Abstract
Stress during pregnancy can induce metabolic disorders in adult offspring. To analyze the possible differential response to a high-fat-sucrose (HFS) diet in offspring affected by prenatal stress (PNS) or not, pregnant Wistar rats (n = 11) were exposed to a chronic mild stress during the third week of gestation. The aim of this study was to model a chronic depressive-like state that develops over time in response to exposure of rats to a series of mild and unpredictable stressors. Control dams (n = 11) remained undisturbed. Adult offspring were fed chow or HFS diet (20% protein, 35% carbohydrate, 45% fat) for 10 weeks. Changes in adiposity, biochemical profile, and retroperitoneal adipose tissue gene expression by real-time polymerase chain reaction were analyzed. An interaction was observed between HFS and PNS concerning visceral adiposity, with higher fat mass in HFS-fed stressed rats, statistically significant only in females. HFS modified lipid profile and increased insulin resistance biomarkers, while PNS reduced insulin concentrations and the homeostasis model assessment index. HFS diet increased gene (mRNA) expression for leptin and apelin and decreased cyclin-dependent kinase inhibitor 1A and fatty acid synthase (Fasn), whereas PNS increased Fasn and stearoyl-CoA desaturase1. An interaction between diet and PNS was observed for adiponutrin (Adpn) and peroxisome proliferator-activated receptor-γ coactivator1-α (Ppargc1a) gene expression: Adpn was increased by the PNS only in HFS-fed rats, whereas Ppargc1a was increased by the PNS only in chow-fed rats. From these results, it can be concluded that experience of maternal stress during intrauterine development can enhance predisposition to obesity induced by a HFS diet intake.
Introduction
Obesity can be described as a clinical condition in which an excess of body fat is accumulated to the extent that it may have an adverse effect on health, leading to reduced life expectancy and/or increased health problems (Haslam and James Citation2005). This chronic disease is one of the most serious public health burdens around the world (Marti et al. Citation2008). The increasing prevalence of obesity is attributable not only to genetic factors (Moleres et al. Citation2009) or to the high intake of foods rich in fat and sugar (Astrup et al. Citation2008), but also to lifestyle and environmental factors such as adverse early life experiences including prenatal stress (PNS), which can program physiological systems and behavior in later life (Brunton and Russell Citation2010).
From human and animal studies, the “Barker Hypothesis” proposed that the intrauterine environment plays a significant role in the health of the offspring, such that exposure to limited resources in utero induces in the offspring maladaptive responses to the ample postnatal nutritional environment, contributing to the development of obesity and diabetic features (Hales and Barker Citation1992). However, not only the nutritional status of the mother can affect her offspring, but also stress conditions arising from socioeconomic or psychosocial factors, which have been associated with the development of obesity-related conditions including excessive visceral fat deposition, insulin resistance, dyslipidemia, hypertension, and cardiovascular disease in humans (Rosmond and Bjorntorp Citation1999).
Indeed, PNS in rodents has been implicated in altered stress response (Brunton Citation2010), increased anxiety-like behavior (Miyagawa et al. Citation2011), and cognitive impairment (Li et al. Citation2008), but little is known about the consequences of PNS on energy homeostasis. Moreover, there are controversial results in experimental studies combining hypercaloric diets and stress, as it has been described that either a reduction (Tamashiro et al. Citation2007; Paternain et al. Citation2011) or an increase (Kuo et al. Citation2007; Tamashiro et al. Citation2009) in body weight may occur in response to stress, which may depend not only on the applied stress paradigms, but also on the species and strains, or the type and composition of the diet. Based on this information, we hypothesized that maternal stress during the prenatal stage could predispose offspring to obesity in adulthood. For this purpose, we have evaluated the peripheral effects of a high-fat-sucrose (HFS) diet intake in adulthood, after experiencing a chronic stressful situation during development in the uterus.
Materials and methods
Animals and experimental design
Timed-pregnant Wistar rats on gestation day 9 supplied by Harlan Iberica (Barcelona, Spain) were individually housed in a temperature (21 ± 1°C) and humidity (55 ± 5%) controlled room on a 12-h light/dark cycle (lights on 08:00–20:00 h) with food and water freely available. Dams were weighed, and food intake was measured daily throughout gestation.
The day all the litters were born was designated postnatal day 0 (PND 0), and all pups were sexed by assessing anogenital distance. In this study, only litters that contained 8–10 pups were included. After all rats delivered, three dams were excluded because they were not pregnant and three litters (two in non-stressed group and one in stressed group) were eliminated because they had less than eight pups. Finally, 14 litters were used for the study: seven in the non-stressed group and seven in the stressed group. Rats were weaned on PND 21.
At 2 months old, offsprings of both genders were randomized by weight and assigned to one of two dietary groups: standard chow diet (20% protein, 67% carbohydrate, 13% fat; 2014 Tekland Global 14% Protein Rodent Maintenance Diet, Harlan Iberica, Barcelona, Spain) [control (C) group, n = 8 and control stress (CE) group, n = 8] and HFS diet (20% protein, 35% carbohydrate, from which 17% was sucrose, 45% fat from Research Diets, Inc., New Brunswick, NJ, USA (D12,451)) [HFS, n = 8 and high-fat-sucrose-stress (HFSE) group, n = 8]. Rats had ad libitum access to water and food during the dietary treatment (10 weeks), and body weight was recorded once a week.
In the 9th week of dietary treatment and after a fasting period of 12 h, rats were intraperitoneally injected with glucose at 1 g/kg body weight (Lomba et al. Citation2010a) in order to carry out an intraperitoneal glucose tolerance test (IPGTT). A drop of blood from a tail vein was collected by needle prick at 0, 15, 30, 60, and 120 min after glucose administration in order to determine glucose concentrations with a glucose meter device (Roche Diagnostic, Mannheim, Germany).
After rats were killed by decapitation without anesthesia (end of week 10 of diet treatment), trunk blood was collected to obtain the serum for biochemical measurements; serum was separated after centrifugation of clotted blood. Tissue samples and serum were immediately collected and frozen ( − 80°C). Liver, different adipose depots [subcutaneous (Sc), retroperitoneal (Rp), perigonadal (periovarian (Peri) in female and epididymal (Epi) in male), and mesenteric (Mes)], gastrocnemius and extensor digitorum longus muscles, and thymus were carefully dissected, weight, and stored immediately at − 80°C for further analysis. Visceral fat was calculated as the sum of perigonadal, Rp and Mes fat pads.
All the procedures were performed according to national and institutional guidelines of the Animal Care and Use Committee at the University of Navarra.
Unpredictable PNS paradigm
Randomly selected pregnant female rats (n = 10) were exposed to an unpredictable stress paradigm during the final eight days of gestation (days 14–21). Each stressor was applied during the light cycle unless noted otherwise. We selected a variable stress paradigm for our studies to prevent the rats from habituating to the stressor (Barnum et al. Citation2007). Stress was restricted to the third week of gestation because the neuronal circuits regulating the hypothalamo-pituitary-adrenal (HPA) axis and energy homeostasis, including the hypothalamus, are rapidly developing during this period (Weinstock Citation2001). The remaining control dams (n = 10) were exposed to normal animal room husbandry practices in the animal facility. The stress paradigm consisted of 1 h restraint in a cage with dimensions of 3.6 × 9 × 10 cm; 10 min swim stress in water at room temperature (22–24°C) in a vertical plexiglass cylinder (height: 45 cm, diameter: 19 cm) filled with water up to 28–30 cm water; 12 h wet bedding; 6 h intermittent bell (10 db, 1 s/10 s); 18 h food deprivation during dark phase of light cycle; and overnight illumination. A schedule for the application of the stressors is shown in . All stressed rats received exactly the same stressors.
Table I. Schedule of variable stress during gestation.
Body composition analysis
On PND 28, at 2 months old (before starting the dietary treatment) and before the day of killing (after the 10 weeks of dietary treatment), whole body composition (fat and lean tissues) was determined using nuclear magnetic resonance technology with an Echo MRI Analyzer system (Echo Medical Systems, Houston, TX, USA) (Nixon et al. Citation2010).
Serum measurements
Circulating glucose was measured with a HK-CP kit (ABX diagnostic, Montpellier, France) using automatized PENTRA C200 equipment (HORIBA Medical, Montpellier, France). Serum leptin (Linco Research, St Charles, MO, USA), insulin (Mercodia AB, Uppsala, Sweden), and monocyte chemotactic protein-1 (MCP-1) concentrations [an inflammatory marker (Melgarejo et al. Citation2009)] (Invitrogen, Carlsbad, CA, USA) were determined by enzyme-linked-immunosorbent assay (ELISA) using an automatized TRITURUS equipment (Grifols International S.A., Barcelona, Spain). Homeostasis model assessment (HOMA) is an index that estimates insulin resistance based on the relationship between the fasting plasma insulin concentration and the glucose concentration. It was calculated as fasting plasma glucose (mM) multiplied by fasting serum insulin (μU/ml) divided by 22.5, as described elsewhere (Paternain et al. Citation2011). Serum corticosterone concentration was determined using a commercially available enzyme immunoassay kit (Enzo Life Sciences, Farmingdale, NY, USA). Inter-assay variability and intra-assay variability for each assay, respectively, were as follows: leptin (Lep), 3.0–5.7% and 2.0–4.6%; insulin, 8.5–9.4% and 1.4–4.6%; and corticosterone, 7.8–13.1% and 6.6–8.0%. The sensitivities of these assays were 0.04 ng/ml for Lep, ≤ 0.15 μg/l for insulin, and 26.99 pg/ml for corticosterone.
Determination of fecal corticosterone
Twenty-four hour fecal samples were collected in the 4th, 5th, 6th, and 7th week of dietary treatment and stored at − 20°C until analyzed. Rats were moved to a clean cage for 24 h, and then moved to another clean cage. The total 24-h fecal samples were powdered, and corticosterone concentrations were determined in 0.2 g of dust-like fecal material by ethanol extraction followed by a corticosterone enzyme immunoassay (EIA kit; Enzo Life Sciences). Inter-assay variability and intra-assay variability for this assay were 7.8–13.1% and 6.6–8.0%, respectively, and it had a sensitivity of 26.99 pg/ml. The results are reported as the total mass of corticoid content in the 24-h fecal sample according to a previously published protocol (Paternain et al. Citation2011).
Real-time polymerase chain reaction (PCR)
Total RNA and DNA were isolated from Rp white adipose tissue (WAT) according to AllPrep® DNA/RNA manufacturer's instruction (Qiagen, Germantown, MD, USA). The cDNA was synthesized using RT2 First Strand Kit (Qiagen). Finally, quantitative real-time polymerase chain reaction (PCR) of 48 genes that are recognized to be related to obesity and glucocorticoid metabolism (Table S2) was performed following manufacturer's recommendations using ABI PRISM 7900 HT Fast Real-Time PCR System. Obesity-related genes were explored with RT2 qPCR Primer Assay (Qiagen), while glucocorticoid metabolism genes were analyzed with Taqman probes for rats (Applied Biosystems, Austin, TX, USA). The gene expression levels were normalized using glyceraldehyde-3-phosphate dehydrogenase mRNA as an internal control. Fold change between the groups was calculated using the 2-ΔΔCt method (Paternain et al. Citation2011). They were considered differentially expressed if the mRNA values showed fold change of at least 1.5 and also satisfied p < 0.05.
Molecular network generation using Ingenuity pathways analysis
Networks were generated with all 48 analyzed genes and corresponding fold changes through Ingenuity pathways analysis (Ingenuity Systems, http://www.ingenuity.com, Redwood City, CA, USA), limiting the number of networks and eligible molecules per network to 25 and 35, respectively. Networks were algorithmically generated based on their connectivity and ranked by score (negative exponent of the right-tailed Fisher's exact test result). Molecules are represented as nodes, and the biological relationship between two nodes as an edge (line). Nodes are displayed using various shapes that represent the functional class of the gene product, whereas edges describe the nature of the relationship between the nodes, as defined in Ingenuity Systems.
Statistical analysis
All results are expressed as mean ± standard error of the mean (SEM). Data were evaluated by two-way ANOVA (Diet and PNS), repeated-measures ANOVA (RM-ANOVA), or Student's t-tests for independent samples as appropriate. Subsequent comparisons between groups were made with Bonferroni test procedures. A level of probability set at p < 0.05 was used as statistically significant. All analyses were carried out using SPSS 15.0 packages for Windows (Chicago, IL, USA).
Results
Dam and offspring measurements
There were no significant differences in maternal body weight between the groups before stress exposure (data not shown). However, the stress paradigm during the last week of gestation (gestation days 14–21) resulted in a lower maternal body weight (g) (mean ± SEM, non-stress: 318.2 ± 13.5 vs. stress: 284.3 ± 2.9, Student's t-test: p < 0.01), although there were no marked differences in food intake (Kcal/day) (non-stress: 82.7 ± 5.4 vs. stress: 75.1 ± 1.8).
On the last day of the stress paradigm, fecal corticosterone content (ng/24 h) was lower in the stressed group of dams than in the control group (non-stress: 2.0 ± 0.2 vs. stress: 1.3 ± 0.1, Student's t-test: p < 0.01).
Maternal stress significantly reduced offspring birth weight, and this effect lasted until the pups were 7 days old. Maternal stress also resulted in shorter body length, only in female offspring, and a lower body fat mass, only in male offspring (Table SI).
Body measurements and food intake in adult offspring
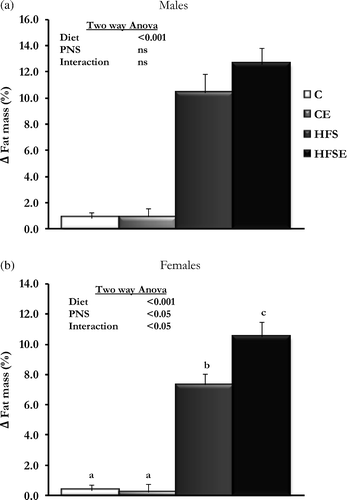
The HFS diet treatment induced the expected obesity model in offspring of both sexes (), which was reflected in a higher final body weight and fat mass deposition. Interestingly, rats subjected to PNS and fed with HFS diet in adulthood (HFSE group) gained more body fat mass than HFS rats (), although statistical significance was reached only in females () (Males: Main effect of diet: F3,30 = 105.471, p < 0.001, n = 7–8; Females: Main effect of diet: F3,31 = 157.425, p < 0.001; Main effect of PNS: F3,31 = 4.901, p < 0.05; Interaction (Diet × PNS): F3,31 = 5.720, p < 0.05, n = 8).
Table II. Body and food intake measurements in adult rats and statistical analysis of the groups.
Figure 1. The effect of HFS diet intake and PNS on body fat mass gain in adult offspring. Results are group mean ± SEM. Different letters (a, b, and c) indicate significant differences p < 0.05, Post-hoc Bonferroni. (a) Males (n = 7–8), (b) Females (n = 8). C, Control; CE, Control stress; HFS, High-fat-sucrose; HFSE, High-fat-sucrose-stress; PNS, Prenatal stress; ns, not significant.
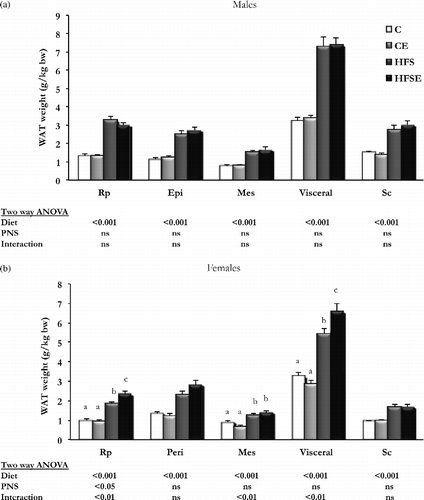
Regarding different WAT depots (), there was an increase in all measured depots with HFS diet in both sexes (Males: Main effect of diet in Rp WAT (F3,30 = 105.471, p < 0.001, n = 8), Epi WAT (F3,30 = 105.471, p < 0.001, n = 8), Mes WAT (F3,30 = 105.471, p < 0.001, n = 8), Visceral WAT (F3,30 = 105.471, p < 0.001, n = 8), and Sc WAT (F3,30 = 105.471, p < 0.001, n = 8); Females: Main effect of diet in Rp WAT (F3,30 = 105.471, p < 0.001, n = 8), Peri WAT (F3,30 = 105.471, p < 0.001, n = 8), Mes WAT (F3,30 = 105.471, p < 0.001, n = 8), Visceral WAT (F3,30 = 105.471, p < 0.001, n = 8), and Sc WAT (F3,30 = 105.471, p < 0.001, n = 8), but a sex-specific response induced by PNS was observed. Hence, there were no changes due to PNS in male rats (), whereas in females () an interaction was observed between HFS diet and PNS in visceral WAT depots (Interaction (Diet × PNS) in Rp WAT (F3,30 = 105.471, p < 0.001, n = 8), Mes WAT (F3,30 = 105.471, p < 0.001, n = 8), and Visceral WAT (F3,30 = 105.471, p < 0.001, n = 8)), indicating that PNS induced more visceral adiposity in female rats.
Figure 2. The effect of HFS diet intake and PNS on different WAT depots. Results are group mean ± SEM. Different letters (a, b, and c) indicate significant differences p < 0.05, post-hoc Bonferroni. (a) Males (n = 8), (b) Females (n = 8); C, Control, n = 8; CE, Control stress, n = 8; HFS, High-fat-sucrose, n = 8; HFSE, High-fat-sucrose-stress, n = 8; WAT, White adipose tissue; Rp, Retroperitoneal; Epi, Epididymal; Peri, Periovarian; Mes, Mesenteric; Sc, Subcutaneous; PNS, Prenatal stress; ns, not significant.
Male and female rats that experienced PNS had a greater food intake than the control groups but interestingly, we only observed a significant effect of the HFS diet in male rats (). Moreover, both sexes that fed the HFS diet had greater energy efficiency than rats on normal diet ().
Regarding other organ weights, the HFS diet intake induced a decrease in liver and total muscle weight (calculated as the sum of gastrocnemius and extensor digitorum longus weight), but only in male rats (Main effect of diet (F3,30 = 40.910, p < 0.001, n = 7–8). There were no differences in thymus weight among the groups (Figure S1).
Biochemical biomarkers in adult offspring
Biochemical measurements after 10 weeks of dietary treatment confirmed that the HFS diet intake induced changes leading to features commonly associated with obesity and metabolic syndrome, although these changes were more evident in female rats. These modifications were significant increases in serum leptin and glucose concentrations and a decrease in lipid profile markers (FFA in males; triglycerides, cholesterol, HDL, FFA in females; ). Insulin resistance biomarkers (serum insulin concentration, HOMA) were also increased by HFS diet in males and females, but PNS decreased these biomarkers significantly, except in PNS males ().
Table III. Biochemical measurements in serum in adulthood and statistical analysis of the groups.
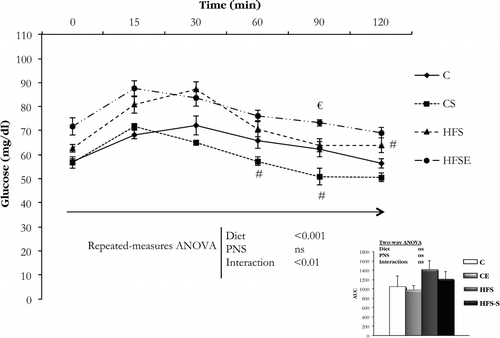
At 9 weeks of dietary treatment, an IPGTT was conducted (). RM-ANOVA of these data demonstrated that blood glucose concentration during the IPGTT varied significantly with time (Main effect of time, F5 = 40.990, p < 0.05). Moreover, a main effect of diet (F1,28 = 68.844, p < 0.001, n = 8) and interaction (Diet × PNS) (F1,28 = 11.059, p < 0.01, n = 8) was observed. Further analysis indicated that after 12 h of fasting rats fed with HFS showed higher baseline glucose concentrations, and this effect was also observed at 15 and 30 min. Regarding chow-fed rats, the glucose clearance was faster in PNS rats. Overall, although the HFS diet intake induced a slower glucose clearance, PNS during the last week of gestation further slowed glucose clearance, indicating that PNS could worsen metabolic alterations in diet-induced obesity.
Figure 3. IPGTT conducted in female rats in the four experimental groups (n = 8 per group). Data are mean ± SEM, and were analyzed with RM-ANOVA. The results of each time point were analyzed with two-way ANOVA (Diet × PNS): When an interaction between two factors was observed (60 min point, p < 0.05; 90 min point, p < 0.01; 120 min point, p < 0.05), multiple comparisons were made with Student's t-test: #p < 0.05 versus C rats, €p < 0.05 versus HFS rats. C, Control; CS, Control stress; HFS, High-fat-sucrose; HFSE, High-fat-sucrose-stress; PNS, Prenatal stress, ns, not significant.Inset: AUC, area under the curve (n = 8 per group). Data are mean ± SEM, and were analyzed with two-way ANOVA (Diet × PNS).
The analysis of serum MCP-1 was assessed as an inflammatory marker, but no significant changes were observed ().
Corticosterone in adult offspring
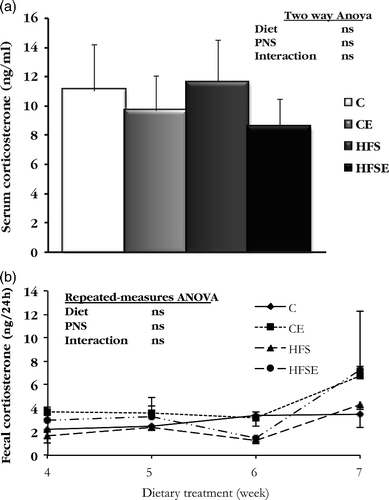
The analysis of corticosterone concentrations and the mRNA expression levels for genes related to glucocorticoid signaling was carried out only in female rats. The results showed that serum corticosterone concentration in females at the end of the experiment was not altered by HFS diet or by the stress paradigm (). Regarding the analysis of corticosterone content in feces over 24 h, in weeks 4, 5, 6, and 7 of dietary treatment were also not affected by treatment ().
Figure 4. The effect of HFS diet intake and PNS on corticosterone concentrations in female rats. Results are expressed by mean ± SEM. (a) Serum corticosterone concentration (n = 8). Data were analyzed with two-way ANOVA (Diet × PNS); (b) Fecal corticosterone content (24 h collection; n = 5–6 per group). Data were analyzed with RM-ANOVA. C, Control; CE, Control stress; HFS, High-fat-sucrose; HFSE, High-fat-sucrose-stress; PNS, Prenatal stress; ns, not significant.
Analysis of mRNA expression for different genes related to the metabolism or action of glucocorticoids in Rp WAT in female rats showed that 11β-hydroxysteroid dehydrogenase (HSD)-2 gene expression was significantly increased by HFS diet (p < 0.01, ); the mRNA levels of 11β-HSD-1, mineralocorticoid receptor and glucocorticoid receptor (GR) was not different among the groups ().
Table IV. Differentially expressed genes in Peri WAT related to obesity and glucocorticoid metabolism in adult female rats.
Obesity-related genes in adult offspring
In this experimental model, 9 out of 48 studied genes were differentially expressed Rp WAT as a result of HFS diet or PNS in female rats (). HFS induced an increase in mRNA expression for Apelin (Apln) and Lep, and a decrease in cyclin-dependent kinase inhibitor 1A (Cdkn1a) and fatty acid synthase (Fasn). Furthermore, PNS increased mRNA expression for Fasn and stearoyl-CoA desaturase1 (Scd1), while it decreased interleukin-6 (Il6) mRNA expression levels. Statistically significant interactions between HFS diet intake and PNS were observed for adiponutrin (Adpn) and peroxisome proliferator-activated receptor-γ coactivator1-α (Ppargc1a) mRNA expression. Regarding Adpn mRNA, an increase in PNS females was observed only in rats fed with HFS diet, and for Ppargc1a mRNA, PNS increased expression only in chow-fed females.
Ingenuity analysis of differentially expressed genes in WAT () showed statistically implicated three main pathways related to the endocrine system, cell cycle, and lipid metabolism in the effects of PNS and HFS diet. Figure S2 shows the integrated network affected by HFS diet intake, and Figure S3 shows the integrated network affected by PNS.
Discussion
The environment during fetal development is important for the health of adult offspring, as exposure to nutritional shortages in pregnancy increases predisposition to development of obesity and diabetes mellitus type 2 in the offspring (Hales and Barker Citation1992). However, stressful situations have also been associated with the development of obesity-related alterations (Rosmond and Bjorntorp Citation1999). Moreover, it has been reported that immune stress in late pregnant rats reduced gestational length and the number of viable pups born (Paris et al. Citation2011). Thus, in the current study, we examined the peripheral effects of a HFS diet intake on adult rats exposed to PNS.
It has been reported that there is a circadian variation in the corticosterone levels throughout rat pregnancy (Atkinson and Waddell Citation1995). In this study, the stress exposure in late pregnancy reduced maternal weight during the period of the treatment and restricted early postnatal growth of the pups in line with previous findings (Woods Citation2006; Franko et al. Citation2010); this reduced maternal weight during the treatment could be due in part to the reduced weight of the fetuses and may also have reflected the differences in concentration of stress hormones (Franko et al. Citation2010). Moreover, lower fecal corticosterone levels in dams were observed in the last day of the stress paradigm in agreement with other studies (Neumann et al. Citation1998; Douglas et al. Citation2003, Citation2005). These studies reported that in the rat corticosterone secretory responses in pregnancy are attenuated as a mechanism for minimizing exposure of the fetuses and neonate to glucocorticoids in the peripartum period and that central mechanisms underlie this reduced response.
In relation to the effects in adult offspring, as expected, the intake of a hypercaloric diet induced changes in several obesity-related phenotypical variables (Lomba et al. Citation2010b). In particular, the weight of white fat pads was increased, leading to a higher final body weight. Regarding the biochemical measurements, HFS diet intake also induced alterations leading to common features associated with obesity and the metabolic syndrome, such as increased serum glucose, insulin, and Lep concentrations, and increased HOMA, although the effects were more apparent in female rats. The decrease in lipid profile observed (decreased serum concentrations of triglycerides, HDL, and FFA), in contrast with the increase detected in human beings, is commonly found in rat models of diet-induced obesity (Lomba et al. Citation2010b). However, the PNS paradigm induced an increase in food intake and a higher fat mass gain only in HFS diet-fed rats, indicating that PNS could worsen diet-induced obesity. PNS induced a decrease in insulin resistance biomarkers, which is in agreement with previous studies (Delaunay et al. Citation1997; Lambillotte et al. Citation1997; Solas et al. Citation2010) that reported an inhibition of insulin secretion from pancreatic β-cells due to glucocorticoids. Although tendencies for these changes were observed in both sexes, statistical significance was reached only in female rats, indicating that PNS can have sex-dependent effects on rats.
Neither ovariectomy nor orchiectomy during adulthood changes the weight gain or food intake response to restraint stress (Garcia-Caceres et al. Citation2010), which suggests that post-pubertal gonadal steroids do not underlie the sex difference observed in this stress response. Indeed, some aspects of neuroendocrine stress axis function are responsive to post-pubertal sex steroid manipulations, while others are not (Patchev et al. Citation1999). However, it is well known that sex steroids have organizational effects on the developing hypothalamus, which results in structural changes that underlie sexually dimorphic endocrine responses including stress, growth, and reproduction (McCarthy et al. Citation2008; Koehl et al. Citation2009). Thus, ovariectomized female rats gain significantly more weight than control females, and orchiectomized males gain less weight than control males (Roepke Citation2009). Moreover, gender is one major variable that appears to confer differential vulnerability to stress. Darnaudery and Maccari (Citation2008) and Simpson and Kelly (Citation2012) commented that males and females differ in physiological and behavioral responses to stressors. Despite the knowledge that in humans, women are more susceptible than men to stress-related mental illnesses such as major depression (Swaab and Hofman Citation1995; Lewinsohn et al. Citation1998; Weinstock Citation1999), many of the relevant studies in this field have been conducted in male rodents, and less information is available on the responses of females to stressors. Therefore, to investigate the effects of PNS on diet-induced obesity, a deeper analysis was conducted only in female rats.
PNS in rodents has also been implicated in altered stress responsivity of adult offspring (Brunton Citation2010), and to analyze the HPA axis response, serum and fecal corticosterone were measured. We did not observe any statistically significant changes in serum corticosterone concentration (after 10 weeks of diet treatment). However, the serum concentrations of corticosterone are of limited value because they were determined after behavioral manipulations. In this context, fecal corticosterone has advantages to study the changes in glucocorticoid release (Cavigelli et al. Citation2005; Paternain et al. Citation2011). Nevertheless, no changes were observed in corticosterone levels in feces over 24 h in any of analyzed weeks (week 4, 5, 6, and 7 of diet treatment). It has been reported that basal plasma corticosterone levels are elevated in prenatally stressed female rats (Weinstock et al. Citation1998; Ward et al. Citation2000). However, Kay et al. (Citation1998) failed to report increased basal HPA activity following PNS. These differences may result from differences between the PNS paradigms used as well as variation in the time of day when blood samples were taken, as PNS has been shown to induce a phase shift in the circadian corticosterone rhythm in adult offspring (Koehl et al. Citation1997; Koehl et al. Citation1999). Also, there were no changes in Rp WAT, after 10 weeks of diet treatment, in mRNA levels of different genes related to the metabolism of glucocorticoids, except that HFS diet intake increased 11β-HSD2 mRNA expression levels. This enzyme is one of the enzymes modulating local glucocorticoid effects, regulating the access of 11β-hydroxyglucocorticoids to its receptor by converting corticosterone to the GR-inactive form dehydrocorticosterone (Kershaw et al. Citation2005). This outcome is consistent with our previous study, which found enhanced 11β-hsd2 gene expression in Sc WAT in male Wistar rats with high-fat diet-induced obesity (Milagro et al. Citation2007).
It is noteworthy that some of the results presented in this study can be affected by the sex hormone cycle of the females, which was not assessed in this study, as in many other studies on early life effects (Lehmann et al. Citation1999; McIntosh et al. Citation1999; Wigger and Neumann Citation1999; Kalinichev et al. Citation2002). Estrogen may either alter or interact with HPA axis regulation of corticosterone release and cognitive function, and it has been suggested that estrogen in females may protect against the effects of corticosterone (Luine Citation2002).
Regarding the obesity-related genes, ingenuity analysis revealed that the expression of genes involved in lipogenesis was affected by both dietary treatment and PNS. Thus, HFS diet intake increased Lep (Milagro et al. Citation2009) and Apln (Fernandez-Galilea et al. Citation2011) mRNA expression. However, Cdkn1a (Boque et al. Citation2009) and Fasn (Lomba et al. Citation2010a) mRNA levels were downregulated by HFS diet. Given that the HFS diet intake increased the body WAT mass, it was unexpected to observe that the expression of these genes was downregulated in adipose tissue. However, these results can be explained, because at the time of measurement (week 10 of HFS diet) the rats could be at a late stage of obesity and the fat mass longer expanding (Diraison et al. Citation2002). Nevertheless, PNS also increased lipogenic genes, such as Fasn (Drake et al. Citation2010) and Scd1 (Hu et al. Citation2004), and decreased Il6 mRNA levels (Ishii-Yonemoto et al. Citation2010). Interestingly, an interaction between the dietary treatment and PNS was observed in Adpn and Ppargc1a gene expression. Adpn is an adipose-specific transmembrane protein that is regulated by energy balance (Baulande et al. Citation2001) and that has been postulated to be the part of the adipose-specific energy homeostasis sensor (Johansson et al. Citation2006). Thus, an increase in Adpn gene expression with PNS was observed only when these rats were fed with HFS diet. Moreover, Ppargc1a gene expression was also increased with PNS, but only when these rats were fed normal chow. Furthermore, Wyrwoll et al. (Citation2008) described that female offspring of dexamethasone-treated mothers had increased skeletal muscle expression of Ppargc1a, which contributes to the insulin-sensitizing effects of Pparg activators as a transcriptional coactivator (Finck and Kelly Citation2006). Hence, the upregulation of this gene may be a homeostatic response to disturbances in insulin signaling, which is in agreement with the current work. Overall, these results reinforce the idea that PNS predisposes offspring to greater WAT gain in adulthood.
Conclusion
There is evidence that the conditions during the intrauterine period are critical for the development of the offspring since disturbances in this stage can induce important biological changes that persist into adulthood (Hales and Barker Citation1992). The general conclusion of the present work is that experience of stress during intrauterine development leads to adult rats having a higher predisposition to obesity induced by HFS diet intake, but this was found to be statistically significant only for females, indicating that PNS can have sex-dependent effects on rats. A deeper analysis conducted in WAT from female rats indicated that altered Fasn, Scd1, Adpn, and Ppargc1a gene expression regulation could be implicated in a permanent effect of PNS in these rats; any epigenetic mechanisms involved remain to be explored.
Supplementary Material
Download PDF (145.6 KB)Supplementary Material
Download PDF (679.3 KB)Supplementary Material
Download PDF (530.4 KB)Supplementary Material
Download MS Word (63 KB)Supplementary Material
Download MS Word (132.5 KB)Acknowledgements
The authors are grateful to Veronica Ciaurriz for her technical laboratory support. The authors also appreciate the careful reading and correction of the last version by Alexandra Simpson for English quality.
Declaration of interest : The authors would like to thank Línea Especial (LE/97) from the University of Navarra and the Carlos III Health Institute (CIBER project, Spain; Grant No. CB06/03/1017) for the financial support of this study and “Asociación de Amigos de la Universidad de Navarra” and IBERCAJA (Spain) for the doctoral fellowships of Ana Laura De la Garza and Laura Paternain. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Astrup A, Dyerberg J, Selleck M, Stender S. 2008. Nutrition transition and its relationship to the development of obesity and related chronic diseases. Obes Rev. 9 Suppl. 1: 48–52.
- Atkinson HC, Waddell BJ. 1995. The hypothalamic-pituitary-adrenal axis in rat pregnancy and lactation: Circadian variation and interrelationship of plasma adrenocorticotropin and corticosterone. Endocrinology. 136:512–520.
- Barnum CJ, Blandino PJr, Deak T. 2007. Adaptation in the corticosterone and hyperthermic responses to stress following repeated stressor exposure. J Neuroendocrinol. 19:632–642.
- Baulande S, Lasnier F, Lucas M, Pairault J. 2001. Adiponutrin, a transmembrane protein corresponding to a novel dietary- and obesity-linked mRNA specifically expressed in the adipose lineage. J Biol Chem. 276:33336–33344.
- Boque N, Campion J, Milagro FI, Moreno-Aliaga MJ, Martinez JA. 2009. Some cyclin-dependent kinase inhibitors-related genes are regulated by vitamin C in a model of diet-induced obesity. Biol Pharm Bull. 32:1462–1468.
- Brunton PJ. 2010. Resetting the dynamic range of hypothalamic-pituitary-adrenal axis stress responses through pregnancy. J Neuroendocrinol. 22:1198–1213.
- Brunton PJ, Russell JA. 2010. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J Neuroendocrinol. 22:258–271.
- Cavigelli SA, Monfort SL, Whitney TK, Mechref YS, Novotny M, McClintock MK. 2005. Frequent serial fecal corticoid measures from rats reflect circadian and ovarian corticosterone rhythms. J Endocrinol. 184:153–163.
- Darnaudery M, Maccari S. 2008. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev. 57:571–585.
- Delaunay F, Khan A, Cintra A, Davani B, Ling ZC, Andersson A, Ostenson CG, Gustafsson J, Efendic S, Okret S. 1997. Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids. J Clin Invest. 100:2094–2098.
- Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. 2002. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 282:E46–E51.
- Douglas AJ, Brunton PJ, Bosch OJ, Russell JA, Neumann ID. 2003. Neuroendocrine responses to stress in mice: Hyporesponsiveness in pregnancy and parturition. Endocrinology. 144:5268–5276.
- Douglas AJ, Meddle SL, Toschi N, Bosch OJ, Neumann ID. 2005. Reduced activity of the noradrenergic system in the paraventricular nucleus at the end of pregnancy: Implications for stress hyporesponsiveness. J Neuroendocrinol. 17:40–48.
- Drake AJ, Raubenheimer PJ, Kerrigan D, McInnes KJ, Seckl JR, Walker BR. 2010. Prenatal dexamethasone programs expression of genes in liver and adipose tissue and increased hepatic lipid accumulation but not obesity on a high-fat diet. Endocrinology. 151:1581–1587.
- Fernandez-Galilea M, Perez-Matute P, Prieto-Hontoria P, Martinez JA, Moreno-Aliaga MJ. 2011. Effects of lipoic acid on apelin in 3T3-L1 adipocytes and in high-fat fed rats. J Physiol Biochem. 67:479–486.
- Finck BN, Kelly DP. 2006. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 116:615–622.
- Franko KL, Forhead AJ, Fowden AL. 2010. Differential effects of prenatal stress and glucocorticoid administration on postnatal growth and glucose metabolism in rats. J Endocrinol. 204:319–329.
- Garcia-Caceres C, Diz-Chaves Y, Lagunas N, Calmarza-Font I, Azcoitia I, Garcia-Segura LM, Frago LM, Argente J, Chowen JA. 2010. The weight gain response to stress during adulthood is conditioned by both sex and prenatal stress exposure. Psychoneuroendocrinology. 35:403–413.
- Hales CN, Barker DJ. 1992. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia. 35:595–601.
- Haslam DW, James WP. 2005. Obesity. Lancet. 366:1197–1209.
- Hu CC, Qing K, Chen Y. 2004. Diet-induced changes in stearoyl-CoA desaturase 1 expression in obesity-prone and -resistant mice. Obes Res. 12:1264–1270.
- Ishii-Yonemoto T, Masuzaki H, Yasue S, Okada S, Kozuka C, Tanaka T, Noguchi M, Tomita T, Fujikura J, Yamamoto Y, Ebihara K, Hosoda K, Nakao K. 2010. Glucocorticoid reamplification within cells intensifies NF-kappaB and MAPK signaling and reinforces inflammation in activated preadipocytes. Am J Physiol Endocrinol Metab. 298:E930–E940.
- Johansson LE, Hoffstedt J, Parikh H, Carlsson E, Wabitsch M, Bondeson AG, Hedenbro J, Tornqvist H, Groop L, Ridderstrale M. 2006. Variation in the adiponutrin gene influences its expression and associates with obesity. Diabetes. 55:826–833.
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. 2002. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 73:131–140.
- Kay G, Tarcic N, Poltyrev T, Weinstock M. 1998. Prenatal stress depresses immune function in rats. Physiol Behav. 63:397–402.
- Kershaw EE, Morton NM, Dhillon H, Ramage L, Seckl JR, Flier JS. 2005. Adipocyte-specific glucocorticoid inactivation protects against diet-induced obesity. Diabetes. 54:1023–1031.
- Koehl M, Barbazanges A, Le Moal M, Maccari S. 1997. Prenatal stress induces a phase advance of circadian corticosterone rhythm in adult rats which is prevented by postnatal stress. Brain Res. 759:317–320.
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. 1999. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 40:302–315.
- Koehl M, Lemaire V, Le Moal M, Abrous DN. 2009. Age-dependent effect of prenatal stress on hippocampal cell proliferation in female rats. Eur J Neurosci. 29:635–640.
- Kuo LE, Abe K, Zukowska Z. 2007. Stress, NPY and vascular remodeling: Implications for stress-related diseases. Peptides. 28:435–440.
- Lambillotte C, Gilon P, Henquin JC. 1997. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest. 99:414–423.
- Lehmann J, Pryce CR, Bettschen D, Feldon J. 1999. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol Biochem Behav. 64:705–715.
- Lewinsohn PM, Gotlib IH, Lewinsohn M, Seeley JR, Allen NB. 1998. Gender differences in anxiety disorders and anxiety symptoms in adolescents. J Abnorm Psychol. 107:109–117.
- Li H, Li X, Jia N, Cai Q, Bai Z, Chen R, Song T, Zhu Z, Liu J. 2008. NF-kappaB regulates prenatal stress-induced cognitive impairment in offspring rats. Behav Neurosci. 122:331–339.
- Lomba A, Martinez JA, Garcia-Diaz DF, Paternain L, Marti A, Campion J, Milagro FI. Weight gain induced by an isocaloric pair-fed high fat diet: a nutriepigenetic study on FASN and NDUFB6 gene promoters. Mol Genet Metab. 2010a; 101:273–278.
- Lomba A, Milagro FI, Garcia-Diaz DF, Marti A, Campion J, Martinez JA. Obesity induced by a pair-fed high fat sucrose diet: methylation and expression pattern of genes related to energy homeostasis. Lipids Health Dis. 2010b; 9:60–70.
- Luine V. 2002. Sex differences in chronic stress effects on memory in rats. Stress. 5:205–216.
- Marti A, Martinez-Gonzalez MA, Martinez JA. 2008. Interaction between genes and lifestyle factors on obesity. Proc Nutr Soc. 67:1–8.
- McCarthy MM, Schwarz JM, Wright CL, Dean SL. 2008. Mechanisms mediating oestradiol modulation of the developing brain. J Neuroendocrinol. 20:777–783.
- McIntosh J, Anisman H, Merali Z. 1999. Short- and long-periods of neonatal maternal separation differentially affect anxiety and feeding in adult rats: Gender-dependent effects. Brain Res Dev Brain Res. 113:97–106.
- Melgarejo E, Medina MA, Sanchez-Jimenez F, Urdiales JL. 2009. Monocyte chemoattractant protein-1: A key mediator in inflammatory processes. Int J Biochem Cell Biol. 41:998–1001.
- Milagro FI, Campion J, Garcia-Diaz DF, Goyenechea E, Paternain L, Martinez JA. 2009. High fat diet-induced obesity modifies the methylation pattern of leptin promoter in rats. J Physiol Biochem. 65:1–9.
- Milagro FI, Campion J, Martinez JA. 2007. 11-Beta hydroxysteroid dehydrogenase type 2 expression in white adipose tissue is strongly correlated with adiposity. J Steroid Biochem Mol Biol. 104:81–84.
- Miyagawa K, Tsuji M, Fujimori K, Saito Y, Takeda H. 2011. Prenatal stress induces anxiety-like behavior together with the disruption of central serotonin neurons in mice. Neurosci Res. 70:111–117.
- Moleres A, Rendo-Urteaga T, Azcona C, Martinez JA, Gomez-Martinez S, Ruiz JR, Moreno LA, Marcos A, Marti A. 2009. Il6 gene promoter polymorphism (-174G/C) influences the association between fat mass and cardiovascular risk factors. J Physiol Biochem. 65:405–413.
- Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ. 1998. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol. 508 Pt 1: 289–300.
- Nixon JP, Zhang M, Wang C, Kuskowski MA, Novak CM, Levine JA, Billington CJ, Kotz CM. 2010. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity. 18:1652–1659.
- Paris JJ, Brunton PJ, Russell JA, Frye CA. 2011. Immune stress in late pregnant rats decreases length of gestation and fecundity, and alters later cognitive and affective behaviour of surviving pre-adolescent offspring. Stress. 14:652–664.
- Patchev VK, Hayashi S, Orikasa C, Almeida OF. 1999. Ontogeny of gender-specific responsiveness to stress and glucocorticoids in the rat and its determination by the neonatal gonadal steroid environment. Stress. 3:41–54.
- Paternain L, Garcia-Diaz DF, Milagro FI, Gonzalez-Muniesa P, Martinez JA, Campion J. 2011. Regulation by chronic-mild stress of glucocorticoids, monocyte chemoattractant protein-1 and adiposity in rats fed on a high-fat diet. Physiol Behav. 103:173–180.
- Roepke TA. 2009. Oestrogen modulates hypothalamic control of energy homeostasis through multiple mechanisms. J Neuroendocrinol. 21:141–150.
- Rosmond R, Bjorntorp P. 1999. Psychosocial and socio-economic factors in women and their relationship to obesity and regional body fat distribution. Int J Obes Relat Metab Disord. 23:138–145.
- Simpson J, Kelly JP. 2012. An investigation of whether there are sex differences in certain behavioural and neurochemical parameters in the rat. Behav Brain Res. 229:289–300.
- Solas M, Aisa B, Mugueta MC, Del Rio J, Tordera RM, Ramirez MJ. 2010. Interactions between age, stress and insulin on cognition: implications for Alzheimer's disease. Neuropsychopharmacology. 35:1664–1673.
- Swaab DF, Hofman MA. 1995. Sexual differentiation of the human hypothalamus in relation to gender and sexual orientation. Trends Neurosci. 18:264–270.
- Tamashiro KL, Nguyen MM, Ostrander MM, Gardner SR, Ma LY, Woods SC, Sakai RR. 2007. Social stress and recovery: implications for body weight and body composition. Am J Physiol Regul Integr Comp Physiol. 293:R1864–R1874.
- Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. 2009. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes. 58:1116–1125.
- Ward HE, Johnson EA, Salm AK, Birkle DL. 2000. Effects of prenatal stress on defensive withdrawal behavior and corticotropin releasing factor systems in rat brain. Physiol Behav. 70:359–366.
- Weinstock LS. 1999. Gender differences in the presentation and management of social anxiety disorder. J Clin Psychiatry. 60 Suppl. 9: 9–13.
- Weinstock M. 2001. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 65:427–451.
- Weinstock M, Razin M, Schorer-Apelbaum D, Men D, McCarty R. 1998. Gender differences in sympathoadrenal activity in rats at rest and in response to footshock stress. Int J Dev Neurosci. 16:289–295.
- Wigger A, Neumann ID. 1999. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 66:293–302.
- Woods LL. 2006. Maternal glucocorticoids and prenatal programming of hypertension. Am J Physiol Regul Integr Comp Physiol. 291:R1069–R1075.
- Wyrwoll CS, Mark PJ, Mori TA, Waddell BJ. 2008. Developmental programming of adult hyperinsulinemia, increased proinflammatory cytokine production, and altered skeletal muscle expression of SLC2A4 (GLUT4) and uncoupling protein 3. J Endocrinol. 198:571–579.
