Abstract
Although there is well-documented evidence for hyperactivity of hypothalamic–pituitary–adrenal (HPA) axis function in anorexia nervosa (AN), there has been little research into secretory patterns of salivary cortisol and dehydroepiandrosterone (DHEA) in this condition. The cortisol awakening response (CAR), a prominent and discrete feature of the cortisol cycle, has not been extensively explored in adolescent AN. Saliva samples were collected at awakening, 30 min and 12 h post-awakening on two consecutive weekdays from eight female adolescents with clinically diagnosed AN and 41 healthy control (HC) age-matched females. Adolescent AN patients had greater salivary cortisol and DHEA concentrations than HC girls at all points. Increased hormone secretion was unrelated to body mass index. However, despite hypersecretion of both hormones, the circadian pattern including the CAR paralleled that of the HC group. Findings from this preliminary study confirm dysregulation of HPA axis function in adolescent AN as evidenced by hypersecretion of both cortisol and DHEA, which share the common secretagogue adrenocorticotropic hormone. However, the parallel diurnal profiles for AN and HC participants, including the CAR, may indicate hypersecretion per se rather than differential regulation of the diurnal pattern of these two adrenal steroids in AN.
Introduction
Anorexia nervosa (AN) is a serious and complex condition reported to affect 0.5% of Western young women (Hsu Citation1996). Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis is associated with the AN condition (Connan et al. Citation2003). Although this may be a consequence of starvation (Södersten et al. Citation2008), recent evidence suggests that higher levels of cortisol (unlike leptin) are associated with disordered eating psychopathology in women across the weight spectrum, independent of body mass index (BMI; Lawson et al. Citation2011). There is agreement that cerebrospinal fluid corticotropin-releasing hormone (CRH), plasma cortisol and dehydroepiandrosterone (DHEA) are hypersecreted in AN, and HPA axis hyperactivity is thought to arise from an increase in the number of secretory bursts (Misra et al. Citation2004) and dysregulation of feedback inhibition at the level of the hypothalamus or at higher centres (Connan et al. Citation2003). To date, there are no published data to support the concept of HPA overdrive in adolescent AN, as investigated by salivary cortisol and DHEA. We report here the first such study monitoring both adrenal steroids in terms of the patterns of their circadian profiles. The diurnal profile of salivary cortisol has recently been examined in adult female outpatients with AN (Monteleone et al. 2011b), however it remains unknown whether hypersecretion of these adrenal steroids is accompanied by aberrant patterns of secretion, for example flat diurnal cycles, in adolescents.
We explored basal HPA axis secretory activity by measuring salivary cortisol and DHEA in parallel samples across the diurnal cycle. The circadian pattern of cortisol secretion (unlike DHEA) is controlled by the paraventricular nucleus and the HPA axis and secondarily via direct sympathetic innervation to the zona fasciculata of the adrenal gland by adrenergic sympathetic pathways (Edwards and Jones Citation1993; Ehrhart-Bornstein et al. Citation1998; Sage et al. Citation2002; Engeland and Arnhold Citation2005; Ulrich-Lai et al. Citation2006). DHEA is secreted from the zona reticularis, which lacks direct sympathetic innervation (Charlton et al. Citation1992; Ehrhart-Bornstein et al. Citation1998). This has been offered as an explanation as to why there is no DHEA awakening response comparable to the cortisol awakening response (CAR; Hucklebridge et al. Citation2005; Clow et al. Citation2010; CitationOskis et al., in press).
Dysregulation of circadian patterns, as indicated by aberrant CARs and flattening of the cortisol diurnal decline, has been linked with chronic stress, major depressive disorder and a range of health conditions in adults (Meerlo et al. Citation2002; Fries et al. Citation2009), as well as in children and adolescents (Alink et al. Citation2008; Shirtcliff and Essex Citation2008; Turner-Cobb et al. Citation2008). Hence, clarification of the nature of HPA axis dysfunction in the early stages of adolescent AN is needed. Furthermore, a recent study reports a dissociation between HPA axis dysfunction and sympathetic nervous system activity in response to acute stress in AN (Monteleone et al. Citation2011a), underscoring the need to clearly define the nature of HPA axis dysfunction in this disorder.
There is a clear rationale for exploring cortisol secretion in AN using saliva samples, as hypersecretion of the hormone beyond the point of saturation of glucocorticoid-binding protein (which can occur in AN) can lead to underestimation of physiologically active levels of the hormone in blood (Dos Santos et al. Citation2007). However, there has been very limited investigation of salivary cortisol in this clinical population. Three studies have reported hypersecretion of cortisol but present conflicting evidence about whether the circadian pattern of HPA activity is preserved (Putignano et al. Citation2001; Schule et al. Citation2006; Dos Santos et al. Citation2007). To date, there has been only one investigation of the CAR in AN, which reported an elevated awakening response in women with the disorder (Monteleone et al. Citation2011b). Adolescence is the peak time of onset for AN (Kessler et al. Citation2005); however, there has been no similar investigation of adolescents with AN, despite the CAR being associated with interpersonal attachment (Luijk et al. Citation2010; Oskis et al. Citation2011), which is considered a risk factor for adolescent AN (Connan et al. Citation2003). DHEA, as measured in saliva, has received little attention in relation to AN. The rationale for parallel measurement of DHEA is that it enables interpretation of aberrant patterns of the CAR, i.e. whether dysregulation in AN affects both cortisol and DHEA (reflecting overall steroidogenic capacity) or cortisol alone (CAR-specific mechanisms).
Adolescence is an important developmental phase for vulnerability to HPA dysregulation and onset of AN (Connan et al. Citation2003). The current study set out to examine salivary cortisol and DHEA in medication-free, inpatient adolescent girls with early-onset AN but no co-morbid psychopathology. The comparator healthy participant data were derived from a parallel larger study investigating HPA axis activity in healthy females (Oskis et al. Citation2009). Our hypotheses were that AN would be characterised by HPA axis dysfunction as indicated by a combination of hypersecretion and aberrant circadian patterns of both hormones, and that these differences would be independent of BMI.
Materials and methods
Participants
Eight female adolescents, mean ( ± SD) age 15.13 ( ± 1.64) years, with AN (restrictive subtype) were recruited from a specialist residential clinic. Upon admission, patients received a clinical assessment and diagnosis by the consultant psychiatrist. All had experienced first menses, but were currently amenorrhoeaic, exhibited no co-morbid psychopathology or any additional acute or chronic illness. None was taking anti-depressants or any other prescribed medication. All had early-onset AN; mean age at first diagnosis was at 12.75 ( ± 1.49) years. The mean duration of condition was 2.38 ( ± 1.06) years and at the time of taking part in the study, the mean length of stay in the clinic was 4.63 ( ± 1.06) months. As such, some individuals were not in the acute phase of the disorder. Participants were all undergoing medium-stay programmes at the clinic, whereby the young person is admitted at a very low weight and the expected outcome is for the individual to reach a healthy weight and demonstrate the capacity to maintain this prior to transferring to outpatient treatment. The duration of a medium stay is usually between 3 and 9 months. Scheduled discharge dates for participants were 1–3 months from time of participation.
Forty-one healthy females, mean ( ± SD) age 15.46 ( ± 1.53) years, were recruited as a matched healthy control (HC) group. All were post-menarche, non-smokers, without acute or chronic illness or taking prescribed medication (including the oral contraceptive). This group had no history of psychopathology.
In terms of the entire sample, 90% were White British. Furthermore, 70% of the participants resided with both parents and all participants had at least one parent who was in employment. All participants provided informed written consent (individually or by parent/guardian if under 16 years). Ethical approval for the study was obtained from the University of Westminster and the Barnet, Enfield and Haringey Local Research Ethics Committees.
Protocol and procedure
Participants received a study pack, which included the participant information sheet, consent form, full written instructions, saliva sampling materials and the Eating Disorder Inventory (EDI; Garner Citation2004). The EDI-3 is a widely used self-report measure of traits and symptom clusters clinically relevant to eating disorders and contains 91 items that form eating disorder risk and psychological subscales.
Recruitment of AN participants was initiated via the clinical team at the centre, and patients were informed about the study via a group presentation by the lead researcher (AO). Upon informed written consent, appropriate individual meeting times to brief each participant about the study procedures were arranged and each was given a study pack. Recruitment of AN participants was challenging and persisted for a year: from a potential pool of 20 participants a total of 8 volunteered. The HC group was mainly recruited by approaching local schools (see Oskis et al. Citation2009 for further details). Recruitment and data collection were conducted by the same researcher (AO: white female aged 23–24 years), except for BMI measurement in the AN group, which was determined by the clinical team on a twice-weekly basis as part of their treatment regime. For the HC group, height and weight were measured by the researcher; equipment was calibrated with that of the clinic and was constant throughout.
Participants collected saliva samples on awakening, 30 min and 12 h post-awakening on two consecutive weekdays. Saliva was collected by passive drooling into an Eppendorf tube. For 30 min prior to the collection of each sample, participants were requested to take nothing by mouth other than water, avoid vigorous exercise and brushing teeth. Participants froze samples on the same day of collection, and insulated packs were used to transfer samples to the laboratory where they were stored at − 20°C until assay. On each study day, participants recorded their awakening time and the exact times of collection of saliva samples.
To maximise adherence to protocol within the HC group, personalised text message reminders were sent to prompt each saliva sample collection (for details, see Oskis et al. Citation2009). For the AN group, the timing of all samples was monitored by the members of the clinical team, as were the other behavioural requirements.
Salivary assays
Samples were thawed and centrifuged at 1500 g (3000 rpm) for 15 min. Cortisol concentration was determined by the expanded range high sensitivity enzyme linked immunosorbent assay from Salimetrics Europe Ltd., Suffolk, UK (USA). Similarly, the Salimetrics Salivary DHEA Enzyme Immunoassay was used to measure DHEA concentrations. Given that freeze–thaw cycles for DHEA are recommended to be kept to a minimum (Schwartz et al. Citation2005), the DHEA assay was carried out first.
The standard range for the cortisol assay was 0.0828–82.80 nmol/l, and the correlation of this assay with serum cortisol (n = 49) was r = 0.91, p < 0.0001. For the DHEA assay, the standard range was 0.03–3.47 nmol/l and the correlation of the assay with serum DHEA (n = 39) was r = 0.857, p < 0.0001. For both cortisol and DHEA, intra- and inter-assay variations were below 5% and 10%, respectively.
Statistical analysis
Cortisol and DHEA concentrations were moderately positively skewed for both groups. A square-root transformation was used to reduce skewness. Mean concentrations represented in figures are original values. Levene's test indicated equality of variance between the groups for each sampling point.
Tests of difference were used to examine differences in demographic, situational and EDI-3 scores between AN and HC groups. Chi-square analyses were used to investigate differences in the qualitative classifications of the EDI between AN and HC groups, i.e. whether responses were ‘low clinical’, ‘typical clinical’ or ‘elevated clinical’. Hormone data comprised three measures on each day: 0, 30 min and 12 h post-awakening. Mixed analyses of variance (ANOVAs) were used to examine hormone secretion, with factors ‘sampling day’ and ‘sampling time’. The between-subjects factor was ‘group’. Pearson's tests of correlation were used to assess relationships between hormone concentrations and EDI-3 scores. Given the difference in BMI between groups, and findings linking BMI and DHEA secretion (e.g. Matchock et al. Citation2007), BMI was partialled in all correlational analysis and included as a covariate in the ANOVAs. As BMI was only related to DHEA data, all results for DHEA are reported following adjustment for BMI. As an additional check on the relationship between BMI and hormone levels, the AN group was also compared to a subgroup of HC participants (n = 8) with equivalent BMIs, using ANOVAs, as outlined above.
Results
Demographic, situational and EDI-3 characteristics of the AN and HC groups are shown in . As expected, the AN group exhibited a lower BMI (range 17.10–20.00 kg/m2) than the HC participants and significantly higher scores on most EDI-3 scales, except bulimia (confirming their status as the restrictive subtype of AN). In terms of qualitative classifications of the EDI, significantly more of the HC group was classified as having a low clinical drive for thinness (χ2 = 19.576, p < 0.0005), body dissatisfaction (χ2 = 35.958, p = 0.0005), eating disorder risk (χ2 = 18.743, p < 0.0005) and general psychological maladjustment (χ2 = 20.045, p < 0.0005).
Table I. Demographic, situational and EDI-3 characteristics for each group (mean ± SD).
The ANOVA on cortisol data revealed a main effect of sampling time (F(1.6,74.9) = 95.616, p < 0.0005, partial η2 = 0.670). The awakening response, followed by declining levels, was observed in both groups (see ). There was a main effect of group (F(1,47) = 15.413, p < 0.0005, partial η2 = 0.247). Post hoc analysis with Bonferroni correction revealed that participants with AN had higher cortisol concentrations at all sampling points (all p's < 0.017). There was no main effect of day (F(1,47) = 0.494, p = 0.486, partial η2 < 0.010) and no interactions between the factors, including no sample by group interaction, which suggested that there was no group difference in the magnitude of increase in cortisol levels following awakening (the CAR) or the diurnal decline (F(1.6,74.9) = 0.140, p = 0.869, partial η2 = 0.003). Including BMI as a covariate in these analyses did not affect any of the reported results.
Figure 1. (a) Mean ( ± SEM) salivary cortisol and (b) DHEA concentrations (nmol/l) over the two sampling days for AN (n = 8) and HC (n = 41) groups (*p < 0.0005).
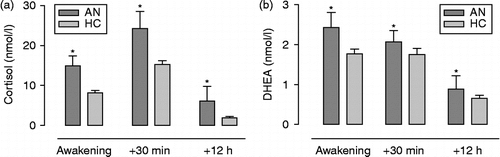
DHEA concentrations revealed a main effect of sampling time (F(2,92) = 52.234, p < 0.0005, partial η2 = 0.526), and a significant linear contrast confirmed that levels declined from awakening to 12 h post-awakening. There was a main effect of group on hormone concentrations, indicating that those with AN had greater levels of DHEA over the diurnal period (F(1,46) = 4.236, p = 0.045, partial η2 = 0.084). Post hoc tests showed the AN group to have higher DHEA concentrations at all sampling points (all p's < 0.05). No main effect of day was present (F(1,46) = 0.024, p = 0.878, partial η2 = 0.001). Also, there were no interactions between any of the factors, suggesting no difference between AN and HC groups in the fall of DHEA levels from morning to evening (). The same results were obtained following inclusion of BMI as a covariate in the analyses.
As an additional check on the influence of BMI on hormone levels, cortisol and DHEA secretion were compared between the AN group and eight HC girls with a mean BMI of 18.40 ( ± 0.68), which was equivalent to the AN group (see ). The same results as above were found, with a main effect of group for both cortisol (F(1,14) = 6.204, p = 0.026, partial η2 = 0.307) and DHEA (F(1,14) = 6.620, p = 0.022, partial η2 = 0.321).
It is interesting to note that within the HC group, mean evening cortisol concentrations were positively associated with drive for thinness (r = 0.245, p = 0.050) and the eating disorder risk composite score (r = 0.350, p = 0.020). Again, this finding was independent of BMI. No other hormone measure was associated with any EDI-3 scales within the healthy participants; r values ranged from 0.007 to 0.219 and all p values were >0.05.
Discussion
Adolescent patients with AN showed evidence of HPA axis dysfunction as indexed by hypersecretion of salivary cortisol and DHEA in the morning and evening. However, despite dysregulation of HPA axis function there were no differences between groups in either the CAR or diurnal decline in circulating hormone, which are dynamic markers of circadian HPA function. Thus, the patients presented with higher but parallel patterns of cortisol and DHEA secretion, indicating that the disorder may be associated with hypersecretion of the common secretogogue adrenocorticotropic hormone (ACTH), rather than aberrance in zona fasciculata adrenocortical innervation (Edwards and Jones Citation1993; Ehrhart-Bornstein et al. Citation1998; Sage et al. Citation2002; Engeland and Arnhold Citation2005; Ulrich-Lai et al. Citation2006).
Our findings are in agreement with the recent study of adult female outpatients with AN by Monteleone et al. (Citation2011b). Their sample exhibited an enhanced salivary CAR compared to the control group, including significantly higher awakening hormone levels. However, the clinical sample of Monteleone et al. displayed a different CAR pattern compared to our AN group: cortisol secretion in their women with AN peaked at 15 min post-awakening and then rapidly decreased, whereas our AN sample showed a rise in cortisol until 30 min after awakening. Taken together, these findings suggest that there may be differences in CAR patterns between adults and adolescents with AN.
Our results regarding increased HPA axis activity in adolescent AN are also consistent with a large body of literature showing hyperactivity of the HPA axis in this condition (Misra et al. Citation2004; Lo Sauro et al. Citation2008) associated with an increased number of secretory bursts (Misra et al. Citation2004). In healthy participants, dexamethasone inhibits ACTH (and downstream hormone) secretion. Reduced or absent dexamethasone suppression is reported in 90% of AN patients (Kling et al. Citation1993; Licinio Citation1995; Monteleone et al. Citation2006). The evidence of hypersecretion presented here is also consistent with less HPA feedback sensitivity in AN, as suggested by Connan et al. (Citation2003). However, the cortisol and DHEA diurnal patterns were preserved and parallel to those of the HCs. These are novel findings that deserve further investigation as they may suggest a difference between AN and other psychopathologies associated with dysregulation of neuroendocrine function as indexed by flattened circadian cycles, e.g. for adolescent mental health symptoms and adult major depressive disorder (Stetler and Miller Citation2005).
Activation of the HPA axis is not the sole determinant of the circadian pattern of cortisol secretion as indicated by the lack of a DHEA awakening response reported here and previously (Hucklebridge et al. Citation2005; CitationOskis et al. in press). These observations can be attributed to the fact that levels of pituitary-derived ACTH can be dissociated from the levels of cortisol (Kalsbeek et al. Citation2006; Ulrich-Lai et al. Citation2006). Changes in adrenal sensitivity to ACTH (both increases and decreases) are linked with time of day and the Suprachiasmatic nucleus (SCN) (Buijs et al. Citation1997; Buijs et al. Citation2003; Bornstein et al. Citation2008), and are mediated via sympathetic innervation of the adrenal zona fasciculata (Edwards and Jones Citation1993; Ehrhart-Bornstein et al. Citation1998; Sage et al. Citation2002; Engeland and Arnhold Citation2005; Ulrich-Lai et al. Citation2006). The data presented here suggest HPA dysfunction, primarily the pituitary drive, rather than any extra-pituitary mediation. Notably, eating disorders are associated with autonomic dysfunction associated with hypervagal activity (Green et al. Citation2009), which may not impact upon autonomic input to circadian rhythmicity due to the lack of vagal input to the adrenal cortex (Ehrhart-Bornstein et al. Citation1998).
The preserved dynamic of the CAR, in combination with hypersecretion of cortisol, is noteworthy, in the light of the extensive literature associating aberrant patterns of the dynamic of the CAR with a wide range of psychopathologies and with risk factors for eating disorders (Stetler and Miller Citation2005; Therrien et al. Citation2008; Fries et al. Citation2009; Luijk et al. Citation2010; Oskis et al. Citation2011). A replication of this study, including measures of sympathetic nervous system activity as well as ACTH, is warranted to establish the robustness of the findings and the legitimacy of the interpretation reported here. However, these data resonate with the findings of a positive association between trait markers of sympathetic nervous system status and the dynamic of the CAR, but not with levels of post-awakening cortisol secretion (Stalder et al. Citation2011).
A significant positive correlation between higher evening cortisol levels and eating disorder risk was observed in the HC group. A cortisol rhythm that remains elevated in the evening compared to the HCs is deemed to be the most robust cross-sectional correlate of psychopathology in adolescents (Adam et al. Citation2008). This may constitute an early phase in the dysregulation of the HPA axis in AN. Indeed, high basal levels of cortisol during adolescence are associated with hyperarousal and a higher biological sensitivity to context; it has been proposed that these individuals may be specifically vulnerable to mental health problems during periods of high stress (Shirtcliff and Essex Citation2008). It would be interesting to follow up this finding with longitudinal studies to explore in more detail the relationship between the development of signs of HPA axis abnormalities during adolescence, mental health problems generally and the onset of AN in particular.
In addition, although the results reported here were all independent of BMI, it is also necessary to study patients after recovery and restoration of normal BMI to fully determine the impact of starvation on neuroendocrine function in this population. Our findings are consistent with those of Lawson et al. (Citation2011) who found that higher levels of cortisol are associated with disordered eating psychopathology independent of BMI. However, it is noteworthy that the methodology of Lawson et al. (Citation2011) differed from that of the current study, and involved overnight serum samples that were pooled for average cortisol levels. An early study of plasma cortisol by Gold et al. (Citation1986) reported hypercortisolism as well as an attenuated release of ACTH to CRH in anorexic patients, both of which return to normal after long-term weight restoration of at least 6 months. These authors interpret this finding as an indication of intact feedback at the pituitary that is overridden by an increased ACTH drive. Another study reported that administration of a glucocorticoid antagonist to anorexic individuals led to an increase in both ACTH and cortisol (Kling et al. Citation1993), which again suggests an increase in the set point for ACTH release.
Despite the use of different methodologies, our findings are consistent with the idea of adrenal hyper-responsiveness to ACTH in anorexia, since levels of both salivary cortisol and DHEA were higher compared to the control group. Although (unlike Gold et al. Citation1986) the mean BMI of our AN group indicates that they were not in the acute stage of the disorder at the time of study, their weight restoration had not persisted for at least 6 months, which may be why their profiles were aberrant. It would be interesting to study our sample 6 months after discharge from the clinic to see whether long-term weight restoration results in restored salivary cortisol and DHEA profiles. A recent study of salivary cortisol in adolescents with AN showed that the change in cortisol level before and after inpatient treatment correlated with increased body weight gain ratio just after treatment, but not with the ratio after 1 year (Shibuya et al. Citation2011).
The adolescent AN sample in this study is small; thus, the findings must be viewed tentatively and the results should be regarded as preliminary. Yet, a strength of the study is the careful attention paid to participant adherence to the saliva sampling protocol and exclusion of comorbid Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) disorders, as high rates of comorbid Axis I and II psychopathology exist among females with eating disorders (O'Brien and Vincent Citation2003). The findings suggest that neuroendocrine hypersecretion, independent of BMI, occurs in adolescent females with early-onset AN but points to preservation of the extra-pituitary sympathetic nervous system's fine-tuning of the circadian pattern of cortisol secretion, in contrast to the findings of Monteleone et al. (Citation2011a) which suggest a dissociation in peripheral regulation of adrenocortical regulation in relation to an acute stress challenge. These data lay the foundation for future investigations regarding different aspects of neuroendocrine secretory activity in AN and risk of AN, especially via longitudinal study designs.
Acknowledgements
The authors would like to thank David Wood and Komal Parekh of the Ellern Mede Centre for Eating Disorders for their assistance with recruitment of participants with AN.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adam EK, Sutton JM, Doane LD, Mineka S. 2008. Incorporating hypothalamic–pituitary–adrenal axis measures into preventive interventions for adolescent depression: Are we there yet?. Dev Psychopathol. 20 3: 975–1001.
- Alink LRA, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. 2008. Cortisol and externalizing behavior in children and adolescents: Mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Dev Psychobiol. 50:427–450.
- Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. 2008. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab. 19:175–180.
- Buijs RM, Wortel J, Van Heerikhuize JJ, Kalsbeek A. 1997. Novel environment induced inhibition of corticosterone secretion: Physiological evidence for a suprachiasmatic nucleus mediated neuronal hypothalamo-adrenal cortex pathway. Brain Res. 758:229–236.
- Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. 2003. The biological clock tunes the organs of the body: Timing by hormones and the autonomic nervous system. J Endocrinol. 177:17–26.
- Charlton BG, McGadey J, Russell D, Neal DE. 1992. Noradrenergic innervation of the human adrenal-cortex as revealed by dopamine-beta-hydroxylase immunohistochemistry. J Anat. 180:501–506.
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. 2010. The cortisol awakening response: More than a measure of HPA axis function. Neurosci Biobehav Rev. 35:97–103.
- Connan F, Campbell IC, Katzman M, Lightman SL, Treasure J. 2003. A neurodevelopmental model for anorexia nervosa. Physiol Behav. 79:13–24.
- Dos Santos E, Dos Santos JE, Ribeir RP, Silva A, Moreira AC, De Sa MFS. 2007. Absence of circadian salivary cortisol rhythm in women with anorexia nervosa. J Pediatr Adolesc Gynecol. 20:13–18.
- Edwards AV, Jones CT. 1993. Autonomic control of adrenal function. J Anat. 183:291–307.
- Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. 1998. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 19:101–143.
- Engeland WC, Arnhold MM. 2005. Neural circuitry in the regulation of adrenal corticosterone rhythmicity. Endocrine. 28:325–331.
- Fries E, Dettenborn L, Kirschbaum C. 2009. The cortisol awakening response (CAR): Facts and future directions. Int J Psychophysiol. 72:67–73.
- Garner DM. 2004. Eating Disorder Inventory-3 professional manual. Lutz, Florida: Psychological Assessment Resources, Inc.
- Gold PW, Gwirtsman H, Avgerinos PC, Nieman LK, Gallucci WD, Kaye W, Jimerson D, Ebert M, Rittmaster R, Loriaux DL, Chrousos GP. 1986. Abnormal hypothalamic–pituitary–adrenal function in anorexia nervosa. Pathophysiologic mechanisms in underweight and weight-corrected patients. N Engl J Med. 314:1335–1342.
- Green MA, Hallengren JJ, Davids CM, Riopel CM, Skaggs AK. 2009. An association between eating disorder behaviors and autonomic dysfunction in a nonclinical population. A pilot study. Appetite. 53:139–142.
- Hsu LKG. 1996. Epidemiology of the eating disorders. Psychiatr Clin N Am. 19:681–700.
- Hucklebridge F, Hussain T, Evans P, Clow A. 2005. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology. 30:51–57.
- Kalsbeek A, Palm IF, La Fleur SE, Scheer F, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. 2006. SCN outputs and the hypothalamic balance of life. J Biol Rhythm. 21:458–469.
- Kessler RC, Berglund P, Demler O, Jin R, Walters EE. 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 62:593–602.
- Kling MA, Demitrack MA, Whitfield HJ, Kalogeras KT, Listwak SJ, Debellis MD, Chrousos GP, Gold PW, Brandt HA. 1993. Effects of the glucocorticoid antagonist ru-486 on pituitary-adrenal-function in patients with anorexia-nervosa and healthy-volunteers–enhancement of plasma ACTH and cortisol secretion in underweight patients. Neuroendocrinology. 57:1082–1091.
- Lawson EA, Eddy KT, Donoho D, Misra M, Miller KK, Meenaghan E, Lydecker J, Herzog D, Kibanski A. 2011. Appetite-regulating hormones cortisol and peptide YY are associated with disordered eating psychopathology, independent of body mass index. Eur J Endocrinol. 164:253–261.
- Licinio J. 1995. Anorexia nervosa: Directions for future research. Int J Eat Disord. 17:235–241.
- Lo Sauro C, Ravaldi C, Cabras PL, Faravelli C, Ricca V. 2008. Stress, hypothalamic–pituitary–adrenal axis and eating disorders. Neuropsychobiology. 57:95–115.
- Luijk MPCM, Saridjan N, Tharner A, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Jaddoe VWV, Hofman A, Verhulst FC, Tiemeier H. 2010. Attachment, depression, and cortisol: Deviant patterns in insecure-resistant and disorganized infants. Dev Psychobiol. 52:441–452.
- Matchock RL, Dorn LD, Susman EJ. 2007. Diurnal and seasonal cortisol, testosterone and DHEA rhythms in boys and girls during puberty. Chronobiol Int. 24:969–990.
- Meerlo P, Sgoifo A, Turek FW. 2002. The effects of social defeat and other stressors on the expression of circadian rhythms. Stress. 5:15–22.
- Misra M, Miller KK, Almazan C, Ramaswamy K, Lapcharoensap W, Worley M, Neubauer G, Herzog DB, Klibanski A. 2004. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 89:4972–4980.
- Monteleone P, Luisi M, Martiadis V, Serritella C, Longobardi N, Casarosa E, Genazzani AR, Maj M. 2006. Impaired reduction of enhanced levels of dehydroepiandrosterone by oral dexamethasone in anorexia nervosa. Psychoneuroendocrinology. 31:537–542.
- Monteleone P, Scognamiglio P, Canestrelli B, Serino I, Monteleone AM, Maj M. Asymmetry of salivary cortisol and α-amylase responses to psychosocial stress in anorexia nervosa but not in bulimia nervosa. Psychol Med. 2011a; 2:1–7.
- Monteleone P, Scognamiglio P, Monteleone AM, Mastromo D, Steardo L, Serino I, Maj M. Abnormal diurnal patterns of salivary α-amylase and cortisol secretion in acute patients with anorexia nervosa. World J Biol Psychiatry. 2011b; 12:455–461.
- O'Brien KM, Vincent NK. 2003. Psychiatric comorbidity in anorexia and bulimia nervosa: Nature, prevalence, and causal relationships. Clin Psychol Rev. 23:57–74.
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A. 2009. Diurnal patterns of salivary cortisol across the adolescent period in healthy females. Psychoneuroendocrinology. 34:307–316.
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A. 2011. Anxious attachment style and cortisol dysregulation in healthy female children and adolescents. J Child Psychol Psychiatry. 52:111–118.
- Oskis A, Clow A, Loveday C, Thorn L, Hucklebridge F. 2012. Differences between the diurnal patterns of cortisol and DHEA in healthy female adolescents. Stress. 15:110–114.
- Putignano P, Dubini A, Toja P, Invitti C, Bonfanti S, Redaelli G, Zappulli D, Cavagnini F. 2001. Salivary cortisol measurement in normal-weight, obese and anorexic women: Comparison with plasma cortisol. Eur J Endocrinol. 145:165–171.
- Sage D, Maurel D, Bosler O. 2002. Corticosterone-dependent driving influence of the suprachiasmatic nucleus on adrenal sensitivity to ACTH. Am J Physiol Endocrinol Metab. 282:E458–E465.
- Schule C, Sighart C, Hennig J, Laakmann G. 2006. Mirtazapine inhibits salivary cortisol concentrations in anorexia nervosa. Prog Neuropsychopharmacol. 30:1015–1019.
- Schwartz EB, Eppihimer L, Nelson VJ, Granger DA. 2005. Effects of sample storage temperature on the measurement of salivary analytes: Degradation of testosterone, estradiol and progesterone. Clin Chem. 51:A48–A49.
- Shibuya I, Nagamitsu S, Okamura H, Komatsu H, Ozono S, Yamashita Y, Matsuishi T. 2011. Changes in salivary cortisol levels as a prognostic predictor in children with anorexia nervosa. Int J Psychophysiol. 82:196–201.
- Shirtcliff EA, Essex MJ. 2008. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev Psychobiol. 50:690–703.
- Södersten P, Nergårdh R, Bergh C, Zandian M, Scheurink A. 2008. Behavioral neuroendocrinology and treatment of anorexia nervosa. Front Neuroendocrinol. 29:445–462.
- Stalder T, Evans P, Hucklebridge F, Clow A. 2011. Associations between the cortisol awakening response and heart rate variability. Psychoneuroendocrinology. 36:454–462.
- Stetler C, Miller GE. 2005. Blunted cortisol response to awakening in mild to moderate depression: Regulatory influences of sleep patterns and social contacts. J Abnorm Psychol. 114:697–705.
- Therrien F, Drapeau V, Lupien SJ, Beaulieu S, Doré J, Tremblay A, Richard D. 2008. Awakening cortisol response in relation to psychosocial profiles and eating behaviors. Physiol Behav. 93:282–288.
- Turner-Cobb JM, Rixon L, Jessop DS. 2008. A prospective study of diurnal cortisol responses to the social experience of school transition in four-year-old children: Anticipation, exposure, and adaptation. Dev Psychobiol. 50:377–389.
- Ulrich-Lai YM, Arnhold MM, Engeland WC. 2006. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol. 290:R1128–R1135.