Abstract
Immune challenge during pregnancy is associated with preterm birth and poor perinatal development. The mechanisms of these effects are not known. 5α-Pregnan-3α-ol-20-one (3α,5α-THP), the neuroactive metabolite of progesterone, is critical for neurodevelopment and stress responses, and can influence cognition and affective behaviours. To develop an immune challenge model of preterm birth, pregnant Long–Evans rat dams were administered lipopolysaccharide [LPS; 30 μg/kg/ml, intraperitoneal (IP)], interleukin-1β (IL-1β; 1 μg/rat, IP) or vehicle (0.9% saline, IP) daily on gestational days 17–21. Compared to control treatment, prenatal LPS or IL-1β reduced gestational length and the number of viable pups born. At 28–30 days of age, male and female offspring of mothers exposed to prenatal IL-1β had reduced cognitive performance in the object recognition task compared to controls. In females, but not males, prenatal IL-1β reduced anxiety-like behaviour, indicated by entries to the centre of an open field. In the hippocampus, progesterone turnover to its 5α-reduced metabolites was lower in prenatally exposed IL-1β female, but not in male offspring. IL-1β-exposed males and females had reduced oestradiol content in hippocampus, medial prefrontal cortex and diencephalon compared to controls. Thus, immune stress during late pregnancy reduced gestational length and negatively impacted birth outcomes, hippocampal function and central neurosteroid formation in the offspring.
Introduction
The relationship between challenges during gestation and foetal outcomes is important and needs to be better understood. Gestational stressors can negatively influence birth outcomes (Giurgescu Citation2009; Latendresse Citation2009), cognitive and/or emotional processes in the offspring (Talge et al. Citation2010; Kerstjens et al. Citation2011; Lind et al. Citation2011; Tanskanen et al. Citation2011) and vulnerability to neuropsychiatric disorders later in life (Taylor Citation1969; Newton Citation1986; Hadders-Algra et al. Citation1988; Hakulinen et al. Citation1988; Holst et al. Citation1989; Sfakianaki and Norwitz Citation2006; Petrini et al. Citation2009; Doyle and Anderson Citation2010). Maternal neuroendocrine stress responses to immune insults are suppressed in late pregnancy (Brunton et al. Citation2005); nevertheless, maternal infection may still compromise birth outcomes. Viral infections during pregnancy have deleterious effects (Vrachnis et al. Citation2011). In women, approximately 25–40% of preterm births (defined as delivery before 37 weeks of gestation) are associated with a maternal immune response and/or infection (Goldenberg et al. Citation2008). Thus, understanding the effects that gestational immune challenge has on birth outcomes that are relevant for the survival of the foetus, as well as the long-term effects on the offspring, is important for improving the quality of life.
Among pregnant women, infections via bacteria that express the endotoxin, lipopolysaccharide (LPS), are associated with preterm birth (Romero et al. Citation1988). Similar effects are observed in rodent models, wherein LPS has been demonstrated to elevate circulating concentrations of cytokines, including tumour necrosis factor-α, interleukin-1β (IL-1β) and interleukin-6 (IL-6; Ashdown et al. Citation2006; Xu et al. Citation2007) and to promote preterm birth (Elovitz and Mrinalini Citation2004). The consequences of gestational immune challenge on the offspring may depend upon several factors including when the exposure occurs during gestation. Early in gestation, exposure to LPS is associated with foetal death and resorption (Gendron et al. Citation1990; Ogando et al. Citation2003), whereas exposure to LPS in mid to late gestation is associated with foetal death and preterm delivery (Leazer et al. Citation2002; Xu et al. Citation2007; Zhao et al. Citation2008). Different consequences of LPS exposure associated with gestational age indicate that hormonal factors may influence the sequelae associated with immune challenge.
Progesterone is essential for the successful maintenance of pregnancy. Immune challenge in early pregnancy may reduce progesterone formation, promoting termination of pregnancy (Erlebacher et al. Citation2004). In rodents, pregnancy is characterised by increased secretion of progesterone by the corpora lutea that maintain progestogen levels. Some of the effects of progesterone on pregnancy may be mediated, in part, by its conversion to 5α-pregnan-3α-ol-20-one (tetrahydroxy-progesterone, or 3α,5α, also called allopregnanolone). Progesterone is converted to 3α,5α-THP by the sequential actions of 5α-reductase, which catalyses the reduction of progesterone to dihydroprogesterone (DHP), and 3α-hydroxysteroid dehydrogenase, which catalyses the hydroxylation of DHP to 3α,5α-THP. Unlike progesterone and DHP, 3α,5α-THP does not act at cognate, intracellular progestin receptors (Kontula et al. Citation1975; Iswari et al. Citation1986) and instead acts via γ-aminobutyric acid A (GABAA) receptors (Majewska et al. Citation1986).
3α,5α-THP plays an important role in the development of the central nervous system. During early development, 3α,5α-THP promotes neuronal growth and later has neuroprotective actions, promotes cognitive function and has anti-anxiety effects. We have previously shown that restraint stress during late pregnancy [gestational day (GD) 17–20] reduces maternal hippocampal 3α,5α-THP levels (Frye and Walf Citation2004). However, the effects of immune challenge with IL-1β during late pregnancy on birth outcomes and central progestogen formation in the offspring are not known.
In the present study, pregnant rat dams were exposed to LPS, IL-1β or saline on GD 17–21. Gestational length and the number of viable offspring born were assessed in each group. We hypothesised that endotoxin or cytokine exposure would reduce the duration of gestation and/or the number of surviving offspring. In humans, preterm birth is a leading cause of infant mortality and birth defects (Taylor Citation1969; McCormick Citation1985; Newton Citation1986; Hadders-Algra et al. Citation1988; Hakulinen et al. Citation1988; Holst et al. Citation1989; Sfakianaki and Norwitz Citation2006). Among surviving children that are born preterm, pre-pubertal socio-cognitive and/or emotional impairments have been reported (Talge et al. Citation2010; Kerstjens et al. Citation2011; Lind et al. Citation2011) and some of these cognitive impairments may persist throughout life (Tanskanen et al. Citation2011). Hence, we assessed cognitive and affective function of juvenile rats born to mothers that were exposed to cytokines during pregnancy at a critical time of hippocampal development. Perturbations in cognitive behaviour, dorsal hippocampus morphology and/or 3α,5α-THP formation in prefrontal cortex have been observed among juvenile rat offspring whose mothers were exposed to psychological stress during late pregnancy (Paris and Frye Citation2011a,Citationb). We hypothesised that offspring that were gestationally exposed to immune challenge would demonstrate perturbed cognitive and affective profiles, concomitant with perturbed progesterone metabolism in the brain.
Materials and methods
Ethical approval
These methods were pre-approved by the Institutional Care and Use Committee at the University at Albany-SUNY and were conducted in accordance with ethical guidelines defined by the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH publications no. 80 23, revised 1978).
Animals and housing
Primiparous, timed-pregnant, adult female Long–Evans rats (n = 21) purchased from Taconic Farms (Germantown, NY, USA) were used. Rats were packed on GD 14, shipped on GD 15 and were housed in a temperature (21 ± 1°C) and humidity controlled room in the Life Sciences Research Building Laboratory Animal Care Facility at the University at Albany-SUNY. Rats were group-housed (3–4/cage) until GD 18, after which they were housed singly. Housing rooms were maintained on a reverse 12:12 h light:dark cycle (lights off at 08:00 h) with ad libitum access to Purina Rat Chow and water.
Evaluation of pregnancy status
To ascertain the pregnancy status and duration of gestation, all rats were handled, examined and weighed daily from GD 15 until the day of parturition. Pregnant rats demonstrated continued weight gain of more than 5 g/day throughout pregnancy, had visible teats and foetuses were palpable. Rats that weighed less than the others upon arrival, did not gain weight and did not appear to be pregnant (no palpable pups) were excluded from the study (n = 4). Rats that were heavier, gained weight upon arrival and appeared to be pregnant but began to lose weight after manipulations remained in the study. The cages of all rats were checked for pups hourly from 06:00 to 22:00 h daily from GD 18 to 23. The number of hours after 00:00 h on GD 18 that pups were first observed was recorded as the latency to deliver. The number of pups that were alive in the nest following the completion of parturition was counted and recorded as a measure of fecundity.
Procedure
Assessment of effects of immunological stressors on gestational outcome
Dams were exposed to an immune insult to assess the contribution of prenatal infection on pregnancy outcome. Dams were administered an intraperitoneal (IP) injection of either the endotoxin, LPS (30 μg/kg/ml; Sigma-Aldrich Corp., St Louis, MO, USA; n = 5) or the cytokine, IL-1β (1 μg/rat; R&D Systems, Inc., Minneapolis, MN, USA; n = 8). LPS, administered during gestation, increases cytotoxic lymphocyte invasion, foetal necrosis, abortion and pup mortality during the first post-natal week (Glockner Citation1992), while IL-1β stimulates the HPA axis in adult rats and, when administered during days 17–21 of pregnancy, it is reported to produce aberrations in the psychomotor development of offspring (Uehara et al. Citation1987; Gotz et al. Citation1993). Control dams (n = 8) were administered a daily dose of vehicle of (0.9% saline injection IP; 0.1 ml/100 kg) on GD 17–21.
Phenotype of offspring
To minimise potential confounds of maternal behaviour influencing later outcomes (Weinstock Citation2005; Lonstein Citation2007; Moore Citation2007), all pups were cross-fostered to non-manipulated dams at post-natal day (PND) 1 and litter sizes were kept constant such that all surrogate dams had 16–20 pups to care for following cross-fostering. Pups remained with surrogate dams until weaning at PND 21 and were housed with same-sex conspecifics (n = 4–5/cage). Between PND 28 and 30, 2–4 pups were taken from each litter (in a counterbalanced manner), and hippocampal and cortical function was assessed by behavioural testing in the open field and object recognition tasks. Each litter represented an experimental group in the present study. As such, in each experimental group (control or IL-1β-exposed), 2–4 pups (one male and two females, two males and one female or two males and two females) were derived from the same litter. Among the noted effects of LPS are febrile response and, potentially, anorexia (Hopwood et al. Citation2009). As such, animals were checked for neurological status and weighed prior to testing; however, indications of sickness behaviour were absent and there were no significant differences in the body weight of offspring across groups.
Open field
The open field test is a hippocampus-mediated, anxiety task (Herman et al. Citation1998; Lamprea et al. Citation2003; Edinger and Frye Citation2006; Walf and Frye Citation2007), which was used according to previously published methods (Pellow and File Citation1986; Frye et al. Citation2000). Briefly, the open field is an arena (76 × 57 × 35 cm), divided into a 48-square grid, that is located in a brightly lit room. Rats were placed in the lower right-hand corner and allowed to explore for 5 min. The number of entries made into all 48 squares (total entries), into the peripheral 24 squares (peripheral entries) and into the inner 24 squares (central entries) was recorded by the ANY-Maze tracking programme. Data are also assessed as a percentage of entries into the centre of the open field [(central entries/total entries) × 100] as an index of anxiety (Pellow and File Citation1986; Frye et al. Citation2000).
Object recognition
The object recognition test is a working memory task that primarily relies on cortical functioning and, to a lesser extent, hippocampal functioning (Ennaceur et al. Citation1997; Broadbent et al. Citation2004). This task was used as modified from previously published methods (Ennaceur and Delacour Citation1988; Frye and Lacey Citation2001; Luine et al. Citation2003). During training, rats were placed singly in an open field (76 × 57 × 35 cm) in a brightly lit testing room. Rats were allowed 3 min to explore the open field, which contained two identical, spherical, plastic objects in adjacent corners (plastic toys in the shape of oranges; 6 cm diameter). After training, rats were placed in a dark, sound-dampened room for 4 h. Following this interval, rats were returned to the open field; however, one of the spherical objects was replaced with a cone-shaped object (a plastic toy in the shape of a triangular buoy; 6.25 cm tall, 6 cm wide at base and 1 cm at apex). Rats were allowed to explore these objects for 3 min. Exploration of objects was recorded via ANY-maze (Stoelting, Chicago, IL, USA) animal tracking software. Although rats did not show a bias towards exploring objects on the right versus left side of the open field, placement of the novel object was counterbalanced across treatment groups and testing sessions to eliminate potential confounds of side preference. A greater percentage of time spent exploring the object in the novel location, as a function of the total amount of time spent exploring both objects during testing [duration spent with novel object/(duration spent with novel object+duration spent with familiar object) × 100], is considered an index of enhanced cognitive performance in this task.
Tissue collection
Offspring brains were collected via non-anaesthetised, rapid decapitation immediately upon completion of behavioural testing. Whole brains were rapidly removed from the skull and frozen on dry ice. Trunk blood was collected following rapid decapitation; serum was separated from clotted blood by centrifugation. All tissue was stored at − 80°C until radioimmunoassay for oestradiol and progestogen levels.
Tissue preparation
The brains of offspring were thawed on ice. The hippocampus, medial prefrontal cortex and diencephalon were dissected as previously described (CitationFrye and Rhodes 2006a,b).
Radioimmunoassays for steroid hormones
Oestradiol, P, DHP and 3α,5α-THP concentrations were measured as described below, using previously reported methods (Choi and Dallman Citation1999; Frye and Bayon Citation1999).
Radioactive probes
[3H]oestradiol (NET-317: specific activity = 51.3 Ci/mmol), [3H]progesterone (NET-208: specific activity = 47.5 Ci/mmol) and [3H]3α,5α-THP (used for DHP and 3α,5α-THP, NET-1047: specific activity = 65.0 Ci/mmol) were purchased from Perkin Elmer (Boston, MA, USA).
Extraction of steroids from brain tissues
Oestradiol, progesterone, DHP and 3α,5α-THP were extracted from brain tissues following homogenisation with a glass/glass homogeniser in 50% methanol (MeOH), 1% acetic acid. Tissues were centrifuged at 3000g and the supernatant was separated by chromatography using Sepak cartridges equilibrated with 50% MeOH:1% acetic acid. Steroids were eluted with increasing concentrations of MeOH (50% MeOH followed by 100% MeOH). Solvents were removed using a speed drier. Samples were reconstituted in 350 μl assay buffer.
Antibodies
The oestradiol antibody (E#244, Dr G.D. Niswender, Colorado State University, Fort Collins, CO, USA), which generally binds between 40% and 60% of [3H]oestradiol, was used at a 1:40,000 dilution and bound 54% in the present study. The progesterone antibody (P#337 from Dr G.D. Niswender, Colorado State University) used at a 1:30,000 dilution typically binds between 30% and 50% of [3H]progesterone and bound 48% in the present study. The DHP (X-947) and 3α,5α-THP antibodies (#921412-5, purchased from Dr Robert Purdy, Veterans Medical Affairs, San Diego, CA, USA) used at a 1:5000 dilution binds between 40% and 60% of [3H] 3α,5α-THP and bound 47% in the present study.
Set-up and incubation of radioimmunoassays
The range of the standard curves was 0–1000 pg for oestradiol and 0–8000 pg for progesterone, DHP and 3α,5α-THP. Standards were added to assay buffer followed by the addition of the appropriate antibody (described above) and [3H]steroid. Total assay volumes were 800 μl for oestradiol and progesterone and 950 μl for DHP and 3α,5α-THP. All assays were incubated overnight at 4°C.
Termination of binding
Separation of bound and free steroid was accomplished by the rapid addition of dextran-coated charcoal. Following incubation with charcoal, samples were centrifuged at 3000g and the supernatant was pipetted into a glass scintillation vial with 5 ml scintillation cocktail. Sample tube concentrations were calculated using the logit-log method of Rodbard and Hutt (Citation1974); interpolation of the standards and correction for recovery was calculated with the assay analysis programme, Assay Zap (Biosoft, Cambridge, UK). The inter- and intra-assay reliability co-efficients were oestradiol 0.07 and 0.06, progesterone 0.11 and 0.10, DHP 0.11 and 0.09 and 3α,5α-THP 0.09 and 0.09.
Statistical analyses
One-way analyses of variance (ANOVA) were used to assess differences in gestational outcomes on the time of birth (expressed in hours after GD 18 to parturition), fecundity (mean number of live pups per litter) and behavioural performance of offspring (open field and object recognition). ANOVAs were utilised to assess the endocrine status of offspring. A ratio of progestogen metabolites reduced from the parent hormone, progesterone (DHP+3α,5α-THP: progesterone), was calculated per previous methods (Kellogg and Frye Citation1999; Paris and Frye Citation2011a), as an index of metabolism. Fisher's Protected Least Significant Difference post hoc tests were utilised to determine group differences. When interactions were present, one-way ANOVAs with p value corrected for multiple comparisons were utilised to determine simple main effects. Data are shown as mean ± SEM. Alpha level for statistical significance was p ≤ 0.05.
Results
Immune challenge during late pregnancy reduces time to parturition and fecundity
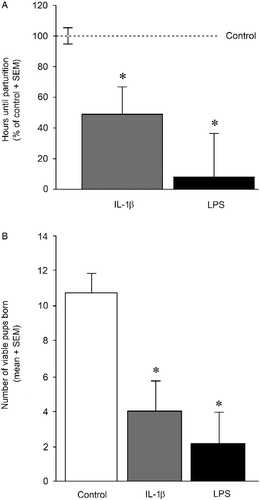
Immune challenge significantly reduced the length of gestation [F(2,18) = 6.86, p < 0.05] and fecundity [F(2,18) = 9.19, p < 0.05]. Dams administered IL-1β (p = 0.03), or LPS (p = 0.002), from GD 17 to 21 gave birth significantly earlier than controls (). The number of viable pups per dam was significantly reduced in the IL-1β- (p = 0.003) and LPS- (p = 0.001) administered groups compared to control dams ().
Figure 1. Length of gestation and viable pups born. (A) The mean length of gestation expressed as the number of hours after 00:00 h on GD 18 (mean ± SEM) that preceded parturition in dams administered either IL-1β (1 μg/rat daily, n = 8), LPS (30 μg/kg daily, n = 5) or vehicle (0.9% saline, n = 8) on GDs 17–21. * Indicates significant difference from control rats, p < 0.05. (B) The mean number of pups/litter present at parturition born to dams administered either IL-1β (1 μg/rat daily, n = 8), LPS (30 μg/kg daily, n = 5) or vehicle (0.9% saline, n = 8) on GDs 17–21. * Indicates significant difference from control rats in one-way ANOVA, p < 0.05.
Prenatal maternal cytokine exposure reduces cognitive performance and promotes sex-dependent aberrations in affective behaviour
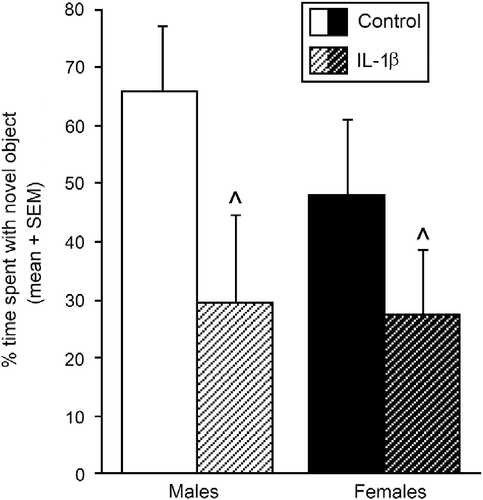
Cognitive performance on the object recognition task was significantly impaired by prenatal cytokine exposure [F(1,37) = 4.83, p < 0.05]. Irrespective of sex, adolescent offspring that were exposed to IL-1β from GD 17 to 21 spent a significantly reduced percentage of time investigating the novel object compared to control offspring ().
Figure 2. Object recognition test and cognitive function. Percentage of time spent investigating a novel object (mean ± SEM) in the object recognition task among pubertal male and female offspring whose mothers were exposed to IL-1β (1 μg/rat daily, males n = 9, females n = 8) or vehicle (0.9% saline, males n = 12, females n = 12) on days 17–21 of gestation. ^Indicates significant main effect in two-way ANOVA for IL-1β-exposed offspring to differ from control offspring, p < 0.05.
Affective behaviour was also influenced by prenatal cytokine exposure; albeit in a sex-specific manner. There was a significant interaction wherein IL-1β-exposed females made a significantly greater percentage [F(1,37) = 7.92, p < 0.05; or frequency [F(1,37) = 6.99, p < 0.05; of entries into the centre of the open field compared to control females (pcentre entry% = 0.0003; pcentre entry frequency = 0.0006). However, no difference in central entries was observed between IL-1β-exposed, and control, males. Notably, sex differences in motor behaviour were observed among adolescent offspring, whereby females made significantly more total [F(1,37) = 6.89, p < 0.05] and peripheral [F(1,37) = 7.44, p < 0.05] entries in the open field than did males ().
Figure 3. Open field test and anxiety-like behaviour. Number of entries (mean ± SEM) into the centre of an open field in pubertal male and female offspring that were exposed prenatally to IL-1β (1 μg/rat daily, males n = 9, females n = 8) or vehicle (0.9% saline, males n = 12, females n = 12) on days 17–21 of gestation. * Indicates significant interaction in two-way ANOVA, wherein IL-1β-exposed females differ from control females, p ≤ 0.05.
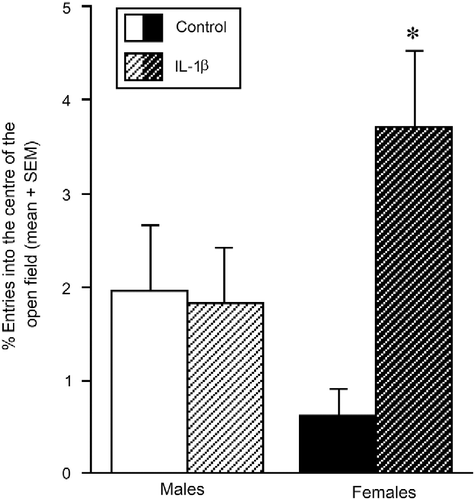
Table I. The open field behaviour (mean ± SEM) of pubertal male and female offspring that were born to mothers exposed to IL-1β (1 μg/rat) or vehicle (saline 0.9%) on GDs 17–21.
Central steroid formation in hippocampus, medial prefrontal cortex and diencephalon is altered by prenatal maternal cytokine exposure
Prenatal cytokine exposure affected 5α-reduced progesterone metabolite formation in the hippocampus of the offspring in a sex-dependent manner [F(1,37) = 3.976, p = 0.05]. The ratio of 5α-reduced metabolites, DHP+3α,5α-THP to progesterone content, in the hippocampus was significantly lower in females that were prenatally exposed to IL-1β compared to that in control females (p = 0.04), with no difference between IL-1β- and vehicle-treated males (). However, there was a significant effect of prenatal IL-1β exposure on hippocampal concentrations of DHP [F(1,37) = 4.25, p < 0.05] in the males (). Hippocampal DHP content was significantly greater in the IL-1β-exposed, compared with control, males (p = 0.0003); however, there were no differences between IL-1β-treated and control females (). Concentrations of 3α,5α-THP in the hippocampus appeared reduced among females, and increased among males, with prenatal IL-1β exposure compared to controls. However, these differences were highly variable and did not reach statistical significance ().
Figure 4. Progesterone utilisation in the hippocampus. Hippocampus progesterone (P) utilisation (ratio of the P metabolites, DHP+3α,5α-THP, to the prohormone, P) among pubertal male and female offspring exposed prenatally to IL-1β (1 μg/rat daily, males n = 9, females n = 8) or vehicle (0.9% saline, males n = 12, females n = 12) on days 15–21 of gestation. * Indicates significant interaction in two-way ANOVA, wherein IL-1β-exposed females differ from control females, p ≤ 0.05.
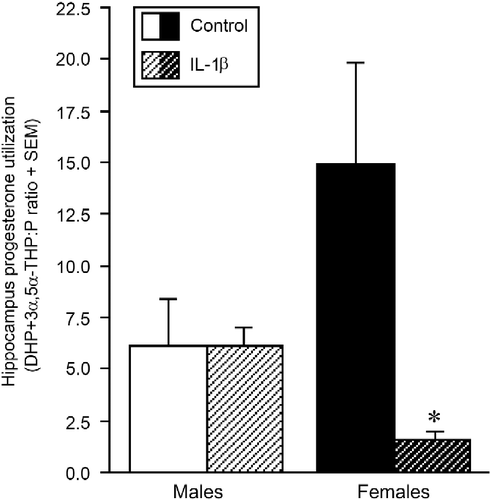
Table II. The concentrations (ng/g, mean ± SEM) of progesterone (P), DHP, 3α,5α-THP and progesterone utilisation (DHP+3α,5α-THP:P ratio) in the hippocampus, medial prefrontal cortex and diencephalon of pubertal male and female offspring of mothers that were exposed to IL-1β (1 μg/rat) or vehicle (saline 0.9%) on GD 17–21.
In the medial prefrontal cortex, there was no effect of prenatal IL-1β treatment on progesterone, DHP or 3α,5α-THP concentrations in either the male or the female offspring. Progesterone concentrations were, however, found to be greater in the medial prefrontal cortex of females versus males [F(1,37) = 4.99, p < 0.05], regardless of prenatal treatment ().
Irrespective of sex, the content of progesterone [F(1,37) = 5.71, p < 0.05] and, to a greater extent, DHP [F(1,37) = 14.75, p < 0.05], in the diencephalon was greater in the prenatally exposed IL-1β offspring compared with that in control offspring (). As with the hippocampus, 3α,5α-THP content seemed to be lower in the diencephalon of IL-1β-exposed females and greater in the IL-1β-exposed males, compared with that in controls; however, this was not significant ().
Prenatal IL-1β treatment resulted in lower oestradiol concentrations in the hippocampus [F(1,37) = 20.63, p < 0.05], medial prefrontal cortex [F(1,37) = 16.50, p < 0.05] and diencephalon [F(1,37) = 4.29, p < 0.05], in both the male and female offspring, compared with that in controls (). Females had significantly higher levels of oestradiol in the hippocampus [F(1,37) = 4.94, p < 0.05] than males ().
Table III. Concentrations of oestradiol (pg/g, mean ± SEM) in the hippocampus, medial prefrontal cortex and diencephalon of pubertal male and female offspring of mothers that were prenatally exposed to IL-1β (1 μg/rat) or vehicle (saline 0.9%) on GD 17–21.
Discussion
The present findings support the hypothesis that immune stress via LPS, or IL-1β, in late gestation negatively impacts birth outcomes in rats. Overall, the length of gestation and the number of viable pups born were reduced among all immune-challenged dams, compared with that in controls. In addition, the hypothesis that IL-1β-exposed offspring would demonstrate perturbed cognitive and affective profiles, concomitant with perturbed progesterone metabolism in the brain was partially upheld. Irrespective of sex, object recognition performance was impaired in the offspring of IL-1β-exposed dams compared with that in the control offspring. These effects did not appear to be due to the differences in motor behaviour given that IL-1β exposure did not significantly alter total, or peripheral, entries in the open-field task. However, IL-1β did produce sex-dependent differences in affective-like behaviour as measured in the open field. IL-1β-exposed females made a significantly greater percentage of entries into the centre of the open field compared to control females, which may indicate reduced anxiety-type behaviour; however, differences in open field behaviour were not observed among the males. These data are consistent with emerging evidence for the protective effects of early adversity on some behavioral measures. Indeed, young adult male mice that were prenatally exposed to LPS have demonstrated reduced anxiety-type behavior in the elevated plus maze (Asiaei et al. Citation2011). Notably, the open field does not provide a definitive measure of anxiety-type behaviour in rodents and future additional measures of anxiety (as well as measures of grooming, rearing and defecation in activity tasks) are warranted.
In addition to altered open field behaviour, IL-1β-exposed females had a significantly reduced ratio of 5α-reduced metabolites to their prohormone precursor, progesterone, in the hippocampus compared to the control females. These data indicate a reduced utilisation of progesterone in IL-1β-exposed females. Notably, IL-1β-exposed offspring also had significant increases in levels of progesterone and DHP (but not 3α,5α-THP) in the diencephalon. Unfortunately, repeated administration of LPS had high toxic effects given that only a few pups survived the treatment, precluding the possibility of examining the consequences of this treatment on neuroendocrine development. Future investigations should aim to assess lower dosages of LPS to mimic the poor perinatal development described in humans following in utero immune challenges. The present regimen may better approximate acute toxic effects during late gestational development with detrimental outcomes for pregnancy. Thus, immune stressors in late gestation can reduce gestational length and fecundity, and perturb later cognitive function and central progestogen formation in the offspring.
Several mechanisms may exist to induce preterm birth in response to immune stress. Activation of the foetal hypothalamic-pituitary-adrenal (HPA) stress axis may be a normative trigger for the onset of parturition. In humans, the foetal HPA is active in the second trimester and can respond independently of the maternal HPA axis (Gitau et al. Citation2001). As the foetus grows within the uterine environment, activation of the foetal HPA axis, concurrent with mechanical stimulation of the uterine wall by the growing foetus, induces changes in the myometrium that enhance excitability and facilitate parturition (Mastorakos and Ilias Citation2003; Shynlova et al. Citation2009; Petraglia et al. Citation2010). HPA activation, via endotoxin immune challenge, facilitates the release of proinflammatory cytokines (including IL-1β), partly in a corticotropin-releasing hormone (CRH) receptor-dependent manner (Theoharides et al. Citation1998; Agelaki et al. Citation2002). The present investigation utilised the administration of LPS or IL-1β to mimic infection and consequent inflammation which may facilitate preterm birth (Mazor et al. Citation1994, Citation1996). In women 30% of preterm birth cases may be due to intra-amniotic infection (Romero et al. Citation1991). As such, HPA activation in response to infection/inflammation may facilitate birth prior to term.
Another putative means for the induction of preterm birth in response to immune challenge may involve oxytocin secretion. In the rat, oxytocin (a potent stimulator of uterine contractions) is secreted in response to systemic administration of LPS (De Laurentiis et al. Citation2010a,Citationb) or IL-1β (Wilson et al. Citation1996; Brunton et al. Citation2006). IL-1β activates the neurohypophysial oxytocin system, at least in part, via brainstem noradrenergic projections that synapse in the supraoptic and paraventricular nuclei of the hypothalamus (Ericsson et al. Citation1994; Brunton et al. Citation2005). Brainstem noradrenergic neurons also relay stimuli from the birth canal to the oxytocin neurons at parturition (Antonijevic et al. Citation2000; Russell et al. Citation2003); as such, premature activation of this pathway (and hence the oxytocin neurons) may increase the risk of preterm labour. In late pregnancy a 3α,5α-THP-dependent inhibitory opioid mechanism does indeed restrain the maternal oxytocin neurons such that the oxytocin secretory response to acute IL-1β administration is dampened (Russell and Brunton Citation2006); however, it is not known whether this mechanism is overcome by repeated IL-1β exposure. The present data suggest that repeated administration of, or prolonged exposure to, LPS or IL-1β can facilitate preterm birth in rats, despite the reduced excitability of the oxytocin neurons to acute IL-1β administration (Brunton et al. Citation2006).
In this study, surviving adolescent rat offspring that were exposed to gestational immune challenge had impaired cognitive function that may be related to foetal programming. Prenatal stress can impair limbic function in surviving offspring (Drago et al. Citation1999; Weinstock Citation2001; Schmitz et al. Citation2002; Frye and Wawrzycki Citation2003; Paris and Frye Citation2011a,Citationb). As in the present investigation, we have previously observed that late-gestational exposure to repeated restraint stress (Paris and Frye Citation2011a), or chronic unpredictable stressors (Paris and Frye Citation2011b) significantly reduces cognitive performance of peri-pubescent males and females concomitant with dysregulation of cortico-limbic neurosteroid formation. Although the concentrations of central steroids observed in the present investigation are lower than those reported in adult Long–Evans rats (Frye et al. Citation2007), they are commensurate with those previously observed in 28–30-day-old rats (Paris and Frye Citation2011a,Citationb). Total pregnane steroid content is reduced in the foetal brain compared to sexually mature adults; particularly when taking into consideration cyclical peaks in pregnane steroid content due to estrous cycle fluctuations (Kellogg and Frye Citation1999). As such, we chose to observe neuroendocrine changes in juvenile rat offspring in the present study, so as to avoid activational steroid effects masking neuroendocrine changes that may have been promoted by prenatal cytokine exposure. Central actions of steroids, such as oestradiol, can promote progestogen neurosteroid formation (Micevych et al. Citation2008). Oestradiol and the pregnane neurosteroid, 3α,5α-THP, have demonstrated trophic effects (Schumacher et al. Citation2000; Herson et al. Citation2009) and can facilitate object recognition in adult rodents (Vaucher et al. Citation2002; Luine et al. Citation2003; Walf et al. Citation2006). Here, we found that gestational IL-1β exposure reduces oestradiol content in hippocampus, mPFC and diencephalon of the male and female offspring, and increases levels of P and DHP, but not their neuroactive metabolite, 3α,5α-THP, in the diencephalon, which may underlie some impaired cognitive function in the offspring. In support, others have observed an elimination of sex differences in aromatase and 5α-reductase activity in the hypothalamus of 10-day-old offspring that were subjected to late-gestational restraint (Reznikov et al. Citation2001; Reznikov and Tarasenko Citation2007). Although the present investigation was limited in that steroid concentrations could not be assessed in circulation, we have assessed these measures in related studies and have found that circulating oestradiol content (mean ng/ml ± SEM) in 28–30-day-old offspring is low among those prenatally exposed to an oil vehicle (males = 4.7 ± 0.7, females 3.8 ± 1.1), 3α,5α-THP (males = 3.0 ± 0.6, females = 2.9 ± 0.8) or a 5α-reductase inhibitor (males = 2.9 ± 1.1, females = 2.1 ± 0.4) on GD 17–21 (Paris et al. Citation2011). However, changes in central oestradiol content in response to prenatal cytokine exposure may be a reflection of changes in peripheral oestradiol synthesis, central oestradiol formation or both. Future studies are required to parse out the important steroid sources that underlie these effects.
The mechanism through which prenatal IL-1β exposure mediates the changes in central progesterone metabolism and reduced oestradiol levels in the offspring brain is unclear. Notably, environmental stress in adulthood has been shown to have sexually dimorphic effects on type-1 5α-reductase mRNA and protein levels in the prefrontal cortex of rats, with an increase in males and a decrease in females (Sánchez et al. Citation2009). Whether prenatal stress has similar effects remains to be elucidated. We also observed a loss of the typical sex difference in open field anxiety-type behaviour, wherein females exposed to IL-1β were observed to make a commensurate percentage of entries into the centre of a brightly lit open field as were males. This occurred concomitant with a reduction in the ratio of 5α-reduced metabolites to progesterone in hippocampus among females that were exposed to IL-1β compared to female controls. As such, the effects of gestational immune challenge on cognitive and open field anxiety-type behaviour of surviving offspring may be influenced by changes in steroid synthesising enzymes.
Whether there is a causative relationship between prenatal cytokine exposure and changes in progestogen synthesis in the pre-adolescent brain is of great interest, but is not yet understood. 3α,5α-THP and progesterone have neuroprotective effects (Schumacher et al. Citation2000; Sayeed and Stein Citation2009) and 5α-reduction is enhanced in a model of foetal hypoxic insult (Yawno et al. Citation2011). Similarly, we found that prenatal IL-1β increased the percentage of central entries in the open field among females, and this behaviour occurred concurrent with greater progesterone utilisation in the hippocampus. Although within-group variation on behavioural measures was greater among IL-1β-exposed compared to control offspring, these data suggest a consistent relationship between central steroid formation and pre-adolescent function. However, it must be noted that causal relationships between prenatal cytokine exposure and steroid-dependent processes have not been delineated. These effects may be directly due to cytokine action, the glucocorticoid promoting effects of cytokines, and/or via another unknown process.
It is an important question as to what extent the observed effects of immune challenge on the offspring's phenotype are due to direct cytokine actions versus the consequences of being born prior to term (potentially due to glucocorticoid-promotion of cytokines). The release of maternal glucocorticoids may play a role and may influence cytokine exposure. Stress alone evokes the release of glucocorticoids (cortisol in humans and corticosterone in most rodents). However, glucocorticoids may also exert divergent effects to acutely enhance pro-inflammatory cytokine action and chronically potentiate the neuroinflammatory response to stressors (reviewed by Frank et al. Citation2011). Excess corticosterone has teratogenic effects on the developing foetus (Setiawan et al. Citation2007; Kapoor et al. Citation2008, Citation2009); however, there are ‘in-built’ mechanisms that act to buffer the effects of excessive levels of maternal glucocorticoid on the foetus(es). The placenta expresses the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which inactivates corticosterone/cortisol, before it can cross the placental barrier (Seckl Citation1997). However, as gestation progresses, placental expression of this enzyme decreases, such that by the final third of pregnancy, the foetus may become vulnerable to maternal glucocorticoid actions. Furthermore, chronic stress during the last week of pregnancy significantly reduces the activity of placental 11β-HSD2 (Welberg et al. Citation2005), decreasing the protective capacity of the placental barrier. As such, foetuses may be particularly vulnerable to repeated maternal stress via glucocorticoid release and/or subsequent inflammatory processes in late gestation. In addition, early parturition may indicate an underdeveloped central nervous system at the time of birth. The blood–brain barrier can be compromised and this may facilitate central nervous system access via inflammatory, or damaging, molecules that would otherwise be precluded (Krizanac-Bengez et al. Citation2006; Willis and Davis Citation2008). Indeed, activation of the foetal immune response has been postulated to underlie white matter brain damage in neonates (Malaeb and Dammann Citation2009). As such, maternal stress responses in late pregnancy may confer vulnerability to preterm birth and its consequences, in particular for brain damage or neurodevelopmental disorder. Cytokine exposure may directly facilitate preterm birth via inflammatory processes (Mazor et al. Citation1994, Citation1996), and may be indirectly facilitated via stress challenge.
The present model demonstrates face validity with consequences for the offspring of preterm birth. In pregnant women, preterm birth is defined as delivery before 37 weeks of gestation (approximately ≥ 10% reduction in the length of gestation). We observed that LPS treatment reduced gestation time in rats by ∼18% and IL-1β treatment reduced gestation time in rats by ∼10%. In light of these findings, the alterations in limbic functioning in the offspring of this (and prior reports) may be considered more salient than other paradigms that have been utilised. However, effects on postnatal development could not be assessed in LPS-exposed offspring given the apparent acute toxic effects of LPS on offspring survival. Future investigations should aim to utilise different dosages and routes of LPS administration to better mimic in utero infection resulting in offspring survival. Moreover, administration of IL-1β to pregnant rat dams is a less physiologically relevant approach than LPS administration, given that viral or bacterial infection during pregnancy is accompanied by induction of many pro- and anti-inflammatory cytokines/chemokines (beyond IL-1β alone) that contribute to sickness behaviour and associated immunological and neurological changes (Hagberg et al. Citation2005). Future investigations should seek to selectively block pro- and anti-inflammatory immune modulators following LPS administration to elucidate the role of these factors in pregnancy outcome and postnatal development in a physiologically relevant model. Additionally, future studies will test the importance of the neuroactive steroids that were found to be reduced in the brains of offspring in the present report. In particular, pharmacological enhancement of 3α,5α-THP in the dorsal hippocampus has been demonstrated to regulate anxiety-type behaviour in adult male rats (Bitran et al. Citation2000); pharmacotherapies aimed at restoring progestogen formation may improve affective disorders associated with this, and other, models of preterm birth. Thus, the present report demonstrates in a rat model of immune challenge during late pregnancy that immunological stressors (LPS and IL-1β) can decrease gestation length, fecundity and/or cognitive/affective functioning in the pre-adolescent offspring. These manipulations would be justified to be replicated with different dosing and routes of administration and full behavioural characterisation on offspring, not only in pre-adolescence, but also at several ages throughout adulthood so that the generalisation, impact and conclusions of the present study can be extended. In particular, future investigations that include measures of cortico-limbic processing such as elevated plus maze, to assess anxiety, and contextual conditioning and/or Y-maze, to assess cognitive performance, would yield more definitive information regarding the neurobiological regions and constructs involved in these effects throughout the lifespan. Thus, these findings may provide a paradigm to investigate further the factors involved in preterm birth and its sequelae.
Declaration of interest: CAF and JJP appreciate technical help by Kassandra Edinger and are supported by funding from the National Institute of Mental Health (MH06769801). PJB and JAR are supported by the Biotechnology and Biological Sciences Research Council (BBSRC). CAF was pregnant at the time the data collection was made. The author reports no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agelaki S, Tsatsanis C, Gravanis A, Margioris AN. 2002. Corticotropin-releasing hormone augments proinflammatory cytokine production frommacrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect Immun. 70:6068–6074.
- Antonijevic IA, Russell JA, Bicknell RJ, Leng G, Douglas AJ. 2000. Effect of progesterone on activation of supraoptic nucleus neurones at parturition. J Reprod Fertil. 120:367–376.
- Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. 2006. The role of cytokines in mediating effects of prenatal infection on the fetus: Implications for schizophrenia. Mol Psychiatry. 11:47–55.
- Asiaei M, Solati J, Salari AA. Prenatal exposure to lps leads to long-lasting physiological consequences in male offspring. Dev Psychobiol (in press). 2011 doi: 10.1002/dev.20568.
- Bitran D, Foley M, Audette D, Leslie N, Frye CA. 2000. Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology (Berl). 151:64–71.
- Broadbent NJ, Squire LR, Clark RE. 2004. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA. 101:14515–14520.
- Brunton PJ, Meddle SL, Ma S, Ochedalski T, Douglas AJ, Russell JA. 2005. Endogenous opioids and attenuated hypothalamic-pituitary-adrenal axis responses to immune challenge in pregnant rats. J Neurosci. 25:5117–5126.
- Brunton PJ, Sabatier N, Leng G, Russell JA. 2006. Suppressed oxytocin neuron responses to immune challenge in late pregnant rats: A role for endogenous opioids. Eur J Neurosci. 23:1241–1247.
- Choi S, Dallman MF. 1999. Hypothalamic obesity: Multiple routes mediated by loss of function in medial cell groups. Endocrinology. 140:4081–4088.
- De Laurentiis A, Fernandez-Solari J, Mohn C, Burdet B, Zorrilla Zubilete MA, Rettori V. The hypothalamic endocannabinoid system participates in the secretion of oxytocin and tumor necrosis factor-alpha induced by lipopolysaccharide. J Neuroimmunol. 2010a; 221:32–41.
- De Laurentiis A, Fernández Solari J, Mohn C, Zorrilla Zubilete M, Rettori V. Endocannabinoid system participates in neuroendocrine control of homeostasis. Neuroimmunomodulation. 2010b; 17:153–156.
- Doyle LW, Anderson PJ. 2010. Adult outcome of extremely preterm infants. Pediatrics. 126:342–351.
- Drago F, Di Leo F, Giardina L. 1999. Prenatal stress induces body weight deficit and behavioural alterations in rats: The effect of diazepam. Eur Neuropsychopharmacol. 9:239–245.
- Edinger KL, Frye CA. 2006. Intrahippocampal administration of an androgen receptor antagonist, flutamide, can increase anxiety-like behavior in intact and DHT-replaced male rats. Horm Behav. 50:216–222.
- Elovitz MA, Mrinalini C. 2004. Animal models of preterm birth. Trends Endocrinol Metab. 15:479–487.
- Ennaceur A, Delacour J. 1988. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 31:47–59.
- Ennaceur A, Neave N, Aggleton JP. 1997. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 113:509–519.
- Ericsson A, Kovács KJ, Sawchenko PE. 1994. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 14:897–913.
- Erlebacher A, Zhang D, Parlow AF, Glimcher LH. 2004. Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system. J Clin Invest. 114:39–48.
- Frank MG, Watkins LR, Maier SF. 2011. Stress- and glucocorticoid-induced priming of neuroinflammatory responses: Potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain Behav Immun. 25:S21–S28.
- Frye CA, Paris JJ, Rhodes ME. 2007. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5alpha-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 133:663–674.
- Frye CA, Rhodes ME. Progestin concentrations are increased following paced mating in midbrain, hippocampus, diencephalon, and cortex of rats in behavioral estrus, but only in midbrain of diestrous rats. Neuroendocrinology. 2006a; 83:336–347.
- Frye CA, Rhodes ME. Infusions of 5α-pregnan-3α-ol-20-one (3α,5α-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3α,5α-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol.. 2006b; 18:960–975.
- Frye CA, Bayon LE. 1999. Mating stimuli influence endogenous variations in the neurosteroids 3α, 5α-THP and 3α-diol. J Neuroendocrinol. 11:839–847.
- Frye CA, Lacey EH. 2001. Posttraining androgens' enhancement of cognitive performance is temporally distinct from androgens' increases in affective behavior. Cogn Affect Behav Neurosci. 1:172–182.
- Frye CA, Petralia SM, Rhodes ME. 2000. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 67:1–10.
- Frye CA, Walf AA. 2004. Hippocampal THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 78:531–540.
- Frye CA, Wawrzycki J. 2003. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav. 44:319–326.
- Gendron RL, Nestel FP, Lapp WS, Baines MG. 1990. Lipopolysaccharide-induced fetal resorption in mice is associated with the intrauterine production of tumour necrosis factor-α. J Reprod Fertil. 90:395–402.
- Gitau R, Fisk NM, Teixeira JM, Cameron A, Glover V. 2001. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab. 86:104–109.
- Giurgescu C. 2009. Are maternal cortisol levels related to preterm birth?. J Obstet Gynecol Neonatal Nurs. 38:377–390.
- Glockner R. 1992. Influence of low birth weight on subsequent reproductive performance of female rats. Exp Toxicol Pathol. 44:421–423.
- Goldenberg RL, Culhane JF, Iams JD, Romero R. 2008. Epidemiology and causes of preterm birth. Lancet. 371:75–84.
- Gotz F, Dorner G, Malz U, Rohde W, Stahl F, Poppe I, Schulze M, Plagemann A. 1993. Short- and long-term effects of perinatal interleukin-1β—application in rats. Neuroendocrinology. 58:344–351.
- Hadders-Algra M, Huisjes HJ, Touwen BC. 1988. Perinatal risk factors and minor neurological dysfunction: Significance for behaviour and school achievement at nine years. Dev Med Child Neurol. 30:482–491.
- Hagberg H, Mallard C, Jacobsson B. 2005. Role of cytokines in preterm labour and brain injury. BJOG. 112:16–18.
- Hakulinen A, Heinonen K, Jokela V, Launiala K. 1988. Prematurity-associated morbidity during the 1st 2 yrs of life. A population-based study. Acta Paediatr Scand. 77:340–348.
- Herman JP, Dolgas CM, Carlson SL. 1998. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 86:449–459.
- Herson PS, Koerner IP, Hurn PD. 2009. Sex, sex steroids, and brain injury. Semin Reprod Med. 27:229–239.
- Holst K, Andersen E, Philip J, Henningsen I. 1989. Antenatal and perinatal conditions correlated to handicap among 4-year-old children. Am J Perinatol. 6:258–267.
- Hopwood N, Maswanganyi T, Harden LM. 2009. Comparison of anorexia, lethargy, and fever induced by bacterial and viral mimetics in rats. Can J Physiol Pharmacol. 87:211–220.
- Iswari S, Colas AE, Karavolas HJ. 1986. Binding of 5α-dihydroprogesterone and other progestins to female rat anterior pituitary nuclear extracts. Steroids. 47:189–203.
- Kapoor A, Kostaki A, Janus C, Matthews SG. 2009. The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behav Brain Res. 197:144–149.
- Kapoor A, Leen J, Matthews SG. 2008. Molecular regulation of the hypothalamic-pituitary-adrenal axis in adult male guinea pigs after prenatal stress at different stages of gestation. J Physiol. 586:4317–4326.
- Kellogg CK, Frye CA. 1999. Endogenous levels of 5α-reduced progestins and androgens in fetal vs. adult rat brains. Brain Res Dev Brain Res. 115:17–24.
- Kerstjens JM, de Winter AF, Bocca-Tjeertes IF, Ten Vergert EM, Reijneveld SA, Bos AF. 2011. Developmental delay in moderately preterm-born children at school entry. J Pediatr. 159:92–98.
- Kontula K, Jänne O, Vihko R, de Jager E, de Visser J, Zeelen F. 1975. Progesterone-binding proteins: In vitro binding and biological activity of different steroidal ligands. Acta Endocrinol (Copenh). 78:574–592.
- Krizanac-Bengez L, Mayberg MR, Cunningham E, Hossain M, Ponnampalam S, Parkinson FE, Janigro D. 2006. Loss of shear stress induces leukocyte-mediated cytokine release and blood-brain barrier failure in dynamic in vitro blood-brain barrier model. J Cell Physiol. 206:68–77.
- Lamprea MR, Cardenas FP, Silveira R, Walsh TJ, Morato S. 2003. Effects of septal cholinergic lesion on rat exploratory behavior in an open-field. Braz J Med Biol Res. 36:233–238.
- Latendresse G. 2009. The interaction between chronic stress and pregnancy: Preterm birth from a biobehavioral perspective. J Midwifery Womens Health. 54:8–17.
- Leazer TM, Barbee B, Ebron-McCoy M, Henry-Sam GA, Rogers JM. 2002. Role of the maternal acute phase response and tumor necrosis factor alpha in the developmental toxicity of lipopolysaccharide in the CD-1 mouse. Reprod Toxicol. 16:173–179.
- Lonstein JS. 2007. Regulation of anxiety during the postpartum period. Front Neuroendocrinol. 28:115–141.
- Luine VN, Jacome LF, Maclusky NJ. 2003. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 144:2836–2844.
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. 1986. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 232:1004–1007.
- Malaeb S, Dammann O. 2009. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol.. 24:1119–1126.
- Mastorakos G, Ilias I. 2003. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann NY Acad Sci. 997:136–149.
- Mazor M, Chaim W, Hershkowitz R, Levy J, Leiberman JR, Glezerman M. 1994. Association between preterm birth and increased maternal plasma cortisol concentrations. Obstet Gynecol. 84:521–524.
- Mazor M, Hershkowitz R, Ghezzi F, Cohen J, Silber A, Levy J, Leiberman JR, Glezerman M. 1996. Maternal plasma and amniotic fluid 17β-estradiol, progesterone and cortisol concentrations in women with successfully and unsuccessfully treated preterm labor. Arch Gynecol Obstet. 258:89–96.
- McCormick MC. 1985. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 312:82–90.
- Micevych P, Soma KK, Sinchak K. 2008. Neuroprogesterone: Key to estrogen positive feedback?. Brain Res Rev. 57:470–480.
- Moore CL. 2007. Maternal behavior, infant development, and the question of developmental resources. Dev Psychobiol. 49:45–53.
- Newton ER. 1986. Antepartum care in multiple gestation. Semin Perinatol. 10:19–29.
- Ogando DG, Paz D, Cella M, Franchi AM. 2003. The fundamental role of increased production of nitric oxide in lipopolysaccharide-induced embryonic resorption in mice. Reproduction. 125:95–110.
- Paris JJ, Brunton PJ, Russell JA, Walf AA, Frye CA. Inhibition of 5α-reductase activity in late pregnancy decreases gestational length and fecundity and impairs object memory and central progestogen milieu of juvenile rat offspring. J Neuroendocrinol. 2011 [DOI:10.1111/j.13652826.2011.02219.x].
- Paris JJ, Frye CA. Juvenile offspring of rats exposed to restraint stress in late gestation have impaired cognitive performance and dysregulated progestogen formation. Stress. 2011a; 14:23–32.
- Paris JJ, Frye CA. Gestational exposure to variable stressors produces decrements in cognitive and neural development of juvenile male and female rats. Curr Top Med Chem. 2011b; 11:1706–1713.
- Pellow S, File SE. 1986. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol Biochem Behav. 24:525–529.
- Petraglia F, Imperatore A, Challis JR. 2010. Neuroendocrine mechanisms in pregnancy and parturition. Endocr Rev. 31:783–816.
- Petrini JR, Dias T, McCormick MC, Massolo ML, Green NS, Escobar GJ. 2009. Increased risk of adverse neurological development for late preterm infants. J Pediatr. 154:169–176.
- Lind A, Korkman M, Lehtonen L, Lapinleimu H, Parkkola R, Matomäki J, Haataja L, PIPARI Study Group. 2011. Cognitive and neuropsychological outcomes at 5 years of age in preterm children born in the 2000s. Dev Med Child Neurol. 53:256–262.
- Reznikov AG, Nosenko ND, Tarasenko LV, Sinitsyn PV, Polyakova LI. 2001. Early and long-term neuroendocrine effects of prenatal stress in male and female rats. Neurosci Behav Physiol. 31:1–5.
- Reznikov AG, Tarasenko LV. 2007. Hormonal protection of gender-related peculiarities of testosterone metabolism in the brain of prenatally stressed rats. Neuro Endocrinol Lett. 28:671–674.
- Rodbard D, Hutt DM. 1974. Statistical analysis of radioimmunoassay and immunoradiometric (labeled antibody) assays: A generalized, weighted, iterative, least-squares method for logistic curve fittingSymposium on RIA and Related Procedures in Medicine Int. Atomic Energy Agency, Vienna. New York: Uni-pub165.
- Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. 1988. Infection in the pathogenesis of preterm labor. Semin Perinatol. 12:262–279.
- Romero R, Avila C, Brekus CA, Morotti R. 1991. The role of systemic and intrauterine infection in preterm parturition. Ann NY Acad Sci. 622:355–375.
- Russell JA, Brunton PJ. 2006. Neuroactive steroids attenuate oxytocin stress responses in late pregnancy. Neuroscience. 138:869–889.
- Russell JA, Leng G, Douglas AJ. 2003. The magnocellular oxytocin system, the fount of maternity: Adaptations in pregnancy. Front Neuroendocrinol. 24:27–61.
- Sánchez P, Torres JM, Olmo A, O'Valle F, Ortega E. 2009. Effects of environmental stress on mRNA and protein expression levels of steroid 5α-reductase isozymes in adult rat brain. Horm Behav. 56:348–353.
- Sayeed I, Stein DG. 2009. Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog Brain Res. 175:219–237.
- Schmitz C, Rhodes ME, Bludau M, Kaplan S, Ong P, Ueffing I, Vehoff J, Korr H, Frye CA. 2002. Depression: Reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Mol Psychiatry. 7:810–813.
- Schumacher M, Akwa Y, Guennoun R, Robert F, Labombarda F, Desarnaud F, Robel P, De Nicola AF, Baulieu EE. 2000. Steroid synthesis and metabolism in the nervous system: Trophic and protective effects. J Neurocytol. 29:307–326.
- Seckl JR. 1997. Glucocorticoids, feto-placental 11β-hydroxysteroid dehydrogenase type 2, and the early life origins of adult disease. Steroids. 62:89–94.
- Setiawan E, Jackson MF, MacDonald JF, Matthews SG. 2007. Effects of repeated prenatal glucocorticoid exposure on long-term potentiation in the juvenile guinea-pig hippocampus. J Physiol. 581:1033–1042.
- Sfakianaki AK, Norwitz ER. 2006. Mechanisms of progesterone action in inhibiting prematurity. Matern Fetal Neonatal Med. 19:763–772.
- Shynlova O, Tsui P, Jaffer S, Lye SJ. 2009. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol. 144:S2–S10.
- Talge NM, Holzman C, Wang J, Lucia V, Gardiner J, Breslau N. 2010. Late-preterm birth and its association with cognitive and socioemotional outcomes at 6 years of age. Pediatrics. 126:1124–1131.
- Tanskanen P, Valkama M, Haapea M, Barnes A, Ridler K, Miettunen J, Murray GK, Veijola JM, Jones PB, Taanila AM, Isohanni MK. 2011. Is prematurity associated with adult cognitive outcome and brain structure?. Pediatr Neurol. 44:12–20.
- Taylor DC. 1969. Differential rates of cerebral maturation between sexes and between hemispheres. Evidence from epilepsy. Lancet. 2:140–142.
- Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E, Chrousos G. 1998. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 139:403–413.
- Uehara A, Gottschall PE, Dahl RR, Arimura A. 1987. Interleukin-1 stimulates ACTH release by an indirect action which requires endogenous corticotropin releasing factor. Endocrinology. 121:1580–1582.
- Vaucher E, Reymond I, Najaffe R, Kar S, Quirion R, Miller MM, Franklin KB. 2002. Estrogen effects on object memory and cholinergic receptors in young and old female mice. Neurobiol Aging. 23:87–95.
- Vrachnis N, Vitoratos N, Iliodromiti Z, Sifakis S, Deligeoroglou E, Creatsas G. 2011. Intrauterine inflammation and preterm delivery. Ann NY Acad Sci. 1205:118–122.
- Walf AA, Frye CA. 2007. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 86:407–414.
- Walf AA, Rhodes ME, Frye CA. 2006. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 86:35–46.
- Weinstock M. 2001. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 65:427–451.
- Weinstock M. 2005. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 19:296–308.
- Welberg LA, Thrivikraman KV, Plotsky PM. 2005. Chronic maternal stress inhibits the capacity to up-regulate placental 11β-hydroxysteroid dehydrogenase type 2 activity. J Endocrinol. 186:R7–R12.
- Willis CL, Davis TP. 2008. Chronic inflammatory pain and the neurovascular unit: A central role for glia in maintaining BBB integrity?. Curr Pharm Des. 14:1625–1643.
- Wilson BC, Fulop K, Summerlee AJ. 1996. Mechanisms of the effect of Icv IL-1β on oxytocin release in the anesthetized, lactating rat. Endocrine. 5:51–57.
- Xu DX, Wang H, Ning H, Zhao L, Chen YH. 2007. Maternally administered melatonin differentially regulates lipopolysaccharide-induced proinflammatory and anti-inflammatory cytokines in maternal serum, amniotic fluid, fetal liver, and fetal brain. J Pineal Res. 43:74–79.
- Yawno T, Yan EB, Hirst JJ, Walker DW. 2011. Neuroactive steroids induce changes in fetal sheep behavior during normoxic and asphyxic states. Stress. 14:13–22.
- Zhao L, Chen YH, Wang H, Ji YL, Ning H, Wang SF, Zhang C, Lu JW, Duan ZH, Xu DX. 2008. Reactive oxygen species contribute to lipopolysaccharide-induced teratogenesis in mice. Toxicol Sci. 103:149–157.