Abstract
Several studies have demonstrated that the presence of stressors during pregnancy induces adverse effects on the neuroendocrine system of the offspring later in life. In the present work, we investigated the effects of early programming on the male reproductive system, employing a prenatal stress (PS) paradigm. This study found that when pregnant dams were placed in a plastic restrainer three times a day during the last week of pregnancy, the offspring showed reduced anogenital distance and delayed testicular descent. Serum luteinising hormone (LH) and follicle-stimulating hormone (FSH) levels were decreased at postnatal day (PND) 28 and testosterone was decreased at PND 75. Increased testosterone plus dihydrotestosterone (T + DHT) concentrations correlated with increased testicular 5α Reductase-1 (5αR-1) mRNA expression at PND 28. Moreover, PS accelerated spermatogenesis at PND 35 and 60, and increased mean seminiferous tubule diameter in pubertal offspring and reduced Leydig cell number was observed at PND 35 and 60. PS offspring had increased androgen receptor (AR) mRNA level at PND 28, and at PND 35 had increased the numbers of Sertoli cells immunopositive for AR. Overall, the results confirm that stress during gestation can induce long-term effects on the male offspring reproductive system. Of particular interest is the pre-pubertal imbalance of circulating hormones that probably trigger accelerated testicular development, followed by an increase in total androgens and a decrease in testosterone concentration during adulthood. Exposure to an unfavourable intrauterine environment might prepare for harsh external conditions by triggering early puberty, increasing reproductive potential.
Introduction
Foetal programming has been defined as the action of several stimuli or events that are capable of inducing permanent changes in the patterns of gene expression of a developing organism when they occur during critical periods of development. As a consequence, lifelong modifications of the physiology, behaviour, morphology and neuroendocrine development have been reported (Kemme et al., Citation2007). In this respect, perinatal environment and maternal well being, especially during pregnancy, are two important factors that exert profound influences on the development of offspring. When one or both of these factors are altered, for example by exposure to stressful events, early and long-term effects on behaviour, reproduction, immune function and the endocrine system have been found (Mastorci et al., Citation2009; Weinstock, Citation2001). Research in this field showed that, in both humans and in experimental animals, the offspring of stressed pregnant mothers displayed, for example, impaired locomotion, and cognitive and sexual behaviour alterations (Darnaudery & Maccari, Citation2008; Maccari & Morley-Fletcher, Citation2007; Pallares et al., Citation2007). As the hypothalamic-pituitary-testicular (HPT) axis is actively functioning during the prenatal period (Huhtaniemi, Citation1995), special attention has recently been focused on the effects of stress during gestation, with particular interest targeted on the long-term consequences of prenatal stress (PS) on HPT function.
In rats, testosterone production by the Leydig cells in the testes is detectable from day 14.5 of gestation. Adequate levels of both testosterone and luteinising hormone (LH) receptor in the testes are essential for the proper masculinisation of the external genitalia structures and for the persistence of the Wolffian duct system (Hughes & Acerini, Citation2008; Huhtaniemi, Citation1995; Knickmeyer & Baron-Cohen, Citation2006). However, LH is not required for initial fetal Leydig cell activity, although the cells are responsive to LH from an early stage. (O'Shaughnessy & Fowler, Citation2011). After genital formation, testicular activity continues until birth and remains active for a few PNDs. A marked increase of testosterone production between gestational days 18.5 and 19.5 has been suggested to be responsible for sexual differentiation of the brain, including gonadotrophin secretion patterns, sexual and aggressive behaviours and morphometric parameters (Seale et al., Citation2005).
Insufficient androgen concentrations during this period might lead to a ‘feminised' brain development. In turn, a developmental impairment of the central nervous system alters the subsequent regulation of endocrine function (Huhtaniemi Citation1995; Knickmeyer & Baron-Cohen, Citation2006; Marshall, Citation1976). Glucocorticoid receptors have been detected in Leydig cells (Weber et al., Citation2000), which are the pre-eminent source of testosterone in male mammals (Gao et al., Citation2002). It has been shown that stress and synthetic glucocorticoid exposure during gestation exert suppressive effects on gonadal steroidogenesis and also diminish LH and testosterone concentrations in the serum of male foetuses. As a result, demasculinisation of the male rat brain and damage to the integrity of the HPT axis has been observed (Arena & Pereira, Citation2002; Oliveira et al., Citation2011; Reznikov et al., Citation2005).
Inappropriate sexual behaviour of adult offspring and gonadal dysfunction have also been reported: PS reduced the number of adult male copulations, decreased the number of ejaculations, enhanced lordotic-like behaviours and increased male partner preference over receptive females (Gerardin et al., Citation2005; Kapoor & Matthews, Citation2011; Shono & Suita, Citation2003). Additionally, a lack of tonic gonadotrophin secretion and altered testosterone secretion profiles were also shown, even during adulthood, in prenatally stressed animals (Gerardin et al., Citation2005; Rodriguez et al., Citation2007; Shono & Suita, Citation2003), and sexual morphological parameters, such as the timing of testicular descent, were also found to be altered (Barros et al., Citation2006). More recently, Schopper et al. (Citation2012) showed that exposing pregnant guinea pigs to mild stress accelerated growth and reproductive maturity in female offspring. In summary, numerous studies indicate that reproductive health and fertility can be ‘programmed' by early events in prenatal life.
Our laboratory has a long standing interest in the effects of PS on development of the midbrain dopaminergic system (DA) (Baier et al., Citation2012). We have previously demonstrated that PS impairs DA metabolism in limbic brain areas, especially after puberty, indicating a particular sensitivity of the DA system to variations in gonadal hormone peaks (Carboni et al., Citation2010; Katunar et al., Citation2010; Silvagni et al., Citation2008) Based on the above background and our own observations, we hypothesised that PS produces an unbalanced hormonal milieu that might be involved in the DA metabolism alterations observed in our studies. Our first approach to evaluate this hypothesis was to study the reproductive axis of the male offspring employing our model of prenatal restraint stress.
In the present study, we evaluated the serum concentrations of pituitary and testicular hormones as well as external morphometric parameters to assess development of the male offspring reproductive system. To further evaluate the mechanisms underlying long-term PS consequences on the male reproductive axis, the effects of PS on the progeny testes were measured, with respect to histomorphometry, androgen receptor (AR) immunoreactivity and mRNA levels for AR and both steroid 5α-reductase (5αR 1 and 2) isozymes, by quantitative real-time reverse transcription-polymerase chain reaction (RT-qPCR).
Materials and methods
Animals
Virgin female Wistar rats weighing 250 g were obtained from highly inbred rats from the animal facility at the University of Buenos Aires. A maximum of five dams were housed per cage with ad libitum access to standard rat chow (Asociación de Cooperativas Argentinas, Buenos Aires, Argentina) and water. A constant light/dark cycle, with lights on at 06:00 h and off at 18:00 h, and a room temperature of 21–25 °C were maintained. Vaginal smears were collected daily prior to mating in order to determine the stage of the oestrous cycle and the day of conception. On the day of proestrus, females were individually mated with a sexually experienced male Wistar rat. Vaginal smears were taken on the following morning. The day on which spermatozoa were found in the smear was designated as day 1 of pregnancy. All procedures were in agreement with the standards for the care of laboratory animals as outlined in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (Facultad de Medicina, UBA).
Prenatal procedures and experimental design
Pregnant dams were randomly assigned to either the control or the PS group and were individually housed with ad libitum access to standard rat chow and water. A constant light/dark cycle (on at 06:00 h, off at 18:00 h) was maintained at a temperature of 21–25 °C. Control rats (n = 8) were left undisturbed in the home cage, while PS dams (n = 8) were subjected to a restraint stress procedure, which involved rats being transferred to an experimental room where the stressor was applied. Pregnant females were individually placed into a transparent plastic restrainer fitted closely to body size for three 45-min periods per day (09:00, 12:00 and 16:00 h) between days 14 and 21 of pregnancy. The restrainer had ventilation holes, and dimensions appropriate for a pregnant rat of 350 g: internal diameter 64 mm, and an adjustable length of 149–208 mm. This type of stressor was chosen because it has an indirect influence on the foetuses via a direct stress on the mother (Maccari et al., Citation1995; Ward & Weisz, Citation1984). The sessions were performed in a lit environment. No other subjects were present in the experimental room during the stress exposure. At the end of the stress session, the rats were returned to the animal housing room and were then individually housed with free access to food and water. On the day of parturition, litter characteristics were recorded, and litters culled to 10 pups, maintaining similar numbers of males and females, wherever possible. The length of gestation, litter size and litter body weight were recorded. Eight litters were maintained for each experimental group. Sexual development markers were recorded until weaning at PND 21. The male and female offspring were housed in separate cages, with no more than five pups per cage, and with standard rat chow and water ad libitum. In this study, only male offspring were used. To avoid litter effects, a maximum of one to two pups from each litter was tested for each experiment. illustrates the experimental design. Different maturational ages (days, D) are designated as prepubertal (PND28), pubertal (PND 35) and adult (PND 60 and over).
Sexual developmental markers
Anogenital distance
Anogenital distance was measured using a vernier-calliper on a randomly selected male pup from each litter at PND 1, 10 and 21. (Gerardin et al., Citation2005; Pereira et al., Citation2006).
Testicular descent
Testicular descent was defined as the day when both testes were fully descended into the scrotal sac and could be palpated while the males were held vertically under their forelimbs. Rats were examined daily, starting on PND 21 (Barros et al., Citation2004; Shono & Suita, Citation2003). Results are expressed as the mean percentage of rats per litter that completed testicular descent, per day.
Tissue collection
In order to avoid possible adverse effects on hormonal measures, the rats were rapidly euthanised by decapitation between 09:00 and 10:00 h. Five to nine males from different litters at PND 28, 35, 45, 60 or 75 were used for this procedure. Trunk blood was collected and serum separated and stored at −80 °C for hormone analysis by radioimmunoassays (RIA).
Testes from offspring at PND 28, 35 and 60 were removed, weighed and processed for histological or immunohistochemistry studies. For histological measures, the testes were fixed in an alcoholic Bouin's solution (0.4% picric acid; 7% acetic acid; 70% ethanol 80°; 30% formaldehyde). For immunohistochemical analysis, 10% formaldehyde was used. In both cases, testes were manually cut into approximately 5-mm coronal slices to facilitate fixative penetration and fixed for 24 h. The testes were then processed by routine histological methods and finally embedded in paraffin wax. From each age and treatment group evaluated, 5-μm thick serial coronal sections from four different levels of the testes were cut using a microtome. Each section was mounted onto a silane-coated slide. The slides were then stained with haematoxylineosin using a routine protocol for histological measures.
Serum hormone assays
LH and follicle-stimulating hormone (FSH) concentrations were determined using kits obtained from the National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases, and Dr A. F. Parlow. The RIA procedure was performed as described (Catalano et al., Citation2010). Results were expressed in terms of RP3 rat LH and FSH standards. Assay sensitivities were 0.015 ng/ml for LH and 0.117 ng/ml for FSH. Intra-assay coefficients of variation (CV) were 7.2% for LH and 8.0% for FSH (Catalano et al., Citation2010).
Measurements of testosterone concentration in serum samples from control and PS rats were determined by employing an antibody obtained from Medicorp Inc. and Immunotech Diagnostics (Montreal, Canada). Assays were performed after extraction of the serum with diethyl ether (Merck). For testosterone, an antibody (testosterone-7α-butyrate-BSA) that has 35% cross-reactivity with dihydrotestosterone (DHT) and 0.04% cross-reactivity with 5α-androstane-3α-17β diol (Diol) was used. Therefore, results are expressed in terms of testosterone and DHT (T + DHT) serum levels (Matzkin et al., Citation2009; Russell et al., Citation1998). The minimum detectable concentration was 0.042 ng/ml and the intra-assay CV was 12%.
In order to further determine specific testosterone concentrations in serum samples, a single assay using the TESTO-RIA-CT Kit (DIA source Immuno Assays), which is more specific for the hormone, showing 0.31% cross-reactivity with DHT, was used. Assay sensitivity was 0.05 ng/ml, and intra-assay CVwas 4.6%.
Measurements of Diol concentrations in serum samples of control and PS rats were made with a specific antibody obtained from Medicorp Inc. and Immunotech Diagnostics (Montreal, Canada), which showed 5% cross-reactivity with testosterone (Rulli et al., Citation1995). Assays were performed after extraction of serum with diethyl ether (Merck). The minimum detectable assay concentration was 0.105 pmol/ml. Intra-assay CV was less than 15%.
The results were expressed as ng of hormone per ml (ng/ml) of serum.
Histological studies
Haematoxylin–eosin-stained sections were examined under a light microscope, and the computational software Imaging software NIS-Elements Basic Research Version 3.0 from Nikon Instruments Inc (Melville, NY) was employed for the analysis. The following characteristics were evaluated:
Seminiferous tubule diameter: the mean seminiferous tubule external diameter in mm was obtained by measuring the 50 most circular seminiferous tubules per rat at 100× total magnification (objective: 10 ×; ocular 10×) (Chandra et al., Citation2010).
Quantification of Leydig cell number: the number of Leydig cells per unit volume of testis was calculated using the Floderus Equation (Tapanainen et al., Citation1984):
where NA is the number of Leydig cell nuclei per unit area of section; T is the thickness of section; D is the average nuclear diameter and 2 h is the height of the smallest recognisable nuclear section. Three rats per age group and three blocks per rat were used. The number of Leydig cell nuclei per unit area (NA) was counted in 100 different grid fields (200 μm on the section) at 400× total magnification (objective: 40 X; ocular: 10×). Nuclear profiles overlapping the boundaries of the grid were ignored. D was obtained by direct measurements of the long and short axes, both in C and EP Leydig cells, and only those nuclei that had both values in a similar range were considered for analysis. Mendis-Handagama & Ewing (Citation1990) demonstrated that when these criteria are met, the use of the Floderus method results in similar Leydig cell numbers to those obtained with the dissector method, which is an unbiased method with respect to the particle shape under study, but dependent on shrinkage.
Spermatogenesis analysis: the morphology and progression of maturation of the germinal epithelium in the seminiferous tubules was categorised according to Johnsen's score, originally devised for human testes (Johnsen, Citation1970). Johnsen's score for the rat was developed by Lewis-Jones & Kerrigan (Citation1985). Briefly, the score applies a grade from 1 to 10 to each tubule cross section according to the following criteria: 10 = complete spermatogenesis and perfect tubules; 9 = many spermatozoa present with disorganised spermatogenesis; 8 = only a few spermatozoa present; 7 = no spermatozoa, but many spermatids present; 6 = only a few spermatids present; 5 = no spermatozoa or spermatids, but many spermatocytes present; 4 = only a few spermatocytes present; 3 = only spermatogonia present; 2 = no germ cells present, but Sertoli cells present; 1 = no germ cells and no Sertoli cells present. The mean Johnsen's score was calculated for 100 seminiferous tubules per rat.
Immunohistochemical analysis of ARs
Paraffin sections were de-waxed and hydrated following the procedure described by Matzkin et al. (Citation2009). AR immunodetection required antigen retrieval, so sections were heated in a microwave oven (4 min at 700 W, 14 min at 280 W followed by 20-min rest) for antigenic recuperation using citrate buffer (0.01 M, pH 6). Endogenous peroxidase activity was blocked by treating the sections with 3% H2O2 in 10% methanol for 20 min. Following this, sections were permeabilised by incubation for 5 min with 0.5% saponin in Tris-buffered saline (TBS) 0.05 M. Non-specific proteins were blocked by incubating the sections with a protein block buffer (10% fetal bovine serum and 1% BSA in TBS) for 60 min. Sections were then incubated overnight at 4 °C in a humidified chamber with the antiserum (polyclonal rabbit anti-AR serum, 1:200, Santa Cruz Biotechnology, Inc., Santa Cruz, CA; Matzkin et al., Citation2009) diluted in incubation buffer (0.02 M NaPO4H2.H2O, 0.15 M NaCl, sodium azide 1% BSA, pH 7.6). On the following day, testicular sections were washed and incubated with biotinylated secondary anti-serum (goat anti-rabbit IgG; 1:200, Sigma) for 2 h at room temperature. Finally, primary antibody binding was visualised by incubating the sections with an avidin-biotin-peroxidase (ABC) kit (Vectastatin ABC; Vector Laboratories) for 1 h, followed by incubation with 0.01% H2O2 and 0.05% 3,3-diaminobenzidine solution (in 0.05 M Tris-HCl, pH 7.6) for 5–10 min. The sections were dehydrated through a graded ethanol series, cleared with xylene and mounted with DPX mountant liquid (Sigma). The negative controls were processed by replacing the primary antibody with TBS. No indication of staining was observed.
mRNA Isolation and quantitative RT-qPCR
Testes from PND 28 and PND 75 rats belonging to control or PS groups were dissected and homogenised in Trizol reagent (Life Technologies, Carlsbad, CA) to isolate total RNA, according to the manufacturer's instructions. PolyA + mRNA was purified using the PolyATract mRNA Isolation System (Promega, Madison, WI). Complementary DNA was synthesised using oligo dT and SuperScriptTM II Reverse Transcriptase (Life Technologies, Carlsbad, CA).
qPCRs were carried out in a 7500 Real-Time PCR System (Applied Biosystems, Life Technologies, Foster City, CA). Quantification of each cDNA was achieved using SYBR Green Master Mix Reagent (Applied Biosystems) in triplicate. Primer sequences are detailed in . Normalisation was accomplished employing succinate dehydrogenase complex subunit A (sdha) and beta-2 microglobulin (b 2 m) as the reference gene. Values shown in all figures were calculated using b 2 m as the reference gene. Normalisations to sdha resulted in almost identical patterns. Relative quantification was performed using a comparative CT method (Guide to Performing Relative Quantitation of Gene Expression Using Real-Time Quantitative PCR. 2004) (Pfaffl, Citation2001). Before each experiment, the calibration curves were validated. Samples with curves amplified out of the calibrated dynamic range were eliminated. All procedures followed the manufacturer's instructions. Values shown are relative to the control animals at PND 28.
Table 1. Characteristic of oligonucleotide primers and amplified products of real-time PCR.
Statistical analysis
Repeated-measure ANOVAs were used to test the differences between prenatal treatments in testicular descent over time. The statistical significance of Johnsen's scores among the groups was determined using the Kruskal–Wallis test. Results for mean diameter of seminiferous tubules, Leydig cell number and immunopositive AR expression were analysed by Student's t-test. LH, testosterone + DHT, specific testosterone and Diol hormone concentrations, anogenital distance and RT-PCR data for AR and 5αR enzyme mRNAs were analysed by two-way ANOVAs in order to evaluate the effects of prenatal treatment, age and possible interactions between both factors. When significant interactions were found, simple effects ANOVA was used. Tukey's post hoc test was performed to test differences between more than two groups. Data for FSH concentrations in serum were also evaluated using the Kruskal–Wallis test followed by the Mann–Whitney U test for comparisons among ages of the same group. This non-parametric statistical method was chosen because it was not possible to check the equality of variances for the data we obtained.
All response variables were transformed for the analysis if normal distributions of residuals were not achieved. Visual inspection of histograms, qq plots and random distribution of fitted values were checked after data transformation. All results are presented as mean ± SEM. The observed differences were considered to be statistically significant when p < 0.05, and n values reported in figures represent the number of litters. Analysis of data was performed by using SPSS 13.0 version and Infostat 2011.
Results
Prenatal stress effects on gestation and male pup physical characteristics
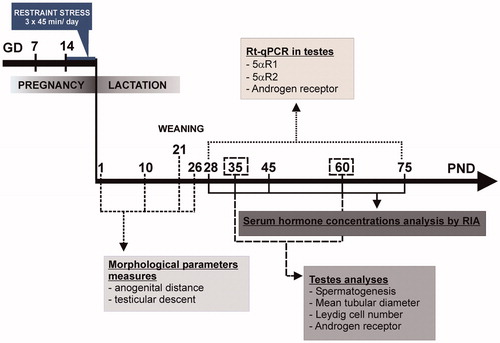
No significant differences were found in the length of gestation, litter size or body weight between control and PS animals, and neither missing limbs nor gross malformations were found in any of the newborn pups. However, the analysis of sexual developmental markers demonstrated that PS males showed a significant decrease of the anogenital distance at all ages evaluated, in comparison to control males (; two-way ANOVA: F1,36 = 16.38, p < 0.001 for prenatal treatment factor effect). Moreover, while most of the control rats completed testis descent at PND 23, PS rats showed a delay by two days (; Repeated-measures ANOVA: F1,28 = 6.582, p < 0.001 for control versus PS at PND 23; F(1,28) = 12.77, p < 0.001 for control versus PS at PND 24).
Figure 2. Effect of prenatal stress on the sexual development landmarks of male pups. C, control; PS, prenatal stress; PND, postnatal day. (A) Values represent the mean anogenital distance ± SEM. Groups with no letters in common are significantly different (two-way ANOVA followed by main effects analyses: p < 0.001 for prenatal treatment factor effects; p < 0.001 for age factor effects; n = 7). (B) Values represent the mean percentage ± SEM of rats per litter in which both testes descended into the scrotal sac. Groups with no letters in common are significantly different (Repeated measures ANOVA followed by simple effects ANOVA analyses: p < 0.05 for C versus PS rats at PND 21; p < 0.001 for C versus PS rats at PND 23; p < 0.001 for C versus PS rats at PND 24; p < 0.001 for age factor effect in C group and p < 0.001 forage factor in PS group; n = 4–5 litters per treatment).
Pituitary and testicular hormone responses to PS
Serum gonadotrophins and androgen hormones were measured at different PNDs.
Serum gonadotrophin concentrations
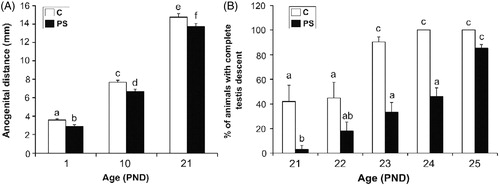
Serum FSH concentrations in control offspring showed a typical profile, with high levels at PND 28 that waned to reach adult levels at PND 75. In contrast, serum FSH concentrations of PS offspring showed a dramatic decrease at PND 28, in comparison to control concentrations. Nevertheless, the concentrations increased to follow the control curve at older ages () (Kruskal–Wallis test: H = 7.37, p < 0.01 for control versus PS at PND 28; H = 15.64, p < 0.01 for age factor effects in control group). A similar effect was found when serum LH concentrations were analysed: PND 28 PS rats showed reduced LH concentrations when compared to control rats. In addition, LH concentrations were significantly lower in PS rats aged 75 days when compared to control rats (; Two-way ANOVA: F1,51 = 25.43, p < 0.001 for PND 28 and F1,51 = 8.66 p < 0.01 for PND 75).
Figure 3. Effect of prenatal stress on serum concentrations of pituitary and testicular hormones. C, control; PS, prenatal stress; PND, postnatal day. Values represent the mean ± SEM serum concentrations of: (A) follicle stimulating hormone (FSH) (Kruskal–Wallis followed by Mann–Whitney U test: p < 0.01 for C versus PS rats at PND 28; p < 0.01 for differences among ages in C group; n = 5–9); (B) luteinizing hormone (LH) (two-way ANOVA followed by simple effects ANOVA analyses: p < 0.001 for C versus PS rats at PND 28; p < 0.01 for C versus PS rats at PND 75; p < 0.001 for age factor effect in PS group; n = 5–9); (C) testosterone + dihydrotestosterone (T + DHT) (two-way ANOVA followed by main effects analysis: p < 0.001 for prenatal treatment factor effects; p < 0.001 for age factor effects; n = 5); (D) specific testosterone (two-way ANOVA followed by simple effects ANOVA analyses: p < 0.001 for C versus PS rats at PND 75; p < 0.001 for age factor effects in C group; p, 0.001 for age factor effects in PS group; p = 5–7). (E) 5α-androstane-3α-17β diol (Diol) (two-way ANOVA followed by simple effects ANOVA analyses: p < 0.001 for C versus PS rats at PND 28; p < 0.001 for C versus PS rats at PND 45; p < 0.001 for age factor effects in C group; p < 0.001 for age factor effects in PS group; n = 5–9). In all cases, the significant differences between groups are indicated with different letters.
Serum androgen concentrations
Testosterone + DHT serum concentrations in control offspring showed a typical inverted V profile with a peak at PND 60 that levelled off at PND 75. Although PS rats showed the same pattern, the androgen concentrations in serum were increased relative to control offspring values (; two-way ANOVA: F1,32 = 46.89 p < 0.001 for prenatal treatment factor effects; F3,32 = 127.09, p < 0.001 for age factor effects). Specific serum testosterone concentrations in PS rats were decreased compared with control offspring at PND 75 (; two-way ANOVA: F1,52 = 10.59, p < 0.001). Serum Diol concentrations in control offspring showed a peak at PND 35 that levelled off to adult concentrations at PND 75. Serum Diol concentrations in PS offspring showed a significant increase at PND 28 and 45 in comparison to control offspring (; two-way ANOVA: F1,56 = 17.02, p < 0.001 for PND 28 and F1,56 = 35.68, p < 0.001 for PND 45).
5αR isoenzyme mRNA expression
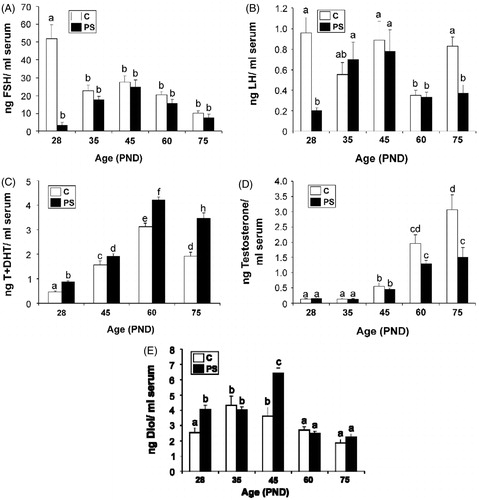
5αR isoenzyme mRNA expression was evaluated in testes at PND 28 and PND 75 in control and PS rats. Only 5αR1 mRNA levels were affected by PS: 5αRl mRNA levels were significantly greater in the PND 28 testes of PS rats compared to the control group. Decreased enzyme mRNA levels were observed at PND 75 with no statistically significant differences between groups at that age (; two-way ANOVA: F1,16 = 288, p < 0.001 for control versus PS at PND 28; FM6 = 10.8, p < 0.01 for age factor effect in control rats; F1,16 = 512, p < 0.001 for age factor effects in PS rats).
Figure 4. Effect of prenatal stress on the testicular steroid 5α-reductase isoform 1 (A) and 2 (B) mRNA expression in 28- and 75-day-old rats. C, control; PS, prenatal stress; PND, postnatal day. The mRNA levels determined by real-time PCR are expressed relative to the housekeeping gene beta-2 microglobulin mRNA expression as arbitrary units and as % of PND 28 control rats. Values are reported as mean ± SEM and different letters depict significant differences between groups (two-way ANOVA followed by simple effects ANOVA analyses: p < 0.001 for C versus PS rats at PND 28; p < 0.01 for age factor effects in C group; p < 0.001 for age factor effects in PS group; n = 4–5).
Prenatal stress on testis histology
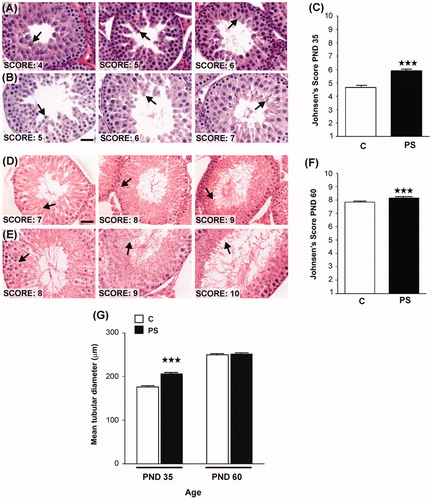
Gross histological analysis of haematoxylin–eosin-stained testis sections demonstrated that testicular histology was similar in the control and PS groups. Both groups showed the albugineous layer covering all of the tissue, which was comprised of seminiferous tubules surrounded by an interstitial space. No malformations or abnormal spermatogenic maturation was evident in either group. Histomorphometric analysis of PND 28 offspring was attempted, but most tubules were closed and the spermatic series completely undifferentiated. No differences in the histomorphometric analysis of PND 28 control and PS rats testes were found (data not shown). Both pubertal (PND 35) and adult (PND 60) PS offspring showed advancement of spermato-genic maturation () in comparison to control rats of the same age. Germinal cell analysis using Johnsen's criteria showed a higher score in PS rats (; Kruskal–Wallis test: H = 313.2, p < 0.001 control versus PS effects for PND 35 and H = 17.66, p < 0.001 control versus PS effects for PND 60). In addition, the seminiferous tubule diameter of PND 35 PS rats was significantly greater than in control rats (; Student t-test: t = 25.82, p < 0.001). However, the number of Leydig cells per cm3 of testis, calculated by using Floderus' equation, was reduced in the PS group at both PND 35 (C: (2.70 ± 0.24) × 107; PS: (1.62 ± 0.21) × 107; Student t-test: t = 5.75, p < 0.01) and 60 (C: (1.44 ± 0.15) × 107; PS: (1.18 ± 0.18) × 107; t = 3.69, p < 0.05).
Figure 5. Effect of prenatal stress on testicular histology of offspring. C, control; PS, prenatal stress. (A), (B)–(D), (E) Images of histological sections showing seminiferous tubules at different stages of spermatogenesis development in postnatal day (PND) 35 rats belonging to C (A) or PS (B) groups or in PND 60 rats belonging to C (D) or PS (E) experimental groups. Scale bar = 50 mm. Arrows show the last stage of maturation of the germinal epithelium. (C–F) Testes were evaluated histologically for the quality ofspermatogenesis according to the Johnsen score based on number and furthest maturation stage present, with a score of ‘10' being optimal with normal numbers of spermatozoa present and a score of ‘1' representing total absence of germ cells and Sertoli cells (Lewis-Jones & Kerrigan, Citation1985). Values are reported as mean ± SEM. Asterisks show the presence of differences in Johnsen's scores between C and PS rats on PND 35 (Kruskal–Wallis test: ***p < 0.001; n = 4) or PND 60 (Kruskal–Wallis test: ***p < 0.001; n = 4). (G) Mean tubular diameter of seminiferous tubules of PND 35 or PND 60 C and PS rats. Values are reported as mean ± SEM. Asterisks show differences in tubular diameter between C animals and PS rats in PND 35 rats (Student t-test: ***p < 0.001; n = 4).
Prenatal stress effects on AR in testis
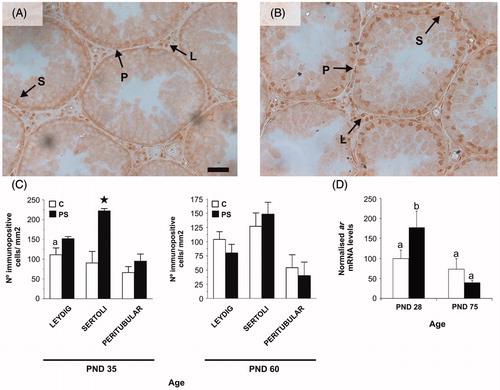
AR immunoexpression was predominant in the nuclei of Leydig, peritubular myoid and Sertoli cells with negative staining in all germ cells (). Stress during gestation increased the number of Sertoli cells with immunopositive AR-expression in PND 35 rats in comparison to control rats (; Student's t-test: t = 4.43, p < 0.05). In addition, PND 28 PS rats showed increased ar mRNA levels in the testes in comparison to control rats at the same age. However, ar mRNA levels of PS rats decreased by PND 75 and were then not statistically different from control rats (; two-way ANOVA: F1,15 = 4.54, p < 0.05 for C versus PS at PND 28; F1,15 = 18.8, p < 0.001 for age factor effects in PS rats).
Figure 6. Effect of prenatal stress on androgen receptor (AR) immunoreactivity (A, B and C) and mRNA levels (D) in rat testes. C, control; PS, prenatal stress; PND, postnatal day. Micrograph shows an example ofAR immunohistochemistry in testes at PND 35 in the (A) or PS (B) groups. Arrows shows positive immunoexpression ofthe AR in Leydig (L), Sertoli (S) and peritubular myoid (P) cells. Scale bar = 50 mm. (C) Number of immunopositive Sertoli, Leydig or peritubular cells per mm2 of testis for PND 35 and 60 rats. Values are mean ± SEM. Symbols represent significant differences between C and PS 35-day-old rats (Student t-test: a,*p < 0.05; n = 4–5). (D) ar mRNA levels in testes are mean ± SEM values, relative to expression of the housekeeping gene beta-2 microglobulin in arbitrary units and as % of PND 28 control rats. Different letters indicate significant differences between groups (two-way ANOVA followed by simple effects ANOVA analyses: p < 0.05 for C versus PS rats at PND 28; p < 0.001 for age factor effects in PS group; n = 4–5).
Discussion
In the present work, the consequences of PS on several aspects of the male offspring reproductive axis were studied. The results demonstrate that repeated exposure to maternal restraint stress prenatally markedly modulates the development of the offspring reproductive axis by inducing long-term changes in sexual maturation, hormone secretion patterns, testicular histology, AR protein and mRNA, and 5α-reductase mRNA expression in the testes.
Morphology
The examination of morphometric parameters, in particular the anogenital distance and testicular descent into the scrotum, provides the simplest and least invasive indicators of normal androgen exposure during the masculinisation programming window and the functioning of the foetal HPT axis, respectively (Scott et al., Citation2009). Anogenital distance is generally two-fold greater in males than in females, and testicular descent normally occurs at PND 21 in rats. In most mammals, the testes must descend from the abdomen to an extracorporeal position to provide a lower ambient temperature for normal spermatogenesis. In the rat, the release and movement of the testes into the scrotum occur in two phases: the transabdominal phase occurs during foetal life and is controlled by the insulin-like 3 factor which is produced by the foetal Leydig cells under the control of LH. The second phase, termed inguinoscrotal phase, is controlled by androgens and takes place after birth with the neonatal surge in testosterone levels. (Hughes & Acerini, Citation2008). If there is insufficient testosterone production or action during the masculinisation programming window in the foetal rat, this can result in disorders of masculinisation, including malformation of the penis, cryptorchidism, an underdeveloped prostate and reduced anogenital distance. Hence, in foetal males, a functional HPT axis is necessary for penile growth and also for proper testicular descent (Scott et al., Citation2009). In this study, PS reduced the anogenital distance in males at all ages recorded and induced a two-day delay in the completion of testicular descent. These results confirm and extend previous findings (Barros et al., Citation2006) and are in agreement with previous studies that have reported alterations of the organisation of the male genital system due to prenatal insults (Gerardin et al., Citation2005; Shono & Suita, Citation2003).
Interestingly, both parameters have been shown to be altered by prenatal exposure to anti-androgens during gestational days 14–19 (Gray et al., Citation2001; Hughes & Acerini, Citation2008; Vinggaard et al., Citation2005), which probably indicates a direct correlation between the testosterone peak on embryonic days 18.5–19.5, the androgen-related biosynthetic enzyme activities and the development of morphometric characteristics.
Pituitary hormones
Production of spermatozoa and secretion of testicular hormones are dependent on stimulation by the pituitary gonadotrophins FSH and LH. FSH plays a key role in the development of the immature testis and its secretion is negatively regulated by the testicular hormones inhibin B, testosterone and oestradiol (McLachlan et al., Citation2002; Whirledge & Cidlowski, Citation2010). In turn, LH binds to its receptor on Leydig cells to stimulate testosterone production (McLachlan et al., Citation2002). In this context, serum FSH concentrations in pre-pubertal control rats decline as the testes mature and testosterone levels increase. In this study, serum FSH concentrations of PS offspring were significantly lower at PND 28, then increased with age to reach control values. Moreover, serum LH concentrations in PS rats were diminished in comparison to control rats aged 28 and 75 days old. As mentioned above, LH stimulates testosterone production; so it is not surprising that this study and others have observed that PS diminished specific testosterone concentrations in adult life following the reduction of LH levels (Rodriguez et al., Citation2007; Shono & Suita, Citation2003; Ward et al., Citation2003; Weinstock Citation2001). It has been reported that PS can alter gonadal steroidogenesis in foetuses (Hughes & Acerini, Citation2008; Pereira et al., Citation2006), but in this model we suggest that PS led to an altered tonic gonadotrophin secretion, which might be responsible for the inappropriate release of testosterone levels during adulthood.
Gonadal hormones
Testosterone is not only essential for the initiation and maintenance of spermatogenesis (Russell et al., Citation1998), but also for the appearance and maintenance of secondary sex characteristics, as well as for the remodelling of the pubertal brain, by orchestrating its plasticity and behavioural maturation (McLachlan et al., Citation2002). However, most of the effects of testosterone on sexual maturation are mediated by the 5-alpha reduced metabolite, DHT, in target tissues (Auchus, Citation2004). Furthermore, in the testes, as in several other tissues including the brain, DHT can be reversibly oxidised to Diol. This metabolic conversion is considered to be a means of inactivating DHT, but is also a source of DHT (Naess et al., Citation1975; Pettersson et al., Citation2009). Although testosterone is the predominant androgen produced in adult mouse and rat testes, DHT and Diol are the dominant steroids secreted by immature testes from neonatal to pubertal stages (Auchus, Citation2004; Killian et al., Citation2003; Podesta & Rivarola, Citation1974; Tapanainen et al., Citation1984).
With the exception of testosterone, little information is available about the effects of PS on concentrations of other androgen hormones. In this study, testosterone + DHT concentrations in serum were increased in PS rats in comparison with controls at all ages. In addition, Diol concentrations were increased in PS offspring at PND 28 and 45. This result can be interpreted as indicating that the augmented concentrations of testosterone + DHT found in PS rats at all ages may be mainly due to an increase in DHT concentrations, which in turn could be reversibly converted to Diol through 3α-hydroxysteroid dehydrogenase (3αHSD). DHT is synthesised by testicular 5αR, which irreversibly converts testosterone to DHT. Stimulation of expression of the enzyme might result in an increase in the synthesis of DHT and thereafter Diol. To test this hypothesis, we investigated the expression of mRNAs for 5αR-1 and 5αR-2 isozymes in testicular homogenates. The increase of 5αR-1 mRNA expression at PND 28 of PS offspring observed supports the hypothesis that the increase in testosterone + DHT concentrations observed in our model might be principally due to an increase in DHT. In turn, DHT is converted to Diol, which was also observed to be increased. Even though both isozyme mRNAs are expressed at PND 28, only 5αR-1 mRNA was observed to be increased after PS, indicating a differential regulation of the isoforms. Although no evidence of this differential regulation has been reported for the immature testis, the 5αR-1 and 5αR-2 isozymes of the adult testis are differentially regulated by FSH and testosterone (Pratis et al., Citation2003). At the time (PND 28) when DHT and Diol were observed to be elevated, both LH and FSH levels were low in our model.
Alternatively, Diol can be synthesised by a second pathway that does not involve testosterone as an intermediate substrate. Auchus (Citation2004) demonstrated that in the testes of pouch young of the tammar wallaby Diol is synthesised by this alternative ‘backdoor pathway'. It can be speculated that in young PS offspring, Diol can be synthesised through this ‘backdoor pathway' as a compensatory mechanism, as the natural testosterone stimulatory hormones FSH and LH were decreased.
Testicular histology
It is well established that stress occurring prenatally or during early pubertal development may also limit the number of Leydig cells, which have increased expression of glucocorticoid receptors, by decreasing their rate of mitosis and inducing apoptosis (Gao et al., Citation2002; Pedrana et al., Citation2008). As expected, in this study the number of Leydig cell per cm3 of testis was diminished in comparison with controls, both in pubertal and adult PS rats.
The seminiferous tubule epithelium showed an advanced level of maturation both at PND 35 and 60 in PS rats. Accordingly, an increase in seminiferous tubular diameter of PS 35-day-old rats was identified. As spermatogenesis depends partially on stimulation by FSH, which was diminished at PND 28, it can be speculated that another mechanism besides gonado-trophin stimulation might be participating in the observed advancement of spermatogenesis. Indeed, several authors have reported that some degree of complete spermatogenesis can be initiated and maintained in the apparent absence of FSH stimulation (McLachlan et al., Citation2002; O'Shaughnessy et al., Citation2010; Russell et al., Citation1998). Previous studies showed that blockage of androgen action in the testes by administration of an AR antagonist, or by inducing LH or intratesticular-testosterone suppression, induces a significant increase in the concentration of testicular DHT and Diol through the up-regulation of 5αR. Therefore, sperm production was found in those animals despite suppression of gonadotrophin and testosterone (McLachlan et al., Citation2002). Moreover, experiments carried out in rats with intratesticular implants of DHT showed that this hormone reduces serum LH concentrations and also decreases intratesticular-testosterone concentrations. In addition, DHT was more effective for the maintenance of spermatogenesis than testosterone at comparable intratesticular concentrations (Chen et al., Citation1994). In fact, it was demonstrated that DHT and Diol, but not testosterone, stimulate meiosis in spermatogonial cells (Chemes et al., Citation1976; Killian et al., Citation2003), which was observed by the detection of a peak of 5αR isozymes and 5α-reduced metabolite production which coincides with the first wave of spermatogenesis in the rat. This indicates a role for 5α-reduced metabolites in the initiation of spermatogenesis. These observations support the hypothesis that the untimely increase of Diol and total androgen production, as a result of the upregulation of the 5 αR-1 isozyme, may be responsible for the advancement of spermatogenesis observed in this model of PS, despite the reduction in FSH, LH levels and the number of Leydig cells. We have no evidence about whether this acceleration prepares for enhanced reproductive functions, but from an evolutionary perspective it can be speculated that exposure to harsh conditions during intrauterine life might trigger fast reproductive maturation when longevity or even survival might be at risk. Although the species, gender and PS protocol are different, Schopper et al. (Citation2012) discuss a similar adaptive response of an early onset of reproduction in female offspring of PS guinea pigs.
Androgen receptor immunoreactivity
This study has shown that prenatally stressed prepubertal rats (PND 28) show increased ar mRNA level in testis homogenates followed by an increased AR immunoreactivity in Sertoli cells at PND 35, indicating increased expression of the receptor in these cells. Interestingly, it has been reported that AR expression in Sertoli cells, but not peritubular or Leydig cells, is essential for androgen action on spermatogonial stimulation and spermatocyte development in knock-out animals with conditionally impaired AR expression in the different cell populations of the testis (O'Shaughnessy et al., Citation2010; Rey et al., Citation2009). These observations add to the hypothesis that the increased 5α-reduced metabolites of testosterone present in pubertal PS rats, in combination with the augmented expression of the AR receptor in Sertoli cells, could be responsible for the advancement of the maturation of these specific cells, thereby accelerating spermatogenesis. However, neither the quantity nor the quality of spermatozoa production was assessed.
Conclusions
In summary, the above data indicate that maternal stress during the last week of gestation in the rat has long-term consequences in male offspring reproductive hormone profile and testis development. PS rats show increased total androgens (testosterone + DHT) concentrations in serum at all evaluated ages. Pre-pubertal PS offspring show decreased gonadotrophin and increased Diol concentrations in serum and increased 5αR1 and ar mRNA levels in the testes. Pubertal PS offspring show an advanced maturation of spermatogenesis with an increase of
AR immunoexpression in Sertoli cells and augmented Diol concentrations in serum. Adult PS offspring show decreased LH concentrations and a marked decrease in specific testosterone levels. It can be hypothesised that the untimely increase of Diol and total androgens together with the increase in AR levels in Sertoli cells could be responsible for the acceleration of the spermatogenesis process in pubertal PS rats. This acceleration might advance reproductive maturity as a strategy to cope with a possible unfavourable environment anticipated by the stress experienced in utero. Our future work will be directed to test the hypothesis that this imbalanced hormonal milieu might be involved in the brain DA metabolism alterations observed in our previous studies.
Declaration of interest
This research was supported by grants from CONICET (PIP 2065) and ANPCYT (PICT 31981). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
We are greatly indebted to Dr Rodolfo Rey and Dr Marcela Venara for their help in designing the morphological protocols and for critical reading of the manuscript and helpful discussions. We appreciate the help of Valentina Sorzzoni and Silvia Trejo in the histological techniques. The skilful technical assistance and bibliographical management of Mrs Susana Buglione is greatly appreciated.
References
- Arena AC, Pereira OC. (2002). Neonatal inhalatory anesthetic exposure: reproductive changes in male rats. Comp Biochem Physiol C Toxicol Pharmacol 133(4):633–40
- Auchus RJ. (2004). The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab 15(9):432–8
- Baier CJ, Katunar MR, Adrover E, Pallares ME, Antonelli MC. (2012). Gestational restraint stress and the developing dopaminergic system: an overview. Neurotox Res 22(1):16–32
- Barros VG, Berger MA, Martijena ID, Sarchi MI, Perez AA, Molina VA, Tarazi FI, Antonelli MC. (2004). Early adoption modifies the effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. J Neurosci Res 76(4):488–96
- Barros VG, Rodriguez P, Martijena ID, Perez A, Molina VA, Antonelli MC. (2006). Prenatal stress and early adoption effects on benzodiazepine receptors and anxiogenic behavior in the adult rat brain. Synapse 60(8):609–18
- Carboni E, Barros VG, Ibba M, Silvagni A, Mura C, Antonelli MC. (2010). Prenatal restraint stress: an in vivo microdialysis study on catecholamine release in the rat prefrontal cortex. Neuroscience 168(1):156–66
- Catalano PN, Di Giorgio N, Bonaventura MM, Bettler B, Libertun C, Lux-Lantos VA. (2010). Lack of functional GABA(B) receptors alters GnRH physiology and sexual dimorphic expression of GnRH and GAD-67 in the brain. Am J Physiol Endocrinol Metab 3(298):E683–96
- Chandra AK, Chatterjee A, Ghosh R, Sarkar M. (2010). Vitamin E-supplementation protect chromium (VI)-induced spermatogenic and steroidogenic disorders in testicular tissues of rats. Food Chem Toxicol 48(3):972–9
- Chemes HE, Podesta E, Rivarola MA. (1976). Action oftestosterone, dihydrotestosterone and 5alpha androstane 3alpha, 17beta diol on the spermatogenesis of immature rats. Biol Reprod 14(3):332–8
- Chen H, Chandrashekar V, Zirkin BR. (1994). Can spermatogenesis be maintained quantitatively in intact adult rats with exogen-ously administered dihydrotestosterone? J Androl 15(2):132–8
- Darnaudery M, Maccari S. (2008). Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev 57(2):571–85
- Gao HB, Tong MH, Hu YQ, Guo QS, Ge R, Hardy MP. (2002). Glucocorticoid induces apoptosis in rat leydig cells. Endocrinology 143(1):130–8
- Gerardin DC, Pereira OC, Kempinas WG, Florio JC, Moreira EG, Bernardi MM. (2005). Sexual behavior, neuroendocrine, and neurochemical aspects in male rats exposed prenatally to stress. Physiol Behav 84(1):97–104
- Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, et al. (2001). Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update 7(3):248–64
- Hughes IA, Acerini CL. (2008). Factors controlling testis descent. Eur J Endocrinol 159(Suppl 1):S75–82
- Huhtaniemi I. (1995). Molecular aspects of the ontogeny of the pituitary-gonadal axis. Reprod Fertil Dev 7(5):1025–35
- Johnsen SG. (1970). Testicular biopsy score count – a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1(1):2–25
- Kapoor A, Matthews SG. (2011). Testosterone is involved in mediating the effects of prenatal stress in male guinea pig offspring. J Physiol 589(Pt 3):755–66
- Katunar M, Saez T, Brusco A, Antonelli M. (2010). Ontogenetic expression of dopamine-related transcription factors and tyrosine hydroxylase in prenatally stressed rats. Neurotoxic Res 18(1):69–81
- Kemme K, Kaiser S, Sachser N. (2007). Prenatal maternal programming determines testosterone response during social challenge. Horm Behav 51(3):387–94
- Killian J, Pratis K, Clifton RJ, Stanton PG, Robertson DM, O'Donnell L. (2003). 5alpha-reductase isoenzymes 1 and 2 in the rat testis during postnatal development. Biol Reprod 68(5):1711–18
- Knickmeyer R, Baron-Cohen S. (2006). Fetal testosterone and sex differences. Early Human Develop 82:755–60
- Lewis-Jones D, Kerrigan D. (1985). A modified Johnsen's count for evaluation of spermatogenesis in the rat. IRCS Med Sci 13:510–11
- Maccari S, Morley-Fletcher S. (2007). Effects of prenatal restraint stress on the hypothalamus-pituitary-adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendocrinology 32(Suppl 1):S10–15
- Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M. (1995). Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci 15(1 Pt 1):110–16
- Marshall WA. (1976). Steroids after birth-puberty. Postgrad Med J 52(612):620–24
- Mastorci F, Vicentini M, Viltart O, Manghi M, Graiani G, Quaini F, Meerlo P, et al. (2009). Long-term effects of prenatal stress: changes in adult cardiovascular regulation and sensitivity to stress. Neurosci Biobehav Rev 33(2):191–203
- Matzkin ME, Gonzalez-Calvar SI, Mayerhofer A, Calandra RS, Frungieri MB. (2009). Testosterone induction of prostaglandinendoperoxide synthase 2 expression and prostaglandin F(2alpha) production in hamster Leydig cells. Reproduction 138(1):163–75
- McLachlan RI, O'Donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, Robertson DM. (2002). Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res 57:149–79
- Mendis-Handagama SM, Ewing LL. (1990). Sources of error in the estimation of Leydig cell numbers in control and atrophied mammalian testes. J Microsc 159(Pt 1):73–82
- Naess O, Attramadal A, Aakvaag A. (1975). Androgen binding proteins in the anterior pituitary, hypothalamus, preoptic area and brain cortex of the rat. Endocrinology 96(1):1–9
- O'Shaughnessy PJ, Fowler PA. (2011). Endocrinology of the mammalian fetal testis. Reproduction 141(1):37–46
- O'Shaughnessy PJ, Verhoeven G, De Gendt K, Monteiro A, Abel MH. (2010). Direct action through the sertoli cells is essential for androgen stimulation of spermatogenesis. Endocrinology 151(5):2343–8
- Oliveira M, Leao P, Rodrigues AJ, Pego JM, Cerqueira JJ, Sousa N. (2011). Programming effects of antenatal corticosteroids exposure in male sexual behavior. J Sex Med 8(7):1965–74
- Pallares ME, Scacchi Bernasconi PA, Feleder C, Cutrera RA. (2007). Effects of prenatal stress on motor performance and anxiety behavior in Swiss mice. Physiol Behav 92(5):951–6
- Pedrana G, Sloboda DM, Perez W, Newnham JP, Bielli A, Martin GB. (2008). Effects of pre-natal glucocorticoids on testicular development in sheep. Anat Histol Embryol 37(5):352–8
- Pereira OC, Bernardi MM, Gerardin DC. (2006). Could neonatal testosterone replacement prevent alterations induced by prenatal stress in male rats? Life Sci 78(24):2767–71
- Pettersson H, Lundqvist J, Oliw E, Norlin M. (2009). CYP7B1-mediated metabolism of 5alpha-androstane-3alpha,17beta-diol (3alpha-Adiol): a novel pathway for potential regulation of the cellular levels of androgens and neurosteroids. Biochim Biophys Acta 1791(12):1206–15
- Pfaffl MW. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
- Podesta EJ, Rivarola MA. (1974). Concentration of androgens in whole testis, seminiferous tubules and interstitial tissue ofrats at different stages of development. Endocrinology 95(2):455–61
- Pratis K, O'Donnell L, Ooi GT, Stanton PG, McLachlan RI, Robertson DM. (2003). Differential regulation of rat testicular 5alpha-reductase type 1 and 2 isoforms by testosterone and FSH. J Endocrinol 176(3):393–403
- Rey RA, Musse M, Venara M, Chemes HE. (2009). Ontogeny of the androgen receptor expression in the fetal and postnatal testis: its relevance on Sertoli cell maturation and the onset of adult spermatogenesis. Microsc Res Tech 72(11):787–95
- Reznikov AG, Nosenko ND, Tarasenko LV. (2005). Opioids are responsible for neurochemical feminization of the brain in prenatally stressed male rats. Neuro Endocrinol Lett 26(1):35–8
- Rodriguez N, Mayer N, Gauna HF. (2007). Effects of prenatal stress on male offspring sexual maturity. Biocell 31(1):67–74
- Rulli SB, Gonzalez-Calvar SI, Campo S, Calandra RS. (1995). Effects of two non-steroidal antiandrogens on testicular function in prepubertal rats. J Androl 16(3):225–32
- Russell LD, Kershaw M, Borg KE, El Shennawy A, Rulli SS, Gates RJ, Calandra RS. (1998). Hormonal regulation of spermatogenesis in the hypophysectomized rat: FSH maintenance of cellular viability during pubertal spermatogenesis. J Androl 19(3):308–19, discussion 341–302
- Scott HM, Mason JI, Sharpe RM. (2009). Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev 30(7):883–925
- Schopper H, Klaus T, Palme R, Ruf T, Huber S. (2012). Sex-specific impact of prenatal stress on growth and reproductive parameters of guinea pigs. J Comp Physiol B182(8):1117–27
- Seale JV, Wood SA, Atkinson HC, Lightman SL, Harbuz MS. (2005). Organizational role for testosterone and estrogen on adult hypothalamic-pituitary-adrenal axis activity in the male rat. Endocrinology 146(4):1973–82
- Shono T, Suita S. (2003). Disturbed pituitary-testicular axis inhibits testicular descent in the prenatal rat. BJU Int 92(6):641–3
- Silvagni A, Barros VG, Mura C, Antonelli MC, Carboni E. (2008). Prenatal restraint stress differentially modifies basal and stimulated dopamine and noradrenaline release in the nucleus accumbens shell: an ‘in vivo' microdialysis study in adolescent and young adult rats. Eur J Neurosci 28(4):744–58
- Tapanainen J, Kuopio T, Pelliniemi LJ, Huhtaniemi I. (1984). Rat testicular endogenous steroids and number of Leydig cells between the fetal period and sexual maturity. Biol Reprod 31(5):1027–35
- Vinggaard AM, Christiansen S, Laier P, Poulsen ME, Breinholt V, Jarfelt K, Jacobsen H, et al. (2005). Perinatal exposure to the fungicide prochloraz feminizes the male rat offspring. Toxicol Sci 85(2):886–97
- Ward IL, Ward OB, Affuso JD, Long WD, 3rd French JA, Hendricks SE. (2003). Fetal testosterone surge: specific modulations induced in male rats by maternal stress and/or alcohol consumption. Horm Behav 43(5):531–9
- Ward IL, Weisz J. (1984). Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology 114(5):1635–44
- Weber MA, Groos S, Hopfl U, Spielmann M, Aumuller G, Konrad L. (2000). Glucocorticoid receptor distribution in rat testis during postnatal development and effects of dexamethasone on immature peritubular cells in vitro. Andrologia 32(1):23–30
- Weinstock M. (2001). Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol 65(5):427–51
- Whirledge S, Cidlowski JA. (2010). Glucocorticoids, stress, and fertility. Minerva Endocrinol 35(2):109–25