Abstract
Mood disorders and chronic stress are frequently associated with gastrointestinal (GI) symptoms including diarrhoea or constipation. Locally produced serotonin [5-hydroxytryptamine (5-HT)] regulates GI motility and is a key factor in the pathophysiology of stress-associated GI disorders. We aimed to establish whether chronic stress can differentially affect faecal output and colon 5-HT concentration in two inbred mouse strains: BALB/c and C57BL/6 which differ in their ability to cope with stress. Adult male BALB/c and C57BL/6 mice were restrained for 2 h daily for 10 days. Defecation was monitored during each stress session. Twenty-four hours after the last session of stress, plasma corticosterone concentration was higher than control in both strains, indicative of a physiological effect of chronic stress; however, stress-induced diarrhoea was more persistent in C57BL/6 mice. Basal concentration of colon 5-HT was higher in C57BL/6 mice, and stress elicited an increase in colon 5-HT only in this strain. Finally, naïve BALB/c mice had a higher sensitivity (incidence of diarrhoea) to 5-HT (0.33 mg/kg, i.p.) than C57BL/6 mice. Our results suggest that differential defecation responses to stress may be associated with colon 5-HT concentration, which may in turn reflect the individual sensitivity to 5-HT. In addition, C57BL/6 mice emerge as a relevant model for studying GI alterations induced by chronic stress.
Introduction
Patients suffering from mood disorders such as anxiety and depression frequently report gastrointestinal (GI) symptoms including abdominal discomfort, pain, diarrhoea or constipation. Interestingly, functional intestinal disorders such as irritable bowel syndrome (IBS) are frequently co-morbid with depression and anxiety (Quigley Citation2006; Clarke et al. Citation2009; Gros et al. Citation2009). In addition, antidepressant medications, especially those that target monoamine reuptake and metabolism, show some efficacy in the treatment of symptoms of IBS and other GI disorders, albeit at doses that favour an analgesic modality (Ford et al. Citation2009).
Serotonin [5-hydroxytryptamine (5-HT)] is one of the key monoamine neurotransmitters regulating GI function (Spiller Citation2002; Sanger Citation2008). 5-HT-containing enteroendocrine cells are abundant in the intestinal mucosa and respond to stimuli such as luminal pressure and various chemical signals. Locally produced 5-HT binds to a variety of receptors on mucosal afferent and myenteric neurons to initiate a secretomotor response, which can cause antral contractions, nausea and vomiting as well as increased intestinal secretion, mechanical propulsion and eventually diarrhoea (Spiller Citation2002; Spiller Citation2008; Bertrand and Bertrand Citation2010). Interestingly, IBS patients have alterations in 5-HT neurotransmitter metabolism, which are consistent with the predominance of either diarrhoea or constipation (Dunlop et al. Citation2005). Another study revealed that enteroendocrine cell numbers are increased in the colon of patients with post-dysenteric IBS (Spiller et al. Citation2000). However, 5-HT levels have also been shown to be higher in the colonic mucosa of constipation-predominant IBS patients compared with diarrhoea-predominant IBS patients or healthy controls (Miwa et al. Citation2001). Moreover, distinct alterations in the genes for serotonin transporter (SERT; Colucci et al. Citation2008), 5-HT3e receptor (Kapeller et al. Citation2008) and the adaptor protein p11 (Camilleri et al. Citation2007) have also been described in IBS. Notably, another study showed that mucosal 5-HT, tryptophan hydroxylase 1 (TPH1) mRNA, SERT mRNA and SERT immunoreactivity were all significantly reduced both in constipation- and diarrhoea-predominant IBS patients, further suggesting some common molecular alterations in the bowel of these patients although enteroendocrine cell numbers were not different between IBS patients and controls (Coates et al. Citation2004).
In recent years, several treatments for IBS have been developed to target serotonergic pathways. 5-HT3 receptor antagonists and 5-HT4 receptor agonists were found to be effective in the treatment of diarrhoea and constipation, respectively, although drugs with these actions were later withdrawn due to adverse side effects (Sanger Citation2008). Although the central serotonergic system is important for maintaining normal emotional and stress responses (Owens and Nemeroff Citation1994; Cryan and Leonard Citation2000; Robinson et al. Citation2010), it is still unclear if there is a direct impact of mood and chronic stress on gut 5-HT neurotransmitter levels. Clearly, a large proportion of patients suffering from mood disorders do not experience colonic alterations (Mussell et al. Citation2008), which may be in part due to variations in genetic vulnerability to stress in the GI neurotransmitter system.
Inbred mouse strains are invaluable tools in dissecting the genetic basis of complex disorders (Cryan and Holmes Citation2005; Jacobson and Cryan Citation2007). BALB/c and C57BL/6 are two inbred mouse strains that differ markedly in the way they cope with stressful stimuli (Brinks et al. Citation2007; O'Mahony et al. Citation2010). BALB/c mice harbour a single nucleotide polymorphism (C1473G) in their TPH2 gene, which results in reduced levels of serotonin compared with C57BL/6 mice, which possess the wild-type C/C allele (Kulikov et al. Citation2005). Moreover, C57BL/6 mice have been used extensively in stress research, and are the background strain on which most transgenic mice are bred, whereas BALB/c mice have been proposed to be a model of pathological anxiety (Belzung and Griebel Citation2001; Cryan and Holmes Citation2005; Jacobson and Cryan Citation2007). Here, we hypothesised that chronic stress can differentially affect gut function, specifically faecal output, in these mouse strains. First, our aim was to analyse the faecal output and colon 5-HT concentration in BALB/c and C57BL/6 mice subjected to chronic restraint stress. Second, in a mechanistic approach, we analysed the effects of exogenous 5-HT on defecation in both strains. We demonstrate here that, in addition to a divergence in defecation responses to stress, C57BL/6 and BALB/c show a dissimilar sensitivity to exogenous 5-HT. Finally, colon 5-HT concentration is also differentially affected by stress in these mice.
Materials and methods
Animals
Adult male BALB/cOlaH (BALB/c) and C57BL/6JOlaHsd (C57BL/6) mice weighing 20–25 g were obtained from the Biological Services Unit, University College, Cork, Ireland and from Harlan, Blackhorn, UK. A total of 96 mice were used in this study. Animal rooms were temperature controlled (21 ± 1°C), with a 12-h light/dark cycle (lights on at 07:00 h). Mice were group-housed (four to eight mice per cage) and cages were cleaned once a week as part of the animal room routine. All procedures were carried out in accordance with EU directive 89/609/EEC and approved by the Animal Experimentation Ethics Committee of University College Cork.
Restraint stress
The restraint stress procedure was carried out in a separate room as previously described (O'Mahony et al. Citation2010; Browne et al. Citation2011). Mice were placed in perforated 50 ml plastic centrifuge tubes, secured horizontally, for 2 h a day during 10 consecutive days. This duration and frequency of stressor has been shown to induce an anxiety and depressive-like behaviour (Govindarajan et al. Citation2006) and the type of restrainer used limits movement without interfering with normal breathing. All stress sessions were carried out between 09.00 and 12.00 h. Unstressed mice were left undisturbed in their home cage.
During each restraint stress session, the occurrence of diarrhoea was assessed using an arbitrary scoring scale ranging from 0 to 3: 0, absence of faecal pellets; 1, well formed, solid pellets; 2, slightly wet and soft to the touch but formed pellets and 3, watery and unformed stool. This scale was adapted from Kawano et al. (Citation2005). This method of faecal scoring was selected owing to the difficulty of obtaining accurate pellet numbers when the mice were in the restrainers.
Mice were killed by cervical dislocation, 24 h after the final day of stress. Tissue and plasma samples were harvested at this time point to account for the effects of chronic 10-day stress rather than the immediate (acute) effects of the last session of stress. All samples were taken between 09.00 and 12.00 h. Trunk blood was mixed with ethylenediaminetetraacetic acid (EDTA), and plasma was separated by centrifugation at 10,000g for 10 min. Colon contents were flushed with cold phosphate-buffered saline, and a 1 cm section of tissue taken from the proximal end was stored at − 80°C for further analyses.
5-HT-induced defecation
We adapted the protocol published by Kadowaki et al. (Citation1993). Briefly, mice were deprived of food overnight before the experiment but had free access to water. The rationale for using the fasting condition was established in a pilot study, in which we observed that this resulted in a higher sensitivity between different doses of 5-HT in comparison with the fed status. In order to habituate mice to handling and minimise novelty stress, mice were handled for a few minutes and weighed daily, for a week prior to the experiments. Evaluation of defecation was made after i.p. administration of 5-HT (from Sigma, Munich, Germany; 0.1, 0.33 or 1 mg/kg). Vehicle was saline containing 0.01% ascorbic acid and the volume of injection was kept constant at 5 ml/kg. Immediately after injection, each mouse was placed in an individual cage without bedding, and defecation was assessed at 0, 5, 10, 15, 20 and 30 min post-injection, using the scoring scale described above. The number of faecal pellets (either well-formed faeces or diarrhoea episodes) was also counted. The procedure was carried out between 10.00 and 12.00 h, and scores were performed in a blinded manner.
Corticosterone immunoassay
Plasma corticosterone concentration was determined using a Corticosterone EIA kit (Assay Designs, MI, USA) according to the manufacturer's instructions. The sensitivity of this method is 27 pg/ml with a range of 32–20000 pg/ml, an intra-assay variation of 8.4% and an inter-assay variation of 8.2%.
High performance liquid chromatography reagents
High performance liquid chromatography (HPLC) grade methanol was obtained from Alkem/Reagecon (Cork, Ireland). HPLC electrochemical grade citric acid, 1 M o-phthalaldehyde, sodium dihydrogen phosphate monohydrate, octane sulphonic acid, EDTA and di-sodium hydrogen orthophosphate were also obtained from Alkem/Reagecon. All other reagents were obtained from Sigma unless otherwise stated.
Tissue homogenisation for HPLC
Full-thickness colon tissue was weighed and placed in cold homogenisation buffer (0.1 M citric acid, 0.1 M sodium dihydrogen phosphate monohydrate, 5.6 mM octane sulphonic acid, 10 μM EDTA in 10% (v/v) methanol solution, pH 2.8). A volume of 1 ml buffer was used per 40–50 mg of colon tissue. Each sample was finely chopped with scissors and homogenised (Polytron dispersing machine, Kinematica, Lucerne, Switzerland), centrifuged at 10,000g for 15 min at 4°C and the supernatant stored at − 80°C until dilution for monoamine analysis.
Monoamine determination
5-HT and its metabolites were assessed as previously described (O'Mahony et al. Citation2008; O'Mahony et al. Citation2011). The mobile phase contained 0.1 M citric acid, 0.1 M sodium dihydrogen phosphate, 0.01 mM EDTA (Alkem/Reagecon), 5.6 mM octane-1-sulphonic acid (Sigma) and 10% (v/v) methanol (Alkem/Reagecon), and was adjusted to pH 2.8 using 4 N sodium hydroxide (Alkem/Reagecon). Mobile phase was filtered through Millipore 0.45 μm HV Durapore membrane filters (AGB, Dublin, Ireland). Sample supernatants were diluted 1:10 in mobile phase immediately prior to analysis. About 20 μl of the diluted supernatant was injected onto the HPLC system, which consisted of a SCL-10Avp system controller, LC-10AS pump, SIL-10A autoinjector (with sample cooler maintained at 4°C), CTO-10A oven, LECD 6A electrochemical detector and an online DGU-20A3 Prominence Degasser (Shimadzu, Duisburg, Germany). A reversed-phase column (Kinetex 2.6u C18 100 × 4.6 mm, Phenomenex, Cheshire, UK), maintained at 30°C, was employed in the separation (flow rate 0.9 ml/min). Chromatograms generated were analysed using Class-VP 5 software. Neurotransmitters were identified by their charac-teristic retention times as determined by standard injections, which were run at regular intervals during sample analysis. Analyte to internal standard peak height ratios were measured and compared with standard injections, and results were expressed as neurotransmitter in micrograms per fresh weight of tissue in grams.
Statistical analysis
Sample size (n = 8 per group) was based on a power calculation aimed at detecting differences at 0.05 level. Values are expressed as mean ± SEM. Changes in corticosterone concentration, daily stress-induced defecation score, neurotransmitter concentrations and 5-HT-induced defecation parameters were evaluated using a two-way ANOVA. Any overall statistical differences were further analysed using post hoc analysis (Bonferroni, GraphPad Prism 4 Software Inc.). The overall stress-induced defecation score was evaluated with a t-test. For the incidence of diarrhoea, differences between strains were evaluated using chi-square test.
Results
Effects of stress on corticosterone and defecation
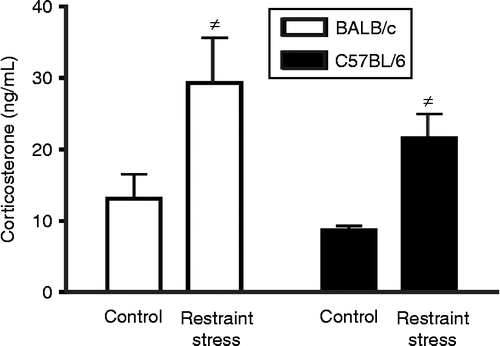
Chronic restraint stress induced an increase in plasma corticosterone concentration in both BALB/c and C57BL/6 mice in comparison with control mice [F(1,23) = 12, p < 0.005; . This observation is indicative of a physiological effect of the chronic restraint stress protocol in both strains; however, there was no differential effect of strain on plasma corticosterone concentration.
Figure 1. Plasma corticosterone concentration as a result of chronic restraint stress. Mice were placed in restrainers for 2 h a day during 10 consecutive days. Plasma corticosterone was analysed 24 h after the last session of stress. Both strains showed a significant increase in plasma corticosterone concentration after chronic stress in comparison with non-stressed mice ( ≠ p < 0.005 by two-way ANOVA). No differences were found between strains. Data are expressed as mean ± SEM; n = 6–8.
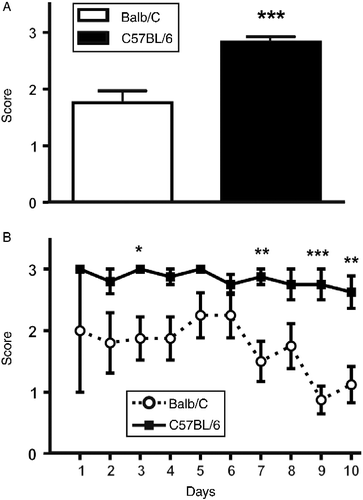
Stress-induced diarrhoea-like response was significantly higher in C57BL/6 mice, as shown in (p < 0.005 by t-test). Analysis of the daily defecation scores also indicates that, at all times, C57BL/6 mice had a more pronounced diarrhoea response [F(1,122) = 61.92, p < 0.005]. Interestingly, towards the end of the experiment, the defecation score for BALB/c mice was closer to 1, whereas scores for C57BL/6 mice remained at an average of 3 (i.e. diarrhoea) throughout the experiment, as shown in .
Figure 2. Stress-induced alterations in defecation. During each restraint stress session, the faecal output was assessed using a scale ranging from 0 (no faeces) to 3 (diarrhoea). (A) Overall stress-induced diarrhoea was more common in C57BL/6 mice (*p < 0.005 by t-test). (B) An analysis of the daily defecation response shows that towards the end of the experiment the defecation score for BALB/c mice was close to 1, whereas scores for C57BL/6 mice remained high (close to a score of 3) throughout the experiment. Asterisks indicate differences between strains on a particular day (*p < 0.05; **p < 0.01; ***p < 0.005 by two-way ANOVA and Bonferroni test). Data are expressed as mean ± SEM; n = 6–8.
Colon monoamines
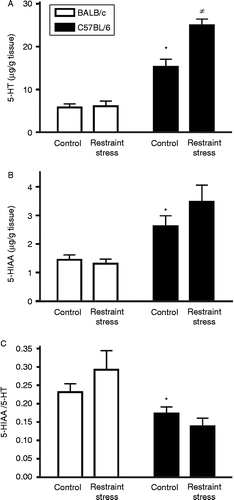
Basal concentration of colon 5-HT was significantly higher in C57BL/6 mice [F(1,23) = 122.37, p < 0.0001; . Furthermore, restraint stress elicited a significant increase in colon 5-HT only in the C57BL/6 strain [F(1,23) = 15.24, p < 0.001], with no changes for BALB/c, as shown in . Interaction between stress and strain was significant at the level p < 0.005 [F(1,23) = 13.62]. The basal concentration of the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) was also different between strains, with a higher 5-HIAA content for C57BL/6 colon [3.48 ± 0.58; F(1,22) = 23.83, p < 0.0001]. No effect of stress was found for 5-HIAA (). Finally, basal 5-HT turnover (i.e. the ratio 5-HIAA/5-HT) was higher in BALB/c mice [F(1,22) = 10.29, p < 0.005], but it was not affected by stress in BALB/c or C57BL/6, as shown in .
Figure 3. Colon monoamines. (A) Basal concentration of colon 5-HT was higher in C57BL/6 mice (*p < 0.0001 by two-way ANOVA), and stress elicited a significant increase in colon 5-HT only in the C57BL/6 strain ( ≠ p < 0.001; interaction p < 0.005 by two-way ANOVA). (B) Basal concentration of colon 5-HIAA was also higher in C57BL/6 mice (*p < 0.0001 by two-way ANOVA), with no effect of stress in either BALB/c or C57BL/6. (C) 5-HT turnover was higher in BALB/c mice (*p < 0.005 by two-way ANOVA), and it was not affected by stress in BALB/C or C57BL/6. Data are expressed as mean ± SEM; n = 6–8.
5-HT-induced defecation
When placed in individual observation cages, vehicle-treated BALB/c mice started defecating within 10 min and kept on producing faeces during the 30 min of the test, as shown in . By contrast, C57BL/6 mice produced the first faecal pellets at 15 min, and by 20 min they stopped defecating (). Although vehicle-treated BALB/c mice in general displayed a significantly higher faecal score [F(1,84) = 23.25, p < 0.0001] and produced more faeces [F(1,84) = 61.50, p < 0.0001] than C57BL/6 mice, individual scores for all vehicle-treated mice were never higher than 1.
Figure 4. Serotonin-induced defecation. Faeces were counted and the occurrence of diarrhoea was scored from 0 (no faeces) to 3 (diarrhoea). (A) and (B) Vehicle-treated BALB/c mice displayed a higher faecal score than C57BL/6 mice (*p < 0.05; **p < 0.01 by two-way ANOVA). I.p. administration of 5-HT significantly increased faecal score in BALB/c and C57BL/6, although no differences were found between strains. (C) and (D) Vehicle-treated BALB/c mice produced more faeces than C57BL/6 mice (**p < 0.01; ***p < 0.005 by two-way ANOVA). 5-HT increased the number of faeces produced by BALB/c and C57BL/6 mice, with no effect of strain. (E) Incidence of diarrhoea at t = 10 min was higher for BALB/c than C57BL/6 mice at 0.33 mg/kg of 5-HT, but lower at 1 mg/kg (*p < 0.05 by chi-square test). Data are expressed as means ± SEM; n = 8.
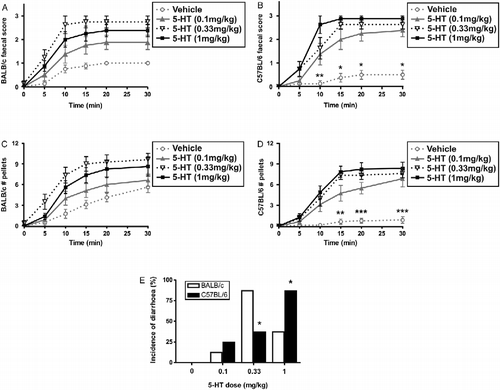
In 5-HT-treated C57BL/6 mice, the highest dose of 5-HT (1 mg/kg) induced the highest response for faecal score (), whereas for number of faeces produced, 1 mg/kg produced a similar effect to 0.33 mg/kg in C57BL/6 mice (). By contrast, 5-HT-treated BALB/c mice showed the greatest effect at 0.33 mg/kg (). In addition, BALB/c had a higher incidence of diarrhoea than C57BL/6 for the dose of 0.33 mg/kg (p < 0.05 by chi-square test), whereas the dose of 1 mg/kg produced a sub-maximal response in BALB/c mice, which was significantly lower than the effect observed in C57BL/6 mice treated with the same dose (p < 0.05 by chi-square test, ).
Discussion
GI function is strongly influenced by mood, and increasing evidence points to a rich communication between the brain and the gut (Taché et al. Citation2001; O'Mahony et al. Citation2009; Forsythe et al. Citation2010). However, a proportion of individuals suffering from depression and anxiety do not report GI symptoms (Mussell et al. Citation2008), suggesting that genetic factors may predispose to susceptibility or resistance to stress-induced colonic dysfunction. Such variability in the sensitivity to stress not only emphasises the complexity of the brain–gut axis but may also be crucial to understanding the mechanisms underlying stress-related GI disorders such as IBS. Here, we show that two inbred mouse strains present a markedly different defecatory response to repeated restraint stress. Whereas C57BL/6 mice have persistent diarrhoea and show enhanced colon 5-HT concentration compared with baseline, the BALB/c strain seems to better adapt to chronic stress being significantly less prone to diarrhoea. At the same time, BALB/c mice do not show increased colon 5-HT concentration as a result of chronic restraint stress. This “adaptation” may only involve the colonic component of stress; it is important to note that BALB/c mice did show increased plasma corticosterone level at the end of the study in comparison with non-stressed BALB/c mice. These observations indicate that the two strains may not only have differences in their colonic function but also in their brain–gut axis as a whole.
BALB/c mice are generally considered more anxious than the C57BL/6 strain (Griebel et al. Citation2000; Cryan and Holmes Citation2005; Brinks et al. Citation2007). They also show differential brain activation patterns when subjected to an acute stressor (O'Mahony et al. Citation2010). A possible cause for such disparity in the behavioural and molecular changes in response to stress is a differential brain 5-HT synthetic capacity in these two mouse strains (Matthes et al. Citation2010; Browne et al. Citation2011). Tryptophan hydroxylase-2 (TPH2) is the rate-limiting enzyme in brain 5-HT biosynthesis, whereas TPH1 is the main isoform present in peripheral tissues (Walther et al. Citation2003). Interestingly, BALB/c mice carry a mutated TPH2 allele, which translates into an enzyme with lower activity, whereas C57BL/6 mice express the normal TPH2 allele (Zhang et al. Citation2004). It is noteworthy that TPH2 knockout mice have similar levels of colon 5-HT than wild-type mice, whereas TPH1 knockout mice exhibit a great reduction in colon 5-HT, confirming that the main enzyme responsible for 5-HT synthesis in the gut is TPH1 (Savelieva et al. Citation2008). Therefore, it is unlikely that TPH2 polymorphisms are directly related to the differences in basal 5-HT content in the colon of BALB/c and C57BL/6. Functional polymorphisms have also been described for the promoter region of the Tph1 gene, which has a higher transcriptional activity in New Zealand White mice than in New Zealand Black mice (Nakamura et al. Citation2006). Such characterisation is not yet available for the BALB/c and C57BL/6 strains. Interestingly, among IBS patients, polymorphisms in the TPH1 gene show some association with the severity of symptoms such as diarrhoea; however, these polymorphisms were not associated with the diagnosis of IBS (Jun et al. Citation2011).
Release of 5-HT by enteroendocrine cells in the gut mucosa can be promoted by luminal pressure as well as by bacterial toxins (Spiller Citation2002). Stimulation of 5-HT secretion is also achieved via beta-adrenergic, purinergic A2A/B and muscarinic receptor activation (Spiller Citation2002). In the rat, acute restraint stress stimulates vagal afferents to the proximal colon and induces the release of 5-HT, which is accompanied by acceleration of colonic transit in a 5-HT3 receptor-dependent manner (Nakade et al. Citation2007; Tsukamoto et al. Citation2007). In contrast, the effects of chronic stress on gut serotonergic neurotransmission remain largely unknown. In this work, we found that a 10-day repeated restraint stress, which increased plasma corticosterone concentration in both BALB/c and C57BL/6 mice, induced a greater diarrhoea-like response in C57BL/6 mice, which is consistent with an increase in the content of 5-HT in the colon. We did not measure the specific source of 5-HT within the mouse gut. However, considering that 95% of colon 5-HT is produced by enterochromaffin cells in the mucosa (Spiller Citation2002), and due to the magnitude of 5-HT changes observed here, we presume it is unlikely that the source would be other than the mucosa. It would be interesting, for future studies, to establish whether the differences in colon 5-HT content between these two mouse strains are associated with the amount of enterochromaffin cells in the colon mucosa, and to investigate if there is a hyperplasia effect in response to restraint stress in C57BL/6 mice.
We hypothesised that if the differential colonic response to stress between BALB/c and C57BL/6 mice relied solely on the different concentration of colon 5-HT, then administration of exogenous 5-HT should induce the same GI effects in both strains. To test for this, we administered different doses of 5-HT to mice and scored the defecation response. Vehicle-treated BALB/c mice displayed a significantly higher faecal score and produced more faeces than C57BL/6 mice. This was not completely unexpected as BALB/c mice are more sensitive to mild stressors such as transfer to a novel cage (Brinks et al. Citation2007). It is important to state that neither BALB/c nor C57BL/6 mice experienced diarrhoea as a result of vehicle administration. When given 5-HT, C57BL/6 mice displayed the most severe diarrhoea behaviour in response to the highest dose (1 mg/kg). In contrast, 5-HT-treated BALB/c mice responded maximally to a lower dose of 0.33 mg/kg, whereas at 1 mg/kg a sub-maximal response was observed. When comparing the incidence of diarrhoea, a similar effect was observed. One drawback of the 5-HT-induced defecation test is that it involves a mild stress due to the novel environment, and interactions between 5-HT and the corticotropin-releasing factor system activated by stress may play a role in the colonic response to the experimental conditions, as previously reported (Nakade et al. Citation2007; Hirata et al. Citation2008). However, the higher sensitivity to 5-HT in BALB/c mice in terms of diarrhoea response is consistent with the finding that at baseline, (1) BALB/c mice have a smaller content of colonic 5-HT than C57BL/6 mice, and (2) there is a higher colon 5-HT turnover in BALB/c than in C57BL/6 mice. All of this may be associated with a differential expression of one or more elements of the colon serotonergic system. Furthermore, the apparent inverse response to 5-HT in BALB/c mice may be associated with a differential regulation of receptor activity. Future studies should also test for differences in colonic TPH activity as well as 5-HT receptors expression and activity in these strains.
Stress is known to induce changes in mouse GI function. Repeated water avoidance stress (WAS) significantly increases the rate of defecation in female C57BL/6 mice (Melgar et al. Citation2008). When additional stressors (either physical or psychological) are incorporated, they can modify the effects of chronic stress on defecation: for instance, chronic WAS induces a greater defecatory response when male mice have been previously exposed to surgery and single housing (Larauche et al. Citation2010). By contrast, when female mice are exposed to dextran sulphate sodium (DSS) for 5 days in order to induce colitis and then to repeated WAS, they show a reduction in faecal output compared with mice that did not receive DSS (Melgar et al. Citation2008; Larsson et al. Citation2009). Interestingly, both BALB/c and C57BL/6 adult mice display decreased faecal output in the open field, when they had been subjected to an early life stressor such as maternal separation (Savignac et al. Citation2011a). However, previous data from our group show that social defeat stress (a resident–intruder protocol for 10 days) induces a constipation-like phenotype in male BALB/c but not in C57BL/6 mice (Savignac et al. Citation2011b). It has been previously shown that different stressors can affect physiology to a different extent (Bowers et al. Citation2008); therefore, future studies should investigate the effects of different stressors, predictable and unpredictable, psychological and physical on GI function.
IBS is a chronic functional GI disorder, which is known to be co-morbid with depression and anxiety. Interestingly, IBS patients have alterations in 5-HT metabolism, which are consistent with the predominance of either diarrhoea or constipation (Dunlop et al. Citation2005). Our results indicate that, in the mouse, differential colonic responses to stress are associated with colon 5-HT concentration, which may in turn reflect the individual sensitivity to 5-HT. This evidence supports the premise that alterations in the brain–gut axis are the key factors to the pathophysiology of functional GI disorders, and it is of great relevance to the development of adequate pharmacology to treat these disorders. Finally, the chronic restraint stress paradigm in C57BL/6 mice emerges as a relevant model for studying IBS or other GI alterations induced by chronic stress.
Acknowledgements
The Alimentary Pharmabiotic Centre is a research centre funded by Science Foundation Ireland (SFI), through the Irish Government's National Development Plan. The authors and their work were supported by Science Foundation Ireland (Grant nos 02/CE/B124 and 07/CE/B1368). JFC is also funded by European Community's Seventh Framework Programme; Grant no. FP7/2007-2013, Grant Agreement 201714.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Belzung C, Griebel G. 2001. Measuring normal and pathological anxiety-like behaviour in mice: A review. Behav Brain Res. 125 1–2: 141–149.
- Bertrand PP, Bertrand RL. 2010. Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci. 153 1–2: 47–57.
- Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. 2008. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun. 22 1: 105–113.
- Brinks V, van der Mark M, de Kloet R, Oitzl M. 2007. Emotion and cognition in high and low stress sensitive mouse strains: A combined neuroendocrine and behavioral study in BALB/c and C57BL/6J mice. Front Behav Neurosci. 1 8: 1–12.
- Browne CA, Clarke G, Dinan T, Cryan JF.. 2011. Differential stress-induced alterations in tryptophan hydroxylase activity and serotonin turnover in two inbred mouse strains. Neuropharmacology. 60 4: 683–691.
- Camilleri M, Andrews CN, Bharucha AE, Carlson PJ, Ferber I, Stephens D, Smyrk TC, Urrutia R, Aerssens J, Thielemans L, Gohlmann H, van den Wyngaert I, Coulie B. 2007. Alterations in expression of p11 and SERT in mucosal biopsy specimens of patients with irritable bowel syndrome. Gastroenterology. 132 1: 17–25.
- Clarke G, Quigley EM, Cryan JF, Dinan TG. 2009. Irritable bowel syndrome: Towards biomarker identification. Trends Mol Med. 15 10: 478–489.
- Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. 2004. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 126 7: 1657–1664.
- Colucci R, Blandizzi C, Bellini M, Ghisu N, Tonini M, Del Tacca M. 2008. The genetics of the serotonin transporter and irritable bowel syndrome. Trends Mol Med. 14 7: 295–304.
- Cryan JF, Holmes A. 2005. The ascent of mouse: Advances in modelling human depression and anxiety. Nat Rev Drug Discov. 4 9: 775–790.
- Cryan JF, Leonard BE. 2000. 5-HT1A and beyond: The role of serotonin and its receptors in depression and the antidepressant response. Hum Psychopharmacol. 15 2: 113–135.
- Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. 2005. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 3 4: 349–357.
- Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. 2009. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: Systematic review and meta-analysis. Gut. 58 3: 367–378.
- Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. 2010. Mood and gut feelings. Brain Behav Immun. 24 1: 9–16.
- Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S. 2006. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci USA. 103 35: 13208–13213.
- Griebel G, Belzung C, Perrault G, Sanger DJ. 2000. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl). 148 2: 164–170.
- Gros DF, Antony MM, McCabe RE, Swinson RP. 2009. Frequency and severity of the symptoms of irritable bowel syndrome across the anxiety disorders and depression. J Anxiety Disord. 23 2: 290–296.
- Hirata T, Keto Y, Nakata M, Takeuchi A, Funatsu T, Akuzawa S, Sasamata M, Miyata K. 2008. Effects of serotonin 5-HT(3) receptor antagonists on CRF-induced abnormal colonic water transport and defecation in rats. Eur J Pharmacol. 587 1–3: 281–284.
- Jacobson LH, Cryan JF. 2007. Feeling strained? Influence of genetic background on depression-related behavior in mice: A review. Behav Genet. 37 1: 171–213.
- Jun S, Kohen R, Cain KC, Jarrett ME, Heitkemper MM. 2011. Associations of tryptophan hydroxylase gene polymorphisms with irritable bowel syndrome. Neurogastroenterol Motil. 23 3: 233–239 e116.
- Kadowaki M, Nagakura Y, Tomoi M, Mori J, Kohsaka M. 1993. Effect of FK1052, a potent 5-hydroxytryptamine3 and 5-hydroxytryptamine4 receptor dual antagonist, on colonic function in vivo. J Pharmacol Exp Ther. 266 1: 74–80.
- Kapeller J, Houghton LA, Monnikes H, Walstab J, Moller D, Bonisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F, Gassler N, Fischer C, Whorwell PJ, Atkinson W, Fell C, Buchner KJ, Schmidtmann M, van der Voort I, Wisser AS, Berg T, Rappold G, Niesler B. 2008. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 17 19: 2967–2977.
- Kawano K, Mori T, Fu L, Ito T, Niisato T, Yoshida S, Shiokawa S, Sato Y, Murakami H, Shishikura T. 2005. Comparison between partial agonist (ME3412) and antagonist (alosetron) of 5-hydroxytryptamine 3 receptor on gastrointestinal function. Neurogastroenterol Motil. 17 2: 290–301.
- Kulikov AV, Osipova DV, Naumenko VS, Popova NK. 2005. Association between Tph2 gene polymorphism, brain tryptophan hydroxylase activity and aggressiveness in mouse strains. Genes Brain Behav. 4 8: 482–485.
- Larauche M, Gourcerol G, Million M, Adelson DW, Taché Y. 2010. Repeated psychological stress-induced alterations of visceral sensitivity and colonic motor functions in mice: Influence of surgery and postoperative single housing on visceromotor responses. Stress. 13 4: 343–354.
- Larsson MH, Miketa A, Martinez V. 2009. Lack of interaction between psychological stress and DSS-induced colitis affecting colonic sensitivity during colorectal distension in mice. Stress. 12 5: 434–444.
- Matthes S, Mosienko V, Bashammakh S, Alenina N, Bader M. 2010. Tryptophan hydroxylase as novel target for the treatment of depressive disorders. Pharmacology. 85 2: 95–109.
- Melgar S, Engstrom K, Jagervall A, Martinez V. 2008. Psychological stress reactivates dextran sulfate sodium-induced chronic colitis in mice. Stress. 11 5: 348–362.
- Miwa J, Echizen H, Matsueda K, Umeda N. 2001. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion. 63 3: 188–194.
- Mussell M, Kroenke K, Spitzer RL, Williams JB, Herzog W, Lowe B. 2008. Gastrointestinal symptoms in primary care: Prevalence and association with depression and anxiety. J Psychosom Res. 64 6: 605–612.
- Nakade Y, Fukuda H, Iwa M, Tsukamoto K, Yanagi H, Yamamura T, Mantyh C, Pappas TN, Takahashi T. 2007. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol. 292 4: G1037–G1044.
- Nakamura K, Sugawara Y, Sawabe K, Ohashi A, Tsurui H, Xiu Y, Ohtsuji M, Lin QS, Nishimura H, Hasegawa H, Hirose S. 2006. Late developmental stage-specific role of tryptophan hydroxylase 1 in brain serotonin levels. J Neurosci. 26 2: 530–534.
- O'Mahony S, Chua AS, Quigley EM, Clarke G, Shanahan F, Keeling PW, Dinan TG. 2008. Evidence of an enhanced central 5HT response in irritable bowel syndrome and in the rat maternal separation model. Neurogastroenterol Motil. 20 6: 680–688.
- O'Mahony CM, Clarke G, Gibney SM, Dinan T, Cryan JF. Strain differences in the neurochemical response to chronic restraint stress in the rat: Relevance to depression. Pharmacol Biochem Behav. 2011 in press.
- O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. 2009. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 65 3: 263–267.
- O'Mahony CM, Sweeney FF, Daly E, Dinan TG, Cryan JF. 2010. Restraint stress-induced brain activation patterns in two strains of mice differing in their anxiety behaviour. Behav Brain Res. 213 2: 148–154.
- Owens MJ, Nemeroff CB. 1994. Role of serotonin in the pathophysiology of depression: Focus on the serotonin transporter. Clin Chem. 40 2: 288–295.
- Quigley EM. 2006. Changing face of irritable bowel syndrome. World J Gastroenterol. 12 1: 1–5.
- Robinson OJ, Cools R, Crockett MJ, Sahakian BJ. 2010. Mood state moderates the role of serotonin in cognitive biases. J Psychopharmacol. 24 4: 573–583.
- Sanger GJ. 2008. 5-hydroxytryptamine and the gastrointestinal tract: Where next?. Trends Pharmacol Sci. 29 9: 465–471.
- Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, Lanthorn TH. 2008. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS One. 3 10: e3301.
- Savignac HM, Dinan TG, Cryan JF. Resistance to early-life stress in mice: Effects of genetic background and stress duration. Front Behav Neurosci. 2011a; 5:13.
- Savignac HM, Finger BC, Pizzo RC, O'Leary OF, Dinan TG, Cryan JF. Increased sensitivity to the effects of chronic social defeat stress in an innately anxious mouse strain. Neuroscience. 2011b 2011 May 27. [Epub ahead of print].
- Spiller R. 2002. Serotonergic modulating drugs for functional gastrointestinal diseases. Br J Clin Pharmacol. 54 1: 11–20.
- Spiller R. 2008. Serotonergic agents and the irritable bowel syndrome: What goes wrong?. Curr Opin Pharmacol. 8 6: 709–714.
- Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. 2000. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 47 6: 804–811.
- Taché Y, Martinez V, Million M, Wang L. 2001. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: Role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol. 280 2: G173–G177.
- Tsukamoto K, Ariga H, Mantyh C, Pappas TN, Yanagi H, Yamamura T, Takahashi T. 2007. Luminally released serotonin stimulates colonic motility and accelerates colonic transit in rats. Am J Physiol Regul Integr Comp Physiol. 293 1: R64–R69.
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. 2003. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 299 5603: 76.
- Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. 2004. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 305 5681: 217.
