Abstract
This study investigated Cortisol responses and perceived stress of 10–15-year-olds to a computerized paradigm including elements of social evaluation, unpredictability, and uncontrollability. Both age and sex differences were examined. Participants were 52 children and adolescents (23 boys, mean age = 12.5 years). Over the course of an approximately 2-h testing session participants were exposed to a computerized testing paradigm, the social evaluative stress test (SEST), that lasted for 50 min and includes elements of social evaluation, unpredictability and uncontrollability. Seven saliva samples were obtained to measure cortisol concentrations before, during and after the SEST, to provide pre-stress values, cortisol reactivity to the stressor and recovery after stress. In addition, subjective emotional stress experiences were recorded. The results showed no effect of age on cortisol responses. Furthermore, although both sexes reported experiencing the paradigm as (equally) stressful, only boys reacted with significant cortisol increases (M = 163%). To our knowledge, this is the first computerized stressor that induces cortisol responses in 10- to 15-year-old boys. Whether the girls' perceived stress results in the activation of other biological systems, such as the sympathetic nervous system as well as in differential activation of brain regions, remains to be determined. Future studies investigating sex differences in stress reactivity during adolescence should include neuroimaging, as well as psychophysiological measures, to unveil some of the mechanisms behind the current findings.
Introduction
In response to stressful challenges, the hypothalamic pituitary adrenal axis (HPA axis) becomes activated, resulting in the release of a cascade of hormones that helps the body to mobilize energy resources to cope more efficiently with a stressful situation. The hormone cortisol, secreted by the adrenal cortex, is the end product of this axis in humans, and the cortisol stress response is considered adaptive and necessary (Sapolsky et al., Citation2000). However, repeated or chronic activation of the HPA axis especially early in life can result in atypical HPA axis reactivity, with blunted or exaggerated HPA axis responses to stress. This, in turn, has been linked to psychopathology later in life (Heim & Nemeroff, Citation2001; Heim et al., Citation2008; Petrowski et al., Citation2010).
Infants are born with a functional HPA axis, reacting with the secretion ofcortisol when confronted with stressors (Jansen et al., Citation2010). During subsequent development and in response to experience, regulation of the HPA axis is further fine-tuned and adapted to each child's individual environment. An important period during development, in which humans are faced with an increasing number of (psychological) challenges, is the transition from middle childhood to adolescence. There is discussion in the literature about whether this period is also accompanied by organizational effects on the regulation of the HPA axis, which might lead to blunted HPA axis reactivity in 11- to 13-year-olds (Gunnar & Vazquez, Citation2006). However, some studies have reported normal HPA axis reactivity during that period (Kudielka et al., Citation2004).
Investigating developmental changes in HPA axis reactivity during adolescence is especially important as adolescence is known to be a vulnerable period for the development of a variety of psychological disorders, including depression, anxiety, and substance dependence (Kessler et al., Citation2005). Moreover, different psychological challenges and physiological changes are faced by male and female adolescents, which could relate to differences in HPA axis reactivity.
A few studies have examined cortisol responses in children during middle childhood and adolescence with the use of laboratory stressors. Social evaluation, combined with uncontrollability and unpredictability, has proven to be effective in eliciting a physiological stress response in laboratory conditions in both adults and children (Dickerson & Kemeny, Citation2004; Gunnar et al., Citation2009). Stroud et al. (Citation2009) examined the stress responses of 7- to 17-year-olds and showed that both children and adolescents reacted with significant increases in cortisol levels during a social performance task. In this study, the cortisol response was larger for the adolescents as compared to the younger children. Another study in children and adolescents aged 11–16 found a significant cortisol increase in reaction to a social performance paradigm only in older boys (13- to 15-year-olds) (Klimes-Dougan et al., Citation2001). These boys showed increases in salivary cortisol concentration of 40% above baseline. In similarly aged children of 12–15 years, Westenberg et al. (Citation2009) also found a significant cortisol increase in reaction to a public speaking task, with no significant main effects of age and sex. Furthermore, a study by Gunnar et al. (Citation2009) examined stress responses in children of four different ages. Nine-year-old children and 15-year-old adolescents reacted with a cortisol increase in response to the test. Of the 13-year-olds, only the girls showed a cortisol increase, while the 11-year-olds and 13-year-old boys showed no increase of salivary cortisol concentration in response to the stressor. Finally, Kudielka et al. (Citation2004) showed significant cortisol increases in 9- to 15-year-olds in response to the Trier Social Stress Test (TSST; Kirschbaum et al., Citation1993).
To summarize, previous research indicates that there might be developmental changes in stress reactivity during the period of middle childhood to adolescence in the physiology ofHPA axis reactivity to stressors. However, only a few studies are available, and the findings are variable. In addition, the role played by additional factors, including sex, in the developmental changes in stress reactivity also remains to be elucidated.
Thus, the aim of the present study was to examine the effects of age and sex on physiological stress reactivity (cortisol) in 10- to 15-year-old children. To this end, we used a newly developed laboratory stress paradigm that includes elements of social evaluation, unpredictability, and uncontrollability. Two important factors led to the decision to develop a new stress paradigm. First, we wished to develop a paradigm that was specifically aimed at adolescents. To achieve this, the evaluation was more focused on personal characteristics than on academic achievement. We hypothesized that this would be stressful for teenagers, because their personal identity and social self are developing and important for acceptance by others (Harter, Citation1990). Also, comparisons with peers were included in the paradigm because of the importance of peer relations in middle childhood and adolescence. The second reason was of a pragmatic nature. Extant effective laboratory stressors for children mostly include public speaking, requiring several experimenters to carry them out (test leader, two or more panel members). The paradigm designed for the present study is computer based, and therefore more efficient than public-speaking tasks.
Based on previous research and the age range of the present study, our hypothesis was that both younger and older children would react with significant increases in salivary cortisol concentration to the paradigm. Furthermore, and because of the contradictory findings in the literature, we examined sex differences in stress reactivity, but had no specific hypotheses on the direction of possible differences.
Methods
Participants
Children were recruited from five primary and three secondary schools in Nijmegen (The Netherlands) by distributing 1075 advertisements and information letters for both children and their parents. This recruitment resulted in 76 applications. Participants who used medication, had a diagnosis for neuropsy-chiatric or mental disorders, and children with recent exposure to traumatic events were excluded from the study (N = 7). Furthermore, 17 children were not included in the study because of scheduling difficulties and illness on the testing day. The final group consisted of 52 children (23 boys) with ages ranging from 10.4 to 15.5 years (M = 12.5 years, SD = 1.21). Written informed consent was obtained from all participants and their parents. The study was approved by the Faculty of Social Sciences' Ethical Committee of the Radboud University Nijmegen, which follows the international Helsinki Declaration.
Procedure
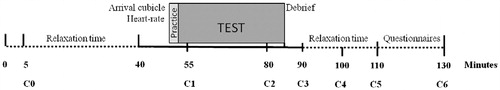
The testing session took place in the Behavioral Science Institute laboratory of the Radboud University Nijmegen. Sessions took place between 13:00 h and 20:00 h and had a total duration of 130 min (). Participants were asked to abstain from eating solids and performing extensive physical activities 45 min before arriving in the laboratory. After arrival, participants were photographed with a neutral facial expression against a neutral background in a black T-shirt. This photograph of their head and shoulders was for later use in the computer task. Afterward, participants relaxed for 40 min in a testing room that was equipped like a living room, including carpet, sofas, and stereo equipment. Participants were then taken to a cubicle where the stress-provoking computer paradigm took place. In the cubicle, participants were verbally instructed by the test leader about the computer paradigm (10 min). After that, the participants completed the computer paradigm (3-min practice, 32-min actual paradigm), followed by one assessment of perceived stress and debriefing (5 min). The cubicle part had a total duration of 50 min. In the final 40 min, participants again relaxed in the ‘living-room' while reading magazines (first 20 min) and filling out extra questionnaires (last 20 min). Saliva was collected for cortisol measurement at – 45, 5, 30, 40, 50, 60, and 80 min relative to the onset ofthe computerized paradigm. At the end of the testing session, each child received a €10 voucher as a gift for participation.
Computer paradigm: Social evaluative stress test
Upon entering the cubicle, participants' two fingers of their non-dominant hand were connected to a "heart-rate device" connected to the computer. This device was actually a dummy, but the participant was told that it registered heart rate and that even small movements of the hand could negatively affect its working. As movements could ruin the data, the child was asked to keep this hand as still as possible. However, and irrespective of the child's actual movements, the alarm light on the screen turned on at two predetermined time points during the answering of the 36 questions (see below) to indicate inappropriate movements.
The investigator then told the child that multiple-choice questions would appear on the computer screen. These could be answered with the dominant hand by means of a button box. A panel would judge the answers as well as the participant's facial expression and posture by means of a camera next to the computer. After that, the children completed the practice session with a total of 28 easy, non-personal questions (e.g. what is the color of this flower?). Thereafter, the following instructions were displayed on the screen in Dutch.
The Dutch Film Association (DFA) has produced a brand new and thrilling 3D film. They want it to be a success! They asked our research institute to find out whether kids of your age like the film. We are looking for kids who represent the youth of today. Their opinion about the film is important to us. They will be the first kids to watch the film and give their opinion about it! From research we know that nice kids represent the youth of today best. To find out whether you are nice and represent the youth of today, we ask you to fill in personal questions on the computer. A strict panel will compare your answers with those of four other children and select the three who represent today's youth the best. Those three kids may watch the film and give their opinion. However, if you belong to the remaining two you will have to give a presentation in front of the panel telling them why you think you are nice and represent the youth. If you are able to convince the panel, you may watch the film as well.
After these instructions, salivary cortisol sample 1 (C1) was taken. To increase the credibility of the story, the photograph of the participant together with photographs of the four other "participating children," as well as a photograph of the "panel" (i.e., four adults sitting in laboratory coats behind a table, with neutral expressions on their faces) were displayed on the screen. Besides that, one of the panel members wearing a laboratory coat in the photograph entered the room before the test began to resolve a supposed "program error that was delaying the start" and introduced him/herself to the participant.
shows the set-up of the computer screen during the test session. The photographs on the right show the position of each child: children in the green zone would watch the movie, while children in the red zone would have to give a presentation to the panel, acting as a jury.
Figure 2. Set up computer screen for the SEST. The photograph is derived from the Radboud Faces Database (Langner et al., Citation2010) and is not of a participant. The position of the photograph on the right changed from the green zone to the red zone during the test. Top left is the alarm light, activated twice during the test.

After the panel member had left, a total of 36 multiple-choice questions was displayed with a fixed time window of 20 s per question (the seconds remaining were shown on the screen). The questions included personal questions (i.e., addressing personal traits, ideas, hobbies, and position in life), and knowledge questions (i.e., addressing knowledge of facts with high levels of difficulty). Personal and knowledge questions were shown in a fixed design; in each block, three personal questions were followed by three knowledge questions. Prior to testing, 20 other children had evaluated the personal questions on age appropriateness, and meaningfulness. Based on their feedback, two test versions were designed: for ages of 10–12 years and 13–15 years. In total, participants were presented with six blocks of questions. After each block, intermediate judgments of the panel changed the participant's position from initially high to low. After answering all the questions, participants looked at a neutral screen for 5 min before being told that their final ranking was the fourth position and that they now had to prepare a presentation for the panel. After another 5 min, perceived stress was assessed and the stress test ended with the debriefing of the participant. In the debriefing, all participants were told that they did not actually have to present in front of the jury. Additionally, they were informed that the SEST was preprogrammed in such a way that it was impossible to win the competition. The SEST was programmed using E-prime version 1.0 (Schneider et al., Citation2002), and was designed to exclude speech and movement in the participant. In this way, it is compatible for use during future neuroimaging sessions. Moreover, the experimental design will enable explicit allocation of neural activation to specific test elements: the control condition (i.e., training session without social evaluation and including only easy, non-personal, knowledge questions), the test session with personal questions, and the test session with knowledge questions.
In sum, the SEST contains elements of social evaluation (i.e., picture of jury, comparison with other children, several judging moments, camera), unpredictability (i.e., unknown paradigm) and of uncontrollability (i.e., "heart-rate device" giving an alarm signal twice irrespective of movements, time pressure for answering questions, falling in the ranking, too difficult questions). In this way, the paradigm capitalized on the elements that are known to induce stress in children and adults in laboratory settings.
Cortisol
Seven saliva samples were taken to obtain cortisol measurements of pre-stress, stress, and recovery concentrations. The saliva samples (C0–C6) were obtained by having the participants spit through a short straw into a 2.5-ml tube at – 45, 5, 30, 40, 50, 60, and 80 min relative to the onset of the computerized paradigm (). All participants provided samples of sufficient volume and quality for assay. The samples were frozen at −20 °C before they were analyzed at the Psychobiology Laboratory of the University of Trier, Germany. The inter-assay coefficients of variation ranged between 7.1% and 9.0%; the sensitivity of the assay was 0.173 nmol/l.
Confounders
Perceived stress
To measure perceived stress, participants rated two items on a 7-point scale (1 = not at all stressful/exciting to 7 = very stressful/exciting), which are as follows: (1) How stressful/exciting did you find the experiment and (2) How stressful/exciting did you find the expectation of giving a presentation in front of the panel? There was a significant correlation between the items: r(49) = 0.62, p < 0.001. Therefore, the mean score of the two items was used in the analyses.
Credibility
Participants rated four items on a 7-point scale to assess the credibility of the social evaluative paradigm, which are as follows: (1) To what extent did you believe the cover story? (2) To what extent did you believe in the presence of other participants? (3) To what extent did you believe in the existence of the jury? (4) To what extent did you believe the heart-rate measures? The scores on the four items were all positively correlated, with r ranging between 0.22 and 0.53. To obtain one measure of credibility, the mean score of the four items was used in the analyses.
Additional confounders
To control the potential confounders that can influence cortisol reactivity, the following variables were included in the analyses: means of transportation (arrival by public transport or car versus arrival by bicycle), school type (primary versus secondary school), and time of day.
Statistical analyses
The first cortisol measurement C0 (t = −45 min) showed an excessive variation between participants, likely indicating a confounding effect from activities taking place prior to the arrival at the laboratory. It was thus excluded from all analyses. The six remaining cortisol samples (C1–C6) were used to study reactivity to and recovery from the stressor. Actual increase (delta) in cortisol concentration was calculated by subtracting the lowest of samples 1 and 2 (5 and 30 min, respectively) from the highest of samples 3, 4, and 5 (40, 50, and 60 min, respectively).
Skewed data were log-transformed, and all measures were checked for outliers. Each cortisol measurement (C1–C6) contained one positive outlier (six in total). Furthermore, one positive outlier in the actual increase in cortisol was detected. All outliers were replaced by the group mean plus three standard deviations (Hasings et al., Citation1947).
To analyze if the paradigm induced a significant increase between the baseline cortisol concentration (lowest of samples 1 and 2) and stress reactivity level (highest of samples 3, 4, and 5) for the group as a whole, a paired samples t-test was computed. A paired samples t-test was also computed between the stress reactivity level (highest of samples 3, 4, and 5) and the recovery level (sample 6) to analyze if the participants' cortisol concentration significantly decreased after the stress period.
To examine the differences between middle childhood and adolescence, two age groups were created (10–12 years and 13–15 years). To analyze the main effect of the paradigm on the cortisol concentrations, a three factor (sex by age group by time) mixed design analyses of variance (RM-ANCOVA) was computed. Greenhouse-Geisser corrections were applied where appropriate. As between-subject factors, child's sex and age (age groups of 10–12 years and 13–15 years) were included in the main analyses. Dependent variables were the cortisol concentrations (with time as the within-subject factor -5, 30, 40, 50, 60, and 80 min relative to the onset of the stressor). Because of the rather small sample size and in order to avoid possible spurious results (Simmons et al., Citation2011), we only included confounders that significantly correlated with the actual increase in cortisol as covariates in the main analyses.
Independent samples t-tests for all separate cortisol measurements were conducted to analyze differences between boys and girls, and differences between middle childhood (age of 10–12 years) and adolescence (age of 13–15 years).
The level for statistically significant differences was p < 0.05.
Results
shows the demographic characteristics and potential confounders of the sample for the total group as well as separated by sex and age groups. Independent sample t-tests showed that the time of testing occurred significantly later for girls than boys (t(50) = −2.48, p < 0.05). Also, the older children were tested significantly later compared to the younger age group (t(50) = −2.20, p < 0.05). Boys showed significantly higher actual cortisol concentration increases (delta) than girls (t(50) = −3.49, p < 0.001), and younger children showed a nonsignificant trend for higher cortisol increases than the older children (t(50) = 1.89, p = 0.07). Furthermore, the children's mean scores on perceived stressfulness and credibility of the test indicated that on average they found the test stressful and believable. Girls rated the credibility of the test higher than boys (t(49) = −2.03, p < 0.05), while there were no significant differences in perceived stressfulness between boys and girls or between younger and older children.
Table 1. Descriptive statistics and possible confounders of the sample (N = 52).
Paired samples t-tests showed a significant difference between pre-stress cortisol concentrations and peak levels (t(51) = −4.04, p < 0.001, d = 0.66), indicating that the paradigm induced a significant increase in childrens' cortisol concentrations for the group as a whole (). Furthermore, there was a significant difference in cortisol between peak concentrations and recovery levels (t(51) = 5.37, p < 0.001, d = 0.62), indicating that the children's cortisol concentrations returned to baseline after the end of the paradigm. Regarding sex differences, for boys, paired samples t-tests showed a significant increase in cortisol concentration (t(22) = −4.04, p < 0.001, d = 1.22), followed by a significant decrease in cortisol concentration (t(22) = 4.37, p < 0.001, d = 0.82). For girls, the increase in cortisol showed only a trend for significance (t(28) = −1.88, p = 0.07, d = 0.27), whereas the ensuing decrease in cortisol concentration was significant (t(28) = 3.54, p < 0.001, d = 0.56).
Figure 3. Salivary cortisol concentrations (means ± SEM) over the stress paradigm: pre-stress concentration (lowest of samples 1 and 2), stress concentration (highest of samples 3, 4, and 5), and recovery concentration (sample 6) for the group as a whole (N = 52), for boys (N = 23) and for girls (N = 29). Paired samples t-tests were used to examine the differences between pre-stress level, stress level and recovery level. ***p < 0.001, †p = 0.07.
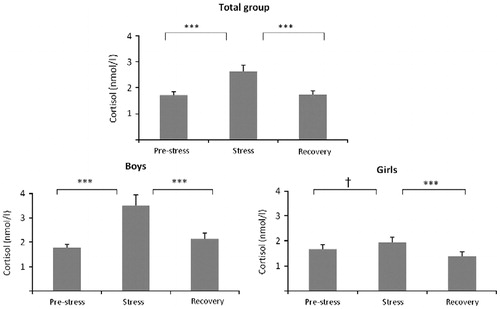
shows the course of the cortisol concentrations separately for girls and boys. Independent samples t-tests showed that boys had significantly higher cortisol values than girls at C3 (t(33.43) = 3.06, p < 0.05, d = 0.93), C4 (t(30.04) = 2.70, p < 0.05, d = 0.83), C5 (t(34.30) = 2.82, p < 0.05, d = 0.85), and C6 (t(50) = 2.80, p < 0.05, d = 0.80).
Figure 4. Salivary cortisol concentrations (means ± SEM) over the social evaluative stress paradigm (SEST) for boys (N = 23; mean increase in cortisol = 163%), and girls (N = 29; mean increase in cortisol = 44%). The gray box indicates the stress phase. Independent samples t-tests were used to examine the difference in the six cortisol measurements between boys and girls. **p < 0.001, *p < 0.05.
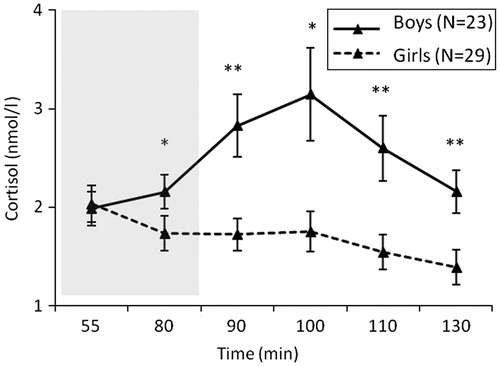
shows the course of cortisol concentrations for the two age groups (10–12 years and 13–15 years). While younger children seemed to react with higher cortisol concentrations as compared to older children, independent samples t-tests showed that, apart from the two non-significant trends, C4 (t(48.63) = 1.89, p = 0.06, d = 0.48) and C5 (t(49.94) = 1.78, p = 0.08, d = 0.46), there were no significant differences between the six cortisol measurements of the younger and older children.
Figure 5. Salivary cortisol concentrations (means ± SEM) over the social evaluative stress paradigm (SEST) for 10- to 12-year-olds (N = 32; mean increase in cortisol = 79.8%), and 13- to 15-year-olds (N = 20; mean increase in cortisol = 30.2%). The gray box indicates the stress phase. Independent samples t-tests were used to examine the difference in the six cortisol measurements between age groups. †1p = 0.06, †2p = 0.08.
The correlations () showed that the actual increase in cortisol concentration (delta) was not correlated with child age, but was significantly correlated with child sex; boys reacted with significantly higher increases in cortisol concentration than girls (r(50) = −0.39, p < 0.01). Of the confounders, means of transportation was significantly correlated with the delta cortisol concentration (r(47) = −0.32, p < 0.05). Children who had cycled to the laboratory showed lower delta values than children who had come by car/bus. Therefore, means of transportation was included as a confounder in the main analyses. Furthermore, the delta cortisol values were significantly correlated with perceived stress, with children reporting higher levels of perceived stress showing higher actual increases in cortisol concentration (r(49) = 0.29, p < 0.05). The delta cortisol values were not correlated with the other confounders (i.e., time of testing, school type, and credibility of the paradigm), and these were therefore excluded from further analyses. Furthermore, the correlations () showed that when the group was split by sex, only means of transportation continued to be significantly positively correlated with the delta cortisol values in girls (r(25) = −0.48, p < 0.05).
Table 2. Correlations between the actual increase in cortisol concentration and potential confounders for the total group (N = 52), boys (N = 23), and girls (N = 29).
Analysis of variance
To analyze the dynamic of the cortisol concentrations across the testing session, a repeated measures ANCOVA was computed, with child sex and age group (10–12 years and 13–15 years) as a between-subject factor, and means of transportation and perceived stress as confounders. The analysis revealed no significant main effect of time for the repeated measurements analysis of cortisol concentrations (F(2.64, 113.66) = 0.20, p > 0.05). However, and in line with the reported t-tests, the cortisol measurements significantly interacted with child's sex (F(2.64, 113.66) = 5.41, p < 0.01), with boys showing higher cortisol reactivity to the paradigm than girls. Children's age did not significantly interact with the cortisol measurements (F(2.64, 113.66) = 0.32, p > 0.05). Furthermore, the confounders' means of transportation (F(2.64, 113.66) = 5.35, p < 0.01) and perceived stress (F(2.64, 113.66) = 3.72, p < 0.05) significantly interacted with the cortisol measurements as described earlier.
Discussion
This study examined cortisol responses of 10- to 15-year-olds to a computerized paradigm (SEST), including elements of social evaluation, unpredictability, and uncontrollability. Both age and sex differences were examined. The results showed no effects of age on cortisol responses. In contrast, the cortisol response was significantly affected by sex: although both sexes reported the paradigm as (equally) stressful, only boys reacted to the paradigm with significant cortisol increases. To our knowledge, this is the first computerized stressor that induces cortisol responses in 10- to 15-year-old boys. The question remains why boys, and not girls, responded with increased cortisol concentrations to the SEST paradigm.
From a psychological point of view, an explanation for finding substantial cortisol reactions in boys can be found in the elements of the paradigm we used. Previous research showed that men are physiologically more affected by achievement type stressors when compared to women (Stroud et al., Citation2002). Our paradigm included achievement (i.e. knowledge questions in competition with peers), which could have led to stronger cortisol increases in boys. However, on the other hand, previous research has also shown that women show stronger physiological responses to interpersonal challenges and social rejection (Stroud et al., Citation2002). The SEST paradigm contained an interpersonal challenge (i.e. competition with peers, personal questions) and social rejection (i.e. ranking based on personal questions), but these elements were perhaps not powerful enough in their present form to trigger stress in the girls. This could be due to the use of an indirect, computerized interpersonal challenge and rejection, instead of a more direct, face-to-face one. However, if the nature of the paradigm was responsible for the sex differences in cortisol responses, one would expect boys to not only show higher cortisol responses, but also to report the task as more stressful. This was not the case: both boys and girls reported the paradigm to be equally stressful. This makes it probable that other than psychological factors are behind the sex differences found in physiological reactivity.
Physiological differences between boys and girls could also (partly) explain why only boys reacted with cortisol reactions to the SEST paradigm. In adults, there is a difference between males and females in their physiological reactivity to acute stressors. Females show greater interoceptive corticolimbic reactivity to acute stress, whereas males show stronger peripheral reactivity, including the HPA axis (Ordaz & Luna, Citation2012). This sex difference emerges in mid-to late-adolescence and is probably caused by gonadal hormones. During puberty, estrogen levels increase in girls with high levels of estrogen receptor density especially in the corticolimbic circuitry (Ter Horst et al., Citation2009). Estrogens block the receptor binding of cortisol by facilitating the production of corticosteroid binding globulin, a molecule that binds to cortisol (McEwen, Citation2002). The increased levels of estrogens during puberty are related to the age of menarche (DiVall & Radovick, Citation2008). Moreover, estrogen levels fluctuate during the menstrual cycle, with the highest concentrations in the follicular phase. Although in adult females the menstrual phase is related to cortisol responses (Kajantie & Philips, Citation2006), there is only one study that examined this during adolescence. Bouma et al. (Citation2009) found that adolescent free cycling girls (aged 15–17 years) reacted with lower cortisol responses to a stressor than boys. However, there were no effects of menstrual phase, possibly because girls of this age had not developed a stable menstrual cycle. Because our sample was quite young, it is likely that not all the girls were already menstruating. Unfortunately, neither the onset of the menstrual cycle, nor the menstrual cycle phase was assessed in the current study. Thus, we can only speculate that the observed sex differences in cortisol responses in the current study might have been caused in part by girls with increased levels of estrogens.
Another factor influencing the concentration of estrogens is the use of oral contraceptives (OC). Previous research in adults showed that OC users show lower cortisol responses to a laboratory stressor than non-OC users, with cortisol levels of OC users similar to women who were in the follicular phase (Kajantie & Philips, Citation2006). A study including adolescents found that OC users showed no response to the stress test (Bouma et al., Citation2009). Given that the mean age of adolescent OC users in the Netherlands is 16.1 years (Stichting Farmaceutische Kengetallen, Citation2009), and that the mean age of our sample was 12.5 years with the oldest participant not yet reaching the age of 16 years, we think it is unlikely that OC use played an important role in this study. However, we did not directly assess OC use in our female participants, so we cannot exclude the possibility that some of the girls might have used OCs.
Despite the finding that the different ages in our sample perceived the SEST paradigm as equally stressful, our results showed an interesting, though non-significant, trend for reactivity differences. The youngest age group (10–12 years) tended to have higher cortisol concentrations in reaction to the paradigm than the older age group (13–15 years). Here also, both psychological and physiological factors could explain this trend. For example, younger children, due to lack of experience, might be more stressed by the anticipation of a presentation in front of a jury, while pubertal stage and hormone differences could account for the trends. However, the sample size was small, and the fact that the oldest age group contained more girls than boys (65% girls) could also explain the trends. Clearly, additional research with larger samples and with broader age ranges is needed in order to detect developmental changes in stress reactivity to specific stressors.
Nonetheless, the current results lead to the conclusion that cortisol reactivity to a social stressor can be observed at all ages, including the transition from childhood to adolescence, as we clearly saw cortisol responses to the SEST in boys. The current results thus support the conclusion that there is consistent HPA axis reactivity across the life span (Kudielka et al., Citation2004), and shed doubt on the suggestion of a hypoactive HPA reactivity period during the transition from childhood to adolescence (Gunnar et al., Citation2009). However, cultural differences may be a possible explanatory factor for the different results among studies, as findings of continuity in HPA axis reactivity are based on European samples (Kudielka et al., Citation2004; Westenberg et al., Citation2009, this issue), while studies showing a lack of cortisol reactivity during the transition from childhood to adolescence are based on North American samples (Gunnar et al., Citation2009; Klimes-Dougan et al., Citation2001; Stroud et al., Citation2009).
Strengths of this study are the inclusion of serial cortisol measures, as well as a pre-stressor resting period of 40 min, in order to obtain a reliable baseline measure of cortisol level. Nonetheless, some limitations should also be noted. The most important limitations are the lack of information on pubertal stage, and in the girls, occurrence and age of menarche, menstrual cycle stage, and OC use. This restricts our capacity for understanding the physiological mechanisms behind our results (e.g., the menstrual cycle phase could explain the lack of a cortisol reaction in some of the girls). Another limitation is that our group was relatively small. This could have, for example, limited our power to detect age differences in cortisol reactivity.
Future research is also needed to examine the difference in physiological stress reactions in adolescent boys and girls. Because girls might react to stress in ways other than cortisol increases, additional measures of stress reactivity would be important, such as measures of the sympathetic nervous system and brain reactivity. The present SEST paradigm can be readily adapted for use during functional neuroima-ging and could therefore be used to measure both cortisol and brain reactivity to the same acute stressor.
Conclusions
This study found that a computerized paradigm including social evaluation, unpredictability and uncontrollability (the SEST) was efficient for increasing cortisol concentrations in 10- to 15-year-old boys. Although girls of the same age did not show increases in cortisol as a reaction to the protocol, both sexes reported experiencing the paradigm as (equally) stressful. These differences in the relations between reported stress and cortisol reactivity are intriguing and an interesting subject for future research on the development of psychophysiological mechanisms during adolescence. Because the paradigm can be easily adapted for use in neuroimaging studies, future mapping of male and female adolescent brain reactivity during the stressor could help illuminate the subject.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
The authors would like to thank the participants of the study, as well as the assistants who helped collect the data.
References
- Bouma EMC, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. (2009). Adolescents' cortisol responses to awakening and social stress; effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology 34:173–81
- Dickerson SS, Kemeny ME. (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 130:355–91
- DiVall SA, Radovick S. (2008). Pubertal development and menarche. Ann NY Acad Sci 1135:19–28
- Gunnar MR, Vazquez D. (2006). Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Dev psychopathology, Vol. 2: dev neuroscience. 2nd ed., Hoboken, NJ: John Wiley & Sons Inc. p 533–77
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. (2009). Developmental changes in HPA activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol 21:69–85
- Harter S. (1990). Processes underlying adolescent self-concept formation. In: Montemayor R, Adams GR, Gullotta TP, editors. From childhood to adolescence: a transitional period? Newbury Park, CA: Sage. p 205–39
- Hasings C, Mosteller F, Tukey JW, Winsor CP. (1947). Lowmoments for small samples: a comparative study of order statistics. Ann Math Stat 18:413–26
- Heim C, Nemeroff CB. (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 49:1023–39
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. (2008). The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33:693–710
- Jansen J, Beijers R, Riksen-Walraven M, de Weerth C. (2010). Cortisol reactivity in young infants. Psychoneuroendocrinology 35:329–38
- Kajantie E, Philips DIW. (2006). The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31:151–78
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. (2005). Lifetime prevalence and age-of-onset distributions of dsm-iv disorders in the national comorbidity survey replication. Arch Gen Psychiatry 62:593–602
- Kirschbaum C, Pirke KM, Hellhammer DH. (1993). The “Trier Social Stress Test”—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. (2001). Adrenocorticol activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev Psychopathol 13:695–719
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. (2004). HPA axis responses to laboratory psycho-social stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology 29:83–98
- Langner O, Dotsch R, Bijlstra G, Wigboldus DHJ, Hawk ST, van Knippenberg A. (2010). Presentation and validation of the Radboud Faces Database. Cognition Emotion 24(8):1377–88
- McEwen B. (2002). Estrogen actions throughout the brain. Endocrine Rev 57:357–84
- Ordaz S, Luna B. (2012). Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuro-endocrinology 37:1135–57
- Petrowski K, Herold U, Joraschky P, Wittchen HU, Kirschbaum C. (2010). A striking pattern of cortisol non-responsiveness to psychosocial stress in patients with panic disorder with concurrent normal cortisol awakening responses. Psychoneuro-endocrinology 35:414–21
- Sapolsky RM, Romero LM, Munck AU. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Rev 21:55–89
- Schneider W, Eschman A, Zuccolotto A. (2002). E-Prime (Version 1.0) [Computer Software]. Pittsburgh, PA: Psychology Software Tools, Inc
- Simmons JP, Nelson LD, Simonsohn U. (2011). False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychological Sci 22(11):1359–66
- Stichting Farmaceutische Kengetallen. (2009). Meer pubers aan de pil. Pharmaceutisch Weekblad 144, available at http://www.sfk.nl/nieuws-publicaties/PW/2009/2009-06.htm (accessed 6 November 2012)
- Stroud L, Foster E, Handwerger K, Papandonatos GD, Granger D, Kivlighan KT, Niaura R. (2009). Stress response and the adolescent transition: performance versus peer rejection stres-sors. Dev Psychopathol 21:47–68
- Stroud LR, Salovey P, Epel ES. (2002). Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry 52:318–27
- Ter Horst GJ, Wichmann R, Gerrits M, Westenbroek C, Lin Y. (2009). Sex differences in stress responses: focus on ovarian hormones. Physiol Behav 97:239–49
- Westenberg M, Bokhorst CL, Miers AC, Sumter SR, Kallen VL, Pelt J, Blote AW. (2009). A prepared speech in front of a prerecorded audience: subjective, physiological, and neuroendo-crine responses to the Leiden public speaking task. Biol Psychol 82:116–24