Abstract
A commonly used method for obtaining blood samples from mice is decapitation. However, there is an obvious need for repeated blood sampling in mice under stress-free conditions. Here, we describe a simple technique to repeatedly collect blood samples from conscious, freely moving mice through a chronically implanted jugular vein catheter. Furthermore, we compare plasma corticosterone (CORT) concentrations in samples obtained through the catheter 1 day after surgery with samples taken from trunk blood obtained under basal or acute stress conditions. CORT concentrations in repeated 100-μl venous blood samples were found to be similar to trunk blood samples both under basal conditions and after stressor exposure collected at identical time points (at 5, 15, and 60 min). Using both techniques, we demonstrate a progressive increase in CORT levels until 15 min after termination of stressor exposure and a decrease towards baseline values 60 min later. Anxiety-related behavior, as assessed on the elevated plus maze 3–4 days after surgery, did not differ between catheterized and non-catheterized mice. Our results provide evidence for application of jugular vein catheterization as a technique for repeated blood sampling in conscious laboratory mice. Use of this technique will greatly reduce the number of animals required for experiments involving endocrine endpoints.
Introduction
The collection of blood from laboratory rats or mice for the estimation of immunological or neuroendocrine parameters, including hormones of the hypothalamo-pituitary-adrenal (HPA) axis, the hypothalamo-neurohypophysial system, or the sympathetic nervous system, is an essential experimental tool in preclinical neurobiological, endocrine, or immunological research. In rodents, this can be achieved by collection of trunk blood (Reber et al. Citation2007), blood from the tail vein (Doeing et al. Citation2003; Pryce et al. Citation2010), or orbital sinus (Rogers et al. Citation1999), resulting in either termination of the experiment or a severe stressor exposure. More elegant methods for monitoring neuroendocrine parameters in rats are blood microdialysis (Neumann et al. Citation1993a) or withdrawal of blood samples from the femoral (Russell et al. Citation1992) or jugular vein through acutely or chronically implanted vein catheters (Neumann et al. Citation1998a; Landgraf et al. Citation1999). Only the latter technique allows chronic sampling of blood under stress-free conditions in freely moving animals. However, to date this powerful method, which provides the opportunity to reveal the dynamics of circulating concentrations of a given substance, has only been effectively applied in rats (Windle et al. Citation1997; Neumann et al. Citation1998a). Despite a similar need in mice, especially given the continuing generation of valuable transgenic lines, and despite repeated approaches to develop a venous blood sampling method in mice (Atkinson et al. Citation2010; Pryce et al. Citation2010), a simplified and reliable method for chronic blood sampling in mice has not been successfully used until now.
Here, we report a simplified technique for repeated or chronic blood sampling in conscious, freely moving mice through an implanted jugular vein catheter, using methods that can be applied in any neuroendocrine laboratory. The sampling method has been validated by comparing concentrations of the stress hormone corticosterone (CORT) in blood samples obtained from the jugular vein and trunk under basal conditions and in response to exposure to two different acute stressors (forced swim, FS; elevated platform, EPF). Both stressors have been described to trigger HPA axis activation in rats (Neumann et al. Citation2000) or mice (Douglas et al. Citation2003). To investigate the effects of catheterization on anxiety-related behavior, we used the well-established elevated plus maze (EPM) test. The EPM is an established unconditioned anxiety test for rodents, which induces a conflict situation (animals choose to remain in the protected closed arms or to explore the open arms of the maze) (Pellow et al. Citation1985).
Materials and methods
Subjects
Adult male C57BL6/N mice (21–23 g body weight) were obtained from a commercial supplier (Charles River, Sulzfeld, Germany), and were single-housed in polycarbonate cages (21 × 15 × 14 cm) for 2 weeks before surgery. Single-housing was previously found to be less stressful compared with group-housing in male mice with respect to body weight gain and anxiety-related behavior during open arm exposure (Singewald et al. Citation2009). Mice were maintained under standard laboratory conditions (12:12 light/dark cycle, lights off at 6 pm, temperature 22°C, 40% humidity, V1535-000 commercial diet for rats/mice maintenance from ssniff®, Soest, Germany, and tap water ad libitum). All experiments were approved by the local Bavarian government and performed in accordance with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health.
Reagents and other materials
Reagents used during surgery included isoflurane (PZN: 6497131, Baxter Deutschland GmbH, Unterschleißheim, Germany) for anesthesia; softasept® N (article number 3887138; B. Braun Melsungen AG, Melsungen, Germany) for disinfection of tissue and instruments; betaisodona® (article number 1970433; Mundipharma GmbH, Limburg-Lahn, Germany) as an antiseptic to reduce the possibility of tissue infection; gentamicin (30,000 IU/ml, PZN: 0536516; 40 HEXAL® SF, Holzkirchen, Germany) for antibiotic treatment; physiological saline (article number 3200950, 0.9% NaCl; B. Braun Melsungen AG, Melsungen, Germany) to replace withdrawn blood volume; and heparinized saline (30 IU/ml, PZN: 3441331; Liquemin® N 25000, Hoffman-La Roche AG, Grenzach-Wyhlen, Germany) to prevent blood clotting in catheter tubing. Volumes are given below where animal treatment procedures are described. Polyethylene (PE-10) tubing (30 m Portex® non-sterile 0.28 mm ID × 0.61 mm OD, Smiths Medical International Ltd, Hythe, Kent, UK, ref: 800/110/100), Dow Corning SILASTIC Silicone Laboratory Tubing (article number 2415496 single 50 coil, 0.30 mm ID × 0.64 mm OD), and chloroform (article number 108749; Merck, Darmstadt, Germany) were used for construction of the catheter, while silk sutures (ref: H4F, 3-0 USP Resorba, Nürnberg, Germany) were used during surgery. Plexiglas cages (38 × 22 × 36 cm) with perforated lids were used for housing the animals after surgery (see ).
Figure 1(a). Schematic representation of the mouse catheter system adapted for repeated blood sampling in conscious mice; a: 1-ml syringe, b: a 15-mm piece of a 27-G cannula, c1: a 400-mm PE-10 extension tubing (ID 0.28 mm; OD 0.61 mm) which is linked to the catheter through another 15-mm piece of 27-G cannula. The catheter is composed of a PE-10 tubing (c2: 90-mm long) connected through a 5-mm overlap (d) to a silicone tubing (e: ID 0.30 mm; OD 0.64 mm, 17-mm long), beveled at its tip to an angle of about 45°. (b). Mouse with already implanted catheter, exteriorized through the nape of the neck. (c). Representation of a catheterized mouse in the Plexiglas cage with the extended PE-10 connection tubing (arrow, c1) attached to the syringe (arrow, a) lying on top of the cage. (d). Schematic representation of the blood sampling protocol: about 24 h after surgery, the indwelling jugular vein catheter is connected to the PE-10 extension tubing and the 1-ml syringe 90 min before the start of the experiment. Thirty minutes after collection of the basal sample, the mouse is exposed for 5 min to the elevated platform (EPF) or (another group of mice) to 60 s of forced swim (FS). At 5, 15, and 60 min after termination of stressor exposure, blood samples 2, 3, and 4 are collected.
Catheter assembly
A 90-mm piece of PE-10 tubing and a 17-mm piece of the silicone tubing were used to prepare catheters. One end of the silicone tubing was expanded by dipping in chloroform for about 15 s to facilitate insertion. The PE-10 tubing was then immediately fitted into the expanded end of the silicone tubing to an overlap of 5 mm, and the free end of the silicone tubing beveled to an angle of about 45° to allow easy insertion into the blood vessel (). The catheter was stored under aseptic conditions in 70% alcohol overnight before surgery.
Surgical procedure: Jugular vein catheterization
Presurgical preparations
Heparinized saline (30 IU/ml), gentamicin (30,000 IU/ml), and sterile saline were prepared before surgery. Before surgery, catheters were repeatedly flushed with distilled water, followed by sterile saline to remove remaining alcohol. They were filled with heparinized saline by attaching the PE-10 end of the catheter through a sterile blunted 27-gauge (27 G) needle (0.45 × 23 mm BL/LB) to a 1-ml syringe (). The filled catheter was placed in sterile saline for at least 10 min before use. All surgical instruments were sterilized with softasept N, and later placed in 70% alcohol for about 10 min before surgery. The instruments were rinsed briefly with sterile saline before use.
Mice were briefly anesthetized using isoflurane. The area around the right clavicle and nape of the neck was shaved and the animal was placed on a heating pad. Continuous supply of inhalant isoflurane was maintained throughout the surgery with careful observation of the breathing frequency of the animal. The absence of a tail and foot withdrawal reflex when pinched was used an index of sufficient anesthesia. The mouse was laid on its back and the hind paws were taped on the surgical board to maintain positioning. Softasept N was applied on the shaved surface, and the surgical procedure was carried out under stereomicroscopic control (Leica MZ6).
Jugular vein catheterization
On average, the entire procedure of catheterization, starting with anesthesia and ending with the return to the home cage, lasted about 25 min. A longitudinal skin incision of about 8–10 mm was made above the right clavicle and the jugular vein was exposed by blunt dissection. After careful isolation from the underlying tissue and exposure of approximately 5 mm of the vein, two silk sutures were placed underneath the vein. One suture was tightly knotted cranially to occlude the vein and interrupt blood flow, while the caudal suture remained untied. Using a microscissors, we made a small v-like incision into the wall of the vein between both ligatures. The beveled end of the catheter was carefully inserted into the jugular vein and advanced with the aid of fine forceps till the tip reached the right atrium. About 13–15 mm of the catheter was inserted into the vein, just including part of the silicone-PE tubing overlap. The caudal suture was then knotted once on both the vein and the catheter at the level of the silicone-PE tubing overlap, holding it in place. About 0.05 ml of heparinized saline was injected into the catheter. An attempt to draw blood through the catheter confirmed the correct positioning of the catheter within the jugular vein and its tip within the right atrium. The small amount of blood withdrawn into the tubing was gently flushed back with heparinized saline. If no blood could be drawn, the catheter was repositioned. The flushing with heparinized saline also prevented blood coagulation within the catheter. Next, the caudal suture was repeatedly knotted on top of the silicone-PE tubing overlap and the free ends of the sutures were cut off.
To exteriorize the catheter, the mouse was laid on its right side and a guide cannula (18 G, 1.2 × 40 mm, BD MicrolanceTM, Fraga, Spain, ref: 304622) guided subcutaneously to the nape of the neck and pushed through the skin behind the ears. The catheter was rapidly detached from the syringe, fitted through the guide cannula, and exteriorized at the other end (). The mouse was turned on its back, the catheter was reconnected to the syringe, and trapped air was drawn into it. Afterwards, 0.03 ml sterile gentamicin was injected intravenously to prevent infection. Heparinized saline (0.05 ml) was next injected to flush down the gentamicin. The syringe was detached, the exteriorized PE-10 tubing bent at an angle of 180°, 20–30 mm away from the skin forming a crimp (see ), and a piece of silicone rubber tubing slipped over this crimp to prevent air entering the tubing. The ventral wound was closed with wound clips. Finally, all surgical incisions were swabbed with betaisodona and the mouse was placed into a clean observation Plexiglas cage () for recovery. Mice remained single-housed in these cages after surgery and during blood sampling, and were observed carefully during the immediate postoperative period.
Blood sampling
To collect blood samples from the undisturbed freely moving mouse, at 8 am of the following day (20–24 h after venous catheter implantation), we connected the jugular vein catheter to a 1-ml plastic disposable syringe filled with heparinized saline through a 400-mm piece of PE-10 tubing that extended outside the cage. The exteriorized free end of the catheter was attached through a 15-mm piece of 27-G cannula to the 400-mm PE-10 extension tubing, which was attached to the 1-ml plastic syringe through a 27-G cannula (). This allowed blood sampling without disturbance of the conscious mouse. The first baseline sample (100 μl) was collected 90 min after connection of the catheter to the syringe. The same volume of blood was replaced with sterile saline before the extension tubing was filled with heparinized saline again. Thirty minutes after collection of the basal sample, the animals were exposed to either the EPF (5 min) or to the FS (60 s). Subsequent samples were collected at 5, 15, and 60 min after termination of stressor exposure (, experimental setup).
Blood samples were collected into EDTA-coated Eppendorf tubes, centrifuged at 5000 rpm for 10 min at 4°C, and plasma aliquots (10 μl) were stored at − 20°C until CORT concentrations were assayed by ELISA (DRG Instruments GmbH, Marburg, Germany).
Stress procedures and behavioral tests
EPF
Exposure of rodents to the EPF has been used as a mild psychological stressor to assess the stress response of the HPA axis (as described in CitationNeumann et al. [2000]). Briefly, the EPF consisted of a circular platform, 25 cm in diameter, and elevated 75 cm above the ground. Mice were exposed to the EPF for 5 min, and blood samples were collected at 5, 15, and 60 min following termination of EPF exposure. The EPF was cleaned thoroughly before each test.
FS
It represents an ethologically relevant combined physical and emotional stressor for rats (Abel Citation1994; Neumann et al. Citation1998b) and mice (Bowers et al. Citation2008). The FS tank consisted of an open top cylinder (25 cm height × 13 cm diameter) filled with tap water (21 ± 1°C) to about 12 cm to the brim. Mice were immersed into the water tank for 60 s, and blood samples were collected at 5, 15, and 60 min following termination of FS exposure. The water was changed after every third FS trial and fecal boli was removed after every swim.
The EPM
It has been used to assess the anxiety-related behavior of rodents (Pellow et al. Citation1985). As described before (Reber et al. Citation2007), our mouse EPM consisted of two open (6 × 30 cm) and two closed (6 × 30 × 17 cm) arms radiating from a central platform (6 × 6 cm) to form a plus-shaped figure. The maze was elevated 130 cm above the floor. Each mouse (with or without the catheter) was placed on the central platform facing the closed arm. All animals were tested between 8 am and 11 am. During the 5-min test period, the latency to the first open arm entry, the number of entries into the open and closed arms, and the time spent on the respective arms were recorded by means of a video/computer setup to allow calculation of the percentage of time spent on the open arm and the percentage of entries performed into open arms as parameters of anxiety-related behavior. Closed arm entries were taken as a parameter of locomotion. The maze was cleaned thoroughly before each test.
Experimental protocols
Plasma CORT concentrations immediately and 24 h after surgery and stressor exposure
In a pilot experiment, we obtained blood samples immediately after surgery (post surgery) and 24 h later (basal), to determine the time point of decreased plasma CORT concentrations after surgery. Further, to determine whether the sampling technique was stressful to the animals, we measured CORT concentrations in blood samples repeatedly collected under basal conditions at 15-min intervals and each replaced by sterile saline (100 μl) in another set of animals 24 h after surgery.
A new set of animals was used to measure plasma CORT responses to an acute stressor as early as 24 h after surgery. During this experiment 30 min after collection of the basal blood sample, mice were exposed to the EPF for 5 min and subsequent blood samples were collected at 5, 15, and 60 min following termination of EPF.
Plasma CORT concentrations in venous and trunk blood in response to EPF or FS exposure
We compared CORT concentrations in plasma samples obtained either repeatedly from the jugular vein catheter or, in separate sets of mice, from trunk blood. Venous or trunk blood samples were taken under basal conditions (Sample 1) or at 5, 15, and 60 min following termination of either EPF or FS exposure. These established stressors differ in the quality (psychological vs. physical) and intensity (mild vs. severe). As the pilot experiment revealed that plasma CORT levels 24 h after surgery were comparable to baseline levels in mice without prior surgery, blood sampling was already performed on the day after surgery. Trunk blood was collected from separate groups of single-housed mice killed at the respective time points of blood sampling from the vein. Mice were briefly (5–10 s) narcotized by CO2 inhalation and decapitated within 50 s from the onset of transportation from the experimental room.
Effects of catheterization on anxiety-related behavior
Three–four days after surgery and blood sampling, mice were tested on the EPM for anxiety-related behavior as described above. Catheterized and non-catheterized control mice received the same stress treatment (5 min EPF 3 days before testing). The mice were transported to the EPM room for habituation in the evening before testing.
Statistical analysis
Data are represented as group means ± SEM. For statistical analysis, the software package SPSS 18.0 (SPSS Inc., Chicago, IL, USA) was used. Comparisons were made using either a two-tailed t-test, one-way analysis of variance (ANOVA; factor time), ANOVA for repeated measures, or a two-way ANOVA (factors sampling technique and factor time) followed by Bonferroni's post-hoc test if appropriate. P ≤ 0.05 was considered statistically significant.
Results
Experimental success
Of all operated mice, 91% survived this procedure, and repeated blood sampling could be successfully performed in 87% of the surviving mice 24 h later. Of catheterized mice, 80% were kept for EPM testing performed 3 days after blood sampling.
Plasma CORT levels immediately and 24 h after stress
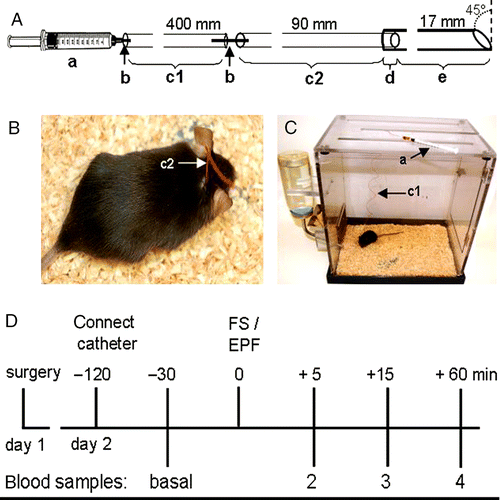
Statistical analysis revealed significantly lower plasma CORT concentrations in catheter blood withdrawn 24 h after surgery compared with blood withdrawn immediately after surgery (P = 0.01; ). Twenty-four hours after surgery, repeated blood sampling and replacement with sterile saline did not affect CORT concentrations in venous blood samples (F2,4 = 0.25, P = 0.79; ). A 5-min exposure to the EPF significantly altered plasma CORT concentrations (F3,15 = 20.74, P < 0.01; ), with increased levels found at 5 and 15 min (P = 0.01) following termination of stressor exposure. Importantly, 24 h after surgery, basal plasma CORT levels in catheter blood were comparable to those in trunk blood samples collected under basal conditions in another set of non-catheterized animals (P = 0.06; ). On the basis of these findings and the high success rate of blood sampling after 24 h, we decided to perform all repeated blood sampling experiments on the day following surgery.
Figure 2. Corticosterone concentrations in mouse venous blood repeatedly collected through a chronically implanted indwelling jugular vein catheter immediately (post surgery; postS) and 24 h after surgery ((a): n = 6), 24 h after surgery under basal conditions at 15-min intervals with replacement of 100 μl sterile saline after each blood sampling ((b): n = 3); and 24 h after surgery under basal conditions as well as 5, 15, and 60 min after exposure to an elevated platform ((c): EPF, arrow, 5 min; n = 6). Data represent means ± SEM; **P < 0.01 versus basal.
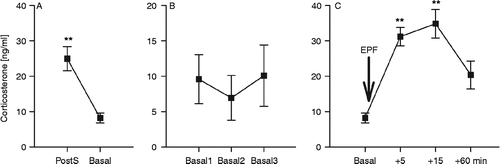
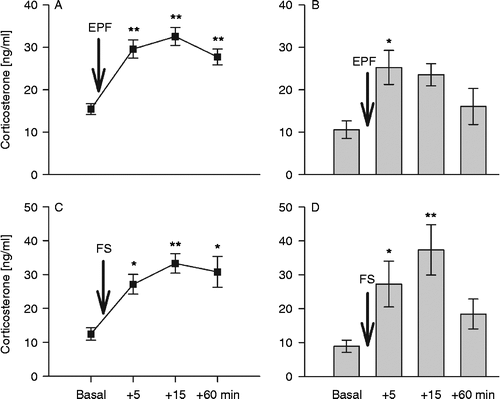
Figure 3. Corticosterone concentrations in mouse blood collected through a chronically implanted indwelling jugular vein catheter ((a,c): n = 10) or from trunk blood ((b,d): n = 7–10) under basal conditions as well as 5, 15, and 60 min after stressor exposure (arrow): (a) and (b) show plasma corticosterone concentrations after exposure to the elevated platform (EPF, 5 min), (c) and (d) after exposure to forced swim (FS, 60 s). Data represent means ± SEM; **P < 0.01, *P < 0.05 versus basal.
Plasma CORT concentrations in venous versus trunk blood samples in response to EPF or FS exposure
Plasma CORT concentrations did not differ between venous and trunk blood samples collected under basal conditions (P = 0.06). Exposure to EPF significantly increased plasma CORT in venous (factor time: F3,27 = 21.62, P < 0.01) and trunk blood (F3,25 = 3.81, P = 0.02) samples. Post-hoc comparison revealed increased CORT levels in venous blood at 5, 15, and 60 min (P = 0.01 vs. basal), whereas, in contrast, in trunk blood samples, elevated CORT concentrations were evident only 5 min after EPF exposure (P = 0.05 vs. basal). However, venous and trunk blood plasma CORT concentrations differed in response to acute EPF exposure (factor sampling technique: F3,61 = 16.71, P < 0.01; factor sampling technique × time: F3,61 = 0.93, P = 0.43; ).
To investigate CORT responses to an acute stressor in venous versus trunk blood samples in detail, we exposed another group of mice to a putatively more severe physical/emotional stressor (FS). Again, basal plasma CORT did not differ between venous and trunk blood samples (P = 0.29; ). The FS increased plasma CORT concentrations in both venous (F3,27 = 9.62, P < 0.01; ) and trunk blood (F3,32 = 5.28, P = 0.01; ), with increased CORT concentrations at 5 min (P = 0.04 for venous, P = 0.05 for trunk blood vs. basal). The FS-induced changes in plasma CORT concentration were independent of the sampling technique (factor sampling technique: F3,68 = 0.81, P = 0.37; factor sampling technique × time: F3,68 = 1.12, P = 0.32; ), and comparable peak concentrations found at 15 min after FS exposure (P = 0.01 vs. basal).
In contrast to trunk blood samples (), plasma CORT levels in venous blood samples were still elevated 60 min following EPF or FS exposure (P = 0.01; , P = 0.03; ). Statistical analysis revealed no significant difference in the area under the curve of plasma CORT concentrations in venous blood samples obtained in response to EPF or FS exposure (P = 0.72; ).
Effects of catheterization on anxiety-related behavior
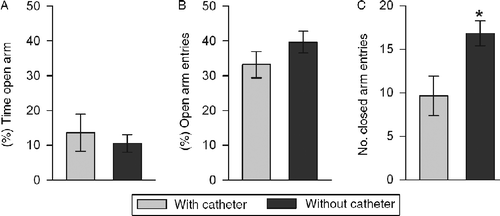
The anxiety-related behavior on the EPM tested 3 days after repeated blood sampling was not different from that found in non-catheterized mice (percentage of time on open arms: P = 0.85, percentage of entries into open arm: P = 0.29). However, a significantly reduced number of closed arm entries performed by catheterized compared with non-catheterized mice (P = 0.02; ) indicated a decreased locomotion in the former.
Figure 4. Anxiety-related behavior of mice with (n = 8) and without (n = 11) a chronic jugular vein catheter 4 days after surgery represented by the percentage of: (a) time spent in the open arms of the elevated plus maze, and (b) entries performed into the open arms of the elevated plus maze. Closed arm entries (c) reflect locomotor activity. Data represent means ± SEM; *P < 0.05 versus catheter group.
Discussion
In this study, we demonstrate that chronic jugular vein catheterization and repeated blood sampling is possible in conscious, freely moving mice. Using our relatively simple catheter and sampling technique, we could repeatedly withdraw as much as 100-μl blood samples up to four times within approximately 120 min; this is sufficient blood volume for the reliable quantification of plasma CORT as well as other plasma hormones that are far less concentrated in plasma than CORT (e.g. corticotrophin (ACTH), oxytocin, and vasopressin). In addition, under these conditions, fluctuations in any given plasma factor can also be monitored in response to pharmacological treatment after completely stress-free intravenous infusion through the implanted jugular vein catheter. Importantly, in three mice (40% of catheterized mice tested at this time point), blood sampling could even be performed up to 14 days after catheterization (data not shown). The jugular vein catheter could also be useful in experiments employing chronic intravenous drug infusion using osmotic minipumps connected to the catheter.
Because of their small size, blood sampling in mice has been frequently limited to non-surgical methods. Thus, repeated blood sampling has been performed from the mouse tail vein or through cardiac puncture among others (http://www.theodora.com/rodent_laboratory/blood_collection.html), often using stressful procedures that do not allow determination of basal hormone levels (Pryce et al. Citation2010). Small amounts of blood can also be collected from the orbital sinus of an anesthetized or firmly restrained mouse (Rogers et al. Citation1999), or from the carotid artery (Niswender et al. Citation1997; Ayala et al. Citation2006). Although studies have been published more than two decades ago showing that serial blood samples can be collected from the right jugular vein of a freely moving mouse (Barr et al. Citation1979), the techniques described in these studies are rather complicated and consequently have not been routinely applied since then. For example, MacLeod and Shapiro (Citation1988) showed that serial blood sampling could only be performed in the mouse when the inserted catheter is tethered to a freely moving wheel system built above the cage. Also, Mokhtarian et al. (Citation1993) reported the possibility of collecting blood samples through a right jugular vein catheter fixed to the skull with dental cement, and Bardelmeijer et al. (Citation2003) designed a special holding cage system that enabled the collection of blood samples through a previously implanted jugular vein catheter. These rather complicated experimental constructions probably prevented the aforementioned protocols from being used more routinely in life science research. They were, however, important steps towards the development of the relatively simple blood sampling technique introduced in this study.
In comparison to arterial catheterization protocols commonly used in metabolic studies, jugular vein catheterization has some advantages, including a relatively simpler catheterization procedure. Moreover, the tip of the catheter rests in the right atrium. This allows “first pass” venous blood collection that should have higher concentrations of plasma hormones originating in the pituitary or the adrenal glands. Moreover, the risk to induce stroke seen after arterial catheterization (Ayala et al. Citation2010) is low after jugular vein catheterization.
Our catheter set up, originally designed for rats, is easily and rapidly prepared, and most importantly, made of inexpensive commercially available materials. We also use special experimental cages (Plexiglas 38 × 22 × 36 cm; ) that are routinely used for blood sampling (Neumann et al. Citation1998a; Landgraf et al. Citation1999) and microdialysis in rats (Neumann et al. Citation1993a,Citationb) and mice (Theodosis et al. Citation2004; Heinzmann et al. Citation2010). These cages are higher (36 cm) than regular mouse cages (21 × 15 × 14 cm) and equipped with a perforated lid allowing unrestricted movement of the mouse with the extension tubing of the catheter and the sampling syringe attached (). However, the sampling system should be transferable to any cage that prevents the freely moving animal from escaping while allowing collection of blood samples from the indwelling catheter through PE-10 extension tubing terminating just above cage. Though relatively simple, it is worth mentioning that our blood sampling technique requires a greater level of expertise for successful experimental performance than obtaining trunk blood.
To further validate the method of repeated blood sampling from the jugular vein, we compared CORT concentrations in venous and trunk blood samples collected under basal conditions and at identical time points following acute stressor exposure. CORT is the main stress hormone of the HPA axis, which is an excellent indicator of any external disturbance. Importantly, we could demonstrate that the plasma CORT concentrations obtained from both the jugular and trunk blood samples were almost identical under basal conditions and following exposure to a physical/emotional stressor (FS), and at least comparable in response to exposure to the EPF. Therefore, the method of blood collection should also be considered in dependence on the stressor applied. Moreover, the observable elevation in plasma CORT at 60 min after stressor exposure in response to EPF and FS in venous blood samples () indicates the possibility of altered HPA axis feedback, for example as a result of repeated sampling or prior surgery. Although further testing is needed at later time points, the technique might be more feasible for assessing acute HPA axis responses, rather than for studying the recovery of the HPA axis. It is also important to mention that mice could require a longer recovery period (maybe 2 h or more), but we did not investigate this in more detail.
Compared with trunk blood collection, serial collection of blood samples from a chronically implanted jugular vein has a lot of advantages. It allows intra-individual comparisons and monitoring of the temporal dynamics of a given blood-born factor. Moreover, a substantial reduction in the number of experimental mice, which has clear ethical and financial benefits, is a major factor.
High CORT concentrations in plasma sampled immediately after surgery () indicate that anesthesia and/or surgery are stressful. This underscores problems with blood sampling under conditions of long-term anesthesia (more than 60 s) or of restraint, routinely performed for blood collection from the tail vein, orbital sinus or through cardiac puncture, as these methods are likely to provide false basal values. Therefore, anesthesia or restraint may result in conflicting basal results relative to those obtained in freely moving non-stressed mice. To our surprise, catheterized mice show low basal plasma CORT levels already 24 h after surgery. Therefore, 1 day of recovery may be sufficient for blood sampling experiments, and studies may be performed the day following surgery. This poses an advantage over rats, in which repeated blood sampling is routinely performed 4–5 days after jugular vein surgery (Neumann et al. Citation1998a).
According to the literature (Hoff Citation2000), the average total volume of blood of an organism is 6–8% of its body weight, i.e. 1.5–2 ml in a 25-g mouse. To avoid serious alterations in cardiovascular functions and hemorrhage of the experimental mouse, not more than 10–15% of total blood volume or 1% of body weight should be collected at one time. Therefore, we chose to withdraw 100-μl blood samples from the catheterized mouse at a time, and immediately replace blood samples with sterile saline, as suggested before (MacLeod and Shapiro Citation1988; Hoff Citation2000). Alternate options include use of donor blood from mice of the same strain (Bardelmeijer et al. Citation2003) or serum from the same mouse (Mokhtarian et al. Citation1993), but this is labor and cost intensive, and interference with the donor's plasma hormones cannot be avoided. This may provide an additional source of (donor's) hormones as discussed above.
It is important to mention that anxiety-related behavior on the EPM did not differ between catheterized mice 4 days after surgery and 3 days after repeated blood sampling and mice tested without surgery and blood sampling (). However, long-term catheterization and prior blood sampling reduced locomotion (); therefore, we would not recommend the use of catheterized mice for detailed behavioral observation and/or assessment of subtle behavioral parameters 4 days after surgery and blood sampling.
In conclusion, repeated or chronic blood sampling is also possible in mice using a simplified technique of indwelling catheterization of the jugular vein. We are convinced that the routine application of catheterization is an advantageous and important technique in the field of endocrine research.
Acknowledgements
The authors are grateful to Andrea Havasi for technical assistance and to Dr Slattery for critically reading the manuscript. These studies were funded by the Bayerische Forschungsstiftung (KN) and DFG (IDN).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abel EL. 1994. A further analysis of physiological changes in rats in the forced swim test. Physiol Behav. 56:795–800.
- Atkinson HC, Leggett JD, Wood SA, Castrique ES, Kershaw YM, Lightman SL. 2010. Regulation of the hypothalamic-pituitary-adrenal axis circadian rhythm by endocannabinoids is sexually diergic. Endocrinology. 151:3720–3727.
- Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. 2006. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 55:390–397.
- Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP. 2010. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 3:525–534.
- Bardelmeijer HA, Buckle T, Ouwehand M, Beijnen JH, Schellens JH, van Tellingen O. 2003. Cannulation of the jugular vein in mice: a method for serial withdrawal of blood samples. Lab Anim. 37:181–187.
- Barr JE, Holmes DB, Ryan LJ, Sharpless SK. 1979. Techniques for the chronic cannulation of the jugular vein in mice. Pharmacol Biochem Behav. 11:115–118.
- Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. 2008. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun. 22:105–113.
- Doeing DC, Borowicz JL, Crockett ET. 2003. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin Pathol. 3:3.
- Douglas AJ, Brunton PJ, Bosch OJ, Russell JA, Neumann ID. 2003. Neuroendocrine responses to stress in mice: hyporesponsiveness in pregnancy and parturition. Endocrinology. 144:5268–5276.
- Heinzmann JM, Thoeringer CK, Knapman A, Palme R, Holsboer F, Uhr M, Landgraf R, Touma C. 2010. Intrahippocampal corticosterone response in mice selectively bred for extremes in stress reactivity: a microdialysis study. J Neuroendocrinol. 22:1187–1197.
- Hoff J. 2000. Methods of blood collection in the mouse. Lab Anim. 29:47–53.
- Landgraf R, Wigger A, Holsboer F, Neumann ID. 1999. Hyper-reactive hypothalamo-pituitary-adrenocortical axis in rats bred for high anxiety-related behaviour. J Neuroendocrinol. 11:405–407.
- MacLeod JN, Shapiro BH. 1988. Repetitive blood sampling in unrestrained and unstressed mice using a chronic indwelling right atrial catheterization apparatus. Lab Anim Sci. 38:603–608.
- Mokhtarian A, Meile MJ, Even PC. 1993. Chronic vascular catheterization in the mouse. Physiol Behav. 54:895–898.
- Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol. 1998b; 508 Pt 1: 289–300.
- Neumann ID, Kromer SA, Toschi N, Ebner K. 2000. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. 96:31–38.
- Neumann I, Ludwig M, Engelmann M, Pittman QJ, Landgraf R. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993a; 58:637–645.
- Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993b; 53:65–75.
- Neumann ID, Wigger A, Liebsch G, Holsboer F, Landgraf R. Increased basal activity of the hypothalamo-pituitary-adrenal axis during pregnancy in rats bred for high anxiety-related behaviour. Psychoneuroendocrinology. 1998a; 23:449–463.
- Niswender KD, Shiota M, Postic C, Cherrington AD, Magnuson MA. 1997. Effects of increased glucokinase gene copy number on glucose homeostasis and hepatic glucose metabolism. J Biol Chem. 272:22570–22575.
- Pellow S, Chopin P, File SE, Briley M. 1985. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 14:149–167.
- Pryce CR, Siegl S, Mayer R, Rahmanzadeh G, McAllister KH. 2010. Endocrine and behavioural responses to acute central CRF challenge are antagonized in the periphery and CNS, respectively, in C57BL/6 mice. Neuropharmacology. 60:318–327.
- Reber SO, Birkeneder L, Veenema AH, Obermeier F, Falk W, Straub RH, Neumann ID. 2007. Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: implications and mechanisms. Endocrinology. 148:670–682.
- Rogers IT, Holder DJ, McPherson HE, Acker WR, Brown EG, Washington MV, Motzel SL, Klein HJ. 1999. Influence of blood collection sites on plasma glucose and insulin concentration in conscious C57BL/6 mice. Contemp Top Lab Anim Sci. 38:25–28.
- Russell JA, Neumann I, Landgraf R. 1992. Oxytocin and vasopressin release in discrete brain areas after naloxone in morphine-tolerant and -dependent anesthetized rats: push–pull perfusion study. J Neurosci. 12:1024–1032.
- Singewald GM, Nguyen NK, Neumann ID, Singewald N, Reber SO. 2009. Effect of chronic psychosocial stress-induced by subordinate colony (CSC) housing on brain neuronal activity patterns in mice. Stress. 12:58–69.
- Theodosis DT, Schachner M, Neumann ID. 2004. Oxytocin neuron activation in NCAM-deficient mice: anatomical and functional consequences. Eur J Neurosci. 20:3270–3280.
- Windle RJ, Brady MM, Kunanandam T, Da Costa AP, Wilson BC, Harbuz M, Lightman SL, Ingram CD. 1997. Reduced response of the hypothalamo-pituitary-adrenal axis to alpha1-agonist stimulation during lactation. Endocrinology. 138:3741–3748.