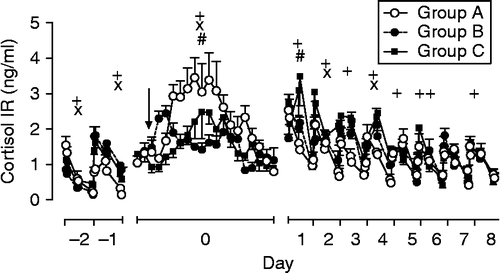
Weaning caused a significant increase in salivary cortisol concentration on day 0 (differences between times p < 0.001, for F-values and df, see ). Differences between groups did not reach statistical significance (p = 0.08), but interactions time × group were significant (p < 0.001, , ). Peak salivary cortisol concentrations in response to weaning were 3.5 ± 0.6, 2.4 ± 0.2, and 2.5 ± 0.7 ng/ml in groups A, B, and C, respectively. On day 1, a peak in cortisol release was detected only in group C (3.5 ± 0.8 ng/ml). On all other days, salivary cortisol concentration showed a circadian rhythm with highest values in the morning and a gradual decline throughout the day (p < 0.001). Differences in salivary cortisol concentrations between groups existed on day − 2 (p < 0.05) and day − 1 (p < 0.001) and on days 2 (p < 0.01) and 4 after weaning (p < 0.05) whereas interactions group × time were significant on day 1 after weaning (p < 0.05; , ).
Figure 6. Salivary cortisol concentration in foals subjected to different weaning protocols (group A: simultaneous weaning without unrelated adult mares, n = 6; group B: simultaneous weaning in the presence of two adult mares unrelated to the foals, n = 5; group C: consecutive weaning without unrelated adult mares) from 1 day before to 8 days after weaning, n = 6. Arrow indicates time of weaning (GLM for repeated measures: +, significant differences between times; × , significant differences between groups; #, interactions time × group for respective day, for details see ). Note changes in scale on x-axis. Data are mean ± SEM.
Beat-to-beat interval and HRV
The RR interval changed significantly over time on the day of weaning (p < 0.01, for F-values and df, see ) and differed significantly between groups (p < 0.01, interactions group × time p < 0.01). The lowest mean RR interval was reached in foals of group A (). In addition to the changes observed on the day of weaning, significant changes over time were found on days 2, 4, 5, 6, 7, and 8 after weaning, with higher RR intervals in the morning than in the afternoon. Differences between groups were found on days 2, 5, and 6 after weaning (, ).
Figure 7. (a) Cardiac RR interval, (b) SDRR, and (c) RMSSD in foals subjected to different weaning protocols (group A: simultaneous weaning without unrelated adult mares, n = 6; group B: simultaneous weaning in the presence of two adult mares unrelated to the foals, n = 5; group C: consecutive weaning without unrelated adult mares) from 1 day before to 8 days after weaning, n = 6. Arrow indicates time of weaning. (GLM for repeated measures, +, significant differences between times; × , significant differences between groups; #, interactions time × group for respective day, for details see ). Data are mean ± SEM.
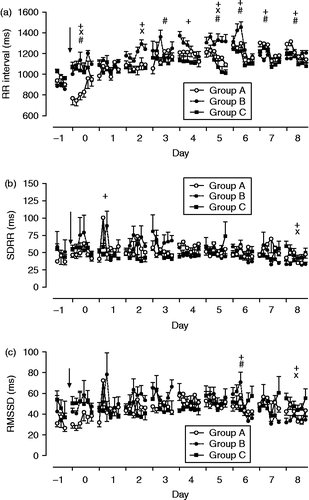
For the HRV variable SDRR, significant differences across times and between groups were found only on individual time points and without a consistent relation to weaning. Within days, there were significant (p < 0.05) changes over time on the day of weaning (increase in group B) and on day 8 after weaning (). For RMSSD, there were significant differences across times and between groups on days 6 and 8 after weaning (p < 0.05; , ).
Discussion
In this study, behavioral and physiological stress responses to weaning in foals were analyzed. Based on salivary cortisol concentration and behavioral parameters, weaning was a stressor but the degree of stress clearly differed between weaning protocols. Cortisol release in the foals was most pronounced in response to simultaneous weaning without the presence of unrelated mares, least pronounced in the group of foals that stayed with two familiar but unrelated mares and in between these two protocols for foals weaned consecutively.
Change in cortisol level is a well-accepted physiological stress parameter and its release increases in association with training (Snow and Rose Citation1981; Schmidt et al. Citation2010b), transport (Baucus et al. Citation1990; Clark et al. Citation1993; CitationSchmidt et al. 2010a,c), and social stress (Alexander and Irvine Citation1998) in horses. Based on salivary cortisol concentrations obtained with the same analytical techniques, transport is a pronounced stressor in horses (CitationSchmidt et al. 2010a,c), whereas cortisol release in response to riding (Schmidt et al. Citation2010b) is less pronounced. Salivary cortisol concentrations in foals after weaning were in the same range as in horses during transport, indicating that weaning is a major stressful event. In contrast to results of the present study, Houpt et al. (Citation1984) found no differences in plasma cortisol concentration of foals between pre- and post-weaning periods. This may be caused by breed differences with ponies, as studied by Houpt et al. (1984), being less excitable than the sport horses in our study or race horses studied by other authors (Malinowski et al. Citation1990; Hoffman et al. Citation1995). A physiological circadian rhythm in cortisol release with high concentrations in the morning and a decrease throughout the day (Hoffsis et al. Citation1970; Bottoms et al. Citation1972) was clearly evident on the days before and after weaning, but was absent on the day of weaning itself. It has been shown previously that even minimal environmental disturbances may disrupt the circadian pattern of cortisol release in horses (Lebelt et al. Citation1996). Although all foals were kept under identical conditions, small and unidentified challenges on the days before weaning might explain small differences in salivary cortisol concentration between groups on these days.
On the days following weaning, in foals weaned consecutively, salivary cortisol concentration was still increased and higher than in the foals of the other groups. Furthermore, a circadian rhythm was not detectable at 1 and 4 days after weaning. Thus, although the initial cortisol response to consecutive weaning was less pronounced than to simultaneous weaning, cortisol release was elevated over a longer time. Evidently, cortisol release increased also in foals already weaned when the dams of other foals were removed from the group on subsequent days.
The frequency of vocalization in response to weaning resembled changes in salivary cortisol concentration with foals weaned simultaneously without the presence of unrelated adult horses whinnying more often than foals weaned in the presence of two unrelated mares. Foals weaned consecutively showed less vocalization per time than foals weaned simultaneously. However, the increase in vocalization occurred for a longer time period, i.e. until 1 day after weaning. Vocalization is considered a behavioral measure of distress in foals (Houpt and Hintz Citation1983; McCall et al. Citation1985) and behavioral data thus support the interpretation of changes in salivary cortisol concentration in the foals of this study. A transient decrease in feeding behavior and increase in defecation was present in all groups of foals. Differences between groups were less evident than for vocalization, but reduced feeding behavior was most pronounced in foals weaned simultaneously without the presence of unrelated adult horses. The increase in defecation was most pronounced in foals weaned consecutively on the day following weaning. On that day salivary cortisol concentrations in these foals were also higher than in foals of the other two groups.
The changes in salivary cortisol concentration and frequency of vocalization indicate that the presence of unrelated but familiar adult mares alleviated the stress of weaning. Under natural conditions, most equid species live in family bands, consisting of one mature stallion and several mares with their offspring (Klingel Citation1967; Linklater Citation2000). The presence of mares other than their mothers thus resembles the natural situation for foals. Observations in rhesus monkeys (Schapiro et al. Citation1996), primates (Hoff et al. Citation1982), and also in elephants (Slotow et al. Citation2000; McComb et al. Citation2001) have shown that the presence of conspecifics enhances success of young animals to cope successfully with future social challenges. In 1 and 2-year-old female horses kept as a group since weaning, the addition of adult horses of the same sex affected behavioral patterns and social relationships of the young horses. One major feature of the behavioral changes was an enlargement of the foals' behavioral repertoire. In addition, the presence of adult horses increased social interactions of the foals both with adults and with other young animals (Bourjade et al. Citation2008). Adults in social groups of young horses could play an important role as a social model through their behavioral responses when interacting with the young horses (Bourjade et al. Citation2008).
Locomotion was determined by pedometers, allowing an accurate quantification of locomotion activity and simultaneous measurements in several animals. In response to weaning, locomotion activity increased for 1 and 2 days in foals weaned either simultaneously or consecutively. Consequently, the time foals spent standing without moving was reduced in foals weaned simultaneously without the presence of unrelated adult horses on the day of weaning. No increase in locomotion time could be demonstrated in the foals weaned in the presence of two familiar but non-related adult mares. In all groups, the time foals spent lying was reduced almost to zero on the day of weaning. Our results demonstrate the least pronounced increase in locomotion activity in response to weaning in the presence of familiar mares. Foals weaned in the presence of familiar mares spent less time lying down on the days after weaning than foals of the other groups. At first this may appear contradictory. However, in foals weaned in the presence of familiar mares, time spent lying down some days after weaning did not, after a transient decrease, differ from the situation before weaning. The presence of adult mares thus could have a positive effect and induce more normal activity in the foals (Bourjade et al. Citation2008). Irrespective of the weaning method, the foals' vocal and locomotion response to separation from the mare were more pronounced in the first 2 h than between 2 and 4 h after weaning. This is in agreement with previous studies, showing the most pronounced behavioral response of foals to weaning within 30 min after separation from their dams (McCall et al. Citation1985). Because pedometers, while precisely recording locomotion time, do not assess the speed and type of locomotion, differences in locomotion activity between groups may have been underestimated in our study. Increased locomotion of foals in response to weaning has been reported previously (Houpt and Hintz Citation1983; McCall et al. Citation1987; Hoffman et al. Citation1995). Foals spent most time walking and moved only a little in trot or canter mode (McCall et al. Citation1985). Calves of beef cattle breeds are often weaned at approximately the same age as horse foals. As in foals, in these calves weaning results in decreased time spent eating and increased walking and vocalization (Veissier and LeNeindre Citation1989; Price et al. Citation2003), and increased vocalization and cortisol release have also been found in young sheep at weaning (Napolitano et al. Citation2008).
Foals of all three groups lost weight after weaning, and a transient weight loss within the first 10 days after weaning in foals is in agreement with a previous study (Rogers Citation2004). In our study, weight loss was most pronounced on day 2 after simultaneous weaning of foals without adult horses. At 2 weeks after weaning, foals staying together with two unrelated mares had gained more weight than foals of the other groups. The foals weaned simultaneously without unrelated adult mares also showed the most pronounced increase in locomotion activity and decrease in feeding, thus explaining the loss of body weight.
In response to weaning, there was no major change in HRV while the RR interval decreased significantly, corresponding to an increase in heart rate in foals weaned simultaneously without unrelated mares present. Part of an acute stress response is the increased release of catecholamines. This leads to a decrease in RR interval, i.e. an increase in heart rate. However, the decreased RR interval in foals weaned simultaneously without unrelated mares is more likely caused by the greater locomotion activity in foals of this group and may thus not be a direct but an indirect stress indicator. An increase in heart rate in response to weaning has been reported previously (Moons et al. Citation2005), but in that study only one mean value was obtained for pre-weaning, weaning, and post-weaning days, respectively. HRV, to our knowledge, has not previously been determined in foals at weaning. In contrast to the results of the present study, stress-induced decreases in RR interval were nearly always accompanied by parallel changes in HRV in previous studies on transport (CitationSchmidt et al. 2010a,c) and training of horses (Schmidt et al. Citation2010b), This supports the interpretation that the decreased RR interval in foals after weaning is mainly an effect of locomotion.
Conclusions
Weaning of foals is a stressful event resulting in increased salivary cortisol concentration, behavioral changes, and weight reduction. The least pronounced stress response was found in foals weaned simultaneously but in the presence of two unrelated but familiar mares. The loss of the mother with regard to security may be compensated in part by the presence of adult and familiar horses.
Supplementary Material
Download MS Word (46.5 KB)Acknowledgements
The authors are grateful to Walter Teske and the team of the Hauptgestüt at the Brandenburg State Stud for help with the mares and foals and to Edith Klobetz-Rassam and Julia Maderner for assistance with the laboratory work.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alexander SL, Irvine CHG. 1998. The effect of social stress on adrenal axis activity in horses: The importance of monitoring corticosteroid-binding globulin capacity. J Endocrinol. 157:425–432.
- Baucus KL, Squires EL, Ralston SL, McKinnon AO, Nett TM. 1990. Effect of transportation on the estrous cycle and concentrations of hormones in mares. J Anim Sci. 68:419–426.
- Bottoms GD, Roesel OF, Rausch FD, Akins EL. 1972. Circadian variation in plasma cortisol and corticosterone in pigs and mares. Am J Vet Res. 33:785–790.
- Bourjade M, Moulinot M, Henry S, Richard-Yris MA, Hausberger M. 2008. Could adults be used to improve social skills of young horses, Equus caballus?. Dev Psychobiol. 50:408–417.
- Clark DK, Friend TH, Dellmeier G. 1993. The effect of orientation during trailer transport on heart rate, cortisol and balance in horses. Appl Anim Behav Sci. 38:179–189.
- Ellis JM, Hollands T. 1998. Accuracy of different methods of estimating weight in horses. Vet Rec. 143:335–336.
- Enriquez DH, Ungerfeld R, Quintans G, Guidoni AL, Hötzel MJ. 2010. The effects of alternative weaning methods on behavior in beef calves. Live Sci. 128:20–27.
- Fraser D, Ritchie JSD, Fraser AF. 1975. The term ‘stress’ in a veterinary context. Br Vet J. 131:653–662.
- Haley DB, Bailey DW, Stookey JM. 2005. The effects of weaning beef calves in two stages on their behavior and growth rate. J Anim Sci. 83:2205–2214.
- Hameister T, Puppe B, Tuchscherer M, Kanitz E. 2010. Effects of weaning age on behavioural and physiological responses of domestic piglets—a review. Berl Münch Tierärztl Wochenschr. 123:11–19.
- Heleski CR, Shelle AC, Nielsen BD, Zanella AJ. 2002. Influence of housing on weanling horse behavior and subsequent welfare. Appl Anim Behav Sci. 78:291–302.
- Hickey MC, Drennan M, Earley B. 2003. The effect of abrupt weaning of suckler calves on the plasma concentrations of cortisol, catecholamines, leukocytes, acute-phase proteins and in vitro interferon-gamma production. J Anim Sci. 81:2847–2855.
- Hoff MP, Nadler RD, Maple TL. 1982. Control role of an adult male in a captive group of lowland gorillas. Folia Primatol. 38:72–85.
- Hoffman RM, Kronfield DS, Holland JL, Greiwe-Crandell K. 1995. Preweaning diet and stall weaning method influences on stress response in foals. J Anim Sci. 73:2922–2930.
- Hoffsis GF, Murdick PW, Tharp VL, Ault K. 1970. Plasma concentrations of cortisol and corticosterone in the normal horse. Am J Vet Res. 31:1379–1387.
- Holland JL, Kronfeld DS, Hoffman RM, Greiwe-Crandell KM, Boyd TL, Cooper WL, Harris PA. 1996. Weaning stress is affected by nutrition and weaning methods. Pferdeheilkunde. 12:257–260.
- Houpt KA, Hintz HF. 1983. Some effects of maternal deprivation on maintenance behavior, spatial relationships and responses to environmental novelty in foals. Appl Anim Ethol. 9:221–230.
- Houpt KA, Hintz HF, Butler WR. 1984. A preliminary study of two methods of weaning foals. Appl Anim Behav Sci. 12:177–181.
- Klingel H. 1967. Soziale organisation und verhalten freilebender steppenzebras (social organisation and behavior in free-ranging plains zebras). Z Tierpsychol. 24:580–624.
- Korte SM, Bouws GAH, Bohus B. 1993. Central actions of corticotropin-releasing hormone (CR-H) on behavioral, neuroendocrine and cardiovascular regulation: Brain corticoid receptor involvement. Horm Behav. 27:167–183.
- Lay DC, Friend TH, Randel RD, Bowers CL, Grissom KK, Mal ME. 1992. Effects of freeze or hotiron branding of Angus calves on some physiological and behavioral indicators of stress. Appl Anim Behav Sci. 33:137–147.
- Lebelt D, Schönreiter S, Zanella AJ. 1996. Salivary cortisol in stallions: The relationship with plasma levels, daytime profile and changes in response to semen collection. Pferdeheilkunde. 12:411–414.
- Lefcourt AM, Elsaesser TH. 1995. Adrenal responses of Angus Hereford cattle to the stress of weaning. J Anim Sci. 73:2669–2676.
- Linklater W. 2000. Adaptive explanation in socio-ecology: Lessons from the equidae. Biol Rev. 75:1–20.
- Malinowski K, Hallquist NA, Helyar L, Sherman AR, Scanes CG. 1990. Effect of different separation protocols between mares and foals on plasma cortisol and cell-mediated immune response. J Equine Vet Sci. 10:363–368.
- Martin P. 1984. The meaning of weaning. Anim Behav. 32:1257–1258.
- McCall CA, Potter GD, Kreider JL. 1985. Locomotor, vocal and other behavioral responses to varying methods of weaning foals. Appl Anim Behav Sci. 14:27–35.
- McCall CA, Potter GD, Kreider JL, Jenkins WL. 1987. Physiological responses in foals weaned by abrupt or gradual methods. J Equine Vet Sci. 7:368–374.
- McComb K, Moss C, Durant SM, Baker L, Sayialel S. 2001. Matriarchs as repositories of social knowledge in African elephants. Science. 292:491–494.
- Momozawa Y, Onob T, Sato F, Kikusui T, Takeuchi Y, Mori Y, Kusunose R. 2003. Assessment of equine temperament by a questionnaire survey to caretakers and evaluation of its reliability by simultaneous behavior test. Appl Anim Behav Sci. 84:127–138.
- Moons CPH, Laughlin K, Zanella AJ. 2005. Effect of short-term maternal separations on weaning stress in foals. Appl Anim Behav Sci. 91:321–335.
- Napolitano F, DeRosa G, Sevi A. 2008. Welfare implications of artificial rearing and early weaning in sheep. Appl Anim Behav Sci. 110:58–72.
- Nicol CJ, Badnell-Waters AJ, Bice R, Kelland A, Wilson AD, Harris PA. 2005. The effects of diet and weaning method on the behavior of young horses. Appl Anim Behav Sci. 95:205–221.
- Palme R, Möstl E. 1997. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Int J Mammal Biol. 62 Suppl II: 192–197.
- Price EO, Harris JE, Borgwardt RE, Sween ML, Connor JM. 2003. Fenceline contact of beef calves with their dams at weaning reduces the negative effects of separation on behavior and growth rate. J Anim Sci. 81:116–121.
- Prunier A, Mounier AM, Hay M. 2005. Effects of castration, tooth resection, or tail docking on plasma metabolites and stress hormones in young piglets. J Anim Sci. 83:216–222.
- Raynaert R, de Paepe M, Peeters G. 1976. Influence of stress, age and sex on serum growth hormone and free fatty acids in cattle. Horm Metab Res. 8:109–114.
- Rogers CW. 2004. The effect of two different weaning procedures on the growth of pasture reared Thoroughbred foals in New Zealand. NZ Vet J. 52:401–403.
- Rose-Meierhöfer S, Standke K, Hoffmann G. 2010. Effect of different group sizes on activity, body condition score, lying and social behaviour of young horses. Züchtungskunde. 82:282–291.
- Schapiro SJ, Bloomsmith MA, Porter LM, Suarez SA. 1996. Enrichment effects on rhesus monkeys successively housed singly, in pairs, and in groups. Appl Anim Behav Sci. 48:159–171.
- Schmidt A, Aurich C, Neuhauser S, Aurich J, Möstl E. 2009. Comparison of cortisol levels in blood plasma, saliva and faeces of horses submitted to different stressors or treated with ACTH, Proceeding of the 5th International Symposium on Equitation Science Sydney, p. 53.
- Schmidt A, Möstl E, Wehnert C, Aurich J, Müller J, Aurich C. Cortisol release and heart rate variability in horse during road transport. Horm Behav. 2010a; 57:209–215.
- Schmidt A, Aurich J, Möstl E, Müller J, Aurich C. Changes in cortisol release and heart rate and heart rate variability during the initial training of 3-year-old sport horses. Horm Behav. 2010b; 58:628–636.
- Schmidt A, Hödl S, Möstl E, Aurich J, Müller J, Aurich C. Cortisol release, heart rate, and heart rate variability in transport-naive horses during repeated road transport. Dom Anim Endocrinol. 2010c; 39:205–213.
- Slotow R, van Dyk G, Poole J, Page B, Klocke A. 2000. Older bull elephants control young males. Nature. 408:425–426.
- Snow DH, Rose RJ. 1981. Hormonal changes associated with long distance exercise. Equine Vet J. 13:195–197.
- Tarvainen MP, Ranta-aho PO, Karjalainen PA. 2002. An advanced detrending method with application to HRV analysis. IEEE Trans Biomed Eng. 49:172–175.
- Veissier I, LeNeindre P. 1989. Weaning in calves: Its effects on social organization. Appl Anim Behav Sci. 24:43–54.
- von Borell E, Langbein J, Despres G, Hansen S, Leterrier C, Marchand-Forde J, Marchand-Forde R, Minero M, Mohr E, Prunier A, Valance D, Veissier I. 2007. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—a review. Physiol Behav. 92:293–316.
- Waran NK, Clarke N, Farnworth M. 2008. The effects of weaning on the domestic horse (Equus caballus). Appl Anim Behav Sci. 110:42–57.
