Abstract
We evaluated how the mild stress-induced increase in endogenous corticosterone affected the pineal gland in Syrian hamsters (Mesocricetus auratus). The animals were maintained under constant light for 1 day, instead of a cycle of 14:10-h, to increase the circulating corticosterone levels during the daytime. The nuclear translocation of nuclear factor kappa B (NFKB), which is the pivotal transcription factor for stress and injury, presented a daily rhythm in normal animals. NFKB nuclear content increased linearly from the onset of light [Zeitgeber Time 0 (ZT0)] until ZT11 and decreased after ZT12 when the plasma corticosterone peak was detected in normal animals. However, the 24-h profiles of the two curves were different, and they did not clearly support an exclusive relationship between corticosterone levels and NFKB content. Therefore, we tested the effect of increased endogenous corticosterone through inducing mild stress by maintaining daytime illumination for one night. This stressful condition, which increased daytime corticosterone levels, resulted in a daytime decrease in NFKB nuclear content, and this was inhibited by mifepristone. Overall, this study shows that NFKB has a daily rhythm in Syrian hamster pineal glands and, by increasing endogenous corticosterone with a stressful condition, NFKB activity is regulated. Therefore, this study suggests that the pineal gland in the Syrian hamster is a sensor of stressful conditions.
Introduction
Melatonin, an indolamine widely distributed in a multitude of taxa, is supposedly ubiquitous in organisms (Reiter et al. Citation2010). Melatonin's primitive function is related to organism protection; however, it has recently emerged as a chemical translator of the light–dark environmental alternation. Melatonin is synthesized by the pineal gland at nighttime. We have recently proposed that both melatonin and the pineal gland are integral players in controlling the defense response in mammals (Markus et al. Citation2007). Accordingly, there is a shuttle between the pineal and extra-pineal synthesis of melatonin. Pineal melatonin synthesis is suppressed to allow an innate immune response to mount and then melatonin is synthesized by immune-competent cells, which provide high concentrations of melatonin to lesion sites. As the defense response progresses, nocturnal melatonin synthesis is restored.
Melatonin synthesis relies on two conserved enzymes: arylalkylamine N-acetyltransferase (AA-NAT) and hydroxyindole-O-methyltransferase. These enzymes convert serotonin into N-acetylserotonin, which is then converted to melatonin. Sympathetic input, which is driven by the circadian clock located in the suprachiasmatic nucleus, strictly controls the translation and activity of AA-NAT. In rodents, the activation of β-adrenoceptors causes phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (phosphoCREB - p-CREB), which promotes translation of the Aa-nat gene; however, phosphorylation of AA-NAT by activated protein kinase A (PKA) inhibits its degradation by the proteasome (for review, Simonneaux and Ribelayga Citation2003).
Pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS) or the pro-inflammatory cytokine tumor necrosis factor (TNF), inhibit the translation of Aa-nat in cultured rat pineal glands (Fernandes et al. Citation2006; da Silveira Cruz-Machado et al. Citation2010). At concentrations comparable to mild stress, corticosterone potentiates the noradrenaline-induced synthesis of melatonin in cultured rat pineal glands (Ferreira et al. Citation2005) and the nocturnal synthesis of melatonin from rat pineal glands perfused in vivo (Fernandes et al. Citation2009). The effect of corticosterone on pineal gland activity is translated into a nocturnal increase in melatonin synthesis in rats that are subjected to restraint stress (Couto-Moraes et al. Citation2009). Higher concentrations of corticosterone may lead to no alteration or inhibition of melatonin synthesis in cultured pineal glands (Yuwiler Citation1989; Ferreira et al. Citation2005), and the nocturnal melatonin surge is reduced in rats subjected to strong stressful conditions (Wu et al. Citation1987).
Nuclear factor kappa B (NFKB) is a pivotal transcription factor for PAMPs, such as pro-inflammatory cytokines, and it also mediates glucocorticoid effects (for a review, see Hayden and Ghosh Citation2008). This transcription factor controls the sequential expression of molecules involved in the mounting and recovery phases of an inflammatory response. This control occurs by initially synthesizing pro-inflammatory cytokines, enzymes, and membrane receptors for mediators of inflammation and inducing the synthesis of NFKB inhibitory proteins, which sequester the NFKB proteins in the cytoplasm.
In the rat pineal gland, LPS and TNF promote the activation of the NKFB pathway; however, corticosterone concentrations that potentiate noradrenaline-induced melatonin synthesis inhibit this pathway (Ferreira et al. Citation2005; Fernandes et al. Citation2006). In addition, NFKB is constitutively activated in the rat pineal gland (Cecon et al. Citation2010). During the day, the nuclear concentration of NFKB increases linearly, but in the light–dark transition, there is a sharp reduction in nuclear NFKB content. Together, these data strongly suggest that the NFKB pathway in the pineal gland is pivotal for mediating its participation in defense responses (Markus et al. Citation2007). Therefore, the pineal gland takes part in mounting an innate immune response because suppressing the nocturnal melatonin rise favors leukocyte migration to injured tissues (Lotufo et al. Citation2001, Citation2006). In addition, it takes part in the immune suppression induced by corticosterone by impairing the migration of cells and reducing the activation of endothelial cells (Tamura et al. Citation2006, Citation2009; Silva et al. Citation2007).
Although these mechanisms in the pineal gland have been extensively studied in rats, no evidence exists on its role in other animals or species that experience seasonal variations in immunity challenges. In this paper, we tested whether endogenous corticosterone modulated the NFKB pathway in the pineal gland in the Syrian hamster, which is a classical seasonal model (Simonneaux and Ribelayga Citation2003). We show that an increase in endogenous corticosterone inhibits NFKB pineal activation, which suggests that the pineal gland in the Syrian hamster can also be considered as a sensor for inflammation mediators.
Materials and methods
Animals
All experiments were conducted in accordance with the European Committee Council Directive 1986 (86/609/EEC). Two-month-old female Syrian hamsters (Mesocricetus auratus) were housed under a long-day photoperiod with a 14-h light (200 lux light intensity from 0500 h) and 10-h dark cycle (2 lux dim red light from 1900 h). They were given water and food ad libitum. Referential ZT0 was defined as the beginning of the light period.
Electromobility shift assay
Electromobility shift assays (EMSAs) were carried out on nuclear extracts from pineal glands as previously described (Ferreira et al. Citation2005). The glands were homogenized in buffer [10 mM 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (HEPES) (pH 7.5), 10 mM KCl, 0.1 mM Ethylenediaminetetraacetic acid (EDTA) (pH 8.0), 10% glycerol, 1 mM Dithiothreitol (DTT), 0.1 mM phenylmethylsulfonyl fluoride (PMSF), and nonyl phenoxypolyethoxylethanol (NP) 40 (10%)] and were kept in ice for 15 min, vortexed for 10 s and centrifuged (12,000g, 1 min, 4°C). The resulting pellets were washed with lysis buffer (50 μl), centrifuged (12,000g, 1 min, 4°C), resuspended [20 μl; 10 mM HEPES (pH 7.5), 0.5 M KCl, 1 mM EDTA (pH 8.0), 10% glycerol, 1 mM DTT, and 0.1 mM PMSF], and kept on a rocking plate for 15 min at 4°C. Samples were then centrifuged (16,000g, 8 min, 4°C), and the resulting supernatants (nuclear extracts) were aliquoted and stored at − 70°C. The protein content was determined by Lowry's method.
A NFKB double-stranded consensus oligonucleotide probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′, Promega, Charbonnières-les-Bains, France) was end-labeled with γ32P-ATP (specific activity 3000 Ci/mmol) in the presence of T4 polynucleotide kinase for 10 min at 37°C (Fermentas, Saint-Rémy-lès-Chevreuse, France). Unincorporated nucleotides were removed by a Sephadex G25 spin column (GE Healthcare Europe GmbH, Velizy-Villacoublay, France). Nuclear extracts (5 μg) were incubated [10 mM HEPES (pH 7.5), 50 mM KCl, 1 mM MgCl2, 0.5 mM EDTA (pH 8.0), 0.5 mM DTT, and 4% glycerol] with poly(dI–dC).poly(dI–dC) (1 μg) for 20 min at room temperature and with a radiolabeled probe (20,000–50,000 cpm) for 30 min at room temperature. Protein–DNA complexes were resolved by nondenaturing 6% acrylamide:bisacrylamide (37.5:1) in 0.25 × Tris–borate/EDTA buffer at 150 V for 1.5 h at room temperature. The gel was dried and exposed to a Biomax MS-1 film (Kodak Co., Rochester, NY, USA) for 24–48 h at − 70°C. Autoradiograms were scanned and analyzed densitometrically. For the competition studies, a NFKB or AP1 (5′-CGTTGATGAGTCAGCCGGAA-3′) unlabeled double-stranded oligonucleotide was included in molar excess over the radiolabeled probe.
Supershift assay
NFKB subunits were identified from a pool of four pineals incubated with 2 μg/ml of rabbit polyclonal affinity-purified antibodies for p50, RelA, p52, cRel, RelB, and Bcl-3 purchased from Santa Cruz Biotechnology, Santa Cruz, CA, USA (sc-114x, sc-109x, sc-298x, sc-70x, sc-226x, and sc-185x, respectively) for 45 min at room temperature before the addition of a γ32P-DNA NFKB probe. EMSA was then carried out as described above.
Corticosterone assay
Corticosterone levels were measured by radioimmunoassay (RIA) using the ImmuChemTM Double Antibody Corticosterone 125I RIA Kit (MP Biomedicals, Loughborough, UK).
Experimental protocols
Characterization of pineal NFKB nuclear content
Animals were killed at ZT11. The pineal glands were removed for immediate preparation of the nuclear extracts from untreated animals or animals treated with the NFKB inhibitor pyrrolidine dithiocarbamate [PDTC, 50–200 mg/kg, intraperitoneal injection (ip), ZT9]. The chosen PDTC concentrations have been shown to inhibit LPS-induced hypotension and inducible nitric oxide synthase expression in rats (Liu et al. Citation1997).
Daily variations in pineal NFKB nuclear content and plasma corticosterone levels
To determine the daily variations in NFKB nuclear content and corticosterone plasma levels, animals were killed at different time periods by decapitation. The pineal glands were kept in eppendorf tubes and maintained at − 20°C before the nuclear extracts were prepared. Blood samples were collected into heparin-containing tubes for corticosterone measurements, and the plasma was stored at − 20°C until analysis.
To determine the effect of constant light
Animals that were kept in the constant light condition (LL) one night before the experiment began were killed at ZT10 or ZT18 after 34 or 42 h in LL, respectively. Pineal glands and blood were collected to determine the nuclear translocation of NFKB and the corticosterone levels, respectively.
Effect of a glucocorticoid receptor antagonist treatment
Glucocorticoid receptors were inhibited by mife-pristone. This is a progesterone analog that inhibits the corticosteroid type II receptor in the hamster; however, it does not inhibit the progesterone receptors as it does in humans (Gray and Leavitt Citation1987). This species difference is due to a glycine to cysteine substitution at position 575 in the hamster progesterone receptor (Benhamou et al. Citation1992).
Animals kept in LL for one night before the start of the experiment were treated with mifepristone (50 mg/kg, ip, according to Munhoz et al. Citation2006) or vehicle at ZT14 on day 1 and ZT6 on day 2. They were killed at ZT10, and the nuclear extracts were immediately obtained.
Chemicals
Ammonium PDTC, mifepristone, and routine reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA). γ32P-ATP (3000 Ci/mmol, 10 Ci/ml) was purchased from Perkin-Elmer Life and Analytical Sciences (Mechelen, Belgium). The NFKB consensus oligonucleotide was purchased from Promega.
Data analyses
Image analyses were carried out with the NIH ImageJ software developed at the US National Institutes of Health (http://rsb.info.nih.gov/nih-image). All data are presented as mean ± SEM of the number of indicated animals. Statistical comparisons were made by a Student's t-test or one-way analysis of variance followed by a Newman–Keuls test.
Results
Three protein-γ32P-NFKB, double-stranded oligonucleotide protein complexes (, lane 1) were found to be blocked by a molar excess of unlabeled probe (, lane 2) in the nuclear extracts of pineal glands from Syrian hamsters that were killed at ZT11. However, no complexes were found using unlabeled oligonucleotides selective for the AP1 transcription factor (, lane 3). In the absence of extracts, no complexes were visualized (, lane 4). Therefore, three different NFKB complexes were observed in the nuclear extracts of pineal glands from Syrian hamsters killed at ZT11.
Figure 1. The nuclear content of NFKB complexes in Syrian hamster pineal glands. An EMSA was carried out with pineal nuclear extracts from hamsters killed at ZT11. Competition assays were conducted in the absence or the presence of a 50 mol excess of an unlabeled, specific (NFKB) or nonspecific (AP1) double-stranded oligonucleotide. (A) Representative autoradiogram of the NFKB-DNA binding in pineal nuclear extracts: Lane 1, basal condition; lane 2, excess of the unlabeled, specific NFKB probe; lane 3, excess of the unlabeled, specific AP-1 probe; lane 4, the absence of nuclear extract. The positions of the 32P-NFKB-DNA protein-binding complexes are indicated. (B) Inhibition of the γ32P-DNA NFKB probe binding to nuclear protein extracts by PDTC (50–200 mg/kg, ip, 2 h before killing). Densitometric analysis of a typical EMSA showing the content of the NFKB complexes (Complex 1, square; Complex 2, circle; and Complex 3, triangle). Data are expressed as mean ± SEM of three glands per time point. (C) Autoradiogram showing the supershift of NFKB with anti-p50 antibody. The antibodies for RelA, p52, cRel, RelB, and Bcl-3 did not supershift the NFKB complexes.
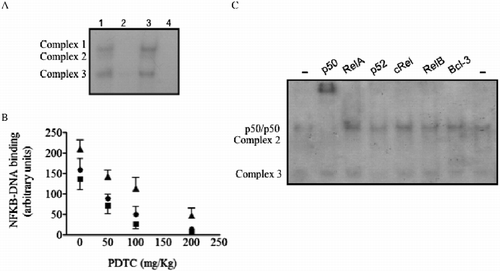
One of the complexes selectively binds p50 subunit antibody (). The remaining antibodies tested (RelA, p52, cRel, RelB, and Bcl-3) did not supershift any complex. Therefore, nuclei from Syrian hamster pineal glands constitutively express the dimer p50/p50.
To confirm that these complexes were NFKB, hamsters were injected with PDTC, a drug that blocks the binding of NFKB complexes to DNA (Hayakawa et al. Citation2003). A dose-dependent inhibition of NFKB complexes was observed in pineal nuclear extracts from animals treated with PDTC (). Therefore, both in vitro competition and in vivo inhibition protocols suggest that NFKB is translocated to the nuclear fraction of the pineal glands in Syrian hamsters killed at ZT11.
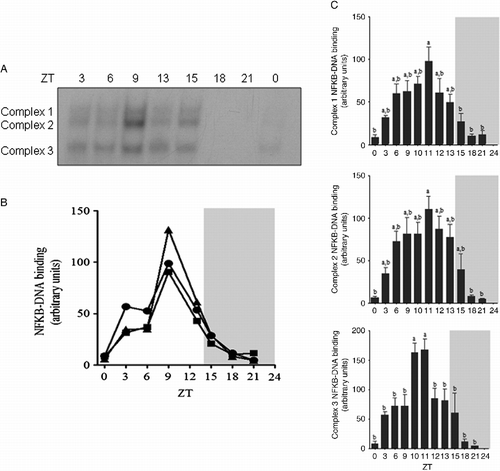
We next evaluated the daily variations in NFKB nuclear content by obtaining nuclear extracts from animals killed at different time points over 24 h. The first group (ZT0) was killed just after the lights were turned on, and the ZT13 and ZT15 groups were killed 1 h before and 1 h after the lights were turned off (ZT14), respectively (). Maximum NFKB nuclear content was observed at ZT9, which was 5 h before the lights were turned off. NFKB nuclear content was already decreasing at ZT13, and the minimum content was attained at ZT18. To obtain accurate measurements for the NFKB peak, we collected new time points at ZT10, ZT11, and ZT12 (). These data showed that the nuclear translocation of NFKB increased until ZT11 for the three complexes. Therefore, the NFKB content in the nuclear fraction of pineal glands from Syrian hamsters is rhythmic, but it is not related to environmental light.
Figure 2. Daily variations in activated NFKB in pineal nuclear extracts from Syrian hamsters. The animals were housed under a 14:10-h light–dark cycle and killed at the indicated time points over 24 h. (A) Representative autoradiogram of a typical EMSA showing the content of NFKB complexes at the indicated ZT. (B) Densitometric analysis of the daily variations in each NFKB complex level (C1, square; C2, triangle; and C3, circle; arbitrary units) from the autoradiogram shown in A. (C) Densitometric analysis of the daily variations in the NFKB complex levels (arbitrary units) in the Syrian hamsters pineal gland. Unshaded areas indicate the light period and shaded areas indicate the dark period. Data are expressed as mean ± SEM of 3–6 glands in each point. a different from b, P < 0.05 tested by one-way analysis of variance followed by Newman–Keuls test.
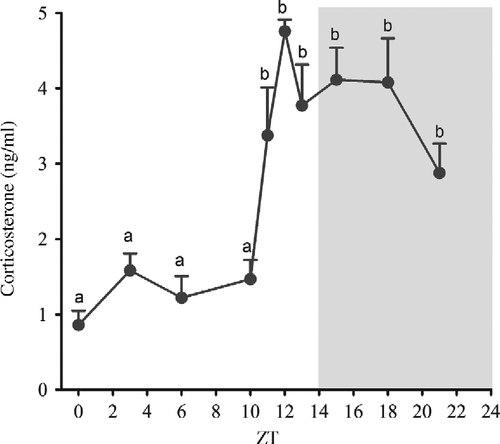
We next compared the daily plasma corticosterone levels with the pineal nuclear NFKB content. Circulating corticosterone varies during the day (), and it is at its minimum at the beginning of the light period (ZT0). A sharp increase in corticosterone levels was observed between ZT10 and ZT12, and this plasma concentration was maintained at a high level during the dark period. The time that the plasma corticosterone levels began to increase (ZT12) was the same time that the NFKB content began to decrease in the nuclear extracts; however, no further similarities were observed in the other parts of the curves ().
Figure 3. Daily variations in plasma corticosterone levels (ng/ml) from Syrian hamsters housed under a 14:10-h light–dark cycle and killed at the indicated time points over 24 h. Corticosterone levels were determined by RIA. The black horizontal bar represents the dark period. Data are expressed as mean ± SEM of six animals in each point. a different from b, P < 0.01 tested by one-way analysis of variance followed by Newman–Keuls test.
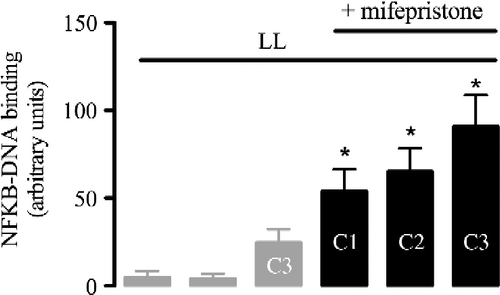
Taking into account the connection between corticosterone and NFKB in stressful conditions, we maintained the animals in constant light for one night, and we evaluated plasma corticosterone levels and pineal nuclear NFKB content the next day at ZT10 and ZT18. These time points were chosen because ZT10 was the point in the light phase with the lowest plasma corticosterone concentration, and ZT18 was in the middle of the dark phase with significantly higher corticosterone concentrations. Maintaining the animals under illumination at night disrupted the corticosterone rhythm and elevated the plasma corticosterone concentrations at ZT10 (). This increase in plasma corticosterone concentrations at ZT10 was accompanied by a marked decrease in the nuclear content of the three NFKB complexes (), an effect that was reversed by treatment with mifepristone, a competitive antagonist of glucocorticoid receptors (Gagne et al. Citation1985). Mifepristone significantly increased the nuclear content of the three NFKB complexes in the pineal gland of the animals killed at ZT10 (). These data suggest that increases in corticosterone may inhibit the NFKB pathway in the pineal glands from Syrian hamsters.
Figure 4. Effects of constant light on plasma corticosterone levels (ng/ml) and the pineal nuclear translocation of NFKB in Syrian hamsters. Animals were kept under a 14:10-h light–dark cycle (LD, white/black bar) or constant light (LL, gray bar) for 34 or 42 h and were killed at ZT10 or ZT18, respectively. (A) Plasma corticosterone levels were measured by RIA. Each bar represents mean ± SEM of the N animals indicated. (B) Representative autoradiogram of a typical EMSA showing the NFKB complex contents (C1, C2, and C3) under LD or LL conditions. (C) Densitometric analysis of the NFKB complex under these conditions. Each bar represents the mean ± SEM of 4–6 glands. Data were analyzed by a Student's t-test and one-way analysis of variance followed by a Newman–Keuls test. *P < 0.05, LL compared to LD at ZT10.
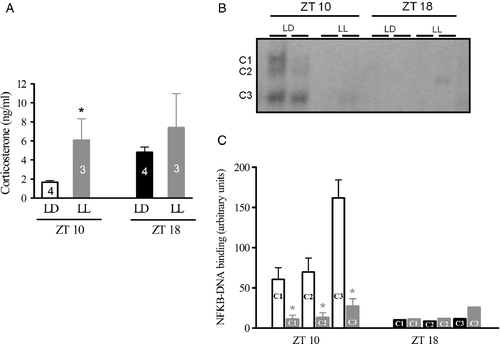
Figure 5. Effect of the glucocorticoid receptor antagonist mifepristone on constant light (LL)-induced inhibition of NFKB activity in the Syrian hamster pineal gland. Animals kept in LL for 34 h were treated with vehicle (gray bars) or mifepristone (gray hatched bar, 50 mg/kg, ip, at ZT14 and ZT6 on the next subjective day) and killed at ZT10. Bars show the densitometric analysis for the binding of the three complexes (C1, C2, and C3) of NFKB nuclear extracts to a nucleotide probe specific for NFKB. Data are expressed as mean ± SEM of five glands. *P < 0.05, LL compared to LL+ mifepristone for each complex.
Discussion
In this study, we showed that maintaining Syrian hamsters in a constant light, which elevated endogenous corticosterone levels, inhibited the NFKB content in the pineal gland. Furthermore, the sharp increase in endogenous, circulating corticosterone levels prior to dark onset was accompanied by a marked decrease in pineal nuclear NFKB content. However, the daily rhythms of NFKB nuclear translocation and corticosterone levels are not identical, suggesting that other factors regulate nuclear NFKB in the pineal gland from Syrian hamsters.
An unlabeled, selective oligonucleotide for NFKB displaced the labeled NFKB probe from the DNA–protein complex; however, an unlabeled, selective oligonucleotide for AP1 did not. In addition, PDTC dose dependently inhibited the nuclear translocation of NFKB, which is similar to observations made in other tissues and cells (Schreck et al. Citation1992; Liu et al. Citation1997). Although PDTC inhibits or stimulates the activation of other transcription factors, such as the signal transducer and activator of transcription protein-3 and AP1 (Muñoz et al. Citation1996; Xie et al. Citation2009), respectively, the use of both biochemical and pharmacological tools strongly suggest that NFKB dimers were detected in this study. Reinforcing that NFKB is translocated to the nucleus of Syrian hamsters pineal glands during the light phase of the day, we showed the presence of the dimer p50/p50, as complex one was supershifted with specific antibody. The other complexes were not identified with the antibody used. Interestingly, the same NFKB constitution was found in nonstimulated rat pineal glands obtained in daytime, but only p50/p50 dimers are translocated into the nucleus (Cecon et al. Citation2010).
Nuclear NFKB has also been found to exhibit a rhythmic expression in rat pineal glands obtained from control animals (Cecon et al. Citation2010) and in cultured glands with or without pro-inflammatory agents (Ferreira et al. Citation2005; da Silveira Cruz-Machado et al. Citation2010). However, the NFKB nuclear content rhythms in the rat and the hamster show different profiles. In the rat, we observed an abrupt reduction in the nuclear content of the NFKB complex at the light–dark transition (Cecon et al. Citation2010), whereas in the Syrian hamster the decrease began at least 2 h before the lights were turned off. The abrupt reduction observed in rats also occurs at the transition between subjective day and subjective night in animals kept in constant darkness. This reduction is not inhibited by propranolol, a β-adrenoceptor antagonist that blocks sympathetic input to the rat pineal gland. Taking into account that the sympathetic input is the signal for the dark period to begin, this suggests that darkness itself was not activating NFKB in the rat pineal gland. The present data reinforce the idea that the light–dark transition has no effect on nuclear NFKB content in the pineal gland because this reduction in Syrian hamsters occurred much earlier than the onset of darkness. Therefore, the drop in nuclear pineal NFKB content appears to be due to these nocturnal rodents anticipating the upcoming, active nighttime state.
The peak plasma corticosterone level anticipates the nighttime activity phase (Tritschler et al. Citation2006; Dickmeis Citation2009). Corticosterone is a soluble hormone that interacts with cytoplasmic glucocorticoid receptors that favor the nuclear translocation of the receptor–hormone complex. This complex acts as a transcription factor to regulate genes that are related to the stress response. Activated glucocorticoid receptors reduce the NFKB nuclear level by two different mechanisms: (1) promoting the transcription of the gene that codes the inhibitory protein to maintain NFKB dimers sequestered into the cytoplasm or (2) by a direct protein–protein interaction with the nuclear NFKB dimer, which decreases its interaction with the kappa B element in the promoter of genes controlled by this transcription factor (for review, Smoak and Cidlovski Citation2004). This interaction will favor the reduction in the NFKB activity and its displacement from DNA. The mutual interaction between NFKB and activated glucocorticoid receptors may explain the inverse correlation between plasma corticosterone levels and nuclear NFKB translocation.
Basal NFKB translocation to the nucleus is reduced by corticosterone in cultured rat pineal glands (Ferreira et al. Citation2005), and the drop in nuclear NFKB is linked to entering the active state in rats maintained in constant darkness (Cecon et al. Citation2010). Therefore, we evaluated the putative relationship between the daily variations in plasma corticosterone levels and nuclear pineal NFKB content. Although our data did not show a clear correlation between the two rhythms, the NFKB content began to reduce at the same time as the plasma corticosterone maximal peak occurred, which was at ZT12. Therefore, the reduction in NFKB activation occurs before the light–dark transition in hamsters and is partially dependent on the increase in corticosterone level. These data reinforce that controlling the pineal nuclear activity is different in Syrian hamster and rats (Sinitskaya et al. Citation2006).
Melatonin synthesis is strongly restricted to the later part of the night in Syrian hamsters; however, the melatonin peak begins earlier in rats (Garidou et al. Citation2003; Sinitskaya et al. Citation2006). This melatonin synthesis delay in Syrian hamsters is attributed to noradrenaline inducing the clock gene Per1 at the beginning of darkness (Wongchitrat et al. Citation2009), which blocks the transcription of key enzymes in melatonin synthesis (Christ et al. Citation2010). NFKB has been reported to inhibit the melatonin biosynthetic pathway in cultured rat pineal glands (Cecon et al. Citation2010). This suggests that the slow decrease in nuclear NFKB content that occurs at the light–dark transition in the hamster pineal gland may participate in blocking melatonin synthesis during the later part of the day. This is in contrast to the sharp reduction in the nuclear NFKB content in the rat pineal gland, which occurs exactly at the light–dark transition.
NFKB nuclear translocation enables the pineal gland to sense stressors and inflammatory stimuli, and therefore, it participates in the inflammatory response and in stressful conditions (Markus et al. Citation2007). In concentrations comparable with the nocturnal surge, melatonin blocked the rolling and adherence of leukocytes that impair the mounting of an inflammatory response (Lotufo et al. Citation2001, Citation2006). This effect is mediated by a direct action of melatonin on endothelial cells (Tamura et al. Citation2006, Citation2009; Silva et al. Citation2007). To mount an efficient inflammatory response at night, the nocturnal pineal surge needs to be shut down. Rat pineal glands express TNF receptors (da Silveira Cruz-Machado et al. Citation2010; Carvalho-Sousa et al. Citation2011), which block the transcription of the Aa-nat gene and the synthesis of melatonin (Fernandes et al. Citation2006). In addition, we showed an inverse correlation between TNF and nocturnal melatonin content during an inflammatory response in humans (Pontes et al. Citation2006, Citation2007). If glucocorticoids potentiate melatonin synthesis, they may be involved in the recovery phase of an inflammatory response or in the response to stress. We reported that exogenous corticosterone increases endogenous melatonin secretion in rats (Fernandes et al. Citation2009), and a mild stress increases plasma corticosterone levels and enhances the nocturnal melatonin surge in rats (Couto-Moraes et al. Citation2009). However, others have shown that high concentrations of corticosterone in vitro (Yuwiler Citation1989) and strong stress-induction (Wu et al. Citation1987) can lead to a reduction in melatonin production.
We evaluated the effect of increased plasma glucocorticoid by inducing a moderate stress. Syrian hamsters were maintained under daytime illumination for one night before the experiment began. The daytime (ZT10) plasma corticosterone levels increased to levels similar to the nighttime values; however, a simultaneous reduction in nuclear NFKB content was observed. To reinforce that this effect was due to corticosterone, animals were treated with mifepristone, which is an inhibitor of glucocorticoid receptors. Mifepristone inhibited the reduction in nuclear NFKB content that was induced by maintaining the animals at daytime illumination for one night. Therefore, the increase in plasma corticosterone which was induced by a mild stress resulted in a reduction in the activity of NFKB in the pineal gland of hamsters.
In conclusion, this study shows that the transcription factor NFKB is activated in the Syrian hamster pineal gland in a rhythmic manner. Its regulation is partly linked to adrenal corticosterone rhythm, as its DNA-binding activity is reduced by the endogenous plasma corticosterone peak that occurs in anticipation of the active period. Furthermore, its activation can also be modulated in mild stressful situations, which reinforces our hypothesis that the pineal gland is a sensor of inflammation.
Acknowledgements
The financial support of FAPESP (2006/57009-6; 2007/07871-6), CAPES/COFECUB (519/05) and CNPq is gratefully acknowledged. RPM is a senior fellow of CNPq. We also acknowledge the Department of Neurobiology of Rhythms at the University of Strasbourg, especially Dr Paul Pévet, Dr Mireille Masson-Pévet, Dr Dominique Ciocca, Laura Ansel, Anthony Salingre, Natasha Sinitskaya, and Christiane Calgari.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Benhamou B, Garcia T, Lerouge T, Vergezac A, Gofflo D, Bigogne C, Chambon P, Gronemeyer H. 1992. A single amino acid that determines the sensitivity of progesterone receptors to RU486. Science. 255:206–209.
- Carvalho-Sousa CE, Da Silveira Cruz-Machado S, Tamura EK, Fernandes PACM, Pinato L, Muxel SM, Cecon E, Markus RP. 2011. Molecular basis for defining the pineal gland and pinealocytes as targets for tumor necrosis factor. Frontiers in Endocrinology. 2:1–11.
- Cecon E, Fernandes PACM, Pinato L, Ferreira ZS, Markus RP. 2010. Daily variation of constitutively activated nuclear factor kappa B (NFkB) in rat pineal gland. Chronobiol Int. 27:52–67.
- Christ E, Pfeffer M, Korf HW, von Gall C. 2010. Pineal melatonin synthesis is altered in Period1 deficient mice. Neuroscience. 171:398–406.
- Couto-Moraes R, Palermo-Neto J, Markus RP. 2009. The immune-pineal axis: Stress as a modulator of pineal gland function. Ann NY Acad Sci. 1153:193–202.
- da Silveira Cruz-Machado S, Carvalho-Sousa CE, Tamura EK, Pinato L, Cecon E, Fernandes PA, de Avellar MC, Ferreira ZS, Markus RP. 2010. TLR4 and CD14 receptors expressed in rat pineal gland trigger NFKB pathway. J Pineal Res.. 49:183–192.
- Dickmeis T. 2009. Glucocorticoids and the circadian clock. J Endocrinol. 200:3–22.
- Fernandes PACM, Cecon E, Markus RP, Ferreira ZS. 2006. Effect of TNF-α on the melatonin synthetic pathway in the rat pineal gland: basis for a ‘feedback’ of the immune response on circadian timing. J Pineal Res. 41:344–350.
- Fernandes PA, Bothorel B, Clesse D, Monteiro AW, Calgari C, Raison S, Simonneaux V, Markus RP. 2009. Local corticosterone infusion enhances nocturnal pineal melatonin production in vivo. J Neuroendocrinol. 21:90–97.
- Ferreira ZS, Fernandes PA, Duma D, Assreuy J, Avellar MC, Markus RP. 2005. Corticosterone modulates noradrenaline-induced melatonin synthesis through inhibition of nuclear factor kappa B. J Pineal Res. 38:182–188.
- Gagne D, Pons M, Philibert D. 1985. RU 38486: A potent antiglucocorticoid in vitro and in vivo. J Steroid Biochem. 23:247–251.
- Garidou ML, Diaz E, Calgari C, Pevet P, Simonneaux V. 2003. Transcription factors may frame Aa-nat gene expression and melatonin synthesis at night in the Syrian hamster pineal gland. Endocrinology. 144:2461–2472.
- Gray GO, Leavitt WW. 1987. RU486 is not an antiprogestin in hamster. J Steroid Biochem. 28:493–497.
- Hayakawa M, Miyashita H, Sakamoto I, Kitagawa M, Tanaka H, Yasuda H, Karin M, Kikugawa K. 2003. Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J. 22:3356–3366.
- Hayden MS, Ghosh S. 2008. Shared principles in NF-kappaB signaling. Cell. 132:344–362.
- Liu SF, Ye X, Malik AB. 1997. In vivo inhibition of nuclear factor kappa B activation prevents inducible nitric oxide synthase expression and systemic hypotension in a rat model of septic shock. J Immunol. 159:3976–3983.
- Lotufo CM, Lopes C, Dubocovich ML, Farsky SH, Markus RP. 2001. Melatonin and N-acetylserotonin inhibit leukocyte rolling and adhesion to rat microcirculation. Eur J Pharmacol. 430:351–357.
- Lotufo CM, Yamashita CE, Farsky SH, Markus RP. 2006. Melatonin effect on endothelial cells reduces vascular permeability increase induced by leukotriene B4. Eur J Pharmacol. 534:258–263.
- Markus RP, Ferreira ZS, Fernandes PA, Cecon E. 2007. The immune-pineal axis: A shuttle between endocrine and paracrine melatonin sources. Neuroimmunomodulation. 14:126–133.
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Scavone C. 2006. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. Neuroscience. 26:3813–3820.
- Muñoz C, Pascual-Salcedo D, Castellanos MC, Alfranca A, Aragonés J, Vara A, Redondo JM, de Landázuri MO. 1996. Pyrrolidine dithiocarbamate inhibits the production of interleukin-6, interleukin-8, and granulocyte-macrophage colony-stimulating factor by human endothelial cells in response to inflammatory mediators: Modulation of NF-kappa B and AP-1 transcription factors activity. Blood. 88:3482–3490.
- Pontes GN, Cardoso EC, Carneiro-Sampaio MM, Markus RP. 2006. Injury switches melatonin production source from endocrine (pineal) to paracrine (phagocytes) – melatonin in human colostrum and colostrum phagocytes. J Pineal Res. 41:136–141.
- Pontes GN, Cardoso EC, Carneiro-Sampaio MM, Markus RP. 2007. Pineal melatonin and the innate immune response: The TNF-alpha increase after cesarean section suppresses nocturnal melatonin production. J Pineal Res. 43:365–371.
- Reiter RJ, Tan DX, Fuentes-Broto L. 2010. Melatonin: A multitasking molecule. Prog Brain Res. 181:127–151.
- Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. 1992. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 175:1181–1194.
- Silva CL, Tamura EK, Macedo SM, Cecon E, Bueno-Alves L, Farsky SH, Ferreira ZS, Markus RP. 2007. Melatonin inhibits nitric oxide production by microvascular endothelial cells in vivo and in vitro. Br J Pharmacol.. 151:195–205.
- Simonneaux V, Ribelayga C. 2003. Generation of the melatonin endocrine message in mammals: A review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev. 55:325–395.
- Sinitskaya N, Salingre A, Klosen P, Revel FG, Pevet P, Simonneaux V. 2006. Differential expression of AP-1 proteins in the pineal gland of Syrian hamster and rat may explain species diversity in Aa-nat gene expression. Endocrinology. 147:5052–5060.
- Smoak KA, Cidlowski JA. 2004. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Ageing Dev. 125:697–706.
- Tamura EK, Silva CL, Markus RP. 2006. Melatonin inhibits endothelial nitric oxide production in vitro. J Pineal Res. 41:267–274.
- Tamura EK, Cecon E, Monteiro AW, Silva CL, Markus RP. 2009. Melatonin inhibits LPS-induced NO production in rat endothelial cells. J Pineal Res. 46:268–274.
- Tritschler M, Saboureau M, Pévet P, Bothorel B. 2006. A functional subdivision of the circadian clock is revealed by differential effects of melatonin administration. Neurosci Lett. 396:73–76.
- Wongchitrat P, Felder-Schmittbuhl MP, Phansuwan-Pujito P, Pévet P, Simonneaux V. 2009. Endogenous rhythmicity of Bmal1 and Rev-erb alpha in the hamster pineal gland is not driven by norepinephrine. Eur J Neurosci. 29:2009–2016.
- Wu WT, Reiter RJ, Troiani ME, Vaughan GM. 1987. Elevated daytime rat pineal and serum melatonin levels induced by isoproterenol are depressed by swimming. Life Sci. 41:1473–1479.
- Xie Y, Kole S, Precht P, Pazin MJ, Bernier M. 2009. S-glutathionylation impairs signal transducer and activator of transcription 3 activation and signaling. Endocrinology. 150:1122–1131.
- Yuwiler A. 1989. Effects of steroids on serotonin-N-acetyltransferase activity of pineals in organ culture. J Neurochem. 52:46–53.