Abstract
Sarcoidosis is an autoimmune disease, and hypothalamic–pituitary–adrenal (HPA) axis activity is blunted in autoimmunity. Exercise stimulates the HPA axis, and we hypothesized that in sarcoidosis patients responses to treadmill exercise would be reduced. Hence, we studied 44 sarcoidosis patients [27 untreated (age, mean ± SD, 42 ± 2 years, 12 males, 15 females) and 17 dexamethasone treated (age, 46 ± 4 years, 7 males, 10 females)] and 20 healthy controls (40 ± 5 years old, 9 males, 11 females). Blood samples were drawn before, at peak (exhaustion), and 15 min after treadmill exercise for adrenocorticotropic hormone (ACTH), cortisol, tumor necrosis factor (TNF), interleukin-1β (IL-1β) and IL-6 measurements. At peak, plasma ACTH (pg/ml) was increased in untreated (mean ± SE, ΔACTH = 162.8 ± 29.9) and treated (ΔACTH = 123.3 ± 48.1) patients and controls (ΔACTH = 112.3 ± 41.7). Post-exercise, cortisol (ng/ml) was increased (p < 0.05) in untreated patients (Δcortisol = 48.4 ± 14.7) and controls (Δcortisol = 46.0 ± 15.9), but not significantly in treated patients (Δcortisol = 1.43 ± 2.56). At baseline, serum IL-6 (pg/ml) and TNF (pg/ml) were higher in untreated (3.02 ± 0.54 and 3.89 ± 0.72) and treated (1.75 ± 0.33 and 2.16 ± 1.00) patients, respectively, than in controls (0.80 ± 0.66 and 1.58 ± 0.32). At peak exercise, IL-6 was increased in untreated (ΔIL-6 = 0.96 ± 0.14) and treated (ΔIL-6 = 0.91 ± 0.47) patients and controls (ΔIL-6 = 0.96 ± 0.18); IL-1β was increased only in controls. Hence, the HPA axis of untreated sarcoidosis patients and controls responded similarly to treadmill exercise. In sarcoidosis patients, increased IL-6 was associated with HPA stimulation. Cortisol concentrations were similar between patients and controls, although IL-6 concentrations were higher in patients. Thus, in the face of chronically elevated IL-6 levels in sarcoidosis, there may be dysfunctional IL-6-induced HPA responses or HPA adaptation to high IL-6 concentrations.
Introduction
Sarcoidosis is a chronic granulomatous disorder of unknown etiology, characterized by the accumulation of T lymphocytes, macrophages, and immune effector cells within the lungs and the lymphatic system, which are most often affected (Zissel et al. Citation2010). Sarcoidosis fulfills the following criteria for an autoimmune disorder: (a) it can be transmitted through the transfer of pathogenic autoantibodies or autoreactive T cells (lung transplants, Kveim–Siltzbach skin test), (b) an autoimmune animal model for the development of sarcoidosis exists, (c) lymphatic infiltrations occur, (d) sarcoidosis co-exists with other autoimmune diseases, (e) immunosuppressive therapy can be effective, and (f) sarcoidosis is associated with particular HLA antigens (Dubaniewicz Citation2010).
In sarcoidosis, CD4+T cells, through interaction with antigen-presenting cells (APCs), initiate and maintain the formation of granulomas (Iannuzzi et al. Citation2007). APCs produce, among other chemokines, high concentrations of tumor necrosis factor (TNF; Iannuzzi et al. Citation2007). However, elevated concentrations of interleukin-6 (IL-6) can be detected by bronchoalveolar lavage and in the serum of patients with sarcoidosis (Sahashi et al. Citation1991; Guntupova et al. Citation2006). In the presence of IL-6, transforming growth factor beta promotes the differentiation of naive T lymphocytes into proinflammatory IL-17 producing T helper 17 cells. The latter, in their turn, promote autoimmunity and inflammation, indicating a central role of IL-6 in the pathogenesis of autoimmunity in sarcoidosis (Mucida et al. Citation2007; Facco et al. Citation2011).
Glucocorticoids inhibit both the cytokine gene expression and the pleiotropic actions of cytokines on target cells, while they suppress effectively the nuclear regulatory factor kappa B (NF-κB) that evidently plays a key role in sarcoidosis, in asthma, rheumatoid arthritis and inflammatory bowel disease (Antoniou et al. Citation2006). Thus, blunted hypothalamic–pituitary–adrenal (HPA) axis responses are associated with enhanced susceptibility to autoimmune inflammatory diseases as has been shown in human autoimmune diseases such as rheumatoid arthritis (Mastorakos and Ilias Citation2000), Sjogren's syndrome (Tzioufas et al. Citation2008), systemic lupus erythematosus (Zietz et al. Citation2000), allergic asthma, and atopy (Buske-Kirschbaum et al. Citation2010).
Exercise is a stressor as activation of many physiological pathways, including the HPA axis, leads to an adaptation to the new conditions (Mastorakos and Pavlatou Citation2005). Treadmill exercise is a potent stimulator of the HPA axis (Luger et al. Citation1987). Circulating adrenocorticotropic hormone (ACTH) concentrations are markers of this stimulation, peaking at maximal exercise, proportionately to the subjects' overall fitness (Mastorakos et al. Citation2005). Furthermore, treadmill exercise is a potent stimulus of IL-6 secretion, an effect mainly mediated via catecholaminergic stimulation (Papanicolaou et al. Citation1996). Interestingly, the IL-6 response to exercise is attenuated after pre-treatment with glucocorticoids (Papanicolaou et al. Citation1996). Previously, we have shown that exogenous IL-6 is a potent stimulator of the HPA axis in humans (Mastorakos et al. Citation1993). In addition, in non-treated rheumatoid arthritis patients, early in the disease course, endogenous IL-6 secretion is positively associated with HPA axis diurnal activity (Crofford et al. Citation1997)
We hypothesized that the HPA axis response (expressed by circulating ACTH and cortisol concentrations) in sarcoidosis patients, to potent stressors such as treadmill exercise would be attenuated, hence facilitating the expression of autoimmune phenomena. To examine this hypothesis, we evaluated circulating concentrations of the pro-inflammatory cytokines (IL-1β, TNF, and IL-6) and ACTH and cortisol at baseline and after an acute session of treadmill exercise in sarcoidosis patients with and without glucocorticoid treatment, and in control healthy volunteers.
Participants and methods
Participants: Patients and controls
Forty-four patients diagnosed with sarcoidosis were consecutively recruited in the Department of Pulmonary and Critical Care of our clinical institution for this study according to the exclusion and inclusion conditions stated below. All study participants received radiological examination, pulmonary function tests, bronchoalveolar lavage, high-resolution computed tomography, and gallium-67 scanning. Diagnosis was confirmed by the histological appearance of typical sarcoid granulomas in transbronchial biopsies of the lung and/or biopsy material obtained from the bronchial mucosa, lymph nodes, and/or skin. All other granulomatous diseases were excluded (Iannuzzi et al. Citation2007). Patients who either had a concurrent illness or had any contraindication to the maximal exercise testing were not included in this study. Among patients with sarcoidosis, 27 were first diagnosed as untreated patients who were not receiving systemic corticosteroid treatment; while 17 were under chronic (more than 1 month) treatment with dexamethasone (3–6 mg daily). Twenty healthy volunteers who did not participate in any exercise training program and had a sedentary lifestyle were selected as healthy control subjects. Special attention was given to match age, body mass index (BMI), and the male to female ratio with those of the sarcoidosis patients, and to exclude adrenal pathology. None of the healthy participants was receiving any treatment. The clinical characteristics of the three groups of participants are shown in . All participants gave their written informed consent before the beginning of the study. Procedures were in accordance with the Helsinki declaration and had the approval of the Institutional Review Board.
Table I. Anthropometric variables of patients and healthy participants.
Protocol
Each patient and control participant performed an acute session of symptom-limited graded exercise on a treadmill (Bruce protocol), as previously described (American College of Sports Medicine Citation2006). The exercise session was performed early in the morning. Patients on dexamethasone did not receive their morning dexamethasone treatment on the test morning. Arterial oxygen saturation of hemoglobin was recorded by a pulse oximeter, and heart rate and rhythm were monitored throughout the exercise period and subsequent 15 min by 12-lead electrocardiography. Patients were encouraged to continue exercise until exhaustion (exercise peak). In case of dyspnea or fatigue and maximal predicted heart rate achieved (calculated as 220 − participant's age in years), the exercise was ended. An intravenous catheter was inserted into an antecubital vein (kept patent by flushing with 0.9% saline) before the test, and blood samples were drawn before, at exercise peak, and 15 min after the test (recovery; three time-points in total) for the measurement of plasma ACTH and cortisol, and serum TNF, IL-1β, and IL-6. At each collection, 10 ml blood was drawn into tubes, containing either gel (serum separation tubes) for serum analyses or ethylenediametetraacetic acid for plasma analysis, and placed on ice immediately. Blood samples were centrifuged (4°C, 1500g, 15 min), and the resulting serum and plasma were removed and stored in aliquots at − 75°C until assayed. Samples were thawed once before analysis. All analyses were carried out in duplicate.
In addition, the metabolic equivalent of task (MET; see later) was measured at baseline and during the duration of treadmill exercise as MET integrated area under the curve (METAUC).
Assays of hormones and cytokines
ACTH was measured using a chemiluminescence immunoassay (Immulite 2000, Siemens, Germany). The intra- and inter-assay coefficients of variation (CVs) and the assay sensitivity (AS) were 6.9%, 9.3%, and 5 pg/ml, respectively. Cortisol was measured using an electrochemiluminescence immunoassay (Roche, Basel, Switzerland). The intra- and inter-assay CVs and AS were 1.3%, < 8%, and 0.20 ng/ml, respectively. The inflammatory cytokines were measured by solid-linked immunosorbent assays using the Quantikine HS Human IL-6, TNF, and IL-1β immunoassay kits (R&D Systems, Minneapolis, MN, USA). For the IL-6 assay, the intra- and inter-assay CVs and AS were 7.9% and 7.1% at 7.94 ng/ml, and 0.094 ng/ml, respectively. For the TNF assay, the intra- and inter-assay CVs and AS were 6.0% and 7.1% at 14.7 pg/ml, and 0.05 pg/ml, respectively. For the IL-1β assay, the intra- and inter-assay CVs and AS were 6.9% and 10.3% at 1.49 pg/ml, and 0.18 pg/ml, respectively. The assays employed are specific and do not exhibit any significant cross-reactivity with several other recombinant human cytokines tested.
Metabolic equivalent of task
MET is a measure of the energy cost of a physical activity such as treadmill exercise. In this case, it was calculated as the ratio of metabolic rate during treadmill exercise to a reference metabolic rate, set by convention to 3.5 ml O2 kg− 1 min− 1 or equivalently 1 kcal kg− 1 h− 1 or 4.184 kJ kg− 1 h− 1; being a ratio MET has no units.
Statistical analysis
All results are expressed as the mean ± standard error (SE) of mean. Statistical intra- and inter-individual comparisons of the studied compounds and the calculated ratios (cortisol to IL-6, cortisol to ACTH, and ACTH to IL-6), for the three time-points of the study protocol (pre-exercise vs. peak exercise vs. recovery) were calculated for the three studied groups of participants (treated vs. untreated vs. controls), by using one-factor repeated measures ANOVA followed by Fisher's post hoc test. In the ratios calculation, cortisol was expressed in ng/ml, whereas ACTH and IL-6 were expressed in pg/ml; cortisol to IL-6 and cortisol to ACTH ratios were divided by a 103 factor to adjust the ratio of their respective units. Linear correlations between either MET or METAUC and IL-6 concentrations were tested using Spearman's rank correlation coefficient. Statistical significance was set at p < 0.05.
Results
IL-6, ACTH, and cortisol concentrations
Interleukin-6
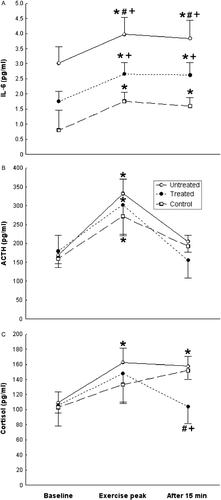
Statistical significance for all comparisons was calculated with one-factor repeated measures ANOVA, followed by Fisher's post hoc test [F(4, 100) = 12.851, p < 0.001; pooled MS = 13.140; df = 50.132; p < 0.001] (). Serum IL-6 concentrations in the untreated patients the dexamethasone-treated patients, and the healthy controls at exercise peak and recovery were significantly higher (p < 0.05) than their baseline concentrations (). IL-6 concentrations were significantly higher (p < 0.05) in the untreated and the dexamethasone-treated patients than in the healthy controls at baseline, exercise peak, and recovery (). Furthermore, IL-6 concentrations at exercise peak and recovery were significantly lower (p < 0.05) in the dexamethasone-treated patients than in the untreated patients (). At peak exercise, IL-6 concentrations were increased from baseline in the untreated (ΔIL-6 = 0.96 ± 0.14 pg/ml) and treated (ΔIL-6 = 0.91 ± 0.47 pg/ml) patients and in the controls (ΔIL-6 = 0.96 ± 0.18 pg/ml).
Figure 1. Serum IL-6 (A), plasma ACTH (B), and plasma cortisol (C) concentrations (mean ± SE) at baseline, exercise peak, and recovery (15 min after exercise) in untreated sarcoidosis patients (n = 27), dexamethasone-treated sarcoidosis patients (n = 17), and healthy control participants (n = 20). *Statistically significant (one-factor repeated measures ANOVA, Fisher's post hoc test, p < 0.05) change from the respective within-group baseline concentrations. #,+Statistically significant (one-factor repeated measures ANOVA, Fisher's post hoc test p < 0.05) difference, respectively, from the dexamethasone-treated sarcoidosis patients and the healthy control participants groups, at the same time-point.
Adrenocorticotropic hormone
Statistical significance for all comparisons was calculated with one-factor repeated measures ANOVA, followed by Fisher's post hoc test [F(4, 68) = 0.844, p = 0.502; pooled MS = 15,895; df = 77.239; p < 0.001]. ACTH concentrations in the untreated and the dexamethasone-treated patients and in the healthy controls at exercise peak were significantly higher (p < 0.05) than their baseline concentrations while no difference was found among the ACTH concentrations of the three studied groups when compared at each time-point (). At peak exercise, plasma ACTH concentrations were increased from baseline (p < 0.05) in the untreated (ΔACTH = 162.8 ± 29.9 pg/ml) and treated (ΔACTH = 123.3 ± 48.1 pg/ml) patients and in the controls (ΔACTH = 112.3 ± 41.7 pg/ml).
Cortisol
Statistical significance for all comparisons was calculated with one-factor repeated measures ANOVA, followed by Fisher's post hoc test [F(4, 68) = 0.848, p = 0.499; pooled MS = 5169.6; df = 56.117; p < 0.001]. Cortisol concentrations in the untreated patients at exercise peak and recovery were significantly higher (p < 0.05) than their baseline concentrations (). At exercise peak in the controls, cortisol concentrations did not differ from baseline, while at recovery they were significantly higher (p < 0.05) than the latter (). In the dexamethasone-treated patients, they did not differ at baseline, exercise peak, and recovery (). Cortisol concentrations at recovery were significantly lower (p < 0.05) in the dexamethasone-treated than in the untreated patients and the healthy controls while they did not differ at the three time-points of the study between untreated patients and controls (). Post-exercise, cortisol concentrations were increased significantly (p < 0.05) in the untreated patients (Δcortisol = 48.4 ± 14.7 ng/ml) and the controls (Δcortisol = 46.0 ± 15.9 ng/ml), but not significantly in the dexamethasone-treated patients (Δcortisol = − 1.43 ± 2.56 ng/ml).
TNF and IL-1β concentrations
Tumor necrosis factor
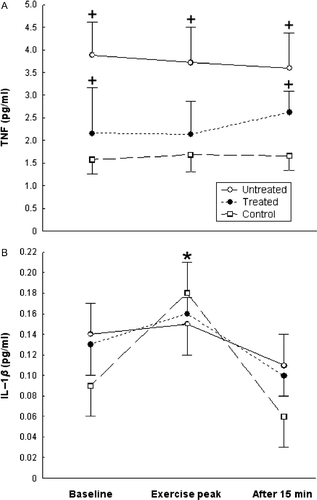
Statistical significance for all comparisons was calculated with one-factor repeated measures ANOVA, followed by Fisher's post hoc test [F(4, 90) = 1.141, p = 0.343 pooled MS = 41.059; df = 46.282; p < 0.05] (). No difference was found among serum TNF concentrations of the three studied time-points in each studied group (). TNF concentrations at baseline, exercise peak, and recovery were significantly higher (p < 0.05 for all time-points) in the untreated patients than in the healthy controls, while at baseline and recovery in the dexamethasone-treated patients they were significantly higher (p < 0.05) than in healthy controls ().
Figure 2. Serum TNF (A) and serum IL-1β (B) concentrations (mean ± SE) at baseline, exercise peak, and recovery (15 min after exercise) in untreated sarcoidosis patients (n = 27), dexamethasone-treated sarcoidosis patients (n = 17), and healthy controls (n = 20). *Statistically significant (one-factor repeated measures ANOVA, Fisher's post hoc test, p < 0.05) change from the respective within-group baseline concentrations. +Statistically significant (one-factor repeated measures ANOVA, Fisher's post hoc test, p < 0.05) difference from the healthy control participants group, at the same time-point.
Interleukin-1β
Statistical significance for all comparisons was calculated with one-factor repeated measures ANOVA, followed by Fisher's post hoc test [F(4, 80) = 1.423, p = 0.234; pooled MS = 0.04911; df = 65.956; p < 0.05]. IL-1β concentrations in the healthy controls were significantly higher (p < 0.05) at exercise peak than at baseline, while they did not differ at baseline, exercise peak, and recovery in the untreated and the dexamethasone-treated patients (). No difference was found among IL-1β concentrations at each studied time-point among the three studied groups ().
ACTH to IL-6, cortisol to IL-6 and cortisol to ACTH ratios
Acth:il-6
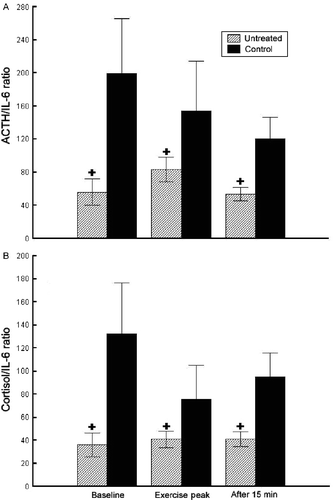
Statistical significance for all comparisons was calculated with one-factor repeated measures ANOVA, followed by Fisher's post hoc test [F(4, 68) = 3.464, p = 0.012; pooled MS = 32,438; df = 59.015; p < 0.05] (). The ACTH to IL-6 ratios were significantly higher in the healthy controls than in the untreated patients at baseline (p < 0.05), exercise peak (p < 0.05), and recovery (p < 0.05; ), while they did not differ significantly in the dexamethasone-treated patients (102.3 ± 43.2, 113.7 ± 29.6, 59.2 ± 28.8, respectively) from either the untreated patients or the healthy controls (data not shown).
Figure 3. ACTH to IL-6 ratios (A) and cortisol to IL-6 ratios (B) at baseline, exercise peak, and recovery (15 min after exercise) in untreated sarcoidosis patients (n = 27) and healthy control participants (n = 20). Cortisol to IL-6 ratio should be divided by a 103 factor to correct for the ratio of their respective units (ng/ml for cortisol; pg/ml for IL-6 and ACTH). +Statistically significant (one-factor repeated measures ANOVA, Fisher's post hoc test, p < 0.05) difference from the healthy control participants group, at the same time-point.
Cortisol:IL-6
Statistical significance for all comparisons was calculated with one-factor repeated measures ANOVA, followed by Fisher's post hoc test [F(4, 100) = 3.714, p = 0.007; pooled MS = 9010.8; df = 60.532; p < 0.05]. The cortisol to IL-6 ratios were significantly higher in the healthy controls than in the untreated patients at baseline (p < 0.05), exercise peak (p < 0.05), and recovery (p < 0.05; ), while they did not differ in the dexamethasone-treated patients (60.3 ± 28.5, 55.7 ± 19.1, 39.7 ± 16.7, respectively) from either the untreated patients or the healthy controls (data not shown).
Cortisol:acth
Statistical significance for all comparisons was calculated with one-factor repeated measures ANOVA, followed by Fisher's post hoc test [F(4, 68) = 0.099, p = 0.982; pooled MS = 0.738; df = 52.38; p < 0.05]. Cortisol to ACTH ratios did not differ at baseline, exercise peak, and recovery among the untreated (0.65 ± 0.22, 0.49 ± 0.22, 0.77 ± 0.17, respectively) and dexamethasone-treated (0.59 ± 0.37, 0.49 ± 0.24, 0.67 ± 0.39, respectively) patients and the healthy controls (0.66 ± 0.09, 0.49 ± 0.17, 0.79 ± 0.09, respectively).
MET and METAUC values and correlations with serum IL-6 concentrations
Statistical significance for all comparisons was calculated with one-factor repeated measures ANOVA, followed by Fisher's post hoc test [MET: F(2, 38) = 0.441, p = 0.646; MS = 9.365; df = 38.000; p>0.05; METAUC: F(2, 38) = 0.966, p = 0.390; MS = 521.15; df = 38.000; p>0.05]. In the untreated and dexamethasone-treated patients, and the healthy controls, the MET (9.91 ± 0.57, 9.19 ± 0.69, 10.55 ± 1.35, respectively) and the METAUC (37.13 ± 5.19, 46.11 ± 6.88, 47.8 ± 6.27, respectively) did not correlate with IL-6 concentrations at baseline (b = − 0.18 and 0.14; b = − 0.18 and − 0.22; b = 0.35 and 0.43, respectively, for the three groups) or exercise peak (b = − 0.18 and 0.16; b = 0.17 and − 0.27; b = 0.18 and 0.29, respectively, for the three groups).
Discussion
By employing an acute treadmill exercise model, we found that ACTH concentrations increased during exercise in the untreated and dexamethasone-treated sarcoidosis patients and in the healthy controls. No significant difference was found in ACTH concentrations among these three groups at any time-point. Treadmill exercise is a potent stimulator of the HPA axis (Luger et al. Citation1987; Mastorakos et al. Citation2005). The increase in ACTH concentration in the dexamethasone-treated patients indicates that exercise overrides the dexamethasone inhibition of the HPA axis via exercise-stimulated factors, such as arginine vasopressin, which induce ACTH release in synergy with or in addition to corticotropin-releasing hormone (CRH) and are minimally influenced by dexamethasone administration (Mastorakos et al. Citation2005).
Cortisol concentrations were increased during exercise and at recovery in the untreated patients and at recovery in the healthy controls, without differing significantly at any time-point. However, in the dexamethasone-treated patients, cortisol concentrations did not increase significantly during exercise, and at recovery levels they were lower than in the untreated patients and the healthy controls. The evidence of lack of cortisol increase during exercise in the latter indicates that their adrenals did not respond normally to the exercise-induced ACTH. This could be attributed to relatively lowered daily ACTH secretion (which exerts trophic activity on the adrenals) in these chronically dexamethasone-treated patients. The finding that ACTH responded to the acute exercise stimulation even in these dexamethasone-treated patients confirms the potent stimulatory effect of this type of exercise on the HPA axis. This dissociation between ACTH and cortisol responses to exercise after chronic dexamethasone administration is not observed after short-term dexamethasone administration in high- and low-intensity exercise protocols (Deuster et al. Citation2000).
Baseline IL-6 plasma concentrations were higher in both dexamethasone-treated and untreated sarcoidosis patients than in healthy controls. Furthermore, IL-6 levels increased during exercise in all three studied groups. During exercise the IL-6 concentrations were higher in the untreated and the dexamethasone-treated patients than in the control group, and higher in the untreated than in the dexamethasone-treated patients. Increased IL-6 concentrations in sarcoidosis patients have already been reported in plasma, sputum, bronchoalveolar lavage, and urine (Sahashi et al. Citation1991; Maniwa et al. Citation1998; Balamugesh et al. Citation2006; Guntupova et al. Citation2006). IL-6 is a cytokine produced from the contracting muscle during exercise and participating in the muscle–adipose tissue cross-talk (Pedersen Citation2009). Treadmill exercise stimulates IL-6 secretion probably via the activation of the sympathetic nervous system (Papanicolaou et al. Citation1996). The latter stimulates IL-6 production and/or secretion, either in a paracrine fashion by norepinephrine and the direct innervation of organs containing IL-6 producing cells, such as the thymus, or in a systemic endocrine fashion, through epinephrine, produced from the adrenal medulla, and exerting distal effects on IL-6 producing cells (Papanicolaou et al. Citation1996). During acute treadmill exercise, the increase in plasma IL-6 concentrations in healthy volunteers pre-treated with glucocorticoids is lower than in untreated healthy volunteers (Papanicolaou et al. Citation1996). Glucocorticoids seem to suppress IL-6 gene transcription through direct and/or indirect inhibition of the NF-κB transcription factor (Papanicolaou et al. Citation1996), thus explaining the trend to lower baseline IL-6 concentrations in the dexamethasone-treated than the untreated sarcoidosis patients in this study. However, the increased IL-6 levels in the dexamethasone-treated sarcoidosis patients compared to controls indicate that exercise remains a potent stimulator of IL-6 secretion even after chronic dexamethasone treatment (Gomez et al. Citation1993). IL-6, a pro-inflammatory cytokine, is a modulator of the immune system (Silver and Hunter Citation2010) and it is implicated in autoimmune pathogenetic mechanisms (Neurath and Finotto Citation2011). Thus, the exercise-associated IL-6 increase in the dexamethasone-treated sarcoidosis patients indicates that treadmill exercise as a stress stimulus could initiate unforeseen autoimmune reactions even in the presence of modest dexamethasone doses.
We found that the ACTH to IL-6 and the cortisol to IL-6 ratios in the healthy controls were higher than in the untreated patients at all three time-points of the treadmill exercise. This finding reflects the higher IL-6 concentrations in the untreated sarcoidosis patients than the control group in the face of the absence of difference in cortisol concentrations among these groups. The cortisol to ACTH ratio did not differ among the untreated and control groups, indicating that cortisol secretion was proportional to the ACTH stimulus in both groups.
Thus, although increased exercise-induced IL-6 concentrations seem to be associated with the potent stimulation of the HPA axis in sarcoidosis patients, the resulting cortisol concentrations were quantitatively similar to those resulting from HPA axis responses of the healthy participants to lower amounts of exercise-induced IL-6. This reflects either a dysfunctional IL-6-induced stimulation of the HPA axis or an adaptation of this axis to the chronically elevated IL-6 concentrations in sarcoidosis patients. This has been described before with exogenous chronic administration of IL-6 to cancerous patients of otherwise good health (Mastorakos et al. Citation1993; Päth et al. Citation2000) as well as with endogenous IL-6 in chronic inflammatory disease such as African trypanosomiasis (Hesse et al. Citation1988; Reincke et al. Citation1994). A truncated HPA axis response to high endogenous IL-6 concentrations has been described in autoimmune diseases such as rheumatoid arthritis (Chikanza et al. Citation1992), and in Sjogren syndrome, a truncated HPA axis response to oral CRH has been reported (Johnson et al. Citation1998).
Furthermore, we found that TNF concentrations did not increase with exercise at any time-point in any of the three studied groups, while they were higher in the untreated patients than in the healthy controls at all time-points. IL-1β concentrations increased significantly from baseline to exercise peak, but only in the control group, while no significant difference was observed among the three groups. In sarcoidosis, TNF, IL-1β, and other cytokines are produced by alveolar macrophages (Hunninghake Citation1984; Pueringer et al. Citation1993; Steffen et al. Citation1993) and can be found in bronchoalveolar lavage cells (Takizawa et al. Citation1997). Previous studies of sarcoidosis report either higher TNF concentrations than controls, with a good correlation with IL-6 (Balamugesh et al. Citation2006), or no difference at all (Bansal et al. Citation1997). The absence of increased baseline IL-1β concentrations in sarcoidosis patients confirms previous studies (Sakito et al. Citation1996; Bansal et al. Citation1997). In healthy volunteers, exercise does not increase TNF or IL-1β plasma concentrations (Ullum et al. Citation1994; Ostrowski et al. Citation1998), while exercise-induced increase in glucocorticoid levels is followed by the suppression of IL-1β and TNF but not of IL-6 production (DeRijk et al. Citation1997). Thus, the absence of TNF and IL-1β stimulation in this study may be the result of the exercise-induced cortisol increase. Of note, in the pro-inflammatory cascade, TNF and IL-1 increases precede increase in IL-6 and are of shorter duration (Suzuki et al. Citation2002). In this study, MET and METAUC values did not differ among the three studied groups. MET is a measure of the energy cost of physical activity expressing the rate of energy consumption (Ainsworth et al. Citation2011). Recently, Ardic et al. (Citation2011) demonstrated lower MET values only in a group of sarcoidosis patients with radiographic stages III and IV compared with patients with radiographic stages 0, I, and II disease. This could explain the absence of difference in MET values in this study between healthy control participants and sarcoidosis patients who were newly diagnosed and demonstrated moderate disease. The absence of any significant correlation between the MET or METAUC values and the serum IL-6 concentrations, a measure of the intensity of the inflammatory state, does not support an association of mild inflammation with acute energy consumption in this case.
In summary, we have shown that baseline plasma ACTH and cortisol concentrations in untreated sarcoidosis patients are similar to those of healthy control participants, while baseline serum IL-6 concentrations are higher in the former. In addition, ACTH and cortisol concentrations of untreated sarcoidosis patients increased during exercise in a similar way to healthy controls.
In conclusion, the HPA axis of untreated sarcoidosis patients reacted to treadmill exercise stimulation similarly to that of healthy control participants. This increase is presumably exerted via the increase in circulating IL-6 (Papanicolaou et al. Citation1996). Although increased IL-6 concentrations seem to be associated with potent stimulation of the HPA axis in sarcoidosis patients, their cortisol concentrations are ‘inappropriately’ similar to those of the healthy participants who demonstrate lower IL-6 concentrations than the former (ACTH to IL-6 and cortisol to IL-6 ratios in untreated sarcoidosis patients were lower than those of healthy control participants during exercise). Thus, in sarcoidosis patients, a dysfunctional IL-6-induced response of the HPA axis or an adaptation of this axis in the face of the chronically elevated IL-6 concentrations might exist (Mastorakos et al. Citation1993; Späth-Schwalbe et al. Citation1994). Consequently, the ‘inappropriately’ normal cortisol concentrations in response to a potent inflammatory stimulus could participate in the pathogenesis of sarcoidosis by not limiting the expression of IL-6-facilitated autoimmune phenomena during stress.
Declaration of interest : The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Disclosure summary: The authors have nothing to disclose.
References
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DRJr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011. Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 43:1575–1581.
- American College of Sports Medicine. 2006. Guidelines for exercise testing and prescription. Philadelphia, PA: Lippincott Williams & Wilkins.
- Antoniou KM, Tzanakis N, Tzouvelekis A, Samiou M, Symvoulakis EK, Siafakas NM, Bouros D. 2006. Quality of life in patients with active sarcoidosis in Greece. Eur J Intern Med. 17:421–426.
- Ardic I, Kaya MG, Yarlioglues M, Dogdu O, Buyukoglan H, Kalay N, Kanbay A, Zencir C, Ergin A.. 2011. Impaired heart rate recovery index in patients with sarcoidosis. Chest. 139:60–68.
- Balamugesh T, Behera D, Bhatnagar A, Majumdar S. 2006. Inflammatory cytokine levels in induced sputum and bronchoalveolar lavage fluid in pulmonary sarcoidosis. Indian J Chest Dis Allied Sci. 48:177–181.
- Bansal AS, Bruce J, Hogan PG, Allen RK. 1997. An assessment of peripheral immunity in patients with sarcoidosis using measurements of serum vitamin D3, cytokines and soluble CD23. Clin Exp Immunol. 110:92–97.
- Buske-Kirschbaum A, Ebrecht M, Hellhammer DH. 2010. Blunted HPA axis responsiveness to stress in atopic patients is associated with the acuity and severeness of allergic inflammation. Brain Behav Immun. 24:1347–1353.
- Chikanza IC, Petrou P, Kingsley G, Chrousos G, Panayi GS. 1992. Defective hypothalamic response to immune and inflammatory stimuli in patients with rheumatoid arthritis. Arthritis Rheum. 35:1281–1288.
- Crofford LJ, Kalogeras KT, Mastorakos G, Magiakou MA, Wells J, Kanik KS, Gold PW, Chrousos GP, Wilder RL. 1997. Circadian relationships between interleukin (IL)-6 and hypothalamic–pituitary–adrenal axis hormones: Failure of IL-6 to cause sustained hypercortisolism in patients with early untreated rheumatoid arthritis. J Clin Endocrinol Metab. 82:1279–1283.
- DeRijk R, Michelson D, Karp B, Petrides J, Galliven E, Deuster P, Paciotti G, Gold PW, Sternberg EM. 1997. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1 beta (IL-1 beta), IL-6, and tumor necrosis factor-alpha (TNF alpha) production in humans: High sensitivity of TNF alpha and resistance of IL-6. J Clin Endocrinol Metab. 82:2182–2191.
- Deuster PA, Petrides JS, Singh A, Chrousos GP, Poth M. 2000. Endocrine response to high-intensity exercise: Dose-dependent effects of dexamethasone. J Clin Endocrinol Metab. 85:1066–1073.
- Dubaniewicz A. 2010. Mycobacterium tuberculosis heat shock proteins and autoimmunity in sarcoidosis. Autoimmun Rev. 9:419–424.
- Facco M, Cabrelle A, Teramo A, Olivieri V, Gnoato M, Teolato S, Ave E, Gattazzo C, Fadini GP, Calabrese F, Semenzato G, Agostini C. 2011. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax. 66:144–150.
- Gomez MT, Magiakou MA, Mastorakos G, Chrousos GP. 1993. The pituitary corticotroph is not the rate limiting step in the postoperative recovery of the hypothalamic–pituitary–adrenal axis in patients with Cushing syndrome. J Clin Endocrinol Metab. 77:173–177.
- Guntupova LD, Borisov SE, Kupavtseva EA, Mikhaîlova LP, Makarova OV. 2006. The cytokine profile in granulomatous lung diseases. Probl Tuberk Bolezn Legk. 6:10–14 (Article in Russian).
- Hesse DG, Tracey KJ, Fong Y, Manogue KR, Palladino MAJr, Cerami A, Shires GT, Lowry SF. 1988. Cytokine appearance in human and endotoxemia and primate bacteremia. Surg Gynecol Obset. 166:147–153.
- Hunninghake GW. 1984. Release of interleukin-1 by alveolar macrophages of patients with active pulmonary sarcoidosis. Am Rev Resp Dis. 129:569–572.
- Iannuzzi MC, Rybicki BA, Teirstein AS. 2007. Sarcoidosis. N Engl J Med. 357:2153–2165.
- Johnson EO, Vlachoyiannopoulos PG, Skopouli FN, Tzioufas AG, Moutsopoulos HM. 1998. Hypofunction of the stress axis in Sjogren's syndrome. J Rheumatol. 25:1508–1514.
- Luger A, Deuster PA, Kyle SB, Gallucci WT, Montgomery LC, Gold PW, Loriaux DL, Chrousos GP. 1987. Acute hypothalamic–pituitary–adrenal responses to the stress of treadmill exercise. Physiologic adaptations to physical training. N Engl J Med. 316:1309–1315.
- Maniwa K, Ogushi F, Haku T, Sone S, Ohmoto Y. 1998. Sarcoidosis associated with acute renal failure and increased levels of interleukin-6 in urine. Intern Med. 37:757–761.
- Mastorakos G, Chrousos GP, Weber JS. 1993. Recombinant interleukin-6 activates the hypothalamic–pituitary–adrenal axis in humans. J Endocrinol Metab. 77:1690–1694.
- Mastorakos G, Ilias I. 2000. Relationship between interleukin-6 (IL-6) and hypothalamic–pituitary–adrenal axis hormones in rheumatoid arthritis. Z Rheumatol. 59:75–79.
- Mastorakos G, Pavlatou M. 2005. Exercise as a stress model and the interplay between the hypothalamus–pituitary–adrenal and the hypothalamus–pituitary–thyroid axes. Horm Metab Res. 37:577–584.
- Mastorakos G, Pavlatou M, Diamanti-Kandarakis E, Chrousos GP. 2005. Exercise and the stress system. Hormones (Athens). 4:73–89.
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 317:256–260.
- Neurath MF, Finotto S. 2011. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 22:83–89.
- Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK. 1998. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol (Lond). 513:889–894.
- Papanicolaou DA, Petrides JS, Tsigos C, Bino S, Kalogeras KT, Wilder R, Gold PW, Deuster PA, Chrousos GP. 1996. Exercise stimulates interleukin-6 secretion: Inhibition by glucocorticoids and correlation with catecholamines. Am J Physiol. 271:E601–E605.
- Päth G, Scherbaum WA, Bornstein SR. 2000. The role of interleukin-6 in the human adrenal gland. Eur J Clin Invest. 30:91–95.
- Pedersen BK. 2009. Edward F. Adolph distinguished lecture: Muscle as an endocrine organ: IL-6 and other myokines. J Appl Physiol. 107:1006–1014.
- Pueringer RJ, Schwartz DA, Dayton CS, Gilbert SB, Hunninghake GW. 1993. The relationship between alveolar-macrophage TNF, IL-1 and PGE2 release, alveolitis, and disease severity in sarcoidosis. Chest. 103:832–838.
- Reincke M, Heppner C, Petzke F, Allolio B, Arlt W, Mbulamberi D, Siekmann L, Vollmer D, Winkelmann W, Chrousos GP. 1994. Impairment of adrenocortical function associated with increased plasma tumor necrosis factor-alpha and interleukin-6 concentrations in African Trypanosomiasis. Neuroimmunomodulation. 1:14–22.
- Sahashi K, Ina Y, Takada K, Sato T, Yamamoto M, Morishita M. 1991. Significance of interleukin 6 in patients with sarcoidosis. Chest. 106:156–160.
- Sakito O, Kadota J, Kohno S, Abe K, Shirai R, Hara K. 1996. Interleukin 1 beta, tumor necrosis factor alpha, and inteleukin 8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis: Potential mechanism of macrolide therapy. Respiration. 63:42–48.
- Silver JS, Hunter CA. 2010. gp130 at the nexus of inflammation, autoimmunity, and cancer. J Leukoc Biol. 88:1145–1156.
- Späth-Schwalbe E, Born J, Schrezenmeier H, Bornstein SR, Stromeyer P, Drechsler S, Fehm HL, Porzsolt F. 1994. Interleukin-6 stimulates the hypothalamus–pituitary–adrenocortiscal axis in man. J Clin Endocrinol Metab. 79:1212–1214.
- Steffen M, Petersen J, Oldings M, Karmeier A, Magnussen H, Thile HG, Reedler A. 1993. Increased secretion of tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 by alveolar macrophages from patients with sarcoidosis. J Allergy Clin Immunol. 91:939–949.
- Suzuki K, Nakaji S, Yamada M, Totsuka M, Sato K, Sugawara K. 2002. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev. 8:6–48.
- Takizawa H, Satoh M, Okazaki H, Matsuzaki G, Suzuki N, Ishii A, Suko M, Okudaira H, Morita Y, Ito K. 1997. Increased IL-6 and IL-8 in bronchoalveolar lavage fluids (BALF) from patients with sarcoidosis: Correlation with the clinical parameters. Clin Exp Immunol. 107:175–181.
- Tzioufas AG, Tsonis J, Moutsopoulos HM. 2008. Neuroendocrine dysfunction in Sjogren's syndrome. Neuroimmunomodulation.. 15:37–45.
- Ullum H, Haahr PM, Diamant M, Palmo J, Halkjaer-Kristensen J, Pedersen BK. 1994. Bicycle exercise enhances plasma IL-6 but does not change IL-1 alpha, IL-1 beta, IL-6, or TNF-alpha pre-mRNA in BMNC. J Appl Physiol. 77:93–97.
- Zietz B, Reber T, Oertel M, Glück T, Schölmerich J, Straub RH. 2000. Altered function of the hypothalamic stress axes in patients with moderately active systemic lupus erythematosus. II. Dissociation between androstenedione, cortisol, or dehydroepindrosterone and interleukin 6 or tumor necrosis factor. J Rheumatol. 27:911–918.
- Zissel G, Prasse A, Müller-Quernheim J. 2010. Immunologic response to sarcoidosis. Semin Respir Crit Care Med.. 31:390–403.
