Abstract
Evidence for a detrimental impact of chronic work stress on health has accumulated in epidemiological research. Recent studies indicate altered hypothalamus–pituitary–adrenal (HPA) axis regulation as a possible biological pathway underlying the link between stress and disease. However, the direction of dysregulation remains unclear, with reported HPA hyper- or hyporeactivity. To disentangle potential effects on different functional levels in the HPA axis, we examined responses using two pharmacological stimulation tests in 53 healthy teachers (31 females, 22 males; mean age: 49.3 years; age range: 30–64 years): a low-dose adrenocorticotrophic hormone (ACTH1–24, Synacthen) test was used to assess adrenal cortex sensitivity and the combined dexamethasone–corticotropin releasing hormone (DEX–CRH) test to examine pituitary and adrenal cortex reactivity. Blood and saliva samples were collected at − 1,+15,+30,+45,+60,+90,+120 min. Emotional exhaustion (EE), the core dimension of burnout, was measured with the Maslach Burnout Inventory. Overcommitment (OC) was assessed according to Siegrist's effort–reward–imbalance model. We found a significant association between EE and higher plasma cortisol profiles after Synacthen (p = 0.045). By contrast, OC was significantly associated with attenuated ACTH (p = 0.045), plasma cortisol (p = 0.005), and salivary cortisol (p = 0.023) concentrations following DEX–CRH. Results support the notion of altered HPA axis regulation in chronically work-stressed teachers, with differential patterns of hyper- and hyporeactivity depending on individual stress condition and the tested functional level of the HPA axis.
Introduction
During the last decade, an increasing number of studies focused on the impact of adverse psychosocial working conditions on health outcomes (Grzywacz and Dooley Citation2003; Butterworth et al. Citation2011). To date, evidence from epidemiological and prospective studies has been accumulated showing that chronic stress at work is a relevant risk factor for the development and progression of manifest disease (Bosma et al. Citation1998; Stansfeld et al. Citation1999; Kivimaki et al. Citation2002; Kumari et al. Citation2004; Siegrist Citation2005). In line with this evidence, recent psychoneuroendocrine research suggests that chronic work stress according to the effort–reward–imbalance/overcommitment (ERI/OC) model and work-related exhaustion manifest in measures of physiological wear and tear, termed allostatic load (Bellingrath et al. Citation2009). In particular, ERI/OC and emotional exhaustion (EE) might lead to alterations in the regulation of the hypothalamus–pituitary–adrenal (HPA) axis, the main endocrine stress responsive system (Chrousos and Gold Citation1992); however, so far results on chronic stress are inconsistent regarding the direction of dysregulation. Generally, HPA axis dysfunction can manifest in hyper- or hypo-(re)activity and considerable divergence exists regarding HPA axis regulation in chronically distressed individuals or patients suffering from stress-related diseases (Yehuda et al. Citation1993; Heim et al. Citation2000; Miller et al. Citation2007). As HPA axis dysregulation represents one potential biological pathway linking ERI/OC or work-related exhaustion to manifest disease (Tsigos and Chrousos Citation1994; McEwen Citation1998a), it is important to understand the psychobiological pathways and, in particular, the physiological sites of such alterations in more detail. Different functional levels of HPA axis regulation might be differentially affected by distinct work stress conditions.
OC, the intrinsic component of the ERI/OC work stress model, captures a permanent motivational or cognitive disposition to react with exaggerated efforts on work obligations (Siegrist Citation1996; Siegrist et al. Citation2004). Overcommitted individuals are prone to overwork, to exhaust their own resources and to show high ambition, high need for control and approval as well as low detachment from work. In the present study, we assessed OC according to the well-established ERI/OC model (Siegrist Citation1996). With this model, we also assessed the extrinsic component of work stress (ERI), capturing the dimensions of perceived efforts spent at work, perceived rewards received in return and the ERI ratio reflecting the amount of work stress experienced by the individual. EE is considered the core dimension or central quality of burnout, a non-psychiatric condition that refers to an individual's feelings of being overextended and of having overly exhausted emotional and physical resources (Maslach et al. Citation2001). To measure work-related EE, we applied the widely used Maslach Burnout Inventory (MBI; Maslach and Jackson Citation1986). The given constructs capture related, although different, aspects of the stress process. OC, which is conceptualized as enduring cognitive-motivational disposition or personality trait, is considered to be a stable psychological risk factor for chronic exposure to stressful experiences due to exaggerated intrinsic work motivation. By contrast, EE as the core component of burnout, and ERI, the extrinsic component of the ERI/OC model, reflect more state-dependent characteristics, as they capture current feelings or symptoms of distress resulting from the perception of work stress. In the present study, distinct constructs were assessed to allow for a broad characterization of individual stress conditions and a differentiated analysis of distinct stress facets on HPA axis regulation.
Available research methods from clinical endocrinology allow addressing different HPA axis functional and structural levels. However, there is still a paucity of studies that investigate the effects of different facets of work-related stress on the various levels of the HPA axis. In a recent experimental study on HPA axis stress reactivity (Bellingrath and Kudielka Citation2008), we applied the Trier Social Stress Test (TSST) to induce moderate psychosocial stress in a highly controlled laboratory setting. In that study, we observed hyporeactive HPA axis responses in individuals overly committed to work. Other studies so far investigated HPA axis feedback sensitivity in relation to burnout using the dexamethasone suppression test (DST). A dexamethasone-induced suppression of endogenous corticosteroid secretion is considered to reflect sensitivity of glucocorticoid receptors in mediating feedback inhibition of the HPA axis. To date, the DST is a standard tool in the assessment of HPA axis alterations, and so far some studies have used it to assess HPA axis functioning in relation to exhaustion due to chronic work stress. While some studies report stronger cortisol suppression in the DST in individuals scoring high in work-related exhaustion (Pruessner et al. Citation1999; Sonnenschein et al. Citation2007; Bellingrath et al. Citation2008), others failed to find this pattern (Mommersteeg et al. Citation2006). However, the DST has been criticized for its low sensitivity. Compared to the DST, a superior sensitivity in the detection of HPA axis alterations in psychiatric conditions has been demonstrated for the combined dexamethasone–corticotropin releasing hormone test (DEX–CRH test; Holsboer et al. Citation1987; Heuser et al. Citation1994). In this protocol, a CRH dose is administered to stimulate adrenocorticotrophic hormone (ACTH) and cortisol release after dexamethasone pre-treatment and to challenge the HPA stress response system after prior suppression. Thus, the paradigm is designed to test joint pituitary and adrenal cortex reactivity. Recently, Wirtz et al. (Citation2010) used the combined DEX–CRH test for the first time to assess potential alterations in HPA axis regulation in individuals with high OC levels. They reported an enhanced total plasma cortisol but not ACTH response to CRH after DEX premedication in individuals with high vs. low levels of OC. This pattern of results was interpreted as heightened reactivity of the adrenal cortex but normal reactivity of the anterior pituitary in overcommitted individuals. However, to the best of our knowledge no study has yet explored potential alterations of adrenal cortex sensitivity in overcommitted or chronically work-stressed individuals by applying the low-dose ACTH1–24 (Synacthen) test. In contrast to the DEX–CRH test, a Synacthen test is applied to trigger directly cortisol secretion from the adrenal cortex. Therefore with the application of a low dose of synthetic ACTH, the response sensitivity of the adrenal cortex can be tested pharmacologically.
Thus, in order to test simultaneously whether potential alterations at different functional levels of the HPA axis are differentially related to chronic work stress in terms of ERI/OC and EE in otherwise healthy individuals, we applied both pharmacological tests in the same study population.
A high risk for chronic work stress and exhaustion is often described for employees in so-called people-oriented occupations requiring high degrees of social interaction. Especially, the teaching profession has gained much attention due to increased rates of early retirements and stress-related disorders (Kyriacou and Sutcliffe Citation1978; Kyriacou and Pratt Citation1985; Weber et al. Citation2001; Weber et al. Citation2004). To capture a wide range of subjective stress levels due to workplace characteristics, we thus selected 53 otherwise healthy school teachers for the current study.
Generally, we expected that our chronic stress measures would be associated with altered HPA axis response patterns to Synacthen or DEX–CRH challenge. More specifically, in line with our previous findings on hyporeactive HPA axis responses to acute psychosocial stress in relation to OC (Bellingrath and Kudielka Citation2008), but at the same time in contrast to findings from the reported Wirtz et al. (Citation2010) study, we hypothesized that OC would be associated with an attenuated HPA axis reactivity if probed by pharmacological stimulation. With regard to the more momentary stress measures, we do not state a directed hypothesis regarding the direction of HPA axis dysregulation because of the obvious paucity of previous studies and inconsistencies in results of available studies.
Taken together, to the best of our knowledge this is the first study exploring whether chronic work stress in terms of ERI/OC and EE is differentially associated with HPA axis responses to a low-dose ACTH1–24 (Synacthen) challenge and combined DEX–CRH test in a sample of healthy school teachers.
Participants and methods
Participants
A sample of 53 currently employed healthy school teachers from the region of Bremen, Germany, was recruited via local newspaper announcements and personal visits to local schools. Before entering the study, all procedures were explained and described thoroughly, and written informed consent was obtained from all participants. An anamnestic medical interview and a brief physical examination were conducted to exclude subjects taking medication or suffering from chronic health problems or diseases potentially interfering with our tests. In particular, volunteers with psychiatric disorders, serious endocrine or heart diseases or intake of corticosteroid or psychotropic medication and persons in psychotherapeutic treatment were not eligible. Pregnant women and habitual smokers were excluded from study participation.
A detailed sample description is given in . Subjects were between 30 and 64 years of age with a normal body mass index (BMI). Twenty-two participants were male. Of the 31 female participants, 11 used oral contraceptives at the time of the study, 10 were in the postmenopausal phase, and 10 were tested during the luteal phase of their menstrual cycle.
Table I. Sample description.
The study protocol was approved by the ethics committee of the German Psychological Society (DGPs) as well as the ethics committee of the Bremen State Medical Association. The principles of the Helsinki Declaration were followed. Subjects received a monetary compensation of 12 Euros per hour for their participation in the study and an individualized feedback about the study results.
Psychological assessment
We applied the 17-item ERI (Siegrist Citation1996; Siegrist et al. Citation2004). The extrinsic component covers the two dimensions perceived efforts (six items on quantitative and qualitative work load as well as increase in work load over time, e.g. “I am often pressured to work overtime”) and experienced or anticipated rewards (11 items on aspects of job security and promotion as well as esteem rewards, e.g. “I receive the respect I deserve from my superiors”). Responders were asked to indicate on a five-point rating scale whether the given assertions apply to their present job situation and, if so, the degree of experienced distress caused by it. The total sum scores derived from each of these two subscales were then used to compute a ratio according to the procedure described by Siegrist et al. (Citation2004). OC, the intrinsic model component, was measured with the one-dimensional six-item scale of the ERI model (Siegrist et al. Citation2004). In this four-point Likert scale, responders indicate their agreement vs. disagreement with statements capturing individual efforts spent on work and inability to withdraw from obligations (e.g. “Work rarely lets me go, it is still on my mind when I go to bed”). The scale results in a single sum score ranging from 6 to 24 with higher scores indicating higher levels of OC. In the present sample, the internal consistency (Cronbach's alpha) was satisfactory for all scales (effort: 0.70, reward: 0.78, OC: 0.74).
Burnout was assessed by a validated German 22-item version (Schwarzer and Jerusalem Citation2001) of the widely applied MBI (Maslach and Jackson Citation1986). The frequency of burnout-related experiences or feelings is rated on a seven-point Likert scale ranging from zero = “never” to six = “daily.” Within three subscales, the domains of EE (nine items), depersonalization (five items), and lack of accomplishment (eight items) are covered. The subscale EE (e.g. “I feel emotionally drained from my work”) is considered the core dimension of burnout (Maslach et al. Citation2001). This subscale results into a sum score ranging from 0 to 54. The following internal consistencies (Cronbach's alpha) were observed in the present sample: 0.89 (EE), 0.78 (depersonalization), and 0.89 (lack of accomplishment).
Depressive symptoms were measured using the validated German version (Herrmann et al. Citation1995; Cronbach's alpha: 0.81) of the Hospital Anxiety and Depression Scale-Depression subscale (HADS-D; Zigmond and Snaith Citation1983), in which participants self-rated frequency and intensity of depressive symptoms by choosing one from four given response alternatives (seven items, e.g. “I still enjoy the things I used to enjoy”).
Experimental protocol
Participants reported twice to our laboratory on afternoons (appointments between 14:00 h and 16:00 h). On the first test day, the ACTH1–24 (Synacthen) sensitivity test was administered, followed by the DEX–CRH test on the second test day. On average, time elapsed between the two tests was 12.2 days ( ± 15.4 standard deviation, SD) with a range of 1–70 days due to individual schedules of subjects. Because of extremely fast elimination of Synacthen from blood (terminal phase reached after 3 h), no spill-over effects are expected for consecutive test days (note: in the present data, time elapsed between test days did not affect any results). On both test days, participants arrived at the laboratory having abstained from alcohol, caffeinated beverages, physical exercise, and a heavy lunch throughout the study day. The first test day involved a short health check by a medical doctor (cardiovascular and anthropometric measures; a chromatographic pregnancy test in premenopausal women). Then, an intravenous catheter was inserted either into the antecubital or the medial cubital vein and a first blood sample (2.7 ml) was taken for the analysis of corticosteroid binding globulin (CBG). Sixty minutes later, at time point − 1 min, a pre-test blood (9 ml) and saliva samples were collected, directly followed by an intravenous injection of a low dose (1 μg) of ACTH1–24 (Synacthen®, Novartis Pharma, Nürnberg, Germany). At time intervals of +15,+30,+45,+60,+90, and +120 min after Synacthen administration, consecutive blood (9 ml) and saliva samples were collected.
The night before the second test day, participants self-administered an oral dose of 1.5 mg dexamethasone (Fortecortin®, Merck, Darmstadt, Germany) at 23:00 h. On the test day, after insertion of the intravenous catheter, participants rested for 45 min. At time point − 1 min, pre-test blood and saliva samples were collected. Immediately afterwards, the HPA axis was challenged with an injection of 100 μg human CRH (Ferring, Kiel, Germany). Further blood (9 ml) and saliva samples were collected +15,+30,+45,+60,+90, and +120 min after hCRH administration.
Biochemical analyses
Venous blood was collected using Monovettes (Sarstedt, Nümbrecht, Germany) for determination of ACTH (Ethylendiaminotetraacetat (EDTA) Monovettes) and total plasma cortisol (EDTA Monovettes) as well as CBG (Serum Monovettes). Saliva samples were collected using Cortisol Salivettes (Sarstedt). Blood samples were immediately stored on ice and centrifuged at 2000 g and 4°C for 15 min in an adjacent room, and plasma or serum was pipetted into aliquots. Aliquots were then frozen at − 20°C until the end of the test day and in the evening at − 80°C until further analysis. Saliva samples were stored at − 20°C. Upon completion of the study, all samples were sent either to the Psychobiological Research Laboratory of the University of Trier, Germany (ACTH, total plasma, and free salivary cortisol) or to IBL International Hamburg, Germany (CBG) on dry ice to be assayed.
Free salivary cortisol was measured in duplicate using a time-resolved immunoassay with fluorometric detection (Delayed Fluorescence Immunoassay (DELFIA), intra-assay variation: 4.0–6.7%, inter-assay variation: 7.1–9.0%). The detection limit was 0.1 nmol/l. For the determination of total plasma cortisol and ACTH concentrations, samples were assayed in duplicate using a commercially available enzyme-linked immunosorbent assay kit (total plasma cortisol: intra-assay variation, 3.2–8.1%; inter-assay variation, 6.5–7.7%; detection limit, 0.1 ng/ml; ACTH: intra-assay variation, 3.1–4.2%; inter-assay variation, 5.8–6.2%). For the determination of CBG concentrations, blood samples from Serum Monovettes were assayed using a commercially available radioimmunoassay kit (intra-assay variation, 2.9–3.9%; inter-assay variation, 2.4–5.5%; detection limit, 0.26 μg/ml).
Statistical analyses
Statistical analyses were carried out using the SPSS statistical software package (IBM SPSS Statistics, Version 19; Chicago, IL, USA). Results are expressed as mean ± SD. For all general linear models (GLMs), F values, degrees of freedom, and p values were corrected according to Greenhouse–Geisser procedure whenever sphericity was violated. The significance level was set at p ≤ 0.05. Effect sizes were calculated by partial eta squared (), expressing the amount of variance explained in the dependent variable by the respective effect. Averages of duplicate values (see biochemical analysis) were used in statistical analyses. Rates below detection limit were set at the lowest detectable value. Cortisol and ACTH concentrations were log-transformed before statistical analysis (the figures present untransformed values for illustration reasons).
Test of potential covariates
At first, potentially occurring main or interaction effects of age, sex, BMI, and CBG concentrations on ACTH, total plasma cortisol, or free salivary cortisol concentrations were assessed in a set of ANOVAs for repeated measures for each stimulation procedure. In this way, we checked for any effects of potential covariates on hormonal responses in our sample including men, women taking hormonal contraception, postmenopausal women, and women in the luteal phase. As age did not yield any significant main or interaction effects for either ACTH or cortisol (all p = n.s.) and to avoid overfitting of our GLMs (Babyak Citation2004), this variable was not included as a covariate in the statistical models (see below). For sex, BMI, and CBG concentrations, significant effects on HPA axis parameters were found and they were thus included as covariates in all subsequent analyses. However, none of these covariates showed significant associations with questionnaire scores (all p = n.s.).
Main models
To analyze hormonal responses to stimulations, ANCOVAs with the repeated measure factor samples ( − 1,+15,+30,+45,+60,+90,+120 min after Synacthen or CRH injection) were run. In the next step, in separate GLMs for repeated measures, effects of the different work stress variables on HPA axis responses were analyzed entering the continuous score of the respective questionnaire. Questionnaire scores were entered into ANCOVAs as continuous stress variables to avoid any reduction of available information by artificial grouping (Aiken and West Citation1991; Royston et al. Citation2006) as applied earlier (Schlotz et al. Citation2004; Kudielka et al. Citation2006; Bellingrath et al. Citation2008).
Results
Sample characteristics with respect to psychological variables
Our sample displayed high, albeit rather heterogeneous levels of work-related stress, as indicated by ERI ratio (). A small ratio close to zero is interpreted as favorable whereas values above 0.72 (Lehr et al. Citation2009), as in our study (), have empirically been identified to indicate adverse working conditions. The OC sum score in our sample covered a broad range (). The EE score in our sample () can be regarded as average according to results from a normative community sample (Schaufeli and Van Dierendonck Citation1995); in our sample, 21 of 53 participants scored above 20 (), which corresponds to the highest tertile of the comparison sample. HADS-D scores (), measuring depressive symptoms, for 35 of our 53 participants (71.1%) were below 8, which is in approximate accordance with values from a normative German sample (Hinz and Brähler Citation2011). Three individuals with a score of 11 and one subject with a score of 12 just reached the cut-off of the HADS-D scale defined by values of 11–21. Other sample characteristics are detailed in .
ACTH1–24 (Synacthen) test
Initial ANCOVAs for repeated measures with the main factor samples confirmed significant responses in both total plasma and free salivary cortisol concentrations to ACTH1–24 injection (total plasma cortisol: F
2.07,107.63 = 173.92; p < 0.001; ; free salivary cortisol: F
2.41,125.55 = 269.93; p < 0.001;
) ().
Figure 1. Mean total plasma cortisol concentration response ( ± SEM) to injection of 1 μg ACTH1–24 in subjects with high vs. low EE. For illustration reasons, the sample was artificially divided by median split into groups with high (N = 27) vs. low (N = 26) EE; statistics are based on continuous EE questionnaire scores; the figure presents untransformed raw values. ANCOVA analysis for repeated measures controlling for sex, BMI, and CBG concentrations revealed that higher EE (entered as continuous variable) was associated with higher concentrations of total plasma cortisol in response to the ACTH1–24 injection (p = 0.045).
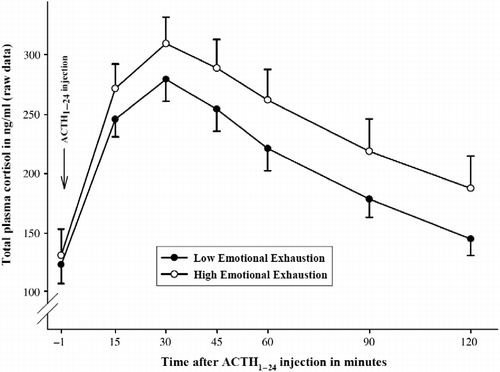
Next, to ascertain whether measures of work-related stress were associated with altered cortisol responses to the test, questionnaire scores were entered one by one as additional continuous independent variables. With respect to burnout, the total MBI score was not associated with total plasma cortisol concentrations following Synacthen injection (main effect total MBI score: F
1,48 = 3.20; p = 0.080; ; interaction samples × total MBI score: F
1.98,94.97 = 1.81; p = 0.170), but the core construct EE was significantly associated with a higher plasma cortisol profile (main effect EE: F
1,48 = 4.25; p = 0.045;
; interaction samples × EE: F
2.00,95.75 = 1.77; p = 0.175) (). The other two subscales (depersonalization and lack of accomplishment) showed no significant associations (main or interaction effects) with total plasma cortisol output (all F < 2.02; all p>0.14). OC was not related to total plasma cortisol concentrations (main effect OC: F
1,48 = 0.10; p = 0.753; interaction samples × OC: F
2.06,98.71 = 1.04; p = 0.358). Neither the ERI subscale effort (main effect effort: F
1,48 = 3.58; p = 0.065;
; samples × effort interaction: F
2.02,96.94 = 0.44; p = 0.65), nor reward (main effect reward: F
1,48 = 1.44; p = 0.237; samples × reward interaction: F
2.03,97.56 = 0.05; p = 0.956), nor the ratio between effort and reward (main effect ERI: F
1,48 = 3.27; p = 0.077;
; samples × ERI interaction: F
2.03,97.53 = 0.23; p = 0.80) were associated with total plasma cortisol concentrations following Synacthen injection. Concerning free salivary cortisol concentrations, no significant associations (main or interaction effects) for any of the questionnaire scores were found (all F < 1.05; all p>0.258).
Dex–crh Test
Initial ANCOVAs for repeated measures with the main factor samples revealed significant responses in all HPA axis parameters to CRH injection (ACTH: F
1.91,99.47 = 112.80; p < 0.001; ; total plasma cortisol: F
1.82,94.00 = 66.69; p < 0.001;
; free salivary cortisol: F
1.49,77.24 = 30.29; p < 0.001;
) ().
Figure 2. Mean ACTH (A), total plasma cortisol (B), and free salivary cortisol (C) concentration responses ( ± SEM) to injection of 100 μg CRH in subjects with high vs. low OC to work. Note: For illustration reasons, the sample was artificially divided by median split into groups with high (N = 26) vs. low (N = 27) OC; statistics are based on continuous OC questionnaire scores; the figures present untransformed raw values. ANCOVA analyses for repeated measures controlling for sex, BMI, and CBG concentrations revealed that higher OC (entered as continuous variable) was associated with lower concentrations of ACTH (p = 0.045), total plasma cortisol (p = 0.005), and free salivary cortisol (p = 0.023) in response to the CRH injection.
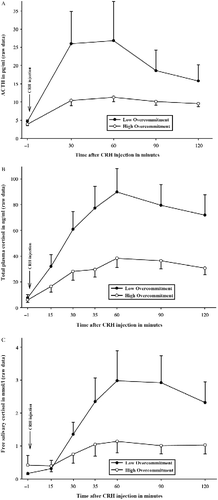
In subsequent analyses, we then added the different questionnaire scores one by one to the GLMs to test for their impact on hormonal responses to the CRH injection. OC yielded significant main effects on all endocrine parameters (): for ACTH and total plasma cortisol, a significant OC main effect emerged (ACTH main effect OC: F
1,48 = 4.24; p = 0.045; ; samples × OC interaction: F
1.82,87.42 = 2.90; p = 0.065;
; total plasma cortisol main effect: F
1,48 = 8.46; p = 0.005;
; samples × OC interaction: F
1.81,86.64 = 2.22; p = 0.120). For free salivary cortisol concentration, significant results were found for both the main effect (F
1,48 = 5.49; p = 0.023;
) as well as the samples × OC interaction (F
1.55,74.50 = 6.45; p = 0.005;
). For all HPA axis parameters, higher OC was associated with lower responses to DEX–CRH. Neither effort, reward, the ERI ratio, the total score of the MBI nor any MBI subscale was significantly related to endocrine responses (all F < 1.40; all p>0.24).
Complementary analyses
In the main analyses, EE yielded a significant effect in the Synacthen test, while OC was significantly associated with HPA axis responses in the DEX–CRH test. As discussed earlier, EE and OC show some symptom overlap with depressive symptomatology (Ahola et al. Citation2005; Bellingrath and Kudielka Citation2008; Bellingrath et al. Citation2008; Wirtz et al. Citation2010). In accordance with these earlier data, EE, OC, and depressive symptomatology showed significant intercorrelations of medium strength (). Thus, in complementary analyses, we tested whether the above reported significant effects are partialled out or remain stable if simultaneously controlling for the other two variables, respectively. Results show that the main effect of EE on total plasma cortisol responses to Synacthen became even stronger when additionally controlling for OC and HADS-D (F
1,46 = 7.20; p = 0.01; ).
Table II. Pearson correlation matrix for MBI-EE, OC, and HADS-D.
The above reported OC main effects in the DEX–CRH test also became more robust (ACTH: F
1,46 = 6.17; p = 0.017; ; total plasma cortisol: F
1,46 = 15.27; p < 0.001;
; free salivary cortisol: F
1,46 = 8.02; p = 0.007;
). Additionally, the respective samples × OC interaction effects gained significance (ACTH: F
1.84,84.64 = 3.20; p = 0.050;
; total plasma cortisol: F
1.85,84.91 = 4.41; p = 0.039;
) or, in the case of free salivary cortisol, became more pronounced (F
1.57,72.40 = 8.02; p = 0.002;
).
Discussion
In a sample of 53 healthy school teachers, we found EE, the core component of burnout, and OC to work to be differentially related to HPA axis functioning as indexed by responses to a low-dose ACTH1–24 challenge and a combined DEX–CRH test. To the best of our knowledge, this is the first study on chronic work stress according to the ERI/OC model and EE that tests for differential effects in stress signal processing at functional anatomical levels of the HPA axis by administering two different endocrine stimulation procedures in the same study population.
First of all, our results show that work-related EE is associated with a higher total plasma cortisol output to a low dose of exogenous ACTH. This finding reflects a heightened sensitivity of the adrenal cortex in emotionally exhausted but otherwise healthy teachers. Second, in line with our hypotheses, OC was associated with a significantly blunted HPA axis response to CRH challenge after dexamethasone premedication. Strikingly, these effects were even stronger when additionally controlling for depression and EE or OC. This means that, by partialling out mutually overlapping facets of the different constructs, the observed effects became even more evident.
To date, the combined DEX–CRH test is considered the most sensitive pharmacological test for the detection of alterations in HPA axis functioning (Heuser et al. Citation1994). This stimulation procedure tests for conjoint pituitary and adrenal cortex reactivity to an exogenous CRH stimulus under feedback inhibition by DEX. It could be argued that the DEX–CRH test primarily acts at a pituitary level because DEX, a synthetic glucocorticoid, only poorly penetrates the blood–brain barrier (De Kloet Citation1997). However, central regulation downstream of the hippocampus is also clearly involved in the response to DEX–CRH provocation due to reduced feedback signaling by suppressed endogenous glucocorticoid concentrations to the brain. In the current study, we observed hyporeactive pituitary as well as adrenal cortex responses as reflected in reduced ACTH, total plasma, and free salivary cortisol concentrations in overcommitted teachers. We therefore deduce that such an attenuated HPA axis response is not solely of pituitary and adrenal origin but also likely involves central mechanisms. This interpretation would also be well in line with findings from two previous studies. In an independent sample of male and female teachers with OC we recently observed reduced ACTH, total plasma, and free salivary cortisol responses to acute psychosocial stress induced by the TSST (Bellingrath and Kudielka Citation2008). Accordingly, Wirtz et al. (Citation2008) reported higher OC to be significantly associated with lower free salivary cortisol and norepinephrine responses after the TSST in working men. Evidence from these studies clearly suggests a hypo-(re)active stress system in overcommitted individuals. Strikingly, these results are in contrast with another recent finding by the same authors (Wirtz et al. Citation2010) who reported an increased total plasma cortisol response to the combined DEX–CRH test in overcommitted subjects. As ACTH reactivity was not related to OC in that study, the authors speculated that an increased response to exogenous CRH may be due to enhanced adrenal cortex sensitivity that might have developed under chronic OC conditions as an adaptive counter-regulatory reaction to compensate for a blunted stress-induced CRH release. Our present empirical results do not support the latter interpretation by Wirtz et al. (Citation2010) as we did not find any indication for increased adrenal cortex sensitivity, as tested in the Synacthen test, in subjects with high OC. We can only speculate on possible reasons for the discrepant findings in the DEX–CRH test in that study and our own study, e.g. on an average younger and less well-educated sample including participants from various occupational fields with an unemployment rate of 20% as well as a lower mean OC score in the Wirtz et al. study (16.57 vs. 13.25). Interestingly, Wirtz et al. generally reported small-to-medium-sized effects, while we found medium-to-large effects in our study.
Strikingly, however, our results in the DEX–CRH test appear to be in agreement with findings by Rydmark et al. (Citation2006) who found markedly attenuated HPA axis responses to DEX–CRH in patients on long-term sick leave with job stress-induced depression compared to a matched healthy control group. The authors of that study speculate that the observed pattern is indicative of a hypoactive central stress-axis circuitry and in this way is comparable to conditions found in atypical depression. In a follow-up study by Wahlberg et al. (Citation2009), the same sample was retested with the DEX–CRH test and the hyporeactive response pattern compared to the control group was found to be stable even after clinical improvement of depressive symptoms and full remission in the majority of patients. Wahlberg and colleagues (Citation2009) interpret this finding as initial evidence for hyporeactivity as manifested in the DEX–CRH test being a trait rather than a state marker of disease in this population. An attenuated response to DEX–CRH would thus reflect a stable marker of a pre-existing vulnerability. Based on these findings, it is hypothesized that in affected individuals, the inability to mount an adequate stress response may impede successful coping with stressful job conditions and ultimately lead to the development of depressive symptoms. Alternatively, it might be speculated whether a sustained hyporeactivity could also reflect a “scar”-marker rather than a vulnerability marker. Individuals could have acquired the hyporeactive response pattern during a period of extended stress or a state of psychopathology and maintained it thereafter. In our present study, we found OC to be associated with dampened responses to DEX–CRH. OC in turn has been identified as a risk factor for the development of depression in earlier studies (Dragano et al. Citation2008). Integrating these various findings, it might either be speculated that both OC and a hyporeactive central stress circuitry are (interrelated) risk factors for the development of depression or that a coping style characterized by high OC results in a hyporeactive stress circuitry, which eventually leads to manifestations of stress-induced depressive symptomatology. As we included only healthy participants on one measurement occasion so far, our present study cannot clarify this question.
How could the differential effects of individual stress condition on HPA axis regulation as observed in the present study be explained? This seems not to be an easy endeavor, especially since OC and EE are significantly intercorrelated. We would like to offer two potential explanations.
First, unlike EE as core component of burnout and also unlike ERI, OC is conceptualized as an underlying and enduring motivational pattern to cope with job demands and is even considered as a stable personality trait. Work-related stress due to OC can thus be assumed to be relatively stable over time and relatively invariant to changes in the work place or the environmental context. By contrast, both EE and ERI might reflect more momentary or even state-dependent characteristics that result as a consequence of present adverse working conditions. It is thus conceivable that a dispositional permanently overcommitted coping style affects the HPA axis differently than subjective stress or feelings of work-related exhaustion. However, previous research has repeatedly shown that EE is also related to dispositional variables like, for example, neuroticism/low emotional stability or low agreeableness (Goddard et al. Citation2004; Cano-García et al. Citation2005; Bakker et al. Citation2006). This suggests that some individuals are more prone to actually experience states of burnout or EE than others. Exhaustion can thus be regarded neither as independent of personality aspects nor as purely a “trait.” Rather, EE seems to be the result of a dynamic interaction of individual disposition and situational factors. Thus, taken together, empirical evidence challenges the idea that OC and EE can be reliably separated by trait vs. state aspects.
However, a second and alternative mechanistic pathway is conceivable. This pathway implicates a time course model of HPA axis hypo- and hyper(re)activity. As outlined above, an overcommitted coping style constitutes a psychological risk factor, e.g. for depression (Dragano et al. Citation2008). Overcommitted individuals are at an increased risk to experience chronic work stress because they expose themselves more frequently and constantly to high demands at work and exaggerate their work-related efforts. This coping style might initially and under sound working conditions be successful. However, it might over time trigger an overload of stressful experiences and eventually promote feelings of exhaustion or depressive symptoms. Due to their high intrinsic needs for control and approval, overcommitted individuals seem likely to be prone to experience feelings of exhaustion when they meet frustrating working conditions. This would explain the high intercorrelations between OC, EE, and HADS-D. From a psychobiological perspective, a hypo-(re)active stress system in overcommitted subjects might be a mechanism to prevent hypercortisolism in an organism frequently exposed to stressful experiences. So far, this pattern would be in line with the time course model of hypocortisolism that has been proposed earlier (Hellhammer and Wade Citation1993; Fries et al. Citation2005; Kudielka et al. Citation2006; Miller et al. Citation2007). It proposes an initial period of enhanced stress responsiveness to be followed by a phase of blunted responsiveness under prolonged stress. An overcommitted coping style, which imposes chronic stress on the body, would over time lead to attenuated HPA axis responses. However, if, as a consequence of frustrated efforts, feelings of EE and subjective distress arise, a counter-regulatory increase in adrenal sensitivity might take place. Conceivably, this could then be interpreted as a bodily attempt to reinstall homeostasis and overall functioning. Our findings would therefore extend the established time course model by a phase in which the organism strives to re-enhance HPA axis responsiveness after a period of blunted functioning.
These scenarios obviously must remain speculations. In this respect, an apparent limitation of the present study is its cross-sectional design. Only longitudinal approaches would be able to assess the assumed gradual alterations of HPA axis regulation over time. Besides important limitations (e.g. cross-sectional design, medium-sized and heterogeneous study sample including men, naturally cycling women in the luteal phase, women using oral contraception, and women in the postmenopausal phase, no use of a standardized clinical interview in recruitment of participants, and no constant time interval between the two test days), there are also some strengths of the current study like the broad characterization of the HPA axis (including ACTH, total plasma cortisol, and free salivary cortisol) as well as the application of both an ACTH1–24 sensitivity and combined DEX–CRH test in one study sample. The simultaneous test administration allowed for testing of potential alterations at different levels of HPA axis functioning under different conditions of chronic stress. Furthermore, our sample of healthy school teachers provided a model for studying the impact of chronic work-stress on physiological functioning. Reported average levels of work-related stress were medium to high with a sufficiently broad range of stress levels covering highly stressed and barely stressed individuals.
Finally, the observation that the effect of EE on cortisol responses to Synacthen reached the level of significance for total plasma cortisol but not for salivary cortisol, warrants some comments. First, the EE effect on salivary cortisol approached the level of significance if controlling for HADS-D and OC (p = 0.07), and additional analyses on net increases or area under the curve responses showed that total plasma cortisol and salivary cortisol after Synacthen stimulation are significantly intercorrelated. Considering the generally lower effect sizes in the Synacthen test compared to the DEX–CRH test raises the idea that the effect on salivary cortisol might have failed to reach significance due to insufficient statistical power. Further, the fact that we applied a low-dose Synacthen sensitivity test might have contributed to the discrepancy. However, the present findings also support the well-known observation that plasma cortisol and salivary cortisol responses are often highly intercorrelated, but can show significant dissociations (Hellhammer et al. Citation2009). Especially under response conditions, there is not necessarily always a linear relationship between the two HPA axis indicators, pointing to the necessity for distinguishing between them (Kirschbaum et al. Citation1999; Kudielka and Kirschbaum Citation2005).
In sum, our results support the notion of HPA axis dysregulation under conditions of chronic work-related stress. While EE is associated with increased adrenal cortex sensitivity, OC is related to hyporeactive pituitary and adrenal cortex responses. Thus, our data show a differential pattern of hyper- and hypo-(re)activity in relation to work-related EE and OC depending on the tested level of HPA axis functioning and individual stress condition. According to McEwen's concept of allostatic load (McEwen Citation1998b), both chronic over- as well as under-activity of functional parts of the stress response system should be regarded as wear and tear of body and brain, such that both patterns of dysregulation contribute to translating adverse psychosocial conditions into bodily changes and ultimately bringing about manifest disease.
Acknowledgements
We would like to thank Heike Pieper and Prof. Dr. Norbert Wrobel for their support in medical questions and during data acquisition. This study was supported by Emmy Noether research grant KU 1401/4-1, KU 1401/4-2, and KU 1401/4-3 of the German Research Foundation (DFG) awarded to Brigitte M. Kudielka.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahola K, Honkonen T, Isometsa E, Kalimo R, Nykyri E, Aromaa A, Lonnqvist J. 2005. The relationship between job-related burnout and depressive disorders—results from the Finnish Health 2000 Study. J Affect Disord. 88:55–62.
- Aiken LR, West SG. 1991. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage.
- Babyak MA. 2004. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 66:411–421.
- Bakker AB, Van der Zee KI, Lewig KA, Dollard MF. 2006. The relationship between the big five personality factors and burnout: A study among volunteer counselors. J Soc Psychol. 146:31–50.
- Bellingrath S, Kudielka BM. 2008. Effort–reward–imbalance and overcommitment are associated with hypothalamus–pituitary–adrenal (HPA) axis responses to acute psychosocial stress in healthy working schoolteachers. Psychoneuroendocrinology. 33:1335–1343.
- Bellingrath S, Weigl T, Kudielka BM. 2008. Cortisol dysregulation in school teachers in relation to burnout, vital exhaustion, and effort–reward–imbalance. Biol Psychol. 78:104–113.
- Bellingrath S, Weigl T, Kudielka BM. 2009. Chronic work stress and exhaustion is associated with higher allostastic load in female school teachers. Stress. 12:37–48.
- Bosma H, Peter R, Siegrist J, Marmot M. 1998. Two alternative job stress models and the risk of coronary heart disease. Am J Public Health. 88:68–74.
- Butterworth P, Leach LS, Strazdins L, Olesen SC, Rodgers B, Broom DH. 2011. The psychosocial quality of work determines whether employment has benefits for mental health: Results from a longitudinal national household panel survey. Occup Environ Med. 68:806–812.
- Cano-García FJ, Padilla-Muñoz EM, Carrasco-Ortiz MÁ. 2005. Personality and contextual variables in teacher burnout. Pers Ind Diff. 38:929–940.
- Chrousos GP, Gold PW. 1992. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 267:1244–1252.
- De Kloet ER. 1997. Why dexamethasone poorly penetrates in brain. Stress. 2:13–20.
- Dragano N, He Y, Moebus S, Jockel KH, Erbel R, Siegrist J. 2008. Two models of job stress and depressive symptoms. Results from a population-based study. Soc Psychiatry Psychiatr Epidemiol. 43:72–78.
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. 2005. A new view on hypocortisolism. Psychoneuroendocrinology. 30:1010–1016.
- Goddard R, Patton W, Creed P. 2004. The importance and place of neuroticism in predicting burnout in employment service case managers. J Appl Soc Psychol. 34:282–296.
- Grzywacz JG, Dooley D. 2003. “Good jobs” to “bad jobs”: Replicated evidence of an employment continuum from two large surveys. Soc Sci Med. 56:1749–1760.
- Heim C, Ehlert U, Hellhammer DH. 2000. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 25:1–35.
- Hellhammer DH, Wade S. 1993. Endocrine correlates of stress vulnerability. Psychother Psychosom. 60:8–17.
- Hellhammer DH, Wüst S, Kudielka BM. 2009. Salivary cortisol as biomarker in stress research. Psychoneuroendocrinology. 34:163–171.
- Herrmann C, Buss U, Snaith RP. 1995. HADS-D hospital anxiety and depression scale—Deutsche VersionEin Fragebogen zur Erfassung von Angst und Depressivität in der somatischen Medizin. Bern: Verlag Hans Huber.
- Heuser I, Yassouridis A, Holsboer F. 1994. The combined dexamethasone/CRH test: A refined laboratory test for psychiatric disorders. J Psychiatr Res. 28:341–356.
- Hinz A, Brähler E. 2011. Normative values for the hospital anxiety and depression scale (HADS) in the general German population. J Psychosom Res. 71:74–78.
- Holsboer F, von Bardeleben U, Wiedemann K, Muller OA, Stalla GK. 1987. Serial assessment of corticotropin-releasing hormone response after dexamethasone in depression. Implications for pathophysiology of DST nonsuppression. Biol Psychiatry. 22:228–234.
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. 1999. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom Med. 61:154–162.
- Kivimaki M, Leino-Arjas P, Luukkonen R, Riihimaki H, Vahtera J, Kirjonen J. 2002. Work stress and risk of cardiovascular mortality: Prospective cohort study of industrial employees. BMJ. 325:857–860.
- Kudielka BM, Bellingrath S, Hellhammer DH. 2006. Cortisol in burnout and vital exhaustion: An overview. G Ital Med Lav Ergon. 28:34–42.
- Kudielka BM, Kirschbaum C. 2005. Sex differences in HPA axis responses to stress: A review. Biol Psychol. 69:113–132.
- Kudielka BM, von Känel R, Preckel D, Zgraggen L, Mischler K, Fischer JE. 2006. Exhaustion is associated with reduced habituation of free cortisol responses to repeated acute psychosocial stress. Biol Psychol. 72:147–153.
- Kumari M, Head J, Marmot M. 2004. Prospective study of social and other risk factors for incidence of type 2 diabetes in the Whitehall II study. Arch Intern Med. 164:1873–1880.
- Kyriacou C, Pratt J. 1985. Teacher stress and psychoneurotic symptoms. Br J Educ Psychol. 55 Pt 1: 61–64.
- Kyriacou C, Sutcliffe J. 1978. Teacher stress: Prevalence, sources, and symptoms. Br J Educ Psychol. 48:159–167.
- Lehr D, Koch S, Hillert A. 2009. Where is (im)balance? Necessity and construction of evaluated cut-off points for effort–reward–imbalance and overcommitment. J Occup Org Psychol. 83:251–261.
- Maslach C, Jackson SE. 1986. Maslach burnout inventory, Manual. Palo Alto, CA: Consulting Psychologists Press.
- Maslach C, Schaufeli WB, Leiter MP. 2001. Job burnout. Annu Rev Psychol. 52:397–422.
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998a; 338:171–179.
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998b; 840:33–44.
- Miller GE, Chen E, Zhou ES. 2007. If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychol Bull. 133:25–45.
- Mommersteeg PM, Heijnen CJ, Verbraak MJ, van Doornen LJ. 2006. Clinical burnout is not reflected in the cortisol awakening response, the day-curve or the response to a low-dose dexamethasone suppression test. Psychoneuroendocrinology. 31:216–225.
- Pruessner JC, Hellhammer DH, Kirschbaum C. 1999. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 61:197–204.
- Royston P, Altman DG, Sauerbrei W. 2006. Dichotomizing continuous predictors in multiple regression: A bad idea. Stat Med. 25:127–141.
- Rydmark I, Wahlberg K, Ghatan PH, Modell S, Nygren A, Ingvar M, Asberg M, Heilig M. 2006. Neuroendocrine, cognitive and structural imaging characteristics of women on longterm sickleave with job stress-induced depression. Biol Psychiatry. 60:867–873.
- Schaufeli WB, Van Dierendonck D. 1995. A cautionary note about the cross-national and clinical validity of cut-off points for the Maslach burnout inventory. Psychol Rep. 76:1083–1090.
- Schlotz W, Hellhammer J, Schulz P, Stone AA. 2004. Perceived work overload and chronic worrying predict weekend–weekday differences in the cortisol awakening response. Psychosom Med. 66:207–214.
- Schwarzer R, Jerusalem M. 2001. Skalen zur Erfassung von Lehrer- und Schülermerkmalen. Dokumentation der psychometrischen Verfahren im Rahmen der wissenschaftlichen Begleitstudie des Modellversuchs selbstwirksame Schulen. Berlin: Online Web Version.
- Siegrist J. 1996. Adverse health effects of high-effort/low-reward conditions. J Occup Health Psychol. 1:27–41.
- Siegrist J. 2005. Social reciprocity and health: New scientific evidence and policy implications. Psychoneuroendocrinology. 30:1033–1038.
- Siegrist J, Starke D, Chandola T, Godin I, Marmot M, Niedhammer I, Peter R. 2004. The measurement of effort–reward imbalance at work: European comparisons. Soc Sci Med. 58:1483–1499.
- Sonnenschein M, Mommersteeg PM, Houtveen JH, Sorbi MJ, Schaufeli WB, van Doornen LJ. 2007. Exhaustion and endocrine functioning in clinical burnout: An in-depth study using the experience sampling method. Biol Psychol. 75:176–184.
- Stansfeld SA, Fuhrer R, Shipley MJ, Marmot MG. 1999. Work characteristics predict psychiatric disorder: Prospective results from the Whitehall II Study. Occup Environ Med. 56:302–307.
- Tsigos C, Chrousos GP. 1994. Physiology of the hypothalamic–pituitary–adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinol Metab Clin North Am. 23:451–466.
- Wahlberg K, Ghatan PH, Modell S, Nygren A, Ingvar M, Asberg M, Heilig M. 2009. Suppressed neuroendocrine stress response in depressed women on job-stress-related long-term sick leave: A stable marker potentially suggestive of preexisting vulnerability. Biol Psychiatry. 65:742–747.
- Weber A, Weltle D, Lederer P. 2001. “Macht Schule krank?” Zur Problematik krankheitsbedingter Frühpensionierung von Lehrkräften. Bayrische Schule. 6:214–215.
- Weber A, Weltle D, Lederer P. 2004. Frühinvalidität im Lehrerberuf: Sozial- und arbeitsmedizinische Aspekte. Deutsches Ärzteblatt. 101:A850–A859.
- Wirtz PH, Siegrist J, Rimmele U, Ehlert U. 2008. Higher overcommitment to work is associated with lower norepinephrine secretion before and after acute psychosocial stress in men. Psychoneuroendocrinology. 33:92–99.
- Wirtz PH, Siegrist J, Schuhmacher A, Hoefels S, Maier W, Zobel AW. 2010. Higher overcommitment to work is associated with higher plasma cortisol but not ACTH responses in the combined dexamethasone/CRH test in apparently healthy men and women. Psychoneuroendocrinology. 35:536–543.
- Yehuda R, Resnick H, Kahana B, Giller EL. 1993. Long-lasting hormonal alterations to extreme stress in humans: Normative or maladaptive?. Psychosom Med. 55:287–297.
- Zigmond AS, Snaith RP. 1983. The hospital anxiety and depression scale. Acta Psychiatr Scand. 67:361–370.
