Abstract
The early-life environment has many long-term effects on mammals. Maternal interaction and early stressful events may affect regulation of the HPA axis during adulthood, leading to differential glucocorticoid secretion in response to stressful situations. These adverse experiences during postnatal development may even sensitize specific neurocircuits to subsequent stressors. Later in life, the overreaction of the HPA axis to stress can constitute a risk factor for metabolic and mental diseases. As tricyclic antidepressants are known to correct glucocorticoid hypersecretion during depression, we treated maternally separated animals with amitriptyline, at a lower dose than habitually used in depression models, to prevent the response to chronic stress during adulthood. Male Wistar rats were separated from the mother for 4.5 h every day for the first 3 weeks of life. From postnatal day 50, animals were subjected to chronic variable stress during 24 d (five types of stressors at different times of day). During the stress, protocol rats were orally administered amitriptyline (5 mg/kg) daily. We observed that maternal separation caused a reduction in plasma ACTH levels (p < 0.05), but evoked hypersecretion of corticosterone (p < 0.05) when it was combined with stress in adulthood. This rise was completely prevented by antidepressant treatment with amitriptyline.
Introduction
In rats, as in other mammals, the development of the HPA response is influenced by the early relationship between the mother and the offspring. It has been suggested that variations in maternal care evoked by maternal separation (MS) can cause long-term effects on the stress system regulation at chemical and behavioral levels (De Kloet et al., Citation2005). This occurs because, during development, early environment promotes differential expression of numerous genes and alterations in neurocircuit organization of the brain. Genetic and structural effects may cause abnormal responses to stress in adults stressed in early life (Ladd et al., Citation2005), and constitute a risk factor for developing an exacerbated stress response during adulthood and aging (Claessens et al., Citation2011; Lehmann & Feldon, Citation2000).
Precise regulation of the HPA axis is essential to preserve a healthy state. Inadequate coping with stressful events can lead to sustained overdrive of the HPA axis causing hypercortisolism (De Kloet et al., Citation2005), which has been proposed as a risk factor for depression, as well as increased abdominal obesity, insulin resistance, accelerating progression towards diabetes Type II, atherosclerosis, hypertension, osteoporosis and cardiovascular problems (Miller et al., Citation2009). Numerous antidepressants are known to correct glucocorticoid hypersecretion during depression. In general, the return of cortisol levels to normal concentrations after antidepressant treatment is a manifestation of remission, while failure to suppress corticosteroid secretion in response to dexamethasone suppression tests predicts an unfavorable clinical course or ineffectiveness of the pharmacological intervention (Gillespie & Nemeroff, Citation2005).
In our laboratory, we evaluate the long-term effects of early life adversity and its interaction with chronic stress during adulthood. We propose this as a model of vulnerability to dysregulation of the HPA axis. In the present study we assessed the effects on the HPA axis by measuring the basal secretion of corticosterone and ACTH after 24 d exposure to chronic variable stress (CVS) in rats previously subjected to early MS for a period of 4.5 h for the first 3 weeks of life. Animals were treated with the tricyclic antidepressant amitriptyline with a lower dose than is habitually used in depression models, with the expectation of correcting the hormone baseline concentrations in the stress model.
Material and methods
Male Wistar derived rats were bred and reared in our animal facility under controlled temperature conditions (22 ± 2 °C) and artificial illumination (12:12 h light/dark; lights on at 07:00 a.m.), with water and food available ad libitum. Animals were housed in 45 cm × 30 cm × 18 cm plastic cages with pine-shaving bedding. Mothers were randomly divided in two groups to be subjected to maternal separation (MS) or not non-maternally separated (NMS). On postnatal day 1, litters were culled to eight pups (four males, four females). For MS, pups were separated daily from their mother for 4.5 h during the first 3 weeks of life (Ogawa et al., Citation1994) by removing the dam from the home cage and placing it alone into another cage in the same room. The litters were kept at room temperature during the separation with water and food ad libitum, allowing them to thermoregulate by gathering them together in the nest. Separations were carried out between 8:00 am and 12:30 pm until postnatal day 21. NMS remained undisturbed in the maternal cage except for a bedding change twice a week, until the weaning age at postnatal day 25.
After weaning, male rats were selected and housed in standard cages in groups of four. They were handled daily by the same researcher to minimize stress reactions to manipulation at the moment of sacrifice. Also, they were habituated to the later oral administration of the antidepressant by administering 0.5 ml of fresh water with a 1 ml syringe, without the needle, to the depth of the entrance of the throat. Both NMS and MS rats were randomly divided into two groups: one that was submitted to CVS, and a second that remained unstressed (NCVS). Unrelated subjects were used to form the groups to avoid confounding litter effects. The CVS protocol as well as the antidepressant and vehicle treatments started at 50 d of age (200–250 g weight). Amitriptyline (AMI) (5 mg/kg) was given with a syringe, diluted in 0.5 ml of saline solution. AMI or vehicle were administered every day between 12:00 and 2:00 pm in order to keep the administration separated in time from the application of the stressors.
The CVS model consists of five stressors with varying intensities that are presented randomly in time and order to the animals during 24 d. The stressors were (a) noise produced for 4 h by an alarm bell (85 dB); (b) loss of consciousness by ether anesthesia and subsequent application for 2 min; (c) two intraperitoneal (i.p.) injections of 0.5 ml isotonic saline; (d) food deprivation for 24 h; (e) restraint for 1 h by placement inside a 6 cm diameter metal cylinder. The stressors used in this paradigm did not affect body weights. With the exception of noise, stressors were assigned randomly in each replication of the experiments. Noise was always used on the day preceding sacrifice.
In summary, subgroups of NMS and MS animals were subjected to the CVS model concomitant with the administration of AMI or vehicle solution while others remained unstressed (NCVS), concomitant with the administration of AMI or vehicle until PND 74. The resulting experimental groups were: NMS-NCVS (Vehicle; AMI); MS-NCVS (Vehicle; AMI); NMS-CVS (Vehicle; AMI); MS-CVS (Vehicle; AMI).
At day 75 (350–400 g weight) rats were decapitated to collect trunk blood, which was then centrifuged to obtain plasma using methods recommended by the manufacturers of the ACTH and corticosterone assay kits. Samples were frozen and stored for the subsequent determination of plasma ACTH using a commercial kit (IMMULITE®1000 ACTH, solid-phase, two-site sequential chemiluminescent immunometric assay from DPC Diagnostic Products Corporation, Los Angeles, CA) and corticosterone concentration by radioimmunoassay (ImmuChem Double Antibody Corticosterone 125I RIA from ICN Biomedicals, Costa Mesa, CA). Sacrifice was carried out between 09:00 and 12:00 pm in order to avoid unwanted variability linked to diurnal fluctuations in circulating hormone levels, measuring the hormones at the basal level. More details are displayed in (Cotella et al., Citation2009). The ratio of plasma corticosterone to the log of plasma ACTH was calculated as an index of adrenal sensitivity to ACTH (Engeland et al., Citation1981; Ulrich-Lai & Engeland, Citation2002).
Data were analyzed by three-way ANOVA (factors: rearing condition × stress × antidepressant treatment) followed by a Tukey post hoc test with the Infostat software (www.infostat.com.ar). When necessary, homogeneity of variance was obtained by performing analysis on the values after square-root transformation. For all comparisons, p values of less than 0.05 were considered statistically significant. The experiments were performed in full accordance with protocols approved by the animal care committee of the National University of Córdoba, Argentina.
Results
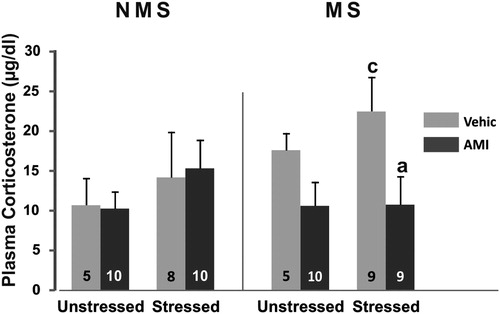
Analysis of plasma corticosterone secretion revealed a significant maternal stress by treatment interaction MS × AMI F(1,57) = 6.38; p < 0.05. Post hoc analysis showed that, in general, MS animals administered vehicle had higher levels of corticosteroid compared to the corresponding AMI groups (p < 0.05) and to the NMS controls (p < 0.05). Regarding individual group differences, when MS was combined with stress, vehicle-treated animals showed a 57% rise in plasma corticosterone compared with the corresponding NMS (p < 0.05). Treatment with amitriptyline in these animals was effective in preventing drive of the adrenal gland (p < 0.05), given that this group had significantly lower levels of plasma corticosteroid than the corresponding vehicle group and the concentration remained at the levels of those NMS animals ().
Figure 1. Plasma corticosterone concentration (µg/dl) in NMS and MS rats subjected to chronic variable stress under AMI (5 mg/kg) or vehicle administration. Mean ± SE are presented. The number of animals per group is included inside each bar. (a) Significant difference (p < 0.05) versus respective vehicle. (c) Significant differences (p < 0.05) versus respective NMS.
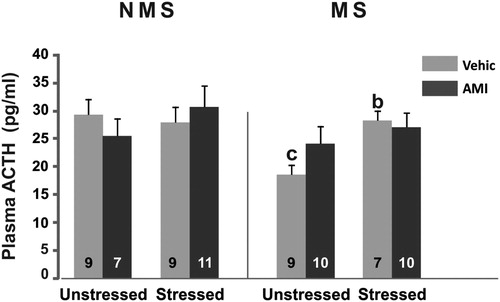
Regarding ACTH, ANOVA showed main effects of MS F(1,64) = 5.15; p < 0.05 and CVS F(1,64) = 5.68; p < 0.05 and a three-way interaction effect MS × CVS × AMI F(1,64) = 4.04; p < 0.05. Post hoc test showed that the unstressed MS group treated with vehicle had significantly lower levels of ACTH compared to the NMS group (p < 0.05). In the MS-CVS groups, the levels of the hormone were higher than those of MS animals not subjected to stress (p < 0.05). AMI did not show effects on any group ().
Figure 2. Plasma ACTH concentration (pg/ml) in NMS and MS rats subjected to chronic variable stress under AMI (5 mg/kg) or vehicle administration. Mean ± SE are presented. The number of animals per group is included inside each bar. (b) Significant difference (p < 0.05) versus respective unstressed. (c) Significant differences (p < 0.05) versus respective NMS.
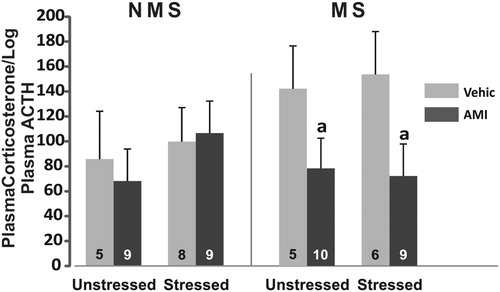
There were no main effects of MS or CVS on adrenal sensitivity to ACTH, estimated as the ratio between plasma corticosterone (ng/ml) over the log of plasma ACTH (pg/ml). ANOVA showed a main effect of AMI F(1,52) = 4.83; p < 0.05. Post hoc analysis showed that MS animals receiving AMI had less reactivity than their respective control group (p < 0.05), and MS–CVS receiving AMI treatment also showed reduced reactivity compared to the saline vehicle group (p < 0.05) ().
Figure 3. Index of adrenal sensitivity to ACTH measured as plasma corticosterone divided by the log of plasma ACTH, in NMS and MS rats subjected to chronic variable stress under AMI (5 mg/kg) or vehicle administration. Mean ± SE are presented. The number of animals per group is included inside each bar. (a) Significant difference (p < 0.05) versus respective vehicle.
Discussion
Our observations indicate that MS influenced the HPA axis in animals tested as adults, causing basal hypersecretion of corticosterone following chronic stress exposure. Increased chronic stress secretion in maternally-stressed animals occurred despite lower resting levels of ACTH in the unstressed state. This stress-induced rise in basal corticosterone was completely prevented by oral low-dose amitriptyline treatment. Antidepressant treatment also reduced adrenal sensitivity to ACTH, which could explain part of the effect of this drug on HPA axis regulation. It should be noted that under CVS, the treatment with the antidepressant was able to regulate the secretion of corticosterone in animals affected by the adverse early environment, which has been suggested as a generator of a stress-vulnerable phenotype in the adult (Claessens et al., Citation2011; Lehmann & Feldon, Citation2000). This means that some long-term outcomes from this phenotype, in this case of altered basal HPA activity following stress, can be normalized by pharmacological treatment, and thus that the deleterious effects of glucocorticoids under certain conditions can, at least, be minimized.
Deeper analysis of the results for all groups showed that stressed animals do not have significantly different levels of basal, i.e. morning concentration, glucocorticoid than those that did not receive stress exposure. This is possibly due to the variability in the dataset as well as inter-individual differences that occur in outbred rat strains (such as Wistar). ACTH also remained at control levels, as noted previously in our laboratory (Renard et al., Citation2007). This lack of the effect of CVS was reported by other authors, arguing that depending on the paradigm of chronic stress applied, basal concentration of corticosterone can be slightly modified and show a small rise, without variations in ACTH levels compared to those observed in control groups. That lack of response of ACTH to chronic stress accompanied by a slight rise of corticosterone could be explained by transient rise in CRH during the paradigm (Aguilera, Citation1994), which means that a response of ACTH could be detected after individual stressors but not as a rise of basal secretion at the end of the protocol.
The significant stress by drug interaction suggested that maternal stress may enhance corticosteroid levels in vehicle treated rats. We detected a 65% increase in corticosterone levels in vehicle-treated MS versus NMS animals. Though the individual post hoc test was not significant, this trend suggests an effect of MS on corticosterone levels, which may be related to the lower levels of ACTH in this group compared with the controls. This would indicate that negative feedback of glucocorticoids on the pituitary gland is intact in this case, i.e. MS would not affect negative feedback of HPA on basal conditions. It has been suggested that animals that have been separated from the mother during the so-called stress hypo-responsive period develop adaptations of the HPA axis to preserve the proactive phase of negative feedback (Ladd et al., Citation2004). This means that the basal regulation of the system works correctly, while the effect would be on the reactive phase of the regulation occurring after a stressor or during the diurnal peak of secretion. Interestingly, amitriptyline reduced adrenal reactivity to ACTH relative to the corresponding vehicle group, even though the levels of corticosterone were not significantly lower than the vehicle group.
As expected, levels of corticosterone observed in separated animals were higher when the animals were also subjected to chronic stress as adults. These animals showed a significant rise of 57% compared to the response to CVS of the non-maternally separated group. Similar results were reported by other studies. For example, with a different separation protocol, a rise in corticosterone concentration has been demonstrated in adult early separated rats when they were exposed to a novel environment (Biagini et al., Citation1998). Other authors observed such augmentation after an acute stressor (Aisa et al., Citation2007). A similar result was found with a combined protocol of MS, but shorter than ours (180 min each day), with chronic stress (Ladd et al., Citation2005). Despite this rise in corticosterone, the ACTH levels in this group remained unchanged compared to the corresponding NMS group and the basal group. Even though this seems to suggest hypertrophy or hyperactivity of the adrenal gland secondary to prolonged stimulation with ACTH (Ulrich-Lai et al., Citation2006), these animals did not reach significant values for the rise of adrenal reactivity. This could be due to the variance in the dataset. Nevertheless, AMI treatment reduced corticosterone concentrations compared to corresponding vehicle groups, with values remaining at levels seen in non-maternally separated and unstressed controls. Despite the fact that ACTH did not change compared to the vehicle group, the index of adrenal reactivity was significantly reduced by AMI, which is concordant with other results that showed that more than 2 weeks of treatment with this antidepressant evoked a decline of adrenal size (Reul et al., Citation1993). The authors explained this as a secondary effect of the chronic increase in the inhibitory control of the HPA axis evoked by the increased expression of the mineralocorticoid receptor (MR) by chronic AMI treatment (Reul et al., Citation1993). Although that process might be underlying the change in adrenal sensitivity observed in our study, we cannot discard effects of the drug on the autonomic drive. It is possible that amitriptyline, through its anticholinergic actions, also influenced splanchnic nerve activation of the adrenal cortex, which is known to affect the reactivity of the cortex to ACTH (Engeland & Arnhold, Citation2005). This could contribute to the effect of the drug on the HPA axis correction.
Even though other reports suggest that not all types of antidepressants have the same effect on the HPA axis (Holsboer & Barden, Citation1996; Scharnholz et al., Citation2010), the prevention of the rise of corticosterone observed in this study is in agreement with previous works of other authors on the effect amitriptyline on the regulation of the HPA axis in different situations: using different doses (Hassan et al., Citation1999; Holsboer & Barden, Citation1996), in different protocols of CVS in rats and mice (Katz & Hersh, Citation1981; Roth & Katz, Citation1981; Soblosky & Thurmond, Citation1986) and in aged rats in which basal levels of corticosterone are naturally augmented (Yau et al., Citation1995).
Taken together, our previous results (Cotella et al., Citation2009) and data presented here indicate that both behavior and corticosterone secretion are regulated by the treatment with the tricyclic antidepressant amitriptyline. However, this regulation occurs in different situatins depending on the previous history of the animal. These results provide evidence that changes evoked by repeated MS as neonates are expressed during adulthood under various contexts (in this case, the chronic stress), and importantly, may be reversed or prevented by pharmacological treatment. Further research is needed to determine the impact of amitriptyline treatment on key regulatory components of stress signaling (expression of the MR and glucocorticoid receptors, as well as CRH (corticotrophin-releasing hormone), AVP (arginine-vasopressin), which are involved in the regulation of the HPA axis and behavioral outcomes.
Declaration of interest
This work was supported by SECyT Grants 214/10 and 26/11, MINCyT PID 2008-Resol 82/09. Doctoral Fellowship CONICET. PhD College of Biological Sciences, Faculty of Exact, Physical and Natural Sciences, National University of Córdoba, Argentina.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Aguilera G. (1994). Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol 15(4):321–50
- Aisa BA, Tordera R, Lasheras B, Del Río J, Ramírez MJ. (2007). Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology 32(3):256–66
- Biagini G, Pich EM, Carani C, Marrama P, Agnati LF. (1998). Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. Int J Dev Neurosci: Off J Int Soc Dev Neurosci 16(3–4):187–97
- Claessens SEF, Daskalakis NP, van der Veen R, Oitzl MS, De Kloet ER, Champagne DL. (2011). Development of individual differences in stress responsiveness: an overview of factors mediating the outcome of early life experiences. Psychopharmacology 214(1):141–54
- Cotella EM, Mestres Lascano I, Levin GM, Suárez MM. (2009). Amitriptyline treatment under chronic stress conditions: effect on circulating catecholamines and anxiety in early maternally separated rats. Int J Neurosci 119(5):664–80
- De Kloet ER, Joëls M, Holsboer F. (2005). Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6(6):463–75
- Engeland W, Arnhold M. (2005). Neural circuitry in the regulation of adrenal corticosterone rhythmicity. Endocrine 28(3):325–31
- Engeland WC, Byrnes GJ, Presnell K, Gann DS. (1981). Adrenocortical sensitivity to adrenocorticotropin (ACTH) in awake dogs changes as a function of the time of observation and after hemorrhage independently of changes in ACTH. Endocrinology 108(6):2149–53
- Gillespie CF, Nemeroff CB. (2005). Hypercortisolemia and depression. Psychosomatic Med 67(17):S26–8
- Hassan AH, Patchev VK, von Rosenstiel P, Holsboer F, Almeida OF. (1999). Plasticity of hippocampal corticosteroid receptors during aging in the rat. FASEB J: Off Publ Fed Am Soc Exptl Biol 13(1):115–22
- Holsboer F, Barden N. (1996). Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocrine Rev 17(2):187–205
- Katz RJ, Hersh S. (1981). Amitriptyline and scopolamine in an animal model of depression. Neurosci Biobehav Rev 5(2):265–71
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. (2004). Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biol Psychiatry 55(4):367–75
- Ladd CO, Thrivikraman KV, Huot RL, Plotsky PM. (2005). Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology 30(6):520–33
- Lehmann J, Feldon J. (2000). Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev Neurosci 11(4):383–408
- Miller G, Chen E, Cole SW. (2009). Health psychology: developing biologically plausible models linking the social world and physical health. Ann Rev Psychol 60:501–24
- Ogawa T, Mikuni M, Kuroda Y, Muneoka K, Mori KJ, Takahashi K. (1994). Periodic maternal deprivation alters stress response in adult offspring: potentiates the negative feedback regulation of restraint stress-induced adrenocortical response and reduces the frequencies of open field-induced behaviors. Pharmacol Biochem Behav 49(4):961–7
- Renard GM, Rivarola MA, Suárez M. (2007). Sexual dimorphism in rats: effects of early maternal separation and variable chronic stress on pituitary-adrenal axis and behavior. Int J Dev Neurosci: Off J Int Soc Devel Neurosci 25(6):373–9
- Reul JM, Stec I, Söder M, Holsboer F. (1993). Chronic treatment of rats with the antidepressant amitriptyline attenuates the activity of the hypothalamic-pituitary-adrenocortical system. Endocrinology 133(1):312–20
- Roth KA, Katz RJ. (1981). Further studies on a novel animal model of depression: therapeutic effects of a tricyclic antidepressant. Neurosci Biobehav Rev 5(2):253–8
- Scharnholz B, Weber-Hamann B, Lederbogen F, Schilling C, Gilles M, Onken V, Frankhauser P, et al. (2010). Antidepressant treatment with mirtazapine, but not venlafaxine, lowers cortisol concentrations in saliva: a randomised open trial. Psychiatry Res 177(1–2):109–13
- Soblosky JS, Thurmond JB. (1986). Biochemical and behavioral correlates of chronic stress: effects of tricyclic antidepressants. Pharmacol Biochem Behav 24(5):1361–8
- Ulrich-Lai Y, Engeland W. (2002). Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology 76:79–92
- Ulrich-Lai Y, Figueiredo H, Ostrander MM, Choi DC, Engeland WC, Herman JP. (2006). Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab 291(5):E965–73
- Yau JL, Olsson T, Morris RGM, Meaney M, Seckl JR. (1995). Glucocorticoids, hippocampal corticosteroid receptor gene expression and antidepressant treatment: relationship with spatial learning in young and aged rats. Neuroscience 66(3):571–81