Abstract
It has been suggested that cognitive impairments exhibited by people with post-traumatic stress disorder (PTSD) result from intrusive, flashback memories transiently interfering with ongoing cognitive processing. Researchers have further speculated that females are more susceptible to developing PTSD because they form stronger traumatic memories than males, hence females may be more sensitive to the negative effects of intrusive memories on cognition. We have examined how the reminder of a naturalistic stress experience would affect rat spatial memory and if sex was a contributing factor to such effects. Male and female Sprague–Dawley rats were exposed, without contact, to an adult female cat for 30 min. Five weeks later, the rats were trained to locate a hidden platform in the radial-arm water maze and given a single long-term memory test trial 24 h later. Before long-term memory testing, the rats were given a 30-min reminder of the cat exposure experienced 5 weeks earlier. The results indicated that the stress reminder impaired spatial memory in the female rats only. Control manipulations revealed that this effect was not attributable to the original cat exposure adversely impacting learning that occurred 5 weeks later, or to merely exposing rats to a novel environment or predator-related cues immediately before testing. These findings provide evidence that the reminder of a naturalistic stressful experience can impair cognitive processing in rats; moreover, since female rats were more susceptible to the memory-impairing effects of the stress reminder, the findings could lend insight into the existing sex differences in susceptibility to PTSD.
Introduction
Individuals who experience life-threatening trauma are at significant risk of developing post-traumatic stress disorder (PTSD). People who develop PTSD experience several debilitating symptoms, such as flashback memories, persistent anxiety, exaggerated startle, hyperarousal and diminished extinction of conditioned fear (Nemeroff et al., Citation2006; Stam, Citation2007). Individuals with PTSD also display significant cognitive impairments, including impaired declarative and working memory and deficits in attention and concentration (Buckley et al., Citation2000; Gilbertson et al., Citation2001). It has been hypothesized that the cognitive impairments observed in PTSD patients could be the result of intrusive, flashback memories transiently interfering with their ability to process new information (Brewin & Smart, Citation2005; McNally, Citation2005; Moradi et al., Citation1999; Zoladz et al., Citation2010). While such speculation has been difficult to investigate in human patients, given the ethical concerns and inherent subjectivity involved with the reactivation of traumatic memories, investigators have used rat models to test such a prediction. For instance, it has been reported previously that placing a rat in a context where it had been shocked (i.e. inhibitory avoidance apparatus) impaired the rat’s memory on a completely different task, which in this case was the location of a hidden platform in a water maze (Zoladz et al., Citation2010). Importantly, these investigators found that the reactivation of an inhibitory avoidance fear memory impaired rat spatial memory in the water maze up to a year after inhibitory avoidance training occurred. This finding supported the notion that invasive fear memories could hinder cognitive processing on other tasks.
An interesting feature of PTSD is that females are significantly more likely to develop the disorder than males (Tolin & Foa, Citation2006). Although little is known as to why females are more susceptible to the disorder, one speculation is that they form stronger emotional memories than males, which results in significantly greater potential for the development of intrusive, traumatic memories. Relevant to this speculation, recent work has reported that females with PTSD exhibit greater fear conditioning than males with PTSD (Inslicht et al., Citation2013). In non-clinical work, women exhibit better recall of emotional information than men (Bloise & Johnson, Citation2007; Canli et al., Citation2002), an effect that has been linked to greater emotion-induced noradrenergic activity in women (Segal & Cahill, Citation2009). In addition, a recent study found that post-learning stress enhanced long-term memory for emotional information in females, but not males (Felmingham et al., Citation2012). If females are more susceptible to the memory-enhancing effects of emotion, it is possible that they form stronger memories of a traumatic event, rendering these memories more intrusive and debilitating.
Sex differences with regards to emotional memory in rodents have been relatively inconsistent and seem to depend on the type of task that is used to assess learning (Dalla & Shors, Citation2009; ter Horst et al., Citation2012). For instance, females outperform males in classical eyeblink conditioning and operant avoidance conditioning paradigms (Beatty & Beatty, Citation1970; Dalla et al., Citation2008; Wood & Shors, Citation1998), while males outperform females on contextual and cue fear conditioning assessments (Maren et al., Citation1994; Pryce et al., Citation1999). At least some of these effects may be explained by sex differences in how the animals respond to aversive stimuli. That is, male rats are more inclined to react to aversive stimuli via passive responses (e.g. freezing), while female rats are more inclined to react to such stimuli via active responses (e.g. attempting to escape shock) (Kirk & Blampied, Citation1985; van Haaren et al., Citation1990). How acute stress affects learning in male and female rats has also been inconclusive (Conrad et al., Citation2004; Park et al., Citation2008). In many cases, what has been reported is that stress causes effects in females that are opposite to the ones observed in males (Shors et al., Citation2004). Also, there is evidence to suggest that, at least in some situations, female rodents are more sensitive to the effects of acute stress manipulations on anxiety-like behavior and learning. Studies have shown that female rats exhibit more vocalizations and greater anxiety in the presence of a predator (Blanchard et al., Citation1991), and they exhibit greater startle responses and a greater impairment of spatial memory when tested at least a week following predator stress (Adamec et al., Citation2006, Citation2008; Mazor et al., Citation2009). These findings provide reasonable evidence to speculate that sex differences in how the memory of a stressor affects cognitive performance may exist in rodents.
The purpose of the present study was 2-fold. First, the previous study reporting that fear memory reactivation impaired water maze memory (Zoladz et al., Citation2010) used the memory of shock, an unnatural experience, as the intrusive reminder. Thus, we wanted to examine how a reminder of a more naturalistic stress experience (e.g. cat exposure) would influence water maze memory in rats. Second, researchers have yet to assess the influence of a stress reminder on cognitive processing in males and females. Thus, we also wanted to examine whether a reminder of the naturalistic stress experience would differentially influence memory in male versus female rats. We hypothesized that rats given the reminder of the stress experience would exhibit impaired spatial memory in the water maze. We also hypothesized that such an effect might depend on the sex of the organism; however, given the inconsistencies in the literature examining sex differences in stress-memory interactions, this hypothesis was non-directional.
Methods
Animals
Sixty-five 1-month-old male (N = 32) and female (N = 33) Sprague–Dawley rats that were bred at Ohio Northern University were used in the present experiment. The rats were housed on a 12 h/12 h light–dark schedule (lights on at 07:00 h) in standard Plexiglas cages (three to four per cage) with food and water provided ad libitum. Both male and female rats were housed in the same room, but they were placed on separate shelves within the room. All experiments were carried out in accordance with the guidelines set forth by the National Institute of Health’s Guide for the Care and Use of Laboratory Animals, and the Institutional Animal Care and Use of Committee at Ohio Northern University approved all procedures.
Apparatus
Predator stress
Rats that were assigned to a stress group were stressed by exposing them to a live cat. During cat exposure, the rats were placed in a perforated wedge-shaped Plexiglas enclosure (Braintree Scientific; Braintree, MA; 20 cm × 20 cm × 8 cm), with a maximum of 6 rats being placed in the enclosure at one time. This Plexiglas enclosure was then placed on the floor of a small room (2.41 m × 1.5 m × 2.26 m) with a spayed adult female cat for 30 min (Diamond et al., Citation2006; Park et al., Citation2008). The Plexiglas enclosure prevented any physical contact between the cat and rats and exposed the rats to only the non-tactile sensory stimuli associated with the cat. Canned cat food was smeared on top of the Plexiglas enclosure to direct cat activity toward the rats. A litter box and a large metal cage (53 cm × 51 cm× 61 cm) were also in the room. The purpose of including these stimuli (e.g. cat food, litter box and metal cage) was to increase the number of salient cues in the environment that could subsequently be used to reactivate the rat’s memory of the cat exposure.
Rats that were assigned to a no stress group either remained in their home cages for the 30 min time frame or were exposed to the same room in which cat exposure took place, except without the presence of the cat. The rats exposed to the room were placed in the Plexiglas apparatus with the cat food smeared on top, which was then placed in the room. Prior to these “non-stress” sessions, previous odors and remains (i.e. fur, urine and feces) left by the cat were removed from the room, and the room was thoroughly cleaned and sanitized.
Predator stress reminder
Prior to water maze testing (described below), the rats either remained in their home cages for 30 min or were exposed to the cat exposure room for 30 min without the presence of the cat. Room exposure consisted of placing the rats in a standard Plexiglas cage and smearing canned cat food on top, which was then placed in the room. The room exposure served as a reminder of the original cat exposure for rats that had been previously exposed to the live cat. As before, prior to these sessions, any previous odors and remains (i.e. fur, urine and feces) left by the cat were removed from the room, and the room was thoroughly cleaned and sanitized by sweeping it and wiping everything down with a bleach cleaning agent.
Learning and memory
Rat learning and memory were assessed in the radial-arm water maze (RAWM) (Park et al., Citation2008; Zoladz et al., Citation2006, Citation2010). The RAWM consisted of a black, galvanized, round tank (168 cm diameter, 56 cm height, 43 cm depth) filled with water (21–22 °C). Using 6 V-shaped stainless steel walls, the tank was divided into 6 arms radiating from an open central area. A black, plastic platform (12 cm diameter) was placed 1 cm below the surface of the water at the end of one arm (the “goal arm”). At the beginning of each trial, the rats were released at the end of one arm (the “start arm”) into the center of the maze and given 60 sec to find the hidden platform. If a rat did not locate the platform within 60 sec, it was gently guided to the platform by the experimenter. Once a rat found or was guided to the platform, it was left there undisturbed for 15 sec. Spatial learning and memory were measured by counting the number of arm entry errors the rats made on each trial. An arm entry error was operationally defined as a rat passing at least halfway down an arm that did not contain the hidden platform or, rarely, when a rat entered and exited the goal arm without climbing onto the platform.
Behavioral procedure and treatment groups
The timeline and procedure for the present experiment is illustrated in . Male and female rats were assigned to stress (i.e. cat exposure) or no stress (i.e. home cage or room exposure) groups. On the first day of the experiment, the rats were given predator stress, room exposure or home cage exposure for 30 min. Five weeks later, the rats were trained in the RAWM. On the first day of training, the rats were given 12 massed acquisition trials, followed 30 min later by 6 massed short-term memory test trials. Twenty-four hours later, the rats were exposed, for 30 min, to the room where cat exposure had previously occurred or remained in their home cages for the same period of time. Subsequently, long-term spatial memory was assessed in the RAWM by giving the rats a single test trial. The 5-week time-gap between the initial cat exposure and the commencement of RAWM training was implemented for two reasons. First, since we were particularly interested in the effects of the stress memory on water maze performance, we wanted to provide a relatively wide time-gap between the predator stress experience and water maze training to limit any negative effects that the stress experience, in and of itself, could have on learning and memory. Second, in humans, PTSD symptoms are expressed long after the original stress experience occurred; thus, we wanted to assess the whether the memory of the cat exposure could exert negative effects on water maze memory relatively long after the cat exposure occurred.
Figure 1. Experimental timeline (top) and groups (bottom). One-month-old rats were exposed to their home cages, a laboratory room or a laboratory room with a cat for 30 min (Manipulation 1). Five weeks later, rats learned to locate a hidden platform in the RAWM; 24 h later, the rats either remained in their home cages or were exposed to the laboratory room for 30 min (Manipulation 2). Exposure to the laboratory room served as a reminder of the stressor for rats previously exposed to the room with a cat; 30 min after Manipulation 2, the rats were given a long-term memory test trial in the RAWM. Experimental groups are outlined below the timeline. Male (M) and female (F) rats were tested.
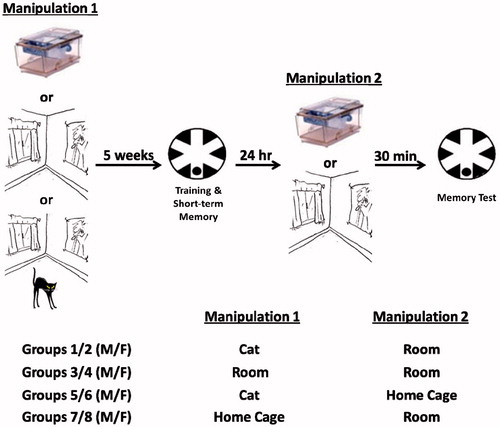
A total of four experimental manipulations were performed in the present study, as illustrated in ; each manipulation was conducted on male and female rats, resulting in a total of 8 groups (N = 8–9 rats per group). The first manipulation consisted of exposing rats to predator stress on Day 1 and then, immediately prior to the water maze memory test, re-exposing them to the room where predator stress had previously occurred (Cat → Room). The second manipulation consisted of exposing rats, on Day 1 and immediately prior to the water maze memory test, to the room where cat exposure occurred in stressed rats, but without the presence of a cat (Room → Room). The third manipulation consisted of exposing rats to predator stress on Day 1 and then, prior to the water maze memory test, leaving them in their home cages for 30 min (Cat → Home Cage). This manipulation controlled for the influence of predator stress, in general, on learning and memory. The fourth manipulation consisted of exposing rats to their home cages on Day 1 and then, immediately prior to the water maze memory test, exposing them to the room where cat exposure had occurred in stressed rats, but without the presence of a cat (Home Cage → Room). This manipulation controlled for the influence of cat-related stimuli (e.g. cat food, litter box and metal cage) and novelty, in general, on learning and memory.
Statistical analyses
Separate analyses were performed for the arm entry errors committed during the acquisition, short-term memory and long-term memory trials in the RAWM. The arm entry errors committed during the acquisition and short-term memory trials were averaged across every two trials to create 2-trial blocks for statistical analysis (). Additionally, since the two cat-exposed groups (i.e. Cat → Room and Cat → Home Cage) had been exposed to identical manipulations up to the point of RAWM training, their acquisition and short-term memory data were combined for statistical analysis. A mixed-model ANOVA was used to analyze arm entry errors made during the acquisition and short-term memory trials, with sex and condition (cat, room, home cage) serving as the between-subjects factors and trial block serving as the within-subjects factor. Since we did not have a fully crossed design with regards to the behavioral manipulations performed prior to the long-term memory test (), the four manipulations that were included in the present study were labeled as a “condition” variable for statistical analysis. A two-way ANOVA was used to analyze arm entry errors made during the long-term memory trial, with sex and condition serving as the between-subjects factors. Alpha was set at 0.05 for all analyses, and Holm–Sidak post hoc tests were employed when the omnibus F test indicated the presence of a statistically significant difference.
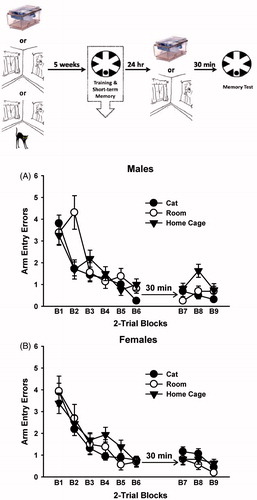
Figure 2. Arm entry errors committed in the radial-arm water maze (RAWM) during acquisition and the short-term memory test trials in male (A; top) and female (B; bottom) rats. The groups are identified in the key by manipulations performed 5 weeks prior to training. The top panel illustrates the experimental timeline, and the box depicts the time point for data. Performance during acquisition (Trials 1–12) and the short-term memory test trials (Trials 13–18) is presented in two-trial blocks (indicated by B1–B9). All rats acquired the spatial learning task, as evidenced by a significant decrease in arm entry errors across trials (p < 0.05, ANOVA). The data are mean ± SEM. N = 8–9 rats per group.
Results
Acquisition
The analysis of arm entry errors during acquisition revealed a significant effect of trial block, F(5,295) = 69.36, p < 0.001, indicating that the rats made fewer errors across trials and thus successfully acquired the task (). There was also a significant condition × trial block interaction, F(10,295) = 3.19, p < 0.05, indicating that rats exposed to the room 5 weeks prior to water maze training made more errors than the other groups on trial block 2. However, there were no group differences on trial blocks 5 and 6, indicating that by the end of training, all groups exhibited statistically equivalent performance. No other significant effects were observed.
Short-term memory
The analysis of arm entry errors committed during the short-term memory trials revealed a significant effect of trial block, F(2,118) = 3.90, p < 0.05, which again showed that the rats made fewer errors across trials (). There was also a significant effect of condition, F(2,59) = 3.49, p < 0.05, indicating that rats exposed to their home cages 5 weeks prior to water maze training made more errors on the short-term memory trials, overall, than rats exposed to the room 5 weeks prior to water maze training. No other significant effects were observed.
Long-term memory
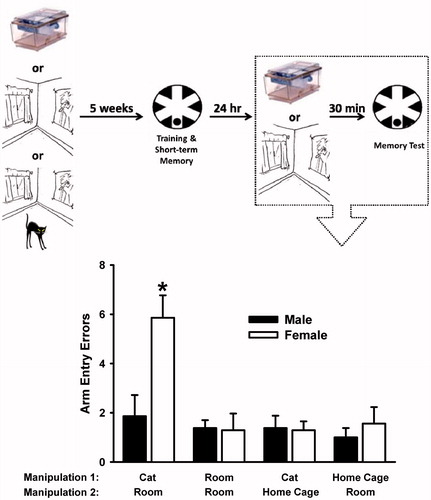
The analysis of arm entry errors during the long-term memory test trial revealed significant effects of condition, F(3,53) = 8.20, and sex, F(1,53) = 6.38, and a significant condition × sex interaction, F(3,53) = 4.83 (all p < 0.05) (). Post hoc tests revealed that the female Cat → Room group committed more arm entry errors on the long-term memory test trial than all other groups.
Figure 3. Arm entry errors committed in the radial-arm water maze (RAWM) during the 24-h long-term memory test. Below the x-axis, the groups are identified by the manipulations that were performed on them during time point 1 (i.e. 5 weeks prior to training) and time point 2 (i.e. immediately prior to water maze testing). The top panel illustrates the experimental timeline, and the box depicts the time point for the data. Female rats exposed to a cat during time point 1 and then given a reminder of that experience immediately prior to memory testing made significantly more arm entry errors than all other groups. Data are mean ± SEM. N = 8–9 rats per group. *p < 0.05 versus all other groups, Holm–Sidak post hoc test.
Discussion
We have shown that the reminder of a naturalistic stress experience (i.e. cat exposure) impairs long-term (24 h) spatial memory retrieval in rats, a finding that extends on previous work reporting a similar effect resulting from the reactivation of fear memory for a shock (Zoladz et al., Citation2010). More important, however, is the finding that, in the present study, such an impairment was observed in female rats only. As stated above, researchers have speculated that females may be more susceptible to PTSD because they form stronger emotional memories than males. This has been supported, at least in part, by the finding of greater fear conditioning in females with PTSD than males with PTSD (Inslicht et al., Citation2013). The memory impairment that we observed could have been limited to female rats because they formed a stronger memory of the cat exposure that occurred 5 weeks earlier, which led to a significantly greater stress response during the reminder. However, this possibility remains purely speculative, as we did not assess the physiological or behavioral responses of the rats to the reminder of the stress experience. Future work will have to examine if female rats exhibit greater physiological (e.g. corticosterone) and/or behavioral (e.g. freezing) responses to such a reminder to determine whether or not this is the case. Although, comparing in rats the strength of memory for a stress experience across the sexes could be difficult, since, as described above, male and female rats exhibit different behavioral responses to aversive stimuli. Another potential explanation for the present findings is that female rats are more susceptible to the memory-impairing effects of stress or a stress reminder, in general; however, the fact that Zoladz et al. (Citation2010) found a similar effect in males, albeit for a different type of stress reminder, does not seem to fit with this speculation. Either way, the present findings may lend insight into why females are more susceptible to developing PTSD. Females may form stronger traumatic memories than males or be more sensitive to the effects of traumatic reminders, either of which would render them more susceptible to PTSD symptomatology and the deleterious effects of intrusive memories on cognitive processing.
The present findings cannot be attributed to a general impairment of spatial learning and memory that resulted from cat exposure 5 weeks prior to training. Control groups that were given cat exposure 5 weeks prior to training and then left in their home cages before the 24-h long-term memory test (i.e. male and female Cat → Home Cage groups) demonstrated intact learning and memory in the RAWM ( and ). In addition, the impairment of spatial memory in the female Cat → Room group does not seem to be due to an acute stress response elicited solely by a novel environment or exposure to predator-related cues. Additional control groups that were exposed, for the first time, to the cat room and predator-related cues immediately prior to the 24-h memory test exhibited intact long-term memory. This finding is consistent with previous work indicating that rat spatial memory is exceptionally resistant to impairment by arousing or distracting stimuli and that for such impairment to occur, arousal in combination with fear is necessary (Woodson et al., Citation2003; Zoladz et al., Citation2010). Collectively, the findings from these control manipulations further support the notion that it was the memory of the stress experience that impaired water maze performance selectively in female rats.
Our findings that the reminder of a stress experience impaired water maze memory is consistent with an extensive literature demonstrating that pre-retrieval stress impairs hippocampus-dependent memory in both humans and rodents (Diamond et al., Citation2007; Roozendaal et al., Citation2009; Sandi & Pinelo-Nava, Citation2007; Schwabe et al., Citation2012). Research has shown that stress impairs electrophysiological measures of hippocampal function, such as long-term potentiation (LTP), which seems to be associated with the observed stress-induced impairments of hippocampus-dependent memory (Diamond et al., Citation2007; Joels & Krugers, Citation2007; Kim & Diamond, Citation2002). Relevant to the present study is the finding that re-exposing rats to a fear-conditioned environment can impair hippocampal LTP and hippocampal memory as well (Garcia et al., Citation1998; Li et al., Citation2005; Zoladz et al., Citation2010). Collectively, these findings resonate with our observed impairment of hippocampus-dependent memory following the reminder of cat exposure in rats.
Importantly, different parts of the hippocampus are differentially involved in cognitive- and fear-related behaviors in rats. For instance, the dorsal hippocampus (DH) is evidently more responsible for cognitive-related behaviors, such as spatial cognition (e.g. water maze navigation), while the ventral hippocampus (VH) is evidently more responsible for fear-related behaviors, such as anxiety and defensive behaviors (e.g. contextual fear and predatory threat responses) (Maggio & Segal, Citation2012). Relevant to the current findings, previous work (Pentkowski et al., Citation2006) has shown that lesions of the VH, but not the DH, impair contextual fear conditioning to a predator threat (although Wang et al. (2013) found that both the DH and VH play a role in predator odor contextual fear conditioning). This raises the question of why the reminder of a predator exposure, which would theoretically activate the VH, would impair spatial memory, a process dependent on the DH. We would argue that a chief involvement of the VH in responding to the reminder of predator exposure would not preclude negative effects of that reminder on DH processing. Indeed, as mentioned above, the reminder of cat exposure was likely a stressful experience for the rats, and there is considerable evidence for such stressors exerting deleterious effects on DH-dependent processes, such as spatial memory. Moreover, researchers have speculated that acute stress results in a region-specific shift in hippocampal function. In particular, Segal and colleagues (Maggio & Segal, Citation2012; Segal et al., Citation2010) have proposed that acute stress reduces the threshold for neuroplasticity in the VH, while at the same time increasing the threshold for neuroplasticity in the DH. This speculation has been supported empirically in several reports (Maggio & Segal, Citation2007, Citation2009). Thus, we would suggest that the cat exposure reminder, while perhaps primarily activating the VH, also led to an increased threshold for neuroplasticity in the DH, which impaired spatial memory.
It is also likely that amygdala-induced modulation of hippocampal function contributed to the impairment of water maze memory that was observed following the stress reminder. The amygdala is significantly involved in fear conditioning and fear memory retrieval, and research has shown that an intact amygdala is necessary for stress-induced alterations of hippocampal synaptic plasticity and learning and memory (Kim et al., Citation2001, Citation2005; Korz & Frey, Citation2005; Zoladz et al., Citation2011). Moreover, direct electrical stimulation of the amygdala has been shown to impair hippocampal LTP (Akirav & Richter-Levin, Citation1999, Citation2002). When the rats were reminded of the original stress experience in the present study, it likely activated the amygdala, which, coupled with the stress-induced increase of corticosteroids, norepinephrine and a host of other transmitter substances (e.g. glutamate), resulted in a saturation of hippocampal plasticity, which rendered the water maze memory inaccessible (Diamond et al., Citation2004, Citation2005, Citation2007).
Perhaps the most important question, particularly for future research, is why females were more affected by the stress reminder than males. This is not the first study to report that females are more susceptible to the emotional modulation of memory than males. Previous work in humans has shown that females recall emotional information better than males (Bloise & Johnson, Citation2007; Canli et al., Citation2002), an effect that has been associated with greater noradrenergic activity in females (Segal & Cahill, Citation2009). Another study reported that post-learning stress enhanced long-term emotional memory in females, but not males (Felmingham et al., Citation2012). In addition, researchers have emphasized important neurobiological differences in how males and females respond to emotional information, such as differential amygdala responsiveness, that may explain why females are more prone to remembering emotionally charged material (Cahill, Citation2006). Research in rodents has also reported sex differences in the neurobiology of emotion; however, this area of research has been less conclusive. One relatively consistent finding is that acute stress differentially affects learning and memory in male and female rats. For instance, acute stress enhances eyeblink conditioning in male rats, while impairing it in female rats (Shors, Citation2004). Importantly, such differential effects of stress on learning and memory depend on an intact amygdala (Waddell et al., Citation2008), and the effects in females have been associated with ovarian hormones (Wood & Shors, Citation1998). Acute stress also produces greater noradrenergic activation in female rats, relative to males (Curtis et al., Citation2006; Valentino et al., Citation2011). These findings indicate that differential neurochemical and neuroanatomical responses to stress across the sexes may be involved in mediating our observed impairment of spatial memory in females.
It is also possible that stress interacts with sex to influence the type of learning strategy that is employed. Research has shown that when spatial and non-spatial (e.g. cue-dependent) strategies are available, male rats tend to adopt spatial strategies, but the preference demonstrated by females can vary, depending on stage of the estrous cycle (Bettis & Jacobs, Citation2009; Korol et al., Citation2004; Pleil & Williams, Citation2010; Tropp & Markus, Citation2001). Furthermore, research has previously shown that stress or amygdala activation causes male rats to adopt a non-spatial, rather than the preferred spatial, strategy to learn and remember the location of a hidden platform in a water maze (Elliott & Packard, Citation2008; Kim et al., Citation2001). Thus, baseline differences in learning strategy between the sexes and/or their interaction with stress could have led to differential effects of the stress reminder on water maze memory.
Conclusions
In summary, we have reported that the reminder of a naturalistic stress experience (i.e. cat exposure) impairs long-term spatial memory in female, but not male, rats. These findings may be explained by the presence of a stronger fear memory in female rats that, when reactivated, impaired their spatial memory. Future work is necessary to determine whether or not this is the case and to assess whether or not male and female rats exhibit differential neurobiological profiles following an acute stress reminder. Our findings establish the present paradigm as a model that may be used in future research to develop a better understanding of the neurobiological mechanisms underlying intrusive memories and their role in the sex differences involved in PTSD.
Declaration of interest
The present study was supported by a Psi Chi undergraduate research grant to HMB.
Acknowledgements
The authors would like to thank Robert Carrothers, Rebecca Brooks, Kassidy Beck and Anna Krivenko for their valuable contribution to the present study.
References
- Adamec R, Head D, Blundell J, Burton P, Berton O. (2006). Lasting anxiogenic effects of feline predator stress in mice: sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiol Behav 88:12–29
- Adamec R, Holmes A, Blundell J. (2008). Vulnerability to lasting anxiogenic effects of brief exposure to predator stimuli: sex, serotonin and other factors-relevance to PTSD. Neurosci Biobehav Rev 32:1287–92
- Akirav I, Richter-Levin G. (1999). Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. J Neurosci 19:10530–5
- Akirav I, Richter-Levin G. (2002). Mechanisms of amygdala modulation of hippocampal plasticity. J Neurosci 22:9912–21
- Beatty WW, Beatty PA. (1970). Hormonal determinants of sex differences in avoidance behavior and reactivity to electric shock in the rat. J Comp Physiol Psychol 73:446–55
- Bettis TJ, Jacobs LF. (2009). Sex-specific strategies in spatial orientation in C57BL/6J mice. Behav Processes 82:249–55
- Blanchard DC, Shepherd JK, De Padua Carobrez A, Blanchard RJ. (1991). Sex effects in defensive behavior: baseline differences and drug interactions. Neurosci Biobehav Rev 15:461–8
- Bloise SM, Johnson MK. (2007). Memory for emotional and neutral information: gender and individual differences in emotional sensitivity. Memory 15:192–204
- Brewin CR, Smart L. (2005). Working memory capacity and suppression of intrusive thoughts. J Behav Ther Exp Psychiatry 36:61–8
- Buckley TC, Blanchard EB, Neill WT. (2000). Information processing and PTSD: a review of the empirical literature. Clin Psychol Rev 20:1041–65
- Cahill L. (2006). Why sex matters for neuroscience. Nat Rev Neurosci 7:477–84
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. (2002). Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci USA 99:10789–94
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. (2004). Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Pharmacol Biochem Behav 78:569–79
- Curtis AL, Bethea T, Valentino RJ. (2006). Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology 31:544–54
- Dalla C, Edgecomb C, Whetstone AS, Shors TJ. (2008). Females do not express learned helplessness like males do. Neuropsychopharmacology 33:1559–69
- Dalla C, Shors TJ. (2009). Sex differences in learning processes of classical and operant conditioning. Physiol Behav 97:229–38
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. (2007). The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast 2007:60803
- Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, Mervis RF. (2006). Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus 16:571–6
- Diamond DM, Park CR, Campbell AM, Woodson JC. (2005). Competitive interactions between endogenous LTD and LTP in the hippocampus underlie the storage of emotional memories and stress-induced amnesia. Hippocampus 15:1006–25
- Diamond DM, Park CR, Woodson JC. (2004). Stress generates emotional memories and retrograde amnesia by inducing an endogenous form of hippocampal LTP. Hippocampus 14:281–91
- Elliott AE, Packard MG. (2008). Intra-amygdala anxiogenic drug infusion prior to retrieval biases rats towards the use of habit memory. Neurobiol Learn Mem 90:616–23
- Felmingham KL, Tran TP, Fong WC, Bryant RA. (2012). Sex differences in emotional memory consolidation: the effect of stress-induced salivary alpha-amylase and cortisol. Biol Psychol 89:539–44
- Garcia R, Tocco G, Baudry M, Thompson RF. (1998). Exposure to a conditioned aversive environment interferes with long-term potentiation induction in the fimbria-CA3 pathway. Neuroscience 82:139–45
- Gilbertson MW, Gurvits TV, Lasko NB, Orr SP, Pitman RK. (2001). Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. J Trauma Stress 14:413–32
- Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr SP, Marmar CR, Neylan TC. (2013). Sex differences in fear conditioning in posttraumatic stress disorder. J Psychiatr Res 47:64–71
- Joels M, Krugers HJ. (2007). LTP after stress: up or down? Neural Plast 2007:93202
- Kim JJ, Diamond DM. (2002). The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci 3:453–62
- Kim JJ, Koo JW, Lee HJ, Han JS. (2005). Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. J Neurosci 25:1532–9
- Kim JJ, Lee HJ, Han JS, Packard MG. (2001). Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci 21:5222–8
- Kirk RC, Blampied NM. (1985). Activity during inescapable shock and subsequent escape avoidance learning: female and male rats compared. NZ J Psychol 14:9–14
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. (2004). Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav 45:330–8
- Korz V, Frey JU. (2005). Bidirectional modulation of hippocampal long-term potentiation under stress and no-stress conditions in basolateral amygdala-lesioned and intact rats. J Neurosci 25:7393–400
- Li Z, Zhou Q, Li L, Mao R, Wang M, Peng W, Dong Z, et al. (2005). Effects of unconditioned and conditioned aversive stimuli in an intense fear conditioning paradigm on synaptic plasticity in the hippocampal CA1 area in vivo. Hippocampus 15:815–24
- Maggio N, Segal M. (2007). Striking variations in corticosteroid modulation of long-term potentiation along the septotemporal axis of the hippocampus. J Neurosci 27:5757–65
- Maggio N, Segal M. (2009). Differential corticosteroid modulation of inhibitory synaptic currents in the dorsal and ventral hippocampus. J Neurosci 29:2857–66
- Maggio N, Segal M. (2012). Steroid modulation of hippocampal plasticity: switching between cognitive and emotional memories. Front Cell Neurosci 6:12 . doi: 10.3389/fncel.2012.00012
- Maren S, De Oca B, Fanselow MS. (1994). Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res 661:25–34
- Mazor A, Matar MA, Kaplan Z, Kozlovsky N, Zohar J, Cohen H. (2009). Gender-related qualitative differences in baseline and post-stress anxiety responses are not reflected in the incidence of criterion-based PTSD-like behaviour patterns. World J Biol Psychiatry 10:856–69
- McNally RJ. (2005). Debunking myths about trauma and memory. Can J Psychiatry 50:817–22
- Moradi AR, Doost HT, Taghavi MR, Yule W, Dalgleish T. (1999). Everyday memory deficits in children and adolescents with PTSD: performance on the Rivermead Behavioural Memory Test. J Child Psychol Psychiatry 40:357–61
- Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. (2006). Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res 40:1–21
- Park CR, Zoladz PR, Conrad CD, Fleshner M, Diamond DM. (2008). Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn Mem 15:271–80
- Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. (2006). Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur J Neurosci 23:2185–96
- Pleil KE, Williams CL. (2010). The development and stability of estrogen-modulated spatial navigation strategies in female rats. Horm Behav 57:360–7
- Pryce CR, Lehmann J, Feldon J. (1999). Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis and Fischer rat strains. Pharmacol Biochem Behav 64:753–9
- Roozendaal B, McEwen BS, Chattarji S. (2009). Stress, memory and the amygdala. Nat Rev Neurosci 10:423–33
- Sandi C, Pinelo-Nava MT. (2007). Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast 2007:78970
- Schwabe L, Joels M, Roozendaal B, Wolf OT, Oitzl MS. (2012). Stress effects on memory: an update and integration. Neurosci Biobehav Rev 36:1740–9
- Segal M, Richter-Levin G, Maggio N. (2010). Stress-induced dynamic routing of hippocampal connectivity: a hypothesis. Hippocampus 20:1332–8
- Segal SK, Cahill L. (2009). Endogenous noradrenergic activation and memory for emotional material in men and women. Psychoneuroendocrinology 34:1263–71
- Shors TJ. (2004). Learning during stressful times. Learn Mem 11:137–44
- Shors TJ, Falduto J, Leuner B. (2004). The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur J Neurosci 19:145–50
- Stam R. (2007). PTSD and stress sensitisation: a tale of brain and body Part 1: human studies. Neurosci Biobehav Rev 31:530–57
- ter Horst JP, de Kloet ER, Schachinger H, Oitzl MS. (2012). Relevance of stress and female sex hormones for emotion and cognition. Cell Mol Neurobiol 32:725–35
- Tolin DF, Foa EB. (2006). Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull 132:959–92
- Tropp J, Markus EJ. (2001). Sex differences in the dynamics of cue utilization and exploratory behavior. Behav Brain Res 119:143–54
- Valentino RJ, Reyes B, Van Bockstaele E, Bangasser D. (2011). Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology 62:13–20
- van Haaren F, van Hest A, Heinsbroek RP. (1990). Behavioral differences between male and female rats: effects of gonadal hormones on learning and memory. Neurosci Biobehav Rev 14:23–33
- Waddell J, Bangasser DA, Shors TJ. (2008). The basolateral nucleus of the amygdala is necessary to induce the opposing effects of stressful experience on learning in males and females. J Neurosci 28:5290–4
- Wang ME, Fraize NP, Yin L, Yuan RK, Petsagourakis D, Wann EG, Muzzio IA. (2013). Differential roles of the dorsal and ventral hippocampus in predator odor contextual fear conditioning. Hippocampus, in press
- Wood GE, Shors TJ. (1998). Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA 95:4066–71
- Woodson JC, Macintosh D, Fleshner M, Diamond DM. (2003). Emotion-induced amnesia in rats: working memory-specific impairment, corticosterone-memory correlation, and fear versus arousal effects on memory. Learn Mem 10:326–36
- Zoladz PR, Campbell AM, Park CR, Schaefer D, Danysz W, Diamond DM. (2006). Enhancement of long-term spatial memory in adult rats by the noncompetitive NMDA receptor antagonists, memantine and neramexane. Pharmacol Biochem Behav 85:298–306
- Zoladz PR, Park CR, Diamond DM. (2011). Neurobiological basis of the complex effects of stress on memory and synaptic plasticity. In: Conrad CD, ed. Handbook on stress: neuropsychological effects of stress on the brain. Oxford, UK: Wiley-Blackwell, 157--78
- Zoladz PR, Woodson JC, Haynes VF, Diamond DM. (2010). Activation of a remote (1-year old) emotional memory interferes with the retrieval of a newly formed hippocampus-dependent memory in rats. Stress 13:36–52