Abstract
The stress-associated activation of the hypothalamus–pituitary–adrenal axis influences memory. Several studies have supported the notion that post-learning stress enhances memory consolidation, while pre-retrieval stress impairs retrieval. Findings regarding the effects of pre-encoding stress, in contrast, have been rather inconsistent. In the current two studies, the impact of an immediate retrieval task on these effects was explored. In the first study, 24 healthy young male participants were exposed to a psychosocial laboratory stressor (Trier Social Stress Test) or a control condition before viewing positive, negative, and neutral photographs, which were accompanied by a brief narrative. Immediate as well as delayed (24 h later) free recall was assessed. Stress was expected to enhance emotional long-term memory without affecting immediate recall performance. Stress caused a significant increase in salivary cortisol concentrations but had no significant effects on immediate or delayed retrieval performance, even though a trend toward poorer memory of the stress group was apparent. Based on these findings, the second experiment tested the hypothesis that the beneficial effects of stress on emotional long-term memory performance might be abolished by an immediate recall test. In the second study (n = 32), the same design was used, except for the omission of the immediate retrieval test. This time stressed participants recalled significantly more negative photographs compared to the control group. The present study indicates that an immediate retrieval attempt of material studied after stress exposure can prevent or even reverse the beneficial effects of pre-encoding stress on emotional long-term memory consolidation.
Introduction
It is well recognized that stress and its associated release of catecholamines and glucocorticoids can influence long-term memory. Research has fostered our understanding of the multiple modulatory variables to be considered (Wolf Citation2008; Wolf Citation2009; Schwabe et al. Citation2010). It is commonly agreed that acute stress can have facilitating as well as impairing effects on long-term memory (Joels et al. Citation2006; Diamond et al. Citation2007; Sandi and Pinelo-Nava Citation2007).
Experimental studies with laboratory animals have been valuable in this respect. Some authors have suggested that stress initially (within minutes) has an enhancing effect on memory mostly driven by noradrenaline, corticotropin releasing factor, and rapid non-genomic effects of corticosterone (Joels et al. Citation2006; Diamond et al. Citation2007; Joels et al. Citation2011). Subsequently, genomic glucocorticoid effects are considered to suppress hippocampal plasticity, thereby impairing the storage of new information acquired minutes to hours after stress exposure (Joels et al. Citation2006; Diamond et al. Citation2007). These studies were thus focused on the temporal [and spatial/contextual (Joels et al. Citation2006)] aspects of the relationship between the stressor and the memory task (rapid beneficial effects vs. slow detrimental effects).
Roozendaal and colleagues (Citation2006) have argued that stress hormones enhance long-term memory consolidation by influencing the basolateral amygdala and the hippocampus. In contrast, stress before memory retrieval has impairing effects (de Quervain et al. Citation1998; Kuhlmann et al. Citation2005). Both effects are more pronounced for emotional material. This model thus focuses on the role of different memory phases (Roozendaal et al. Citation2006), but the issue of pre-learning stress is not addressed explicitly in the Roozendaal model.
Experimental stress studies in humans have supported some of these assumptions. Some studies have reported impairing effects on memory retrieval (Kuhlmann et al. Citation2005; Buchanan et al. Citation2006; Smeets et al. Citation2008; Tollenaar et al. Citation2008b; Merz et al. Citation2010). Other studies demonstrated beneficial effects of post-learning stress on long-term memory consolidation (Cahill et al. Citation2003; Smeets et al. Citation2008; Preuß and Wolf Citation2009).
Results for pre-encoding (pre-learning) stress have been heterogeneous. Several authors have reported enhancing (Nater et al. Citation2007; Schwabe et al. Citation2008), impairing (Kirschbaum et al. Citation1996; Takahashi et al. Citation2004; Smeets et al. Citation2005; Smeets et al. Citation2006), or absent (Smeets et al. Citation2008) effects. Emotionality (Payne et al. Citation2006; Payne et al. Citation2007), the size of the stress response (Wolf et al. Citation2001; Nater et al. Citation2007) as well as circadian influences (Maheu et al. Citation2005) have been discussed as modulatory variables.
In addition to the variables mentioned above, retrieval delay might be important. Effects of pre-learning stress on immediate recall (tested at times when the stress-induced cortisol concentrations are still elevated) might differ from the effects on delayed recall (tested at times when cortisol concentrations have returned to baseline). In the former situation, the impairing effects of stress on memory retrieval (Kuhlmann et al. Citation2005; Buchanan et al. Citation2006; Merz et al. Citation2010) and working memory (Oei et al. Citation2006; Luethi et al. Citation2008; Schoofs et al. Citation2008; Schoofs et al. Citation2009) might prevail, while in the latter condition, the beneficial effects on consolidation can unfold (Roozendaal et al. Citation2006). Moreover, the presence of an immediate recall could have an impact on the consolidation (or reconsolidation) of the material acquired immediately beforehand (Tollenaar et al. Citation2008a; Schwabe and Wolf Citation2010). This issue has not been addressed systematically yet.
The goal of the present two experiments was thus to investigate the effects of pre-encoding stress on long-term memory for stimuli with different valence, with special attention paid to the possible modulatory influence of immediate recall. In the first study, stress was expected to enhance emotional long-term memory consolidation, leading to enhanced emotional memory when tested 24 h later (delayed retrieval), but not when tested immediately after encoding. Based on the findings of the first study, the second study tested the hypothesis that the beneficial effects of pre-encoding stress on emotional long-term memory consolidation occur only when no immediate recall test (as in the first study) is conducted. Based on the previous findings, it was expected that stress would primarily influence emotional arousing material, independent of its valence (see Wolf Citation2009).
Materials and methods
Study 1: Effects of stress on immediate and delayed recall of emotional photographs
Participants
Initially, 26 healthy male students participated. They were recruited via flyers on the campus or postings on several homepages of the university. Participants were excluded if they reported any use of medication that could influence the stress response (e.g. antibiotics, antihistamines, and glucocorticoids). Two participants had to be excluded after the completion of the study due to outlier values (defined as more than 2.5 SDs away from the mean) in the hormonal and/or affective measures. The mean age of the remaining 24 was 24.0 ± 0.66 (mean ± SE) years. The mean body mass index (kg/m2) was 23.32 ( ± 0.53). All provided written informed consent. The study was approved by the ethics committee of the German Psychological Association, and the guidelines of the Declaration of Helsinki were followed.
Study 2: Effects of stress on delayed recall of emotional photographs in the absence of an immediate recall test
Participants
Thirty-two healthy men participated in the second study. The mean age was 24.84 ± 0.82 (mean ± SE). The mean body mass index (kg/m2) was 23.99 ( ± 0.54). Inclusion criteria were identical to Study 1.
Materials
Stimuli
The stimuli used were developed and validated by Buchanan and colleagues (Citation2001, Citation2003). The material, translated by our group, had been used in two studies investigating the influence of basal cortisol concentrations or post-learning stress on memory (Preuß et al. Citation2008; Preuß and Wolf Citation2009). The stimuli consisted of five positive (e.g. two happy girls eating ice-cream; a boy with his favorite toy animal), five negative (e.g. a diseased African child with bandages; a dead man and a dead dog on a dirt road), and five neutral (e.g. people leaving or entering a building; people with books sitting at a table) color photographs, each presented in a random order for a duration of 10 s on a computer screen. Each picture was accompanied by a single narrative sentence which contained information that was not obvious in the picture (e.g. the names of the two girls and the flavor of ice-cream they were eating).
Immediate recall
The immediate free recall test took place directly after the presentation of the pictures. Participants had to write down everything they remembered. Answers were evaluated by two independent judges. Differences in test scores were discussed and were resolved by a third judge. High inter-rater reliability of this procedure has been established (Preuß et al. Citation2008). In line with our previous scoring method (Preuß and Wolf Citation2009), a participant received three points if the information noted could be clearly associated with one of the pictures and was correct in details (e.g. two girls eating ice-cream). It was not a prerequisite for the achievement of three points that parts of the information provided in the narrative were written down (even though this was typically the case). Two points were given for information that could be clearly associated with one of the pictures but contained some incorrect details (e.g. two girls eating candies; a boy and a girl eating ice-cream). If the information was completely wrong or could not be clearly linked to one picture, participants received one point. Thus, 5–15 points could be reached for each valence category.
The design of the second study was identical to the first study, except for the omission of the immediate recall test on day one.
Delayed recall
On the second day, 24 h after the presentation of the pictures, the delayed free recall test was conducted. The procedure as well as the scoring was exactly the same as during the immediate recall task (see above).
Ratings of the pictures
After the delayed retrieval, participants were asked to evaluate the pictures with respect to arousal and valence. All pictures were presented again and participants marked their rating for arousal and valence on two separate five-point Likert scales. The valence scale ranged from very unpleasant to very pleasant (with neutral in the middle). The arousal scale ranged from no arousal to very strong arousal (with weak, moderate, and strong arousal occupying the remaining three points).
Affect measurement (positive and negative affective schedule)
This questionnaire consists of 10 items for negative affect and 10 items for positive affect (Watson et al. Citation1988). This questionnaire, translated into German, has been validated by Krohne and colleagues (Citation1996). Internal consistencies of the two scales are high (Cronbach's α>0.84). Based on previous observations from our laboratory indicating that the negative but not the positive affect scale is sensitive to Trier Social Stress Test (TSST)-induced mood changes (e.g. Schoofs et al. Citation2008; Wolf et al. Citation2009), only the negative affect scale was used. Participants filled out the positive and negative affective schedule (PANAS) before the experimental treatment and a second time after the stress or control treatment.
Stressor and control condition
TSST (Kirschbaum et al. Citation1993) was used. It consists of an oral presentation and an arithmetic task in front of a panel (one woman and one man) acting very reserved, and is videotaped. This stressor, with a total duration of 15 min, reliably elicits a cortisol stress response. The non-stressful control condition (Placebo-TSST) also consists of an oral presentation and an arithmetic task but participants do not perform in front of audience and are not videotaped. It lacks the stressful components of the TSST and does not elicit a cortisol stress response (Het et al. Citation2009).
Saliva samples
Saliva was collected using Salivette collection devices (Sarstedt, Nümbrecht, Germany). Subjects were instructed to refrain from eating and drinking anything except water for 1 h prior to participation. Moreover, physical exercise was to be avoided and potentially stressful days were avoided during the scheduling of the appointments. Four saliva samples were collected (see below). Cortisol was assessed using a commercially available chemiluminescence immuno assay kit (IBL, Hamburg, Germany). Inter- and intra-coefficients of variation were below 10%. Assay sensitivity was 0.16 ng/ml.
Procedure
Participants were tested on 2 days 24 h apart. Testing started between 14:00 and 16:00 h. After arrival, participants filled out the PANAS for the first time. Subsequently, the first saliva sample was collected (base), followed by the TSST or the control condition. It was only after entering the TSST or control condition room that participants found out whether or not they would be part of the stress or control condition. After the treatment (15 min later), the second saliva sample was collected (+01 min) and subjects filled out the PANAS for the second time. Thereafter, the third saliva sample was collected (+10 min). The pictures and the narratives were presented next. After the presentation, participants in Study 1 took the immediate free recall test. The last saliva sample (+25 min) was collected 25 min after the treatment. Participants were returned to the laboratory 24 h later for the delayed recall test and the ratings. The experimental time line is presented in .
Table I. Timeline of experiments.
Statistical analyses
ANOVAs for repeated measurements followed by post hoc t-tests were conducted. The Greenhouse–Geisser corrected F- and p-values were used when indicated. Student's t-tests were conducted in order to follow up significant interaction effects of the ANOVAs. The Bonferroni corrections were implemented when indicated. A value of p < 0.05 was considered significant and a value of p < 0.10 was considered to represent a non-significant trend. Data are presented as means ± SEM.
Results
Study 1: Effects of stress on immediate and delayed recall of emotional photographs
Salivary cortisol concentrations
An ANOVA with the within-subject factor TIME (base vs. +01 min vs. +10 min vs. +25 min) and the between-subject factor STRESS (control vs. TSST) was conducted. Results revealed a significant TIME by STRESS interaction [F(1.4,30.3) = 27.2, p < 0.001]. In the TSST group (n = 12), cortisol concentrations increased from, mean ± SEM, 10.64 ± 0.88 nmol/l (baseline) to 18.03 ± 1.28 (+1 min) to 24.23 ± 1.98 (+10 min) nmol/l before decreasing to 18.76 ± 2.26 (+25 min) nmol/l. In controls (n = 12), cortisol concentrations declined steadily throughout the experiment: 8.37 ± 0.32 nmol/l (baseline), 7.24 ± 0.67 (+1 min), 6.31 ± 0.58 (+10 min), and 5.40 ± 0.43 (+25 min) nmol/l. t-Tests revealed that the TSST group displayed higher cortisol concentrations than the controls at all of the three times post-treatment (all p < 0.001), but did not differ at baseline.
Affect
An ANOVA with the inner-subject factor time (pre-treatment vs. post-treatment) and the between-subject factor stress (control vs. TSST) was conducted for the negative mood scale. Results revealed a non-significant interaction between TIME and STRESS [F(1,22) = 2.48, p = 0.13]. In the stress group, a significant (p < 0.05) increase in negative affect occurred (mean ± SEM, pre 1.32 ± 0.09; post 1.67 ± 0.19). No changes in negative affect were apparent in the control group (pre 1.46 ± 0.12; post 1.48 ± 0.19).
Effects of pre-encoding stress on immediate and delayed memory retrieval
Results are shown in . An ANOVA was conducted with the inner-subject factors RECALL (immediate vs. delayed) and VALENCE (positive vs. negative vs. neutral) and the between-subject factor STRESS (control vs. TSST).
Figure 1. Effects of pre-encoding stress on immediate and delayed recall of emotionally positive (pos), neutral (neu), and negative (neg) photographs (Study 1). No significant differences between the stress group (n = 12) and the control group (n = 12) emerged; however, stressed participants tended to perform poorer overall (ANOVA revealed only a trend towards a main effect of STRESS, p = 0.07). Data are expressed as mean ± SEM.
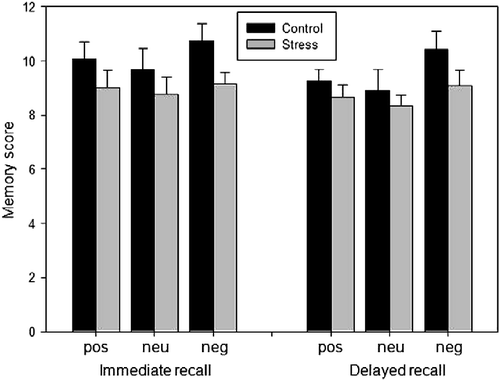
Results revealed only a trend toward a main effect of RECALL [F(1,22) = 3.53, p = 0.07], with better recall during the immediate recall test. In addition, only a trend toward a main effect of STRESS [F(1,22) = 3.74, p = 0.07] was apparent. Overall, stressed participants tended to remember fewer items. None of the other main effects or interactions reached a trend level. If memory data were analyzed with two separate ANOVAs (one for immediate and one for delayed recall), similar results emerged (p = 0.08 and 0.11, respectively).
Subjective ratings
The valence data were analyzed with an ANOVA with the inner-subject factor VALENCE (positive vs. negative vs. neutral) and the between-subject factor STRESS (control vs. TSST). Results revealed a significant main effect of VALENCE (p < 0.01) in the absence of an effect of STRESS (main effect or interaction). Positive pictures were rated (mean ± SEM, 3.82 ± 0.11) as significantly more positive than neutral pictures (3.03 ± 0.06), which in turn were rated as significantly more positive than negative pictures (1.77 ± 0.08).
The arousal data were analyzed with an ANOVA with the inner-subject factor VALENCE (positive vs. negative vs. neutral) and the between-subject factor STRESS (control vs. TSST). Results revealed a significant main effect of VALENCE (p < 0.01) in the absence of an effect of STRESS (main effect or interaction). Negative pictures were rated (3.39 ± 0.12) as significantly more arousing than positive pictures (2.44 ± 0.15), which in turn were rated as significantly more arousing than neutral pictures (1.86 ± 0.15).
Study 2: Effects of stress on delayed recall of emotional photographs in the absence of an immediate recall test
Salivary cortisol concentration
Results revealed a significant TIME by STRESS interaction [F(1.41, 42.26) = 22.05, p < 0.001). In the TSST group (n = 15), cortisol concentrations increased from, mean ± SEM, 12.48 ± 1.52 (baseline) to 19.84 ± 1.85 (+1 min) to 27.54 ± 2.85 (+10 min) nmol/l before decreasing to 20.56 ± 1.98 (+25 min) nmol/l. In controls (n = 17), cortisol concentrations declined steadily throughout the experiment: 9.02 ± 0.59 (baseline), 8.24 ± 0.66 (+1 min), 7.93 ± 0.68 (+10 min), and 6.27 ± 0.72 (+25 min) nmol/l. Planned t-tests revealed that the TSST group displayed higher cortisol concentrations than the controls at all three times post-treatment (all p < 0.001).
Affect
Results revealed a significant interaction between TIME and STRESS [F(1,56) = 8.12, p < 0.01]. In the stress group, a significant (p < 0.05) increase in negative affect occurred (from 1.47 ± 0.09 to 1.94 ± 0.09, mean ± SEM). In the control group, a decrease was apparent (from 1.27 ± 0.09 to 1.12 ± 0.05).
Effects of pre-learning stress on delayed memory retrieval
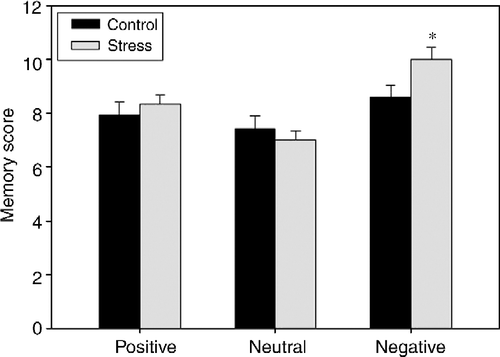
Results are shown in . Results revealed a significant main effect of VALENCE [F(1.95, 58.60) = 17.01, p < 0.001]. Negative items were remembered better than positive items, which in turn were remembered better than neutral items. The main effect STRESS was not significant [F(1,30) = 1.10, p>0.10]. However, an interaction between VALENCE and STRESS was found [F(1.95,58.60) = 3.12, p = 0.05]. Planned t-tests indicated that stressed participants remembered significantly more (p < 0.05) negative items compared to controls. For positive items, a similar descriptive trend was apparent, which, however, failed to reach significance. For neutral items, the opposite descriptive non-significant trend emerged (worse performance of the stressed participants).
Figure 2. Effects of pre-encoding stress on delayed recall of emotionally positive, neutral, and negative photographs (Study 2). The stress group (n = 15) compared to the control group (n = 17) showed enhanced delayed retrieval of negative pictures. ANOVA revealed a STRESS by VALENCE interaction. Data are expressed as mean ± SEM. *p < 0.05, post hoc t-tests.
Subjective ratings
The valence data were analyzed with an ANOVA with the inner-subject factor VALENCE (positive vs. negative vs. neutral) and the between-subject factor STRESS (control vs. TSST). Results revealed a significant main effect of VALENCE (p < 0.01) in the absence of an effect of STRESS (main effect or interaction). Positive pictures were rated (mean ± SEM, 3.97 ± 0.09) as significantly more positive than neutral pictures (2.99 ± 0.05), which in turn were rated as significantly more positive than negative pictures (1.75 ± 0.08).
The arousal data were analyzed with an ANOVA with the inner-subject factor VALENCE (positive vs. negative vs. neutral) and the between-subject factor STRESS (control vs. TSST). Results revealed a significant main effect of VALENCE (p < 0.01) in the absence of an effect of STRESS (main effect or interaction). Negative pictures were rated (mean ± SEM, 3.43 ± 0.13) as significantly more arousing than positive pictures (2.44 ± 0.11), which in turn were rated as significantly more arousing than neutral pictures (1.83 ± 0.12).
Comparison of Study 1 and Study 2
In order to directly test the presence or the absence of differences between the two studies, two additional ANOVAs were conducted (for cortisol concentrations and delayed memory retrieval).
For cortisol, the ANOVA contained the between-subject factor STUDY (Study 1 vs. Study 2), STRESS (control vs. TSST), and the inner-subject factor TIME (base vs. +01 min vs. +10 min vs. +25 min). As expected, there was a significant TIME by STRESS interaction (p < 0.001). Importantly, the factor STUDY was not significant, neither as a main effect nor as an interaction term (STUDY by TIME, STUDY by STRESS, STUDY by STRESS by TIME). All p-values were larger than 0.50.
For delayed memory retrieval, the ANOVA contained the between-subject factor STUDY (Study 1 vs. Study 2), STRESS (control vs. TSST) and the inner-subject factor VALENCE (positive vs. negative vs. neutral). Results revealed a significant effect of the factor STUDY (p < 0.01). Participants in Study 1 retrieved overall more pictures than participants in Study 2. The STUDY by STRESS interaction tended to be significant (p = 0.058). The STUDY by STRESS by VALENCE interaction was not significant (p = 0.11). When the three valence categories were put into separate ANOVAs with the factors STUDY and STRESS, a significant STUDY by STRESS interaction was observed for the negative photographs (p < 0.05), but not for the neutral or positive photographs.
Discussion
The two studies investigated the effects of an immediate recall test on the influence of pre-encoding stress on emotional long-term memory consolidation.
In the first study, pre-encoding stress had no significant effect on the immediate recall of negative, positive, and neutral episodes. At only a trend level, stressed participants performed more poorly compared to controls. A similar pattern was observed for the delayed retrieval test conducted 24 h later. Again, no significant effect of stress could be detected, but rather an indication for impaired recall was apparent. The findings of this study thus failed to detect a beneficial effect of pre-encoding stress on the consolidation of emotional memories.
Beneficial effects on emotional long-term memory consolidation have been observed with pre-learning stress (Payne et al. Citation2007), post-learning stress (Cahill et al. Citation2003; Smeets et al. Citation2008) and with pre-learning cortisol administration (Buchanan and Lovallo Citation2001; Kuhlmann and Wolf Citation2006b). However, several studies have failed to find effects of pre-learning manipulations, or have reported an enhancement for neutral but not for emotional material, or an enhancement that was not further modulated by stimulus emotionality (Abercrombie et al. Citation2003; Smeets et al. Citation2008). In studies investigating immediate recall after stress, the empirical picture is also mixed (Kirschbaum et al. Citation1996; Jelicic et al. Citation2004; Takahashi et al. Citation2004; Smeets et al. Citation2005; Payne et al. Citation2006; Smeets et al. Citation2006). Reasons for the heterogeneous literature are manifold. The studies cited used different memory tasks, different stressors (or cortisol doses), and different retrieval delays, and were conducted at different times of the day. Moreover, the testing-induced arousal might have been different between these studies (Okuda et al. Citation2004; Kuhlmann and Wolf Citation2006a). All these variables have been shown to influence the effects of stress-induced cortisol elevations on memory (Het et al. Citation2005; Wolf Citation2008; Wolf Citation2009).
The current study used (at least for the negative category) highly emotional pictures; however, the number of stimuli (15) was rather low. The task can thus be described as highly emotional but rather easy. It is conceivable that less emotional tasks or tasks containing more stimuli can lead to different results. Some studies have reported that pre-encoding stress enhances emotional, but impairs neutral material (Jelicic et al. Citation2004; Payne et al. Citation2006; Smeets et al. Citation2006). This effect might reflect attentional- and/or state-dependent influences of the stress-induced increase in anxiety and negative affect. In contrast, beneficial effects of stress or cortisol treatment on emotional long-term memory consolidation are considered to develop more slowly over time (Quevedo et al. Citation2003; Kuhlmann and Wolf Citation2006b; Roozendaal et al. Citation2006). The tentative conclusion presented above appears to be supported by pharmacological cortisol studies. Here, several studies report impaired immediate memory retrieval after cortisol treatment (Kirschbaum et al. Citation1996; Monk and Nelson Citation2002; Tops et al. Citation2003). By contrast, studies with a longer retention interval (24 h or more) have reported beneficial effects (Buchanan and Lovallo Citation2001; Abercrombie et al. Citation2003; Het et al. Citation2005).
Stimulated by the lack of effects observed in the first study, the second study was conducted with an identical design except for the omission of the immediate recall test in the second study. The rationale for this study was based on the hypothesis that an immediate recall at times when cortisol concentrations are still elevated might interfere with the beneficial effects of stress on memory consolidation. Roozendaal has postulated that stress puts the brain into a consolidation mode which is accompanied by impaired memory retrieval and impaired working memory (Roozendaal et al. Citation2006). An immediate retrieval attempt at times when cortisol concentrations are still elevated might thus be less successful. Moreover, the poorly retrieved memories rather than the originally acquired memories might be consolidated afterwards. The results of the second experiment revealed that, as predicted, stress enhanced long-term memory consolidation for emotional material. A significant beneficial effect was only observed for the negative images. This most likely reflects the fact that negative photographs induce the strongest arousal, which was supported by the subjective ratings conducted after retrieval testing. The current findings thus support the notion that stress modulates emotionally arousing material in particular (Wolf Citation2009). The results of the second study mirror previous human findings on post-encoding stress, which repeatedly have shown a stress-induced enhancement of emotional memories (Cahill et al. Citation2003; Smeets et al. Citation2008). The results of the current study are thus most likely caused by the effects of glucocorticoids on emotional memory consolidation, even though effects on the initial acquisition process cannot be ruled out.
A direct statistical comparison of the two studies provided additional insight. With respect to salivary cortisol levels, the analysis indicated that the two studies did not differ in overall or stress-induced cortisol concentrations. Thus, the different effects of stress observed in these two studies do not reflect differences in cortisol concentrations between the two experiments. With respect to memory retrieval, the analysis demonstrated overall better memory in Study 1 compared to Study 2. This most likely relates to the beneficial effects of memory retrieval on learning (Karpicke and Roediger Citation2008). Importantly, however, this analysis provided evidence (but only at a trend level) for a study by stress interaction. Thus, depending on the presence or the absence of an immediate recall task, the effects of stress differed in their directions (tending to impair in Study 1, enhancing in Study 2). Moreover, this interaction was observed for negative, but not for neutral or positive photographs. In sum, the direct comparison of the two studies supported the main conclusions derived from the independent analysis and interpretations of the two studies.
The present findings show remarkable similarities to observations made in rodents, from studying effects of corticosterone injected directly after the acquisition of an object recognition task (Okuda et al. Citation2004). The results revealed that memory tested 24 h later was enhanced by this manipulation, which is in line with the second study presented here. In contrast, memory tested 1 h later was impaired, which is in line with the first study presented here. Interestingly, both effects only occurred when the testing situation was arousing (novel) to the animal but not when it was non-arousing (habituated). This additional finding is in line with the current finding that memory of primarily negative photographs was influenced by stress. Taken together, these results indicate that glucocorticoids enhance emotional memory consolidation, but only when memory is tested at a time when consolidation is completed and cortisol concentrations are no longer elevated. In contrast, if retrieval is tested at times when cortisol concentrations are still elevated, the impairing effects on memory retrieval seem to prevail. Importantly, these effects appear to persist, possibly reflecting that the poorly retrieved information (and not the originally learned information) is consolidated. This tentative conclusion would predict that the effects of post-encoding stress on immediate and delayed emotional memory retrieval should be quite similar to the effects observed in the present study. There are ample human studies showing that post-learning stress enhances long-term memory (Cahill et al. Citation2003; Beckner et al. Citation2006; Preuß and Wolf Citation2009; Smeets et al. Citation2008). In contrast, post-encoding stress followed relatively soon (within less than an hour) by a retrieval test is associated with impaired memory retrieval (Buchanan et al. Citation2006; Merz et al. Citation2010).
The present experiments have important limitations. The sample sizes were comparable to similar previous studies, but only provided enough power to detect medium to large effects. Only males were tested in this experiment, so the issue of possible sex differences in the effects found remains for future study (Andreano et al. Citation2008; Schoofs and Wolf Citation2009; Cornelisse et al. Citation2011). The finding that positive and negative images were rated differently with regard to arousal is a limitation, and it remains an open question whether the lack of effects of stress on memory of positive photographs reflects valence effects or differences in arousal. Future studies might use less arousing negative slides or more arousing (e.g. erotic) positive slides. A potential influence of rehearsal (set between encoding and delayed retrieval) cannot be excluded with the current design. It would be of interest to test whether a negative influence of an immediate retrieval test after stress exposure is evident a week later.
Taken together, the present two studies show that beneficial effects of pre-encoding stress on emotional long-term memory consolidation are abolished or even reversed by an immediate recall test conducted at times of elevated cortisol concentrations. The findings are in line with several previous human studies and can be explained by current models on the influence of stress on emotional long-term memory consolidation and long-term memory retrieval.
Acknowledgements
The author would like to thank Diana Preuss for data collection and Tony Buchanan (Department of Psychology Saint Louis University) for providing me with the emotional memory task used in this study. Moreover, I am thankful to Eve-Mariek Hessas for language editing.
Declaration of interest: This study was supported by grants from the German Research Foundation (DFG) WO 733/7-1 and SFB 874 (Project B4) awarded to O. T. Wolf. The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. 2003. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behav Neurosci. 117:505–516.
- Andreano JM, Arjomandi H, Cahill L. 2008. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 33:874–882.
- Beckner VE, Tucker DM, Delville Y, Mohr DC. 2006. Stress facilitates consolidation of verbal memory for a film but does not affect retrieval. Behav Neurosci. 120:518–527.
- Buchanan TW, Lovallo WR. 2001. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 26:307–317.
- Buchanan TW, Denburg NL, Tranel D, Adolphs R. 2001. Verbal and nonverbal emotional memory following unilateral amygdala damage. Learn Mem. 8:326–335.
- Buchanan TW, Karafin MS, Adolphs R. 2003. Selective effects of triazolam on memory for emotional, relative to neutral, stimuli: Differential effects on gist versus detail. Behav Neurosci. 117:517–525.
- Buchanan TW, Tranel D, Adolphs R. 2006. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learn Mem. 13:382–387.
- Cahill L, Gorski L, Le K. 2003. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learn Mem. 10:270–274.
- Cornelisse S, van Stegeren AH, Joels M. 2011. Implications of psychosocial stress on memory formation in a typical male versus female student sample. Psychoneuroendocrinology. 36:569–578.
- de Quervain DJ, Roozendaal B, McGaugh JL. 1998. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 394:787–790.
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. 2007. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes–Dodson law. Neural Plast. 2007:78970.
- Het S, Ramlow G, Wolf OT. 2005. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 30:771–784.
- Het S, Rohleder N, Schoofs D, Kirschbaum C, Wolf OT. 2009. Neuroendocrine and psychometric evaluation of a placebo version of the ‘Trier Social Stress Test’. Psychoneuroendocrinology. 34:1075–1086.
- Jelicic M, Geraerts E, Merckelbach H, Guerrieri R. 2004. Acute stress enhances memory for emotional words, but impairs memory for neutral words. Int J Neurosci. 114:1343–1351.
- Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. 2006. Learning under stress: How does it work?. Trends Cogn Sci.. 10:152–158.
- Joels M, Fernandez G, Roozendaal B. 2011. Stress and emotional memory: A matter of timing. Trends Cogn Sci.. 15:280–288.
- Karpicke JD, Roediger HLIII. 2008. The critical importance of retrieval for learning. Science. 319:966–968.
- Kirschbaum C, Pirke KM, Hellhammer DH. 1993. The ‘Trier Social Stress Test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 28:76–81.
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. 1996. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 58:1475–1483.
- Krohne HW, Egloff B, Kohlmann CW, Tausch A. 1996. Untersuchungen mit einer deutschen Form der Positive and Negative Affect Schedule (PANAS). Diagnostica. 42:139–156.
- Kuhlmann S, Piel M, Wolf OT. 2005. Impaired memory retrieval after psychosocial stress in healthy young men. J Neurosci. 25:2977–2982.
- Kuhlmann S, Wolf OT. A non-arousing test situation abolishes the impairing effects of cortisol on delayed memory retrieval in healthy women. Neurosci Lett. 2006a; 399:268–272.
- Kuhlmann S, Wolf OT. Arousal and cortisol interact in modulating memory consolidation in healthy young men. Behav Neurosci. 2006b; 120:217–223.
- Luethi M, Meier B, Sandi C. 2008. Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Front Behav Neurosci. 2:5.
- Maheu FS, Collicutt P, Kornik R, Moszkowski R, Lupien SJ. 2005. The perfect time to be stressed: A differential modulation of human memory by stress applied in the morning or in the afternoon. Prog Neuropsychopharmacol Biol Psychiatry. 29:1281–1288.
- Merz CJ, Wolf OT, Hennig J. 2010. Stress impairs retrieval of socially relevant information. Behav Neurosci. 124:288–293.
- Monk CS, Nelson CA. 2002. The effects of hydrocortisone on cognitive and neural function: A behavioral and event-related potential investigation. Neuropsychopharmacology. 26:505–519.
- Nater UM, Moor C, Okere U, Stallkamp R, Martin M, . 2007. Performance on a declarative memory task is better in high than low cortisol responders to psychosocial stress. Psychoneuroendocrinology. 32:758–763.
- Oei NY, Everaerd WT, Elzinga BM, van Well S, Bermond B. 2006. Psychosocial stress impairs working memory at high loads: An association with cortisol levels and memory retrieval. Stress. 9:133–141.
- Okuda S, Roozendaal B, McGaugh JL. 2004. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci USA. 101:853–858.
- Payne JD, Jackson ED, Ryan L, Hoscheidt S, Jacobs JW, Nadel L. 2006. The impact of stress on neutral and emotional aspects of episodic memory. Memory. 14:1–16.
- Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, Nadel L. 2007. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learn Mem. 14:861–868.
- Preuß D, Wolf OT. 2009. Post-learning psychosocial stress enhances consolidation of neutral stimuli. Neurobiol Learn Mem. 92:318–326.
- Preuß D, Schoofs D, Wolf OT. 2008. Associations between endogenous cortisol levels and emotional memory in young women: Influence of encoding instructions. Stress. 12:379–387.
- Quevedo J, Sant'Anna MK, Madruga M, Lovato I, de Paris F, Kapczinski F, Izquierdo I, Cahill L. 2003. Differential effects of emotional arousal in short- and long-term memory in healthy adults. Neurobiol Learn Mem. 79:132–135.
- Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. 2006. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 138:901–910.
- Sandi C, Pinelo-Nava MT. 2007. Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plast. 2007:78970.
- Schoofs D, Wolf OT. 2009. Stress and memory retrieval in women: No strong impairing effect during the luteal phase. Behav Neurosci. 123:547–554.
- Schoofs D, Preuss D, Wolf OT. 2008. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology. 33:643–653.
- Schoofs D, Wolf OT, Smeets T. 2009. Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behav Neurosci. 123:1066–1075.
- Schwabe L, Wolf OT. 2010. Stress impairs the reconsolidation of autobiographical memories. Neurobiol Learn Mem. 94:153–157.
- Schwabe L, Bohringer A, Chatterjee M, Schachinger H. 2008. Effects of pre-learning stress on memory for neutral, positive and negative words: Different roles of cortisol and autonomic arousal. Neurobiol Learn Mem. 90:44–53.
- Schwabe L, Wolf OT, Oitzl MS. 2010. Memory formation under stress: Quantity and quality. Neurosci Biobehav Rev. 34:584–591.
- Smeets T, Jelicic M, Merckelbach H. 2005. Stress-induced cortisol responses, sex differences, and false recollections in a DRM paradigm. Biol Psychol. 72:164–172.
- Smeets T, Jelicic M, Merckelbach H. 2006. The effect of acute stress on memory depends on word valence. Int J Psychophysiol. 62:30–37.
- Smeets T, Otgaar H, Candel I, Wolf OT. 2008. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology. 33:1378–1386.
- Takahashi T, Ikeda K, Ishikawa M, Tsukasaki T, Nakama D, Tanida S, Kameda T. 2004. Social stress-induced cortisol elevation acutely impairs social memory in humans. Neurosci Lett. 363:125–130.
- Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd W. Long-term outcomes of memory retrieval under stress. Behav Neurosci. 2008a; 122:697–703.
- Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd WA. The effects of cortisol increase on long-term memory retrieval during and after acute psychosocial stress. Acta Psychol (Amst). 2008b; 127:542–552.
- Tops M, van der Pompe G, Baas D, Mulder LJ, Den Boer JA, Meijman TF, Korf J. 2003. Acute cortisol effects on immediate free recall and recognition of nouns depend on stimulus valence. Psychophysiology. 40:167–173.
- Watson D, Clark LA, Tellegen A. 1988. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 54:1063–1070.
- Wolf OT. 2008. The influence of stress hormones on emotional memory: Relevance for psychopathology. Acta Psychol (Amst). 127:513–531.
- Wolf OT. 2009. Stress and memory in humans: Twelve years of progress?. Brain Res. 1293:142–154.
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. 2001. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 26:711–720.
- Wolf OT, Minnebusch D, Daum I. 2009. Stress impairs acquisition of delay eyeblink conditioning in men and women. Neurobiol Learn Mem. 91:431–436.