Abstract
The body's adaptive reaction to a stressful event, an allostatic response, involves vigorous physiological engagement with and efficient recovery from stress. Our aim was to investigate the influence of individual predispositions on cardiac responses to and recovery from a standardized psychosocial stress task (Trier Social Stress Task) in peacekeepers. We hypothesized that those individuals with higher trait resilience and those with higher resting vagal control would be more likely to present an allostatic response: a vigorous cardiac response to stress (i.e., reduction in interbeat intervals and heart rate variability (HRV)) coupled with a significant cardiac recovery in the aftermath. Fifty male military personnel with a mean age of 25.4 years (SD ± 5.99) were evaluated after returning from a peacekeeping mission. Electrocardiogram recordings were made throughout the experimental session, which consisted five conditions: basal, speech preparation, speech delivery, arithmetic task, and recovery. Mean interbeat intervals and HRV were calculated for each condition. An Ego-Resilience Scale and resting vagal control assessed individual predispositions. Stress tasks reduced interbeat intervals (tachycardia) and HRV in comparison with basal, with return to basal in the aftermath (p < 0.001, for all comparisons). Resilience and resting vagal control correlated positively with cardiac parameters for both stress reactivity and recovery (r ≥ 0.29; p < 0.05). In conclusion, peacekeepers showing higher trait resilience and those with higher resting vagal control presented a more adaptive allostatic reaction characterized by vigorous cardiac response to stress (i.e., tachycardia and vagal withdrawal) and efficient cardiac recovery after stress cessation.
Introduction
Allostasis is an extension of the concept of homeostasis and represents the adaptation process of complex physiological systems to physical, psychological, and environmental challenges, or stress. Allostasis, described as the ability to achieve stability through change, is critical to survival. The core of the body's response to a challenge is twofold: turning on an allostatic response that initiates a complex adaptive pathway and then shutting off this response when the threat is over (McEwen, Citation1998; McEwen & Gianaros, Citation2011).
Resilience is a dynamic process involving an interaction between both risk and protection factors, internal and external to the individual, that act to modify the effects of an adverse life event (Rutter, Citation1999). It corresponds to the process of adapting well in the face of adversity, trauma, tragedy, threats of harm, or other significant sources of stress (Yehuda et al., Citation2006). Resilience has been considered not so much as an invulnerability to stress but rather as an ability to recover from negative events (Garmezy, Citation1991). Trait resilience is characterized by high flexibility in response to changing situational demands and by the ability to bounce back from negative emotional experiences (Block & Kremen, Citation1996). In the context of allostasis, resilience denotes the ability of an organism to respond to stressors in the environment by means of the appropriate engagement and efficient termination of allostatic responses (Karatsoreos & McEwen, Citation2011).
Although the heart has an intrinsic automaticity in its pacemaker tissues, heart rate and rhythm are largely under the control of the parasympathetic and sympathetic branches of the autonomic nervous system. Heart rate variability (HRV) comprises the oscillation in the interval between consecutive heartbeats. Under resting conditions, vagal tone prevails and variations in heart period are largely dependent on parasympathetic modulation (Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology, Citation1996). Mounting research points out that those individuals with resting vagal control higher than average tend to be more resilient, adapting well across a number of different situations. Higher resting vagal control is linked to social and psychological well-being (Kok & Fredrickson, Citation2010), trait positive emotionality (Oveis et al., Citation2009), decreased maladaptive coping (El-Sheikh et al., Citation2001), and increased recovery from stress (Souza et al., Citation2007; Weber et al., Citation2010). Hence, resting vagal control is a promising measure of autonomic flexibility, the capacity of the parasympa-thetic nervous system to adapt to changes in circumstance by modifying arousal, respiration, heart rate, and attention (Porges, Citation1995; Porges, Citation2007). In addition, decreased resting vagal control has been associated with increased allostatic overload, as indexed by high levels of proinflammatory cytokines and cortisol, poor glucose regulation (Thayer & Sternberg, Citation2006), high incidence of mental disorders (Kemp et al., Citation2010), and cardiovascular risk factors such as obesity, hypertension, and diabetes (Thayer et al., Citation2010).
High-risk professionals such as peacekeepers are well suited to the study of resilience as they are routinely exposed to traumatic and stressful events with the consequent possibility of allostatic overload. Although the role of a peacekeeper in modern peacekeeping operations is supposed to be less life-threatening and stressful than a soldier in combat, their work involves many additional demands that go beyond military practice. Among those demands is the fact that no visible and direct enemy may be identified. It is not rare during this kind of operation that peacekeepers are attacked by the same population they are supposed to be helping. This kind of threat leads to a situation that is one of the most notable differences between soldiers as warriors and soldiers as peacekeepers: the need to maintain restraint and neutrality. A wide variety of psychopathological conditions have been reported after peacekeeping missions, such as depression, alcoholism, drug abuse, somatization disorders, acute stress disorder, and posttraumatic stress disorder (Mehlum et al., Citation2006; Orsillo et al., Citation1998). Some studies have proposed that negative personality traits in peacekeepers are associated with an increased risk for developing stress-related symptoms (Bramsen et al., Citation2000; Souza et al., Citation2008). Moreover, one of the main risk factors for stress symptoms in peacekeepers is having more deployment-related exposures (Hotopf et al., Citation2003). Coping with stress is important for the success of a peacekeeping mission, and adaptive allostasis is important for minimizing stress-related symptoms after deployment. The body's adaptive reaction to a stressful event involves vigorous physiological engagement and efficient shutting off when the threat is over. To prevent maladaptive coping, it is important to identify individual predispositions associated with this flexible reaction. Hence, it is important to investigate in peacekeepers if allostatic flexibility when facing acute stress could be related to differential psychological (trait resilience) and physiological (resting vagal control) predispositions. Peacekeepers provide a good opportunity to test for these associations, as other possible intervening factors such as differences in gender, age, health, and physical conditioning can be readily controlled.
In a wide variety of acute psychological stress paradigms, a significant increase in heart rate seems to be a consistent response (Haynes et al., Citation1991; Kudielka et al., Citation2004a; Souza et al., Citation2007). More recently, some studies also found decreased HRV during stress, indicating a reduction in vagal activity (Egizio et al., Citation2008; Weber et al., Citation2010). The Trier Social Stress Test (TSST) (Kirschbaum et al., Citation1993) is one of the most useful protocols to induce acute psychological stress in laboratory settings. In the TSST, the participant is asked to deliver a speech followed by an arithmetic task in front of an evaluating audience and a camera. This protocol consistently evokes subjective stress and activation of both the hypothalamus-pituitary-adrenocortical axis and sympatho-adrenal-medullary system (Kelly et al., Citation2008; Kudielka et al., Citation2004b; Nater et al., Citation2006; Schommer et al., Citation2003).
The aim of the present work was to investigate the association between trait resilience and resting vagal control with the cardiac reactivity to and recovery from acute psychological stress in peacekeepers. We hypothesized that those individuals with higher trait resilience and those with higher resting vagal control would be more likely to present a more flexible allostatic response: a vigorous cardiac response to stress (i.e., tachycardia and reduction in HRV) coupled with a significant cardiac recovery in the aftermath. Indeed, a key aspect of an allostatic response is the efficient turning on and shutting off of the responses of the mediators of allostasis (e.g., cortisol, increased blood pressure, and inflammatory response) minimizing the wear and tear associated with allostatic overload. This efficiency of response reduces the overall exposure of the body to those mediators by confining them to the time window in which an adaptive response to an acute stressor should occur.
Methods
Participants
The study was conducted with 50 male Brazilian Army personnel (soldiers, corporals, and sergeants) with a mean age of 25.4 years (SD ± 5.99) and mean body mass index of 23.2 kg/m2 (SD ± 4.34), who volunteered for the United Nations Mission for Stabilization in Haiti (MINUSTAH). They were recruited to take part in the study after responding to an invitation posted in their units. Before embarking on the 6-month peacekeeping mission in Haiti, all participants received the same preparatory instructions and physical training, delivered by a specialized training team from the Brazilian Army Physical Fitness Research Institute. The experiments were conducted 4–10 months after the return to Brazil from the peacekeeping mission in Haiti. Inclusion criteria required that participants were nonsmokers, reported no mental disorders, and were not taking any medication at the time of the experiment. The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Ethics Institutional Review Board of the Federal University of Rio de Janeiro. Written informed consent was provided by the participants.
Psychometric measure
To evaluate the resilience trait of peacekeepers, we applied the Ego-Resiliency Scale (ER-89) (Block & Kremen, Citation1996). This is a 14-item instrument consisting of phrases that describe trait resilience. It measures trait resilience by assessing the way people manage fluctuations in daily life and what they usually do about their own experiences. Participants rated each phrase on a 1–4 scale (1, does not apply at all to 4, applies very strongly). A high score in the ER-89 suggests that the respondent has a secure personality marked by energy, enthusiasm, and ability to recover from stress, to enjoy the company of others, and to be generous.
Cardiac measures
One PC compatible computer controlled data acquisition of the electrocardiographic parameters and instructions to volunteers, running Acknowledge (BIOPAC Systems Inc.) and Presentation (Neurobehavioral Systems) software programs, respectively. Electrocardiographic recordings were collected at a sampling frequency of 1000 Hz through an electrocardiograph ECG100C module coupled to the MP150 system (BIOPAC Systems Inc.).
An off-line peak detection algorithm (derivative plus threshold) was used to estimate R-wave fiducial points, after which the series was screened by hand and corrected for artifacts. Successive RR intervals were estimated in milliseconds. Two cardiac parameters were extracted for the first 2 min of each experimental condition (see below): the mean interbeat interval (IBI) averaged from the successive R–R series and the HRV, calculated by the root mean square of successive differences between adjacent RR intervals (RMSSD2min). In addition, RMSSD was calculated for the whole 5-min basal period (RMSSD5min) to characterize the “resting vagal control,” considering a form of individual physiological predisposition. In resting conditions, the RMSSD has been associated with parasympathetic outflow to the heart and used to reliably index “vagally” mediated HRV independent of the individual fluctuations in breathing rate (Penttila et al., Citation2001). Data processing followed the recommendations of the Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology (Citation1996). Matlab software (KARDIA) was used to analyze cardiac parameters (de Carvalho et al., Citation2002; Perakakis et al., Citation2010).
Stress task
The TSST was used (Kirschbaum et al., Citation1993), as adapted for the peacekeeping troops (Mendonça-de-Souza, Citation2010). The TSST is a standardized protocol for the induction of moderate to extreme psychological stress in laboratory settings. In this adapted version, the participant had to prepare (10 min) and deliver a speech (5 min) about his qualities as a peacekeeper. He was expected to make a statement about why he would be a good candidate for a future peacekeeping mission. To assure participant engagement, the task was performed in front of two army officers. When the participant finished his speech ahead of time, the officers argued with him until he completed the 5-min period. After the speech, the participant performed an arithmetic task (5 min) in which he was asked to count backwards from 910 by subtracting 7 sequentially (e.g., 910, 903, 896, 889, and so on). After each wrong answer, he was warned and had to restart the test from the beginning. The whole session was video recorded, and the participants were informed that the video would be evaluated later.
Procedure
After completing the written informed consent, participants sat in front of a table in a sound-attenuated and temperature-controlled (22–24 °C) room. The ECG electrodes were placed on the chest at lead II and they completed the Ego-Resilience Scale. After a 20-min period of adaptation to the experimental set-up, a 5-min basal ECG was registered. Next, two army officers entered the room, instructed the volunteer about the speech preparation, and left the room. Then, the participant had 10 min to prepare his speech. After that, the officers came back and sat in front of the volunteer to conduct both stress tasks (speech, 5 min, and arithmetic, 5 min). Finally, the participant was instructed to relax for the next 5 min, while the recovery phase recordings were made. The whole procedure was recorded by a video camera. At the end, participants were thanked and debriefed. All experimental sessions started between 13:00 and 17:00 h.
Statistical analysis
To investigate the reactivity profile to the experimental challenge, two one-way repeated-measures analysis of variance (RM-ANOVA) were conducted separately for the IBI and the HRV (RMSSD2min) with conditions (basal, preparation, speech task, arithmetic task, and recovery) as within-subject factor. Green-house -Geisser tests were conducted to correct the violation of sphericity. Tukey's HSD post hoc tests were carried out to identify the differences whenever necessary.
To investigate the associations between individual predispositions with cardiac reactivity and with cardiac recovery, we employed Spearman correlations. Individual predispositions were measured by resilience trait (ER-89 scores) and by resting vagal control (RMSSD5min). The two parameters of cardiac activity: IBI and RMSSD2min were entered separately in each analysis. For cardiac reactivity analysis, we employed specific indexes calculated by subtraction of the mean values of IBI and RMSSD2min between basal and speech task (basal–speech task) and between basal and arithmetic task (basal–arithmetic task). Recovery from stress was investigated by the indexes generated through subtractions of the mean values of IBI and RMSSD2min between recovery and speech task (recovery–speech task) and between recovery and arithmetic task (recovery–arithmetic task).
Data were analyzed using the Statistica 7.0 software program (StatSoft Inc, OK). In all analyses, statistical significance was taken as p ≤ 0.05.
Results
Acute stress: Reactivity and recovery
RM-ANOVA for mean IBI revealed the main effect of conditions (F(4,164) = 101.34, p < 0.001, ϵ = 0.55). Tukey's post hoc analysis for stress reactivity showed reduction in IBIs (tachycardia) during preparation, speech, and arithmetic conditions in comparison with basal (p < 0.001, for all comparisons). Comparison between mean IBIs for speech and arithmetic conditions was not significant (p = 0.98), but both showed shorter mean IBIs (more tachycardia) compared to preparation condition (p < 0.001, for both comparisons). Post hoc analysis for stress recovery revealed increased IBIs (toward basal levels) for the recovery condition in comparison with preparation, speech, and arithmetic conditions (p < 0.001, for all comparisons). Comparison between mean IBIs for recovery and basal conditions was not significant (p = 0.99). In summary, participants presented tachycardia during each stress condition (preparation, speech, and arithmetic) in relation to basal that recovered toward basal levels after stress cessation ().
Figure 1. Mean ± standard error of interbeat interval (a) and heart rate variability (b). Both measures were calculated for the first 2 min of the five conditions: basal, preparation, speech task, arithmetic task, and recovery (N = 50 participants). RMSSD: root mean square of successive differences between adjacent RR intervals in milliseconds. A one-way repeated-measured analysis of variance (RM-ANOVA) was conducted with conditions as within-subject factor, separately for interbeat interval and RMSSD, with Greenhouse–Geisser correction. *p < 0.05 for comparison with basal and with recovery conditions; #p < 0.05 for comparison with preparation condition; Tukey's HSD post hoc test.
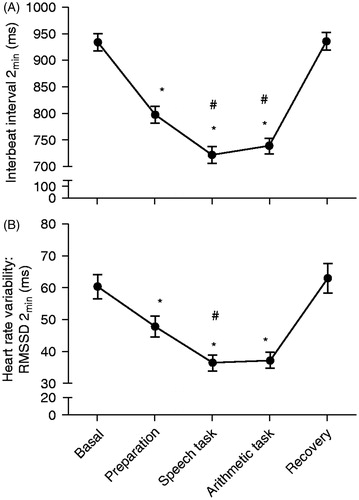
RM-ANOVA for HRV (RMSSD2min) revealed the main effect of conditions (F(4,164) = 17.63, p < 0.0001, ϵ = 0.78). Tukey's post hoc analysis for stress reactivity showed a reduction in RMSSD2min during preparation (p = 0.02), speech, and arithmetic (p < 0.0001, for both comparisons) conditions in comparison with basal. RMSSD2min during speech showed deeper attenuation compared to preparation (p = 0.48). Comparison between arithmetic and preparation and between speech and arithmetic conditions was not significant (p = 0.11 and p = 0.99, respectively). Post hoc analysis for stress recovery revealed that RMSSD2min during recovery increased (toward basal levels) compared to preparation (p = 0.003), speech, and arithmetic conditions (p < 0.0001, for both comparisons). RMSSD2min comparisons between recovery and basal were not significant (p = 0.98). In summary, HRV (RMSSD2min) was significantly lower in stress conditions (preparation, speech, and arithmetic) in comparison with basal and recovery ().
Allostasis and individual predisposition
Psychological and physiological measures of individual predispositions were correlated. Spearman correlation coefficient showed a positive association between trait resilience (measured by ER-89) and resting vagal control (measured by RMSSD during the 5-min basal–RMSSD5min) (p = 0.28, p = 0.05), indicating that participants with increasingly higher scores on trait resilience showed progressively higher level of resting vagal control.
Resting vagal control
Spearman correlation coefficients showed that resting vagal control correlated with reactivity and recovery indexes. Resting vagal control had a significant positive association with IBI reactivity index for speech task (basal–speech task: r = 0.50, p = 0.0003), and a significant positive association with IBI reactivity index for arithmetic task (basal–arithmetic task: r = 0.54, p < 0.0001). Furthermore, resting vagal control had a significant positive association with IBI recovery index for speech task (recovery-speech task: r = 0.45, p = 0.001), and a significant positive association with IBI recovery index for arithmetic task (recovery–arithmetic task: r = 0.53, p < 0.0001). These results indicated that participants with higher resting vagal control reacted more to, and recovered more efficiently from stress, in terms of IBI fluctuations ().
Table 1. Trier Social Stress Test tasks: Spearman correlations between reactivity or recovery indexes (interbeat interval [ms] and heart rate variability [ms]) and individual predispositions (resting vagal control or trait resilience).
Spearman correlation coefficients showed that resting vagal control had a significant positive association with RMSSD2min reactivity index for speech task (basal–speech task: r = 0.76, p < 0.0001), and a significant positive association with RMSSD2min reactivity index for arithmetic task (basal–arithmetic task: r = 0.72, p < 0.0001). Furthermore, resting vagal control had a significant positive association with RMSSD2min recovery index for speech task (recovery–speech task: r = 0.60, p < 0.0001) and a significant positive association with RMSSD2min recovery index for arithmetic task (recovery–arithmetic task: r = 0.53, p < 0.0001). These results indicated that participants with higher resting vagal control had a lower RMSSD during both the stress tasks and a higher RMSSD after these tasks ().
Trait resilience
The mean score on trait resilience measured by ER-89 was 39.0 (SEM ± 0.71). Spearman correlation coefficients showed a significant positive association between trait resilience and IBI reactivity index for speech task (basal–speech task: r = 0.29, p = 0.047) and between trait resilience and IBI reactivity index for arithmetic task (basal–arithmetic task: r = 0.38 p = 0.007). Trait resilience had a significant positive correlation with IBI recovery index for arithmetic task (recovery–arithmetic task: r = 0.29, p = 0.04), and there was no significant correlation between trait resilience and IBI recovery index for speech task (recovery–speech task: r = 0.22, p = 0.13). These results indicated that participants with higher trait resilience react and recover more efficiently from stress, considering the IBI fluctuations ().
Spearman correlation coefficients showed a significant positive association between the trait resilience and RMSSD2min reactivity index for speech task (basal–speech task: r = 0.32,p = 0.03). Trait resilience had a significant positive correlation with RMSSD2min recovery index for speech task (recovery–speech task: r = 0.32, p = 0.03). Considering RMSSD2min reactivity and recovery indexes for arithmetic task, there were no significant associations with trait resilience ().
Discussion
This study investigated the impact of individual physiological and psychological predispositions on the cardiac response to acute psychological stress in Brazilian peacekeepers. Results showed a significant reduction in IBIs (i.e., tachycardia) and in HRV (i.e., vagal withdrawal) during the TSST tasks (preparation, speech, and arithmetic) and a significant recovery of both measures in the aftermath. Moreover, we showed that those individuals with higher resting vagal control and those with higher trait resilience presented a more significant allostatic response with robust tachycardia and vagal withdrawal during stress and more efficient recovery after the end of stress.
Previous studies have demonstrated that the TSST is a potent inductor of a cardiovascular response. Such studies observed a significant acceleration of heart rate (Birkett, Citation2011; Kudielka et al., Citation2004a; Kudielka et al., Citation2004b; Schommer et al., Citation2003) and a reduction in vagal activity (Klinkenberg et al., Citation2009; Petrowski et al., Citation2010) during the stress tasks. Likewise, in this study, a robust reduction in IBI and HRV during both the speech and arithmetic tasks was found. To the best of our knowledge, this is the first study to apply the TSST in a sample of professional peacekeepers.
Our findings regarding the correlation of resting vagal control with stress reactivity and recovery showed that individuals with higher resting vagal control presented a more flexible pattern of response to stress challenge. These participants showed a larger reduction in IBIs and HRV during stress and a larger increase in IBIs and HRV after the cessation of stress. Souza et al. (Citation2007) found that undergraduate students with higher resting vagal control displayed better cardiac recovery from a speech task compared to those with lower resting vagal control, which presented significantly less recovery. Many studies have linked high and low resting vagal control, respectively, with well- and maladaptive functions. Concerning low resting vagal control, Weber et al. (Citation2010) found an association with impaired recovery of cardiovascular, endocrine, and immune markers from stress. Thayer & Sternberg (Citation2006), reviewing the role of vagal activity in allostatic processes, showed that low vagal activity is associated with poor glucose regulation, increased hypothalamic-pituitary-adrenal axis activation, and increased inflammatory processes. In addition, low vagal control has been associated with depression (Thayer et al., Citation1998) and generalized anxiety disorder (Thayer et al., Citation1996). As for high vagal control, several studies have shown that this is associated with self-reports of enhanced regulatory control to moderate/intense stressors (Fabes & Eisenberg, Citation1997) and with active coping in bereavement (O'Connor, Citation2002). Those studies along with the results found in the present work support the emergence of resting vagal control as an important measure of autonomic flexibility and a physiological marker of a well-adaptive allostatic response.
In this study, individuals with higher trait resilience were more flexible (i.e., showed more tachycardia and vagal withdrawal to stress and efficient recovery after the cessation of stress). A possible limitation of this study is that the back-translation and cultural adaptation of the ER-89 resilience scale to Portuguese has not yet been published. However, convergent validity of the scale seems adequate because the scores were correlated with vigorous cardiac response to stress (i.e., tachycardia and vagal withdrawal) and efficient cardiac recovery after the cessation of stress, which is part of resilience construct. In line with present results, Souza et al. (Citation2007), Tugade et al. (Citation2004), and Tugade & Fredrickson (Citation2004) showed that high-resilient participants had greater heart rate recovery from stress than low-resilient participants. Together, these results corroborate the idea that resilient individuals are characterized by high flexibility in response to changing situational demands and by the ability to bounce back from negative emotional experiences. We suggest that trait resilience could be a psychological marker of allostatic response to stress. However, our results indicated that the physiological marker (resting vagal control) had a more consistent association with reactivity and recovery indexes than the psychological marker (trait resilience).
A relevant applicability of this study results is the possibility to increase the level of resting vagal control to help people cope with stress more efficiently. Indeed, some studies have proposed that it is possible to increase resting vagal control through different types of interventions. HRV biofeedback reliably increases resting vagal control in basketball players (Paul et al., Citation2012) and in patients with depression (Siepmann et al., Citation2008). Exercise has also been considered as a strategy to improve autonomic control. Results concerning the effects of aerobic fitness and endurance training on resting markers of parasympathetic autonomic control indicate higher levels of resting vagal modulation of heart rate in fit compared to unfit individuals (Aubert et al., Citation2003; Berkoff et al., Citation2007; Buchheit & Gindre, Citation2006). Furthermore, an intervention study (Duarte, Citation2010), consisting of a 3-month routine of physical exercises, conducted on Brazilian military personnel, who were already fit at the starting point, resulted in significant increase in resting cardiac vagal control compared to those who underwent ordinary activities. Regarding longitudinal studies, some authors have proposed that cardiac vagal control improves with chronic exercise (Buchheit et al., Citation2010; Kiviniemi et al., Citation2006; Nummela et al., Citation2010). Cognitive behavior therapy has also been considered to promote an increase in resting vagal control in posttraumatic stress disorder (Norte et al., Citation2011) and in depressed patients with coronary heart disease (Carney et al., Citation2000). A review has concluded that in healthy subjects, and those with medically diagnosed conditions, acupuncture increased resting vagal control (Anderson et al., Citation2012).
Conclusions
Participants presenting high trait resilience and high resting vagal control seem more flexible, inasmuch as they react and recover more efficiently from stress. In doing so, they potentially reduce the allostatic overload in stress response systems due to an optimal homeostasis-restoration process. By understanding the role of trait resilience and resting vagal control in promoting a more adaptive response and better coping with stress situations, our findings could help the selection, training, and treatment of professionals involved in risk-taking activities, as is the case for peacekeepers.
Declaration of interest
This work was supported by the National Council for Scientific and Technological Development (CNPq); the Carlos Chagas Filho Foundation of Research Support in Rio de Janeiro (FAPERJ); and the Coordination for the Improvement of Higher Education Personnel (CAPES). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Anderson B, Nielsen A, Mckee D, Jeffres A, Kligler B. (2012). Acupuncture and heart rate variability: a systems level approach to understanding mechanism. Explore 8:99–106
- Aubert AE, Seps B, Beckers F. (2003). Heart rate variability in athletes. Sports Med 33:889–919
- Berkoff DJ, Cairns CB, Sanchez LD, Moorman CT. (2007). Heart rate variability in elite American track-and-field athletes. J Strength Cond Res 21:227–31
- Birkett MA. (2011). The Trier Social Stress Test protocol for inducing psychological stress. J Vis Exp., Oct 19;(56). pii: 3238. doi: 10.3791/3238
- Block J, Kremen AM. (1996). IQ and ego-resiliency: conceptual and empirical connections and separateness. J Pers Soc Psychol 70:349–61
- Bramsen I, Dirkzwager AJE, van der Ploeg HM. (2000). Predeployment personality traits and exposure to trauma as predictors of posttraumatic stress symptoms: a prospective study of former peacekeepers. Am J Psychiatry 157:1115–19
- Buchheit M, Chivot A, Parouty J, Mercier D, Al Haddad H, Laursen PB, Ahmaidi S. (2010). Monitoring endurance running performance using cardiac parasympathetic function. Eur J Appl Physiol 108:1153–67
- Buchheit M, Gindre C. (2006). Cardiac parasympathetic regulation: respective associations with cardiorespiratory fitness and training load. Am J Physiol Heart Circ Physiol 291:H451–H458
- Carney RM, Freedland KE, Stein PK, Skala JA, Hoffman P, Jaffe AS. (2000). Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom Med 62:639–47
- de Carvalho JLA, da Rocha AF, Nascimento FAO, Neto JS, Junqueira LF. (2002). Development of a Matlab software for analysis of heart rate variability. In: Yuan B, Tang X, editors. 6th International Conference on Signal Processing. 2. Beijing, China: Institute of Electrical and Electronics Engineers, Inc. p 1488–92
- Duarte AF. (2010). Effects of aerobic training and cardiac vagal modulation upon cardiorespiratory fitness, autonomic control and cardiovascular responses to exercise in Brazilian Army soldiers, PhD Thesis. Gama Filho University, Rio de Janeiro, Brazil (in Portuguese)
- Egizio VB, Jennings JR, Christie IC, Sheu LK, Matthews KA, Gianaros PJ. (2008). Cardiac vagal activity during psychological stress varies with social functioning in older women. Psycho-physiology 45:1046–54
- El-Sheikh M, Harger J, Whitson SM. (2001). Exposure to interparental conflict and children's adjustment and physical health: the moderating role of vagal tone. Child Dev 72:1617–36
- Fabes RA, Eisenberg N. (1997). Regulatory control and adults' stress-related responses to daily life events. J Pers Soc Psychol 73:1107–17
- Garmezy N. (1991). Resilience in Childrens adaptation to negative life events and stressed environments. Pediatr Ann 20(459–460):463–6
- Haynes SN, Gannon LR, Orimoto L, O'Brien WH, Brandt M. (1991). Psychophysiological assessment of post-stress recovery. Psychological assessment: J Consult Clin Psychol 3:356–65
- Hotopf M, David AS, Hull L, Ismail K, Palmer I, Unwin C, Wessely S. (2003). The health effects of peace-keeping in the UK Armed Forces: Bosnia 1992–1996. Predictors of psychological symptoms. Psychol Med 33:155–62
- Karatsoreos IN, McEwen BS. (2011). Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci 15:576–84
- Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL. (2008). Sex differences in emotional and physiological responses to the Trier Social Stress Test. J Behav Ther Exp Psychiatry 39:87–98
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. (2010). Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry 67:1067–74
- Kirschbaum C, Pirke KM, Hellhammer DH. (1993). The Trier Social Stress Test: a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81
- Kiviniemi AM, Hautala AJ, Makikallio TH, Seppanen T, Huikuri HV, Tulppo MP. (2006). Cardiac vagal outflow after aerobic training by analysis of high-frequency oscillation of the R-R interval. Eur J Appl Physiol 96:686–92
- Klinkenberg AV, Nater UM, Nierop A, Bratsikas A, Zimmermann R, Ehlert U. (2009). Heart rate variability changes in pregnant and non-pregnant women during standardized psychosocial stress. Acta Obstet Gynecol Scand 88:77–82
- Kok BE, Fredrickson BL. (2010). Upward spirals of the heart: autonomic flexibility, as indexed by vagal tone, reciprocally and prospectively predicts positive emotions and social connectedness. Biol Psychol 85:432–6
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. (2004a). Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: the impact of age and gender. Int J Behav Med 11:116–21
- Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. (2004b). Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology 29:983–92
- McEwen BS. (1998). Protective and damaging effects of stress mediators. N Engl J Med 338:171–9
- McEwen BS, Gianaros PJ. (2011). Stress- and allostasis-induced brain plasticity. Annu Rev Med 62:431–45
- Mehlum L, Koldsland BO, Loeb ME. (2006). Risk factors for long-term posttraumatic stress reactions in unarmed UN military observers: a four-year follow-up study. J Nerv Ment Dis 194:800–4
- Mendonça-de-Souza AC. (2010). Impact of traumatic events in Brazilian Army soldiers that went to Haiti: Prospective study of salivar cortisol, PhD Thesis, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil (in Portuguese)
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, Ehlert U. (2006). Stress-induced changes in human salivary alpha-amylase activity – Associations with adrenergic activity. Psychoneuroendocrinology 31:49–58
- Norte CE, Souza GGL, Pedrozo AL, Mendonca-de-Souza ACF, Figueira I, Volchan E, Ventura PR. (2011). Impact of cognitive-behavior therapy on resilience-related neurobiological factors. Rev Psiquiatr Clin 38:43–5
- Nummela A, Hynynen E, Kaikkonen P, Rusko H. (2010). Endurance performance and nocturnal HRV indices. Int J Sports Med 31:154–9
- O'Connor K. (2002). A cognitive-behavioral/psychophysiologicaI model of tic disorders. Behav Res Ther 40:1113–42
- Orsillo SM, Roemer L, Litz BT, Ehlich P, Friedman MJ. (1998). Psychiatric symptomatology associated with contemporary peacekeeping: an examination of post-mission functioning among peacekeepers in Somalia. J Trauma Stress 11:611–25
- Oveis C, Gruber J, Haidt J, Cohen AB, Shiota MN, Keltner D. (2009). Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion 9:265–70
- Paul M, Garg K, Sandhu JS. (2012). Role of biofeedback in optimizing psychomotor performance in sports. Asian J Sports Med 3:29–40
- Penttila J, Helminen A, Jartti T, Kuusela T, Huikuri HV, Tulppo MP, Coffeng R, Scheinin H. (2001). Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: effects of various respiratory patterns. Clin Physiol 21:365–76
- Perakakis P, Joffily M, Taylor M, Guerra P, Vila J. (2010). KARDIA: a Matlab software for the analysis of cardiac interbeat intervals. Comput Methods Programs Biomed 98:83–9
- Petrowski K, Herold U, Joraschky P, Mück-Weymann M, Siepmann M. (2010). The effects of psychosocial stress on heart rate variability in panic disorder. German J Psychiatry 13:66–73
- Porges SW. (1995). Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology 32:301–18
- Porges SW. (2007). The polyvagal perspective. Biol Psychol 74:116–43
- Rutter M. (1999). Resilience concepts and findings: implications for family therapy. J Fam Ther 21:119–44
- Schommer NC, Hellhammer DH, Kirschbaum C. (2003). Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosom Med 65:450–60
- Siepmann M, Aykac V, Unterdorfer J, Petrowski K, Mueck-Weymann M. (2008). A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl Psychophysiol Biofeedback 33:195–201
- Souza GG, Mendonca-de-Souza AC, Barros EM, Coutinho EF, Oliveira L, Mendlowicz MV, Figueira I, Volchan E. (2007). Resilience and vagal tone predict cardiac recovery from acute social stress. Stress 10:368–74
- Souza WF, Figueira I, Mendlowicz MV, Volchan E, Mendonca-de-Souza AC, Duarte AF, Monteiro da Silva AM, et al. (2008). Negative affect predicts posttraumatic stress symptoms in Brazilian volunteer United Nations peacekeepers in Haiti. J Nerv Ment Dis 196:852–5
- Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology (1996). Heart rate variability – Standards of measurement, physiological interpretation, and clinical use. Circulation 93:1043–65
- Thayer JF, Friedman BH, Borkovec TD. (1996). Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry 39:255–66
- Thayer JF, Smith M, Rossy LA, Sollers JJ, Friedman BH. (1998). Heart period variability and depressive symptoms: gender differences. Biol Psychiatry 44:304–6
- Thayer JF, Sternberg E. (2006). Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci 1088:361–72
- Thayer JF, Yamamoto SS, Brosschot JF. (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 141:122–31
- Tugade MM, Fredrickson BL. (2004). Resilient individuals use positive emotions to bounce back from negative emotional experiences. J Pers Soc Psychol 86:320–33
- Tugade MM, Fredrickson BL, Barrett LF. (2004). Psychological resilience and positive emotional granularity: examining the benefits of positive emotions on coping and health. J Pers 72:1161–90
- Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmermann-Viehoff F, Thomas A, Perschel FH, et al. (2010). Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur J Appl Physiol 109:201–11
- Yehuda R, Flory JD, Southwick S, Charney DS. (2006). Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann N Y Acad Sci 1071:379–96
