Abstract
Allostatic load theory implies a relationship between exposure to psychological stress and multi-system physiological dysregulation. We used data from population-based samples of men and women in Russia (Moscow; n = 1800; age, mean 68.6 years), Taiwan (n = 1036; 65.6 years) and the United States (US; n = 1054; 58.0 years) -- which are likely to vary widely with respect to levels of stress exposure and biological markers -- to determine the magnitude of the association between perceived stress and physiological dysregulation. The measure of overall dysregulation was based on 15 markers including standard cardiovascular/metabolic risk factors as well as markers of inflammation and neuroendocrine activity. Subjective psychological stress was measured by the perceived stress scale. Only the Moscow sample demonstrated a positive association with overall dysregulation in both sexes. In the US, we found an association among women but not men. Among the Taiwanese, who report the lowest perceived stress, there was no association in women but an unexpected inverse relationship in men. The effects also varied across system-level subscores: the association with perceived stress was most consistent for standard cardiovascular/metabolic factors. Perceived stress was associated with inflammation and neuroendocrine activity in some samples. Although the evidence that perceived stress is the primary source of physiological dysregulation is generally modest, it was stronger in Russia where the level of perceived stress was particularly high. For Russia only, we had information about heart function based on a 24 h ambulatory electrocardiogram; perceived stress was consistently associated with heart rate dysregulation in Russian men and women.
Introduction
Allostatic load theory implies that multi-system physiological dysregulation is the cumulative result of repeated or chronic exposure to stressors (McEwen, Citation2002; McEwen & Stellar, Citation1993). The few studies that have directly tested that implication generally find only a modest association. One explanation for the low correlation may be that this relationship is complicated because responses to stressors are shaped by a wide range of individual and social factors. The combination and interaction of these influences determine a person’s perception of the challenge, and in turn, the physiological response (McEwen, Citation1998). Thus, the psychological mediator, perceived stress, is likely to have a more direct link to physiological dysregulation than the actual stressor.
A few previous studies have tested the relationship between perceived stress and a multi-system measure of physiological dysregulation (or “allostatic load”). Studies in Taiwan (Glei et al., Citation2007; Goldman et al., Citation2005; Weinstein et al., Citation2003), the United States (US) (Gallo et al., Citation2011), Australia (Clark et al., Citation2007) and Germany (Schnorpfeil et al., Citation2003) have found modest association. One advantage of allostatic-load type measures is that they capture dysregulation across multiple interrelated systems with a simple summary score, but the disadvantage is that the results may hide variation in the effects across different systems (McDade, Citation2008). We are aware of only one study that evaluated whether the effect of perceived stress varied across systems. Clark et al. (Citation2007) reported that perceived stress was associated with neuroendocrine dysregulation, but not cardiovascular/metabolic dysregulation. Two other studies examined only a particular system or clinical syndrome. Gersten (2008) found that current perceived stress was associated with neuroendocrine dysregulation in women, but not men; the duration of perceived stress was not significant for either sex. Another study found no association between perceived stress and the collection of markers that define metabolic syndrome (Raikkonen et al., Citation2002).
We selected three countries (Russia, Taiwan and the US) that are likely to vary with respect to levels of perceived stress and physiological dysregulation. We expected Russians to exhibit much higher levels of physiological dysregulation than their counterparts in Taiwan and the US. Although there is little comparative information regarding levels of dysregulation across these three countries, population differences in mortality are likely to reflect, at least in part, underlying levels of biological parameters. Measures of allostatic load have been shown to predict mortality (Juster et al., Citation2010). Thus, our prediction regarding differences in dysregulation was based on observed patterns of survival. In terms of recent mortality decline, Taiwan represents a success story, whereas Russia has experienced a mortality crisis. Between 1970 and 2007, Taiwan made a larger gain in life expectancy (9.4 years) than the US (7.4 years), whereas average lifespan declined by 1.3 years in Russia (Human Mortality Database, Citation2011). By 2007, levels of life expectancy in Taiwan (78.1 years) were on a par with the US (78.3 years), while Russia lagged by more than 10 years (67.6 years). Among men, the gap in life expectancy between Russia and the other two countries was close to 15 years.
Russia is also likely to represent an extreme case with respect to levels of perceived stress. Its transition from a centrally-planned economy to a market-based one has entailed dramatic social, economic and political changes. The erosion of social norms and cohesion after the collapse of the Soviet Union and the ensuing psychological stresses are often blamed for exacerbating the mortality reversal (from a trend of increasing life expectancy to one of stagnation or even decline) that began in the mid-1960s (Shkolnikov et al., Citation2004). The increasing inequality within Russian society has created a polarization between a few “haves” with enormous wealth and a large population of “have nots”, who are increasingly embittered and alienated (Field, Citation1995; Shkolnikov et al., Citation2004).
In contrast, Taiwan’s transformation from an agricultural to an industrial-oriented economy over recent decades has fueled rapid economic growth that is likely to have been favorable for most Taiwanese. Levels of perceived stress are likely to be lower in Taiwan relative to the other two countries for socio-cultural reasons as well. In particular, the collectivistic culture in Taiwan values interdependence, group loyalty and social harmony, whereas the US exemplifies an individualistic culture that favors self-expression and independence in relationships with others. Russia ranks closer to the middle on Hofstede’s individualism-collectivism index (http://www.geert-hofstede.com/hofstede_dimensions.php?culture1=73&culture2=89#compare, accessed 18 Aug 2011). In Taiwan, family remains a key source of support (Son et al., Citation2008), divorce rates are low and the majority of older Taiwanese still live with or in close proximity to their married children or other relatives. High levels of social integration in Taiwan may enhance people’s ability to cope with stressors. If so, we would expect Russia to exhibit the highest levels of perceived stress and Taiwan the lowest, with the US somewhere in between.
In this article, we used data from large population-based samples in each country to determine the magnitude of the association between perceived stress and multi-system physiological dysregulation. Cardiovascular disease is a major contributor to excess Russian mortality, particularly among men, and psychological stress is considered to be a root cause (Bobak & Marmot, Citation1996; Cornia & Paniccià, Citation2000; Shapiro, Citation1995; Walberg et al., Citation1998). The measure of overall dysregulation was based on 15 markers representing three system-level clusters: (1) standard cardiovascular/metabolic risk factors; (2) inflammation, which is thought to play a role in the development of cardiovascular disease and (3) neuroendocrine activity (i.e. stress hormones). We examined subscores based on these three clusters to assess whether the association with perceived stress is driven by particular groups of biological parameters. Although previous studies in Australia and the US reported no relationship between perceived stress and cardiovascular/metabolic dysregulation (Clark et al., Citation2007; Raikkonen et al., Citation2002), we expected to find an association in Russia given the purported role that psychological stressors have played in excess cardiovascular mortality in Russia. Furthermore, we are better equipped to detect this association because our measure of dysregulation incorporates a broader set of biomarkers than previous studies, including indicators of heart function for the Russian sample, based on a 24 h ambulatory electrocardiogram, which are data that are rarely collected in a population-based survey. Thus, for Russia, we have data on three groups of biomarkers (i.e. standard cardiovascular/metabolic risk factors, inflammatory markers and heart rate parameters) that may affect or reflect the function of the cardiovascular system.
We hypothesized that: the association between perceived stress and multi-system physiological dysregulation is stronger in Russia than in the US or Taiwan and that in Russia, perceived stress is associated with dysregulation of standard cardiovascular/metabolic markers and heart rate parameters.
Methods
Participants and data
The data came from the Survey on Stress Aging and Health in Russia (SAHR), the Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan and the Midlife Development in the United States (MIDUS) National Study. All study protocols were conducted in accord with the Declaration of Helsinki. Before being interviewed and medically tested, all participants received information about the study and provided written informed consent.
Russia
The Russian sample included 1800 Muscovites aged 55 years and older who participated in the SAHR (Shkolnikova et al., Citation2009). The survey was conducted between 1 December 2006 and 30 June 2009. The study protocols were approved by the Ethics Committee of the National Research Center for Preventive Medicine (NRCPM) in Moscow, Russia and the Institutional Review Board at Duke University, USA.
Most of the study participants were selected randomly from seven epidemiological cohorts (such as Lipid Research Clinics and Monitoring Trends and Determinants in Cardiovascular Disease (MONICA) cohorts) formed in Moscow between the mid-1970s and the 1990s. Because most of the individuals in the epidemiological cohorts were residents of Moscow before the mid-1980s, an additional small part of the SAHR sample was designed to represent those who moved to Moscow after 1985. These newer residents of Moscow were identified from the Moscow Outpatient Clinics’ registry. The final SAHR sample included 1800 respondents who agreed to participate and who completed an interview and medical testing (response rate = 64%). The sample was aged 68.6 years on average with 1763 individuals originating from the epidemiological cohorts and 37 individuals originating from the Moscow Outpatient Clinics’ registry. presents descriptive statistics for the analysis variables by sex and country.
Table 1. Descriptive statistics for analysis variables, by sex and studya.
In most cases (92%), the interview and medical tests were administered during a hospital visit, but participants who were unable or unwilling to come to the hospital (8%) were interviewed and examined in their homes. The clinical data included anthropometry; measurements of blood pressure and resting pulse; a fasting blood specimen and a 12 h overnight urine specimen (20:00 h to 08:00 h; cortisol and creatinine were measured the night prior to the first appointment; catecholamines were measured in a second specimen that was collected the night following the first visit). Detailed information about laboratory assays are given in Supplemental Tables S-1 and S-2. In addition, respondents completed continuous 24 h heart monitoring (96% participation) using the Schiller Holter system (manufactured by Schiller AG (Baar, Switzerland) in accordance with American College of Cardiology/American Heart Association guidelines (Crawford et al., Citation1999)) and a 3-channel MICROVIT MT-101 digital recording device.
Taiwan
The Taiwan sample included 1036 persons aged 53 years and older who completed an in-home interview as well as the hospital-based medical examination component of the 2006 SEBAS. The research was approved by Institutional Review Boards at the Bureau of Health Promotion within the Taiwan Department of Health and at Georgetown University and Princeton University, USA.
The sampling frame for the 2006 SEBAS included those who completed the 2000 SEBAS examination (aged 60+) and a random sample from a younger cohort (aged 53–60) first interviewed in the 2003 wave of the parent study, the Taiwan Longitudinal Study of Aging (TLSA). The home interview was completed by 1284 respondents (87% response rate) and 1036 (81% of those interviewed) participated in the physical examination; 3 died before the examination, 32 were not eligible because of a health condition, and 213 declined. Analyses presented elsewhere (Chang et al., Citation2012; Goldman et al., Citation2010) indicated that examination of participants did not differ significantly from non-participants in terms of sex, employment status or average self-reported health status, but participation was lower among both the youngest (53–59 years) and oldest respondents (80+ years). Participants were also better educated, less likely to have activities of daily living limitations, more likely to live with a family member and more socially active than non-participants. Several weeks after the household interview, participants collected a 12 h overnight urine specimen (19:00 h to 07:00 h), fasted overnight and visited a nearby hospital the following morning for a physical examination that included collection of a blood specimen and measurements of blood pressure, height, weight and waist and hip circumference. Additional details about the study are provided elsewhere (Chang et al., Citation2007; Glei et al., Citation2011).
United States
The US sample included 1054 persons aged 35–86 years who participated in the biomarker component of MIDUS during 2004–2009 (Ryff et al., Citation2007). The study was approved by Institutional Review Boards at the University of Wisconsin-Madison, the University of California-Los Angeles and Georgetown University, USA.
MIDUS began in 1995 with a sample of non-institutionalized, English speaking residents of the contiguous US, aged 25–76 years. Between 2004 and 2006, a follow-up telephone interview was conducted and respondents were asked to complete a mail-in self-administered questionnaire (SAQ). Respondents from the original national sample (including a subsample of twin pairs) who completed the MIDUS II telephone interview and SAQ (n = 3018) were recruited for the biomarker component. Of these, 338 respondents were deemed ineligible because existing health information indicated that travel to the clinic would entail excessive risk to the respondent or project staff (Love et al., Citation2010). Clinic visits were completed by 1054 participants (39% of those eligible), not including the city-specific (Milwaukee) oversample of African-Americans added in MIDUS II (201 of whom participated in the clinic visit). Although the MIDUS sibling subsample and city oversamples were not generally recruited for the biomarker component, a small number (n = 26) of clinic participants came from these sources. Analyses presented elsewhere (Love et al., Citation2010, Table 3) indicate that respondents in the biomarker sample did not differ significantly from those in the interview sample in terms of age, sex, race, marital status, income or various health indicators, but they were more likely to have a college degree, less likely to be a current smoker and more likely to use alternative medicine compared with the national sample (p < 0.01). Participants in the biomarker component of MIDUS II made a two-day visit to one of three clinical research centers (East coast, Midwest, West coast) where they completed health assessments, a fasting blood draw and an overnight 12 h urine collection (19:00 h to 07:00 h).
Measures
Physiological dysregulation
The measure of overall physiological dysregulation (allostatic load) was based on 15 biomarkers that have been linked with all-cause mortality (see Supplemental Material for details). Standard cardiovascular and metabolic risk factors comprised eight markers: (1) systolic blood pressure (SBP); (2) diastolic blood pressure (DBP); (3) total cholesterol (TC); (4) high-density lipoprotein (HDL); (5) triglycerides; (6) glycoslyated hemoglobin (HbA1c); (7) body mass index (BMI) and (8) waist circumference. Blood pressure was based on the average of two measurements taken by a clinician or survey staff. We included three inflammatory markers: interleukin-6 (IL-6); C-reactive protein (CRP) and fibrinogen. Neuroendocrine markers comprised dehydroepiandrosterone sulfate (DHEAS) and three measures based on the 12 h urine collection: cortisol, epinephrine and norepinephrine. Values of cortisol, epinephrine and norepinephrine were standardized for diuresis by dividing by the level of urinary creatinine.
In order to make dysregulation scores comparable across countries, we use a common set of cutoffs to define high-risk for all countries. High-risk was defined by established clinical cutoffs where available; these included all of the standard cardiovascular and metabolic factors as well as CRP (see Supplemental Table S-3). For the remaining biomarkers, where there were no generally accepted clinical cutoffs, we defined high risk levels based on the sex-specific distribution of the pooled samples (see Supplemental Table S-4). Mortality is associated with high levels of IL-6, fibrinogen, cortisol and norepinephrine; thus, high risk was defined as the top quartile. Low levels of DHEAS are associated with high mortality and thus, the bottom quartile was defined as high risk. In the case of epinephrine, for which both low and high values have been linked with increased mortality, the top and bottom 12.5% were coded as high risk. We created subscores for the cardiovascular/metabolic, inflammatory and neuroendocrine markers by counting the number of markers in each group that fell within high risk levels; these three subscores were summed to obtain the overall physiological dysregulation score.
SAHR is one of the few population-based studies to have included a 24 h ambulatory electrocardiogram. Biomarkers based on these recordings indicate long-term functioning of the heart and its regulation by the autonomic nervous system. Elevated heart rate predicts all-cause and cardiovascular-related mortality even after controlling for arterial hypertension and other potential confounders; it is considered as an independent cardiovascular risk factor (Cook et al., Citation2006; Gillman et al., Citation1993; Palatini et al., Citation2006). Heart regulation is crucial to assuring adequate circulatory response to changing physical loads, activities and stressful challenges of everyday life. Insufficient heart regulation is reflected by depressed heart rate variability (HRV), which is associated with various diseases and mortality (Stein & Kleiger, Citation1999; Tsuji et al., Citation1994;). Thus, for the Russian sample we used the unique electrocardiogram data to construct an additional score based on four markers of heart function: (1) 24 h mean heart rate; (2) ratio of day/night heart rate averages; (3) standard deviation of the normal-to-normal (NN) interval (SDNN) and (4) square root of the mean of the sum of the squared differences between successive NN intervals (RMSSD). SDNN represents a time-domain measure of overall HRV, while RMSSD provides a time-domain estimate of short-term components of HRV. Respondents with a pacemaker (n = 10) were coded as missing for all four markers. Those with fewer than 18 h of recording (n = 9) or more than 20% artifacts (n = 3) were coded as missing for SDNN and RMSSD. The HRV parameters were calculated using only the normal intervals from the recording. High risk was defined by the clinical cutoff for SDNN (Table S-3), the sex-specific top quartile for mean heart rate and the sex-specific bottom quartile for the ratio of day/night heart rate and RMSSD (Table S-4). The heart rate score was created by counting the number of markers that fell at high risk levels.
Perceived stress scale
Subjective psychological stress was measured by the widely used and well-validated perceived stress scale (PSS) (Cohen, Citation1988; Cohen et al., Citation1983). The PSS includes ten questions that measure the extent to which respondents perceive their lives as unpredictable, uncontrollable and overloaded (e.g. “In the past month, how often have you felt that you were unable to control the important things in your life?”, “In the past month, how often have you felt nervous or stressed?”); see Supplementary Material for information regarding translations. Responses were coded on a five-point scale (0–4), with positive items reverse-scored so that higher values reflected greater perceived stress. The index was calculated by summing across all 10 items (potential range = 0 to 40). Alpha reliability was 0.78 in Moscow, 0.82 in Taiwan and 0.86 in the US. If one or two of the items were missing, we took the average across all valid items and multiplied by 10.
Analytical strategy
Analyses are based on the samples of Muscovites aged 55 years and older who participated in SAHR (n = 1800), Taiwanese aged 53 years and older who completed the 2006 SEBAS examination (n = 1036) and Americans aged 35–86 years who participated in the biomarker component of MIDUS II (n = 1054). A total of 7% of Muscovites (n = 122), 6% of Taiwanese (n = 59) and 4% of Americans (n = 39) were missing data for at least one of the 15 principal biomarkers. Among the Russians, 6% (n = 105) were missing data for one of the heart rate parameters. Perceived stress data were missing for 11 Muscovites (0.6%), 41 Taiwanese (4%) and 3 Americans (0.3%). To evaluate whether missing data may have biased the results, we used multiple imputation (Rubin, Citation1996; Schafer, Citation1999) to re-estimate the models on the full samples.
For descriptive statistics, data for Moscow and Taiwan were weighted using post-stratification weights; data for the US were unweighted. We used a two-group t test with sampling weights to compare means across subgroups. Then, linear regression was used to model physiological dysregulation (including the overall score, the three subscores and the heart rate score; see measures section for details) as a function of perceived stress controlling for age (linear and quadratic terms) and education. Models were fitted separately by sex and country. In addition, we fitted an auxiliary model that included other potential confounders: marital status, employment and living arrangements.
Standard errors were calculated using the robust estimator of variance (StataCorp, Citation2007). To account for the multi-stage sampling design of SEBAS, models for Taiwan controlled for urban residence; standard errors were adjusted with the cluster option (StataCorp, Citation2007) to correct for intragroup correlation within primary sampling units (PSU). To account for sampling multiple individuals from the same family for MIDUS, we used the cluster option to correct for family-level correlation in the US models. Statistical significance was defined as p < 0.05.
Results
The younger age of the US sample reflected differences in the sampling frame (ages 35–86 years in the US; 53 years and older in Taiwan; 55 years and older in Moscow). As hypothesized, the reported levels of perceived stress were highest in Moscow and lowest in Taiwan; women reported more stress than men in all three countries. Among men, 18% of Muscovites scored 20 or higher (out of 40 possible) on the perceived stress scale compared with only 10% of Americans and 6% of Taiwanese. The corresponding figures among women were 36% in Moscow, 12% in the US and 9% in Taiwan. Among both sexes, the mean levels of overall dysregulation were highest in Moscow and lowest in Taiwan [Men: Moscow versus US, t(1230) = 5.43, p < 0.001; US versus Taiwan, t(984) = 7.74, p < 0.001; Women: Moscow versus US, t(1459) = 9.79, p < 0.001; US versus Taiwan, t(1004) = 5.67, p < 0.001]. Thus, for both sexes, levels of dysregulation followed the same ordering by country as the PSS. These comparisons implicitly assume that the absolute levels of a given marker are comparable across countries; because the assays were performed by different laboratories, this may not be the case (e.g. a value of 3 mg/L on CRP in SAHR may not be equivalent to the same value in MIDUS or SEBAS).
With respect to dysregulation of standard cardiovascular/metabolic markers, men in Moscow had higher mean levels than their Taiwanese counterparts [t(1370) = 12.12, p < 0.001], but did not differ significantly from men in the U.S [t(1294) = 1.76, p ∼ 0.08]. For women, cardiovascular/metabolic dysregulation was much higher, on average, in Moscow compared with Taiwan [t(1414) = 14.88, p < 0.001] and the US [t(1511) = 11.40, p < 0.001]. Levels of inflammation among men were higher in Moscow compared with Taiwan [t(1375) = 2.11, p ∼ 0.035] and the US [t(1292) = 3.90, p < 0.001]. For women, inflammation was similar in Moscow and the US, but lower in Taiwan [versus Moscow: t(1419) = 3.20, p ∼ 0.001; versus US: t(1042) = 3.01, p ∼ 0.003]. For both sexes, levels of neuroendocrine dysregulation were highest in Moscow and lowest in Taiwan [Men: Moscow versus US, t(1262) = 7.79, p < 0.001; US versus Taiwan, t(993) = 5.72, p < 0.001; Women: Moscow versus US, t(1487) = 6.65, p < 0.001; US versus Taiwan, t(1016) = 5.64, p < 0.001]. In sum, on most dysregulation subscores, Muscovites exhibited the highest levels and Taiwanese the lowest levels with Americans in the middle.
shows the relationship between perceived stress and dysregulation adjusted for age and education. As shown graphically in Figures 1 and 2, perceived stress was positively associated with overall dysregulation among both sexes in Moscow and among women in the US. There was no significant association in American men [β = 0.01, t = 0.25, p ∼ 0.80] or Taiwanese women [β = −0.01, t = −0.14, p ∼ 0.89]. Among men in Taiwan, higher levels of perceived stress appeared to be associated with lower dysregulation.
Figure 1. Standardized coefficients for perceived stress from models predicting physiological dysregulation, men, by country.
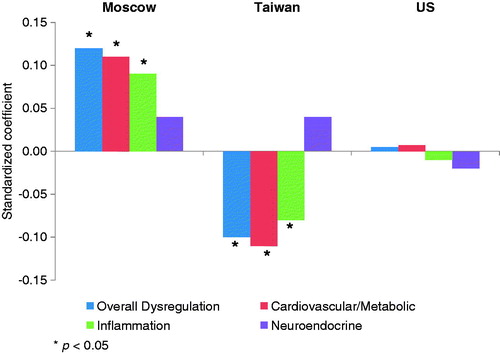
Figure 2. Standardized coefficients for perceived stress from models predicting physiological dysregulation, women, by country.
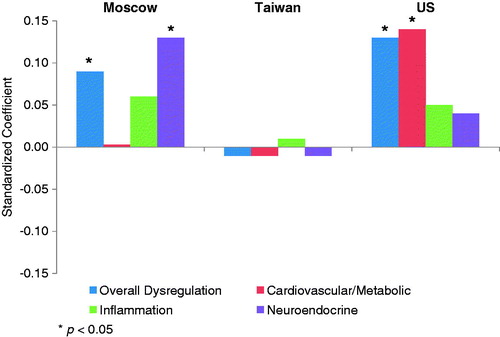
Table 2. Coefficients for perceived stress scale from models predicting physiological dysregulationa, by sex and study.
The estimates for system-level subscores revealed that the effect of perceived stress among US women was driven primarily by cardiovascular/metabolic dysregulation. Perceived stress was associated with both cardiovascular/metabolic and inflammatory markers among men in Moscow and Taiwan (although the effects were in opposite directions). For women in Moscow, perceived stress was most strongly associated with neuroendocrine dysregulation. Scores for heart rate parameters were available only for the Moscow sample. For both men and women, there was a positive association between perceived stress and heart rate dysregulation.
Robustness of the results to alternative specifications
The results were unchanged when other potential confounders were added to the model (see Supplementary Table S-5). We used multiple imputation to re-estimate the results based on the full sample for each country (see Supplementary Material for details). The results were generally similar to those presented here, although the counterintuitive negative association between perceived stress and overall dysregulation in Taiwanese men was weaker and not significant [β = −0.07, t = −1.89, p ∼ 0.07].
Allostatic load has been operationalized in a variety of ways in the literature. To assess the robustness of the results to the formulation of the dysregulation measure, we considered three alternative formulations, which differed in the number of markers included, whether markers were dichotomous or continuous, whether they were one- or two-tailed and whether the same cutoff/standard was used across sexes and countries (see Supplementary Table S-7). The first formulation incorporated the same 15 markers, but the cutoffs defining high-risk were based on the sex-specific distribution for all markers and all were coded as one-tailed: bottom quartile for HDL and DHEAS and top quartile for all other markers. When we used this formulation, the results were generally similar to those presented in .
The second formulation was again based on the same 15 markers, but they were specified as continuous measures. Markers with a skewed distribution were log-transformed to better approximate normality (DBP, waist and fibrinogen remained untransformed). Then, each marker was converted to a Z-score (standardized to have a mean of 0 and SD of 1 for the pooled distribution of the three samples); HDL and DHEAS were reverse-scored. The overall Z-score was calculated as the mean of the standardized markers. This formulation also yielded results that were similar to those in (although coefficients were somewhat weaker).
Finally, we used the original operationalization of allostatic load (Seeman et al., Citation1997) based on 10 markers. This formulation did not include any of the inflammatory markers. It also excluded several cardiovascular/metabolic markers (TC, triglycerides, BMI and waist circumference), although it included the ratio of TC/HDL and waist–hip ratio. High risk was defined by the high-risk quartile (based on the country- but not sex-specific distribution) for all markers (bottom quartile for HDL and DHEAS; top quartile for all others). The association with perceived stress was generally weaker with this formulation.
Use of beta-blockers can influence the heart rate. Thus, we explored whether adjusting for the use of these medications had any effect on the results for the heart rate score in Moscow. The coefficient for perceived stress was unchanged.
Discussion
This study tested whether the expected association between perceived stress and multi-system physiological dysregulation is found in three populations that are likely to vary widely with respect to levels of both stress exposure and biological markers. Only the Moscow sample demonstrated a positive association with overall dysregulation in both sexes. In the US, we found an association among women but not men. Among the Taiwanese, who reported the lowest levels of perceived stress, there was no association in women but an unexpected inverse relationship in men. In all of these cases, the magnitude of the effect was small. Furthermore, we found little consistency between the sexes within a given country and between countries for a given sex. In general, it seems that there was no common pattern of association between perceived stress and overall physiological dysregulation.
The effects also varied across system-level subscores: the association with perceived stress was strongest (albeit still modest) and most consistent for standard cardiovascular/metabolic factors, particularly lipids and measures of obesity markers. Heart rate parameters, which were available only for the Moscow population, also yielded a small, but consistent association for men and women. Inflammation was associated with perceived stress only among men in Moscow and Taiwan, and the relationship was in the opposite direction in the two populations. Finally, only Muscovite women showed a significant association (p < 0.001) between perceived stress and neuroendocrine dysregulation.
If physiological dysregulation reflects accumulated stress, as the allostatic load framework suggests, then why was the association with perceived stress weak and inconsistent? Other studies have yielded, at most, a modest association and suggest that the magnitude of the effect may vary across different domains of perceived stress. Gallo et al. (Citation2011) found that perceived stress in some domains (e.g. work, finances, caregiving) were modestly associated with dysregulation, but other domains (e.g. health, relationships) had no significant effect. The fact that we observed the expected association in Muscovites of both sexes, who also reported the highest levels of perceived stress among the three populations in this study, but not in the Taiwanese, who reported the lowest levels, raises the possibility that the relationship is non-linear. That is, adverse effects of perceived stress on biomarkers may not be evident until the level of stress reaches some threshold.
Transitional Russia and a few other post-Soviet countries are the only populations for which there is some aggregate-level evidence indicating that psychological stress may be driving mortality changes (Bobak & Marmot, Citation1996; Cornia & Paniccià, Citation2000; Shapiro, Citation1995; Walberg et al., Citation1998). Yet, so far there is no individual-level evidence that demonstrates a link between psychological stress and Russian mortality. Moreover, there is no research highlighting the biological mechanisms by which psychological stress might kill Russians. Nevertheless, nearly every researcher studying mortality and health in Russia mentions psychological stress as a likely contributor to excess mortality. Our results provide the first direct evidence linking perceived stress in Russia with biological markers that may affect health and mortality.
In Taiwanese men, the inverse association between perceived stress and dysregulation is puzzling. The result does not appear to be the product of a few outliers. The association was driven primarily by the difference between men who report high levels of stress (PSS ≥ 20) and those with low (PSS < 10) or medium levels of stress. However, it is important to note that men in Taiwan reported the lowest levels of perceived stress of any group: only 6% acknowledged a high level and more than half scored low. The effect was most apparent for IL-6 and fibrinogen, although the coefficients were negative for the metabolic markers as well (see Supplementary Table S-8). The association between high perceived stress and lower dysregulation among Taiwanese men persisted even if we controlled for other potential confounders, health behaviors and health indicators (see Supplementary Table S-5). There was some indication that the reliability and validity of the PSS may be lower for less educated Taiwanese than for their more educated counterparts. Furthermore, when we tested an interaction between education and the PSS, we found that the negative association appeared only among men with little or no education (β = −0.17, p < 0.05); the effect was much smaller and not significant for men with at least some post-primary education (β = −0.02, p ∼ 0.64).
It is also surprising to find so little evidence that perceived stress is associated with neuroendocrine dysregulation (which was measured by DHEAS, cortisol, epinephrine and norepinephrine levels). Two other studies that looked at the association between perceived stress and neuroendocrine dysregulation found mixed results (Clark et al., Citation2007; Gersten, Citation2008). Given the assumption that physiological dysregulation reflects cumulative exposure to stressors, one might expect a stronger association with these stress hormones. One possible explanation is that our measures did not capture the neuroendocrine variation that represents physiological stress. Overnight values for cortisol and catecholamines represent resting levels, but perhaps the differences that matter pertain to variation throughout the day or the response to stressful conditions. Yet, even studies that measured diurnal patterns of salivary cortisol have yielded mixed results. Researchers have found perceived stress associated with increased (Edwards et al., Citation2003), decreased (O'Connor et al., Citation2009) or no significant difference (Lovell et al., Citation2011) in the cortisol waking response. Some studies have shown perceived stress associated with a flattened diurnal slope (Farag et al., Citation2008; Lovell et al., Citation2011), but others have found no significant effect (Abercrombie et al., Citation2004; Kurina et al., Citation2004). Results are also conflicting for the effects on average diurnal output (Edwards et al., Citation2003; Kurina et al., Citation2004; Lovell et al., Citation2011; O'Connor et al., Citation2009).
We note several limitations to this study. First, with cross-sectional data we cannot establish the direction of the relationships. Biological parameters and associated health conditions may affect an individual’s perception of their stress level and thus, the estimated effect may be biased by reverse-causality. Second, as with any study of an older population, mortality selection can affect the results. Increased mortality among those with high levels of perceived stress could attenuate differences in physiological dysregulation among survivors. Given exceptionally high rates of mortality among middle-aged Russian men, this effect was likely to be particularly salient for that population. Yet, the effects of perceived stress were small and inconsistent even in US sample, which is much younger (35–86) and less likely to have been heavily affected by mortality attrition. Third, the measurements of biomarkers capture only a snapshot of intricate processes that are inherently dynamic in nature and subject to measurement error. Finally, current levels of perceived stress are not likely to represent earlier exposures, whereas observed levels of physiological dysregulation are likely to reflect a myriad of influences experienced over the life course.
Conclusions
We need to obtain a better understanding of the determinants of physiological dysregulation. There is ample evidence that physiological dysregulation is an important predictor of mortality and subsequent health decline, but, if we have any hope of preventing such dysregulation, we need to understand its root causes. So far, the evidence that exposure to stressors or level of perceived stress is the primary source of physiological dysregulation is modest. Perhaps it is time to reconsider the underlying premise of the allostatic load framework. A variety of factors, some of which are unrelated to psychological stress, may contribute to multi-system dysregulation. To identify the causal linkages, we may need to develop new frameworks.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This work was supported by the National Institute on Aging (grant numbers R01AG026786, R01AG16790, R01AG16661, P01-AG020166), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number R24HD047879) and the Dynasty Foundation (Russia).
SAHR was funded by the National Institute on Aging (grant number R01AG026786).
Funding for the TLSA came from the Taiwan Department of Health, the Taiwan National Health Research Institute [grant number DD01-86IX-GR601S] and the Taiwan Provincial Government. SEBAS was funded by the Demography and Epidemiology Unit of the Behavioral and Social Research Program of the National Institute on Aging [grant numbers R01 AG16790, R01 AG16661]. The Bureau of Health Promotion (BHP, Department of Health, Taiwan) provided additional financial support for SEBAS 2000.
The original MIDUS study was supported by the MacArthur Foundation Research Network on Successful Midlife Development. The MIDUS longitudinal follow-up was supported by the National Institute on Aging [grant number P01-AG020166]. The specimen collection was also facilitated by the General Clinical Research Centers Program [grant numbers M01-RR023942 to Georgetown University; M01-RR00865 to UCLA] and by the Clinical and Translational Science Award program of the National Center for Research Resources, National Institutes of Health [grant number 1UL1RR025011 to University of Wisconsin-Madison].
Supplementary Material
Download PDF (480 KB)Acknowledgements
The fieldwork and data processing for SAHR were conducted jointly by the National Research Center for Preventive Medicine (NRCPM, Moscow), the Max Planck Institute for Demographic Research (MPIDR, Rostock, Germany) and Duke University (Durham, NC, USA). Collaborators at the Moscow Institute of Pediatry and Pediatric Surgery coordinated analysis of the 24 h electrocardiogram recordings. We express our gratitude to James W. Vaupel at MPIDR for his leadership in designing the SAHR and for encouraging investigation of the associations between socioeconomic status and biomarkers. We are also grateful to: Evgeny Andreev at the New Economic School (Moscow) and Alexander Deev at NRCPM, who were responsible for the massive data cleaning and processing work for the SAHR; Svetlana Shalnova at NRCPM, who made major contributions to the methodology for collecting, handling and processing various biological markers and helped ensure the quality of these data and Viktoria Metelskaya at NRCPM for providing consultation on the biochemical measurements.
We acknowledge the hard work and dedication of the staff at the Center for Population and Health Survey Research (BHP), who were instrumental in the design and implementation of the SEBAS and supervised all aspects of the fieldwork and data processing.
We thank the staff of the Clinical Research Centers at the UW-Madison, UCLA and Georgetown University for their support in conducting the MIDUS study.
References
- Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. (2004). Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology 29:1082–92
- Bobak M, Marmot M. (1996). East-west mortality divide and its potential explanations: proposed research agenda. Br Med J 312:421–5
- Chang M, Glei D, Goldman N, Weinstein M. (2007). The Taiwan biomarker project. In: Weinstein M, Vaupel JW, Wachter KW, editors. Biosocial surveys. Committee on advances in collecting and utilizing biological indicators and genetic information in social science surveys, committee on population, division of behavioral and social sciences and education. Washington, DC: The National Academies Press. p 3-1–3-16
- Chang MC, Lin HS, Chuang YL, Goldman N, Peterson CE, Glei DA, Weinstein M, et al. (2012). Social environment and biomarkers of aging study (SEBAS) in Taiwan, 2000 and 2006: main documentation for SEBAS longitudinal public use data (released 2012) No. ICPSR03792-v5). Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]
- Clark MS, Bond MJ, Hecker JR. (2007). Environmental stress, psychological stress and allostatic load. Psychol Health Med 12:18–30
- Cohen S. (1988). Psychosocial models of the role of social support in the etiology of physical disease. Health Psychol 7:269–97
- Cohen S, Kamarck T, Mermelstein R. (1983). A global measure of perceived stress. J Health Soc Behav 24:385–96
- Cook S, Togni M, Schaub MC, Wenaweser P, Hess OM. (2006). High heart rate: a cardiovascular risk factor? Eur Heart J 27:2387–93
- Cornia GA, Paniccià R. (2000). The transition mortality crisis: evidence, interpretation and policy responses. In: Cornia GA, Paniccià R, editors. The mortality crisis in transitional economies. Oxford: Oxford University Press. p 3–37
- Crawford MH, Bernstein SJ, Deedwania PC, DiMarco JP, Ferrick KJ, Garson A, Jr Green LA, et al. (1999). ACC/AHA guidelines for ambulatory electrocardiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee to revise the guidelines for ambulatory electrocardiography). Circulation 100:886–93
- Edwards S, Hucklebridge F, Clow A, Evans P. (2003). Components of the diurnal cortisol cycle in relation to upper respiratory symptoms and perceived stress. Psychosom Med 65:320–7
- Farag NH, Moore WE, Lovallo WR, Mills PJ, Khandrika S, Eichner JE. (2008). Hypothalamic-pituitary-adrenal axis function: relative contributions of perceived stress and obesity in women. J Womens Health (Larchmt) 17:1647–55
- Field MG. (1995). The health crisis in the former Soviet Union: a report from the ‘post-war' zone. Soc Sci Med 41:1469–78
- Gallo LC, Jimenez JA, Shivpuri S, Espinosa de los Monteros K, Mills PJ. (2011). Domains of chronic stress, lifestyle factors, and allostatic load in middle-aged Mexican-American women. Ann Behav Med 41:21–31
- Gersten O. (2008). Neuroendocrine biomarkers, social relations, and the cumulative costs of stress in Taiwan. Soc Sci Med 66:507–19; discussion 520–35
- Gillman MW, Kannel WB, Belanger A, D'Agostino RB. (1993). Influence of heart rate on mortality among persons with hypertension: the Framingham Study. Am Heart J 125:1148–54
- Glei DA, Goldman N, Lin Y, Weinstein M. (2011). Age-related changes in biomarkers: longitudinal data from a population-based sample. Res Aging 33:312–26
- Glei DA, Goldman N, Chuang YL, Weinstein M. (2007). Do chronic stressors lead to physiological dysregulation? Testing the theory of allostatic load. Psychosom Med 69:769–76
- Goldman N, Glei DA, Lin YH, Weinstein M. (2010). The serotonin transporter polymorphism (5-HTTLPR): allelic variation and links with depressive symptoms. Depress Anxiety 27:260–9
- Goldman N, Glei DA, Seplaki C, Liu IW, Weinstein M. (2005). Perceived stress and physiological dysregulation in older adults. Stress 8:95–105
- Human Mortality Database (HMD). (2011). University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany), available at www.mortality.org (accessed 30 August 2011)
- Juster RP, McEwen BS, Lupien SJ. (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev 35:2–16
- Kurina LM, Schneider B, Waite LJ. (2004). Stress, symptoms of depression and anxiety, and cortisol patterns in working parents. Stress and Health 20:53–63
- Love GD, Seeman TE, Weinstein M, Ryff CD. (2010). Bioindicators in the MIDUS National Study: protocol, measures, sample, and comparative context. J Aging Health 22:1059–80
- Lovell B, Moss M, Wetherell MA. (2011). Perceived stress, common health complaints and diurnal patterns of cortisol secretion in young, otherwise healthy individuals. Horm Behav 60:301–5
- McDade TW. (2008). Challenges and opportunities for integrative health research in the context of culture: a commentary on Gersten. Soc Sci Med 66:520–4
- McEwen BS. (1998). Protective and damaging effects of stress mediators. N Engl J Med 338:171–9
- McEwen BS. (2002). Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging 23:921–39
- McEwen BS, Stellar E. (1993). Stress and the individual. Mechanisms leading to disease. Arch Intern Med 153:2093–101
- O'Connor DB, Hendrickx H, Dadd T, Elliman TD, Willis TA, Talbot D, Mayes AE, et al. (2009). Cortisol awakening rise in middle-aged women in relation to psychological stress. Psychoneuroendocrinology 34:1486–94
- Palatini P, Benetos A, Grassi G, Julius S, Kjeldsen SE, Mancia G, Narkiewicz K, et al. (2006). Identification and management of the hypertensive patient with elevated heart rate: statement of a European Society of Hypertension Consensus Meeting. J Hypertens 24:603–10
- Raikkonen K, Matthews KA, Kuller LH. (2002). The relationship between psychological risk attributes and the metabolic syndrome in healthy women: antecedent or consequence? Metabolism 51:1573–7
- Rubin DB. (1996). Multiple imputation after 18+ years (with discussion). J Am Statist Assoc 91:473–89
- Ryff C, Almeida DM, Ayanian JS, Carr DS, Cleary PD, Coe C, Davidson R, et al. (2007). Midlife Development in the United States (MIDUS II), 2004–2006 [Computer file]. No. ICPSR04652-v1). Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]
- Schafer JL. (1999). Multiple imputation: a primer. Stat Methods Med Res 8:3–15
- Schnorpfeil P, Noll A, Schulze R, Ehlert U, Frey K, Fischer JE. (2003). Allostatic load and work conditions. Soc Sci Med 57:647–56
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. (1997). Price of adaptation – allostatic load and its health consequences. MacArthur Studies of Successful Aging. Arch Intern Med 157:2259–68
- Shapiro J. (1995). The Russian mortality crisis and its causes. In: Aslund A, editor. Economic reform at risk. London: Pinter. p 149–78
- Shkolnikov VM, Andreev EM, Leon DA, McKee M, Mesle F, Vallin J. (2004). Mortality reversal in Russia: the story so far. Hygiea Internationalis 4:29–80
- Shkolnikova M, Shalnova S, Shkolnikov VM, Metelskaya V, Deev A, Andreev E, Jdanov D, Vaupel JW. (2009). Biological mechanisms of disease and death in Moscow: rationale and design of the survey on Stress Aging and Health in Russia (SAHR). BMC Public Health 9:293
- Son J, Lin N, George LK. (2008). Cross-national comparison of social support structures between Taiwan and the United States. J Health Soc Behav 49:104–18
- StataCorp. (2007). Stata Statistical Software: Release 10. College Station, TX: StataCorp LP
- Stein PK, Kleiger RE. (1999). Insights from the study of heart rate variability. Annu Rev Med 50:249–61
- Tsuji H, Venditti FJ, Jr Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. (1994). Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation 90:878–83
- Walberg P, McKee M, Shkolnikov V, Chenet L, Leon DA. (1998). Economic change, crime, and mortality crisis in Russia: regional analysis. Br Med J 317:312–18
- Weinstein M, Goldman N, Hedley A, Yu-Hsuan L, Seeman T. (2003). Social linkages to biological markers of health among the elderly. J Biosoc Sci 35:433–53
