Abstract
Assessing long-term cortisol secretion presents difficulties when cortisol measurement is carried out by saliva, plasma and urine analyses. Hair cortisol has gained increased interest as an alternative biological marker. So far, one study has been published studying hair cortisol in endurance athletes, showing higher levels compared to controls. Using accelerometer data in the present study, we cross-sectionally explored the relationship between moderate physical activity (MPA) and vigorous physical activity (VPA) levels and hair cortisol concentrations after taking into account age, gender, and perceived stress. Hair specimens were collected from 46 university students (20 males, 26 females, Mage ± SD =21.2 ± 1.87 years). Participants provided information about their socio-demographic background and levels of perceived stress. Accelerometer data were collected to assess physical activity. Cortisol concentrations were measured in the first 3-cm hair segment nearest to the scalp. MPA was not correlated with hair cortisol content (r = −0.08). A significant correlation was found between VPA and hair cortisol (r = 0.34, p < 0.05). A regression analysis revealed that participants with higher VPA had elevated hair cortisol concentrations even after taking into account age, gender and perceived stress (β = 0.33, p < 0.05, ΔR2 = 0.106). This is the first study showing that objectively assessed VPA is associated with increased hair cortisol levels in young adults. As VPA can be regarded as a physical stressor, it seems imperative that researchers consider participants’ levels of VPA if they examine the relationship between stress exposure, hair cortisol and health.
Introduction
Measuring cortisol in stress research is extremely common as the hypothalamus-pituitary-adrenal (HPA) axis is believed to play a large role in explaining some of the health impairments related to chronic psychosocial stress (Holsboer & Ising, Citation2010; Miller et al., Citation2007). In the literature, salivary, plasma or serum, and urinary specimens have been used to analyze cortisol levels. These indicators have merit in various research contexts (e.g. assessment of acute responses to laboratory stressors) and are convenient measurements (e.g. non-invasive, cost-effective, can be collected by non-medical personnel or participants). However, they are mere snapshots of HPA activity and only reveal acutely circulating cortisol levels (e.g. saliva or plasma) or average cortisol secretion over a short period (usually 24 h; urine) (Dettenborn et al., Citation2012a; Karlen et al., Citation2011).
Recently, researchers have proposed hair cortisol as an alternative biochemical marker that avoids many of the informational complications associated with serum, salivary and urinary cortisol (Dowlati et al., Citation2010; Steudte et al., Citation2011) and that might fill the methodological void associated with the assessment of cortisol over longer periods of time (Gow et al., Citation2010; Stalder & Kirschbaum, Citation2012). The evidence that hair cortisol is a valid indicator of long-term cortisol secretion has grown rapidly over the past few years, hence, during a rather short period, hair cortisol measurement has become a widely accepted approach in biopsychology and endocrinology (Gow et al., Citation2010; Stalder & Kirschbaum, Citation2012).
Stalder and Kirschbaum (Citation2012) mentioned that there is still limited understanding about how cortisol is incorporated into the hair shaft. Possible mechanisms include: (a) passive diffusion from blood capillaries into the growing hair cells (originating from adrenal cortex secretion), (b) sebum secretion from the sebaceous gland into the completed hair shaft, (c) incorporation of substances from external sources (contamination through cortisol-containing creams or medical ointments on the hands), (d) local synthesis through intrafollicular production, and (e) sweat. Additionally, Slominski et al. (Citation2005) have shown in vitro that normal human epidural melanocytes from moderately pigmented skin can stimulate cortisol production, and that stress leads to a similar activation sequence in the single cell and in the whole body.
Additionally, using this method in exercise and sport science (particularly with elite athletes) may present new challenges regarding the interpretation of cortisol levels even though hair cortisol measurement offers great possibilities in stress research (Gerber et al., Citation2012). Exercise itself can be regarded as a physical stressor, and there is a plethora of studies that examined the influence of acute bouts of exercise on physiological stress reactions in children and adults, as well as in healthy and clinical populations (Delcorral et al., Citation1994; Negrao, Citation2000; Shephard & Sidney, Citation1975). Taken together, these studies show that acute bouts of exercise (above ∼70% VO2max) result in a substantial increase in salivary and plasma cortisol concentrations (Borer, Citation2003).
Skoluda et al. (Citation2012) discovered that endurance athletes have increased hair cortisol levels compared to controls. Thus, these researchers compared hair cortisol concentrations in the first three 3-cm head-hair segments (reflecting the previous three months) of 304 amateur athletes including long-distance runners (10-km runners, half-marathon and marathon runners), triathletes, cyclists, and 70 controls. Independent of sex, endurance athletes had 46% higher cortisol levels in the first 3-cm hair segment. Elevated cortisol levels were also found in the second and third segments. Importantly, they showed a dose response relationship between training volume and hair cortisol levels. In conclusion, these findings indicate that repeated exercise-induced HPA axis activation coincides with heightened hair cortisol levels. However, this pioneer study on hair cortisol in athletes raises important issues regarding the complexity of interpreting cortisol data in both elite athletes and leisure-time exercisers. Specifically, a large part of the retrospective cortisol measured in hair is likely due to repeated cortisol elevations associated with participation in vigorous physical activity and, to a lesser extent to exposure to psychological stress.
While Skoluda et al. (Citation2012) compared hair cortisol levels between endurance athletes and controls, we examine in the current study how objectively assessed levels of moderate physical activity (MPA) and vigorous physical activity (VPA) are associated with hair cortisol levels in young adults after taking into account age, gender, and levels of self-perceived stress.
Thus, the present study extends prior research in that Skoluda et al. (Citation2012) compared extreme groups, whereas the aim of this work was to explore linear associations between physical activity and hair cortisol concentration in a group of young adults using hierarchical regression analyses. Furthermore, we aimed at objectively measuring levels of physical activity via accelerometer data, whereas Skoluda et al. (Citation2012) compared discrete groups of participants who self-identified as endurance athletes or inactive.
Our hypotheses were that levels of VPA, but not MPA are associated with the participants’ hair cortisol concentrations (Hypothesis 1). We further expected that VPA explains variance in hair cortisol beyond that explained by age, gender and subjectively perceived stress (Hypothesis 2).
Materials and methods
Participants
Participants were 46 undergraduate students from the University of Basel, Switzerland (26 women, 20 men; total sample: Mage = 21.2 years, SD = 1.87; women: Mage = 20.6 years, SD = 1.39; men: Mage = 21.8 years, SD = 2.65) who were recruited via word-of-mouth recommendation.
Procedures
These voluntary participants gave written informed consent before providing information about their demographic background and levels of perceived stress. Additionally, they wore an accelerometer device for seven consecutive days. Participants received a food voucher (worth 10 Euro) as an incentive. The local ethics committee approved the study protocol and the study was conducted in accordance with the Declaration of Helsinki.
Assessment of physical activity
Physical activity was assessed with an accelerometer (GT1M, Actigraph, Shalimar, FL) worn around the hip. The measurement period lasted seven consecutive days (Trost et al., Citation2000) and the sampling epoch was set at 15 s (Rowlands, Citation2007). Participants were instructed not to wear the accelerometer during the night. Time per day spent in MPA (1952–5724 cpm [counts per minute], 3–6 MET [standard metabolic unit; 1 MET = energy used by the body at rest]) and VPA (>5724 cpm, >6 MET) were determined based on the raw accelerometer counts and the ActiLife® computer software, with cut-off values derived from Freedson et al. (Citation1998). The reliability and validity of the Actigraph instruments has been documented previously (e.g. Leenders et al., Citation2003; Rothney et al., Citation2008; Silva et al., Citation2010). All participants assured that their physical activity during the 7-d measurement period was representative for the 3-months that is reflected in the hair cortisol concentration.
Measurement of hair cortisol
Methods to quantify hair cortisol include enzyme linked immunosorbent assays (ELISA) and high performance-liquid chromatography-mass spectrometry (HPLC/MS) (Gow et al., Citation2010). Gow et al. (Citation2010) have described mass spectrometry methods as the “gold standards” of hair analysis, but these methods are relatively expensive. This may explain why few researchers have used them to quantify hair cortisol (e.g. Cirimele et al., Citation2000; Raul et al., Citation2004). Most researchers have used ELISA methods to establish hair cortisol levels in humans (Kalra et al., Citation2007; Kirschbaum et al., Citation2009; Thomson et al., Citation2010; van Uum et al., Citation2008; Yamada et al., Citation2007). The techniques used by different research groups are quite similar, as described in detail elsewhere (Kirschbaum et al., Citation2009; van Uum et al., Citation2008).
Hair collection and treatment
In the present study, on the last accelerometer day, hair strands of approximately 3 mm (diameter) were taken as near as possible to the scalp from a posterior vertex position. Trained study personnel using fine-tipped surgical scissors collected the hair samples. Cortisol concentrations were measured for the first 3-cm hair segment most proximal to the scalp, providing a retrospective index of cortisol secretion over the past 3 months. Due to insufficient amount of hair (<3 cm or <25 mg), data from three participants were excluded from the analyses. One participant used corticosteroid medication, which caused much higher hair cortisol concentrations (117.7 pg/mg) than peers (M = 25.17 pg/mg, SD = 12.93, range: 6.89–63.67 pg/mg), hence, we excluded these data. The wash procedure and steroid extraction was carried out at the biochemical laboratory of the University of Dresden, Germany, and as described in the laboratory protocol by Kirschbaum et al. (Citation2009). That is, each hair segment was put into a 15 ml Falcon tube, then 2.5 ml isopropanol was used to wash each hair segment twice for 3 min. After the hair had dried (for at least 12 h), the hair was powdered in a Retsch ball mill (5 min at 30.0 Hz). Twenty-five milligrams of the powdered hair were transferred into a 2 ml cryo vial. 1.5 ml pure methanol was added to the vial and rotated slowly for 45 min to extract steroids. The sample was then centrifuged at 10 080 × g 3 min; 1 ml of the clear supernatant was transferred into a new vial before the methanol (alcohol) was evaporated under a constant stream of nitrogen (60 °C) until samples were completely dried.
Hair cortisol assay
In the next step, 0.4 ml phosphate buffer, pH 7.1, containing 0.1% bovine serum albumen (IBL-International, Hamburg, Germany) was added and vials were vortexed for 15 s. Cortisol was determined by analyzing 80 µl of the reconstituted sample by commercially available immunoassay (CLIA, IBL-Hamburg, Germany). In accordance with the study of Skoluda et al. (Citation2012), the minimal amount of powdered hair was 25 mg (instead of 50 mg as in the Kirschbaum et al. (Citation2009) protocol). Intra- and interassay coefficients of variance were below 10% in the present study.
Measurement of perceived stress
General perceived stress was assessed with the widely used Perceived Stress Scale (PSS: Cohen et al., Citation1983), which is based on the cognitive-transactional stress theory (Lazarus & Folkman, Citation1984). The PSS consisted of ten short items measuring the frequency that respondents found their lives unpredictable, uncontrollable, and overloading (e.g. “How often have you felt that you could not control the important things in your life?”). Answers were given on a 5-point Likert scale from 1 (never) to 5 (very often). Items were summed to obtain an overall score, with higher scores reflecting higher stress perceptions. Adequate validity and reliability of this instrument have been established previously (Cohen & Williamson, Citation1988). The Cronbach’s alpha in the present sample was α = 0.89.
Statistical analyses
Kolmogorov–Smirnov and Levene’s tests were used to test for violations of homogeneity of variance. As cortisol measurements are often positively skewed (Manenschijn et al., Citation2011; Skoluda et al., Citation2012), log transformations were performed in case of non-normally distributed data. In the current study, a log transformation provided normally distributed data. Log-transformed cortisol data were used for computing inferential statistics, whereas descriptive findings (mean values and standard deviations) are presented in original units (pg/mg).
After inspection of the descriptive statistics, Pearson’s correlation coefficients were calculated between all study variables. To test whether level of physical activity is associated with hair cortisol levels after examining potential extraneous factors, separate hierarchical regression analyses were performed with hair cortisol concentration as a dependent variable and MPA or VPA as independent factors. Age and gender were entered in the first step of the regression equation, followed by subjectively perceived stress (step 2). Finally, MPA or VPA were entered in the third step. Cohen’s (Citation1988) recommendations were used to interpret the magnitude of the relationships found in the regression analyses with R2 ≥ 0.01 corresponding to a weak relationship, R2 ≥ 0.06 to a moderate relationship, and R2 ≥ 0.14 to a strong relationship.
Results
shows the descriptive statistics of all study variables. In total, participants reported between 164 and 775 min of MPA per week and between 0 and 213 weekly minutes of VPA. Participants’ hair cortisol concentrations varied between 6.89 and 63.7 pg/mg with a mean of 25.2 pg/mg (SD = 12.93).
Table 1. Descriptive statistics, psychometric properties and correlations between all study variables.
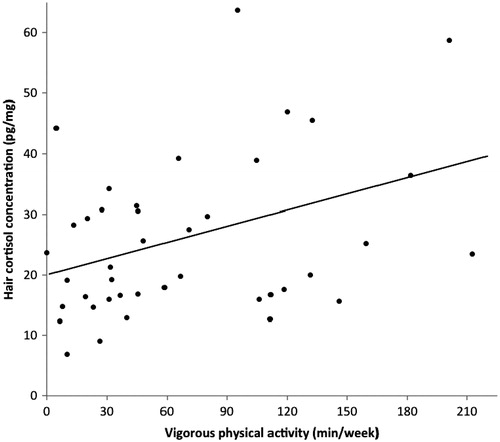
further reveals that significant bivariate relationships only existed between MPA and VPA (r = 0.33, p < 0.05), and between VPA and hair cortisol concentrations (r = 0.34, p < 0.05). The bivariate relationship between VPA for the whole group and hair cortisol is illustrated in . Although not statistically significant, the strength of the correlation between hair cortisol and VPA was similar for women (r = 0.37, p = 0.09) and men (r = 0.33, p = 0.15) when analyzed separately by gender. Moreover, no significant correlation was found between perceived stress and MPA (r = 0.15, p = 0.34), and perceived stress and VPA (r = − 0.19, p = 0.24). Finally, participants’ hair cortisol concentrations were unrelated to their perceived stress levels.
Figure 1. Scatterplot illustrating the bivariate relationship (Pearson product-moment correlation: r = 0.34, p < 0.05) between hair cortisol concentration and vigorous physical activity. N = 42 (20 males, 22 females).
represents the results of the two separate hierarchical regression analyses with MPA or VPA as predictor variables. Neither age nor gender was significantly associated with participants’ hair cortisol concentrations (step 1). Similarly, levels of self-perceived stress did not explain significant levels of variance in participants’ hair cortisol concentrations (step 2). In the third step, a significant positive association emerged for VPA (β = 0.33, p < 0.05); following Cohen (Citation1998), the amount of additionally explained variance (10.6%) pointed towards a moderate to strong relationship. In contrast, no significant influence was found for MPA.
Table 2. Hierarchical regression analyses with hair cortisol concentration as outcome and vigorous and moderate physical activity as independent variables.
Discussion
The key finding of the present study is that in support of Hypothesis 1 increasing levels of VPA are associated with elevated hair cortisol concentrations. Moreover, in line with Hypothesis 2, a regression analysis showed that the relationship between VPA and hair cortisol concentrations persisted even after examining participants’ age, gender, and subjectively perceived stress. The present findings broaden the extant literature because they establish a link between objectively measured physical activity (accelerometer data) and hair cortisol concentrations, and distinguish an effect of VPA from no effect of MPA.
Our study revealed that VPA was associated with high hair cortisol levels, whereas MPA was not. This result was expected as VPA (with intensity above ∼70% VO2max) leads to a significant increase in salivary and plasma cortisol concentrations (Luger et al., Citation1988; Negrao, Citation2000; Shephard & Sidney, Citation1975). Hence, repeated exercise-induced HPA activation seems to be reflected in overall heightened hair cortisol levels. Given Cohen’s (Citation1988) effect size recommendations, the 10.6% of explained variance in the regression analysis was considered a moderate to strong effect. Despite considerable sample differences, our findings corroborate the results of Skoluda et al. (Citation2012) who demonstrated that endurance athletes have higher hair cortisol levels than controls, and who revealed a dose response relationship between training volume and hair cortisol levels. Our sample was relatively homogeneous (young adults, university students), however, the sample of the Skoluda et al. (Citation2012) study varied more in the area of social and demographic background. Students are typically a relatively active group (Steptoe et al., Citation1997), which is why few students had very low physical activity levels in the present population. For instance, only one student had 0 min/wk of VPA in our sample, and only five students had less than 10 min/wk of VPA. Therefore, we believe that the effect sizes found in our study are relatively conservative estimates, and that the magnitude of the relationships would have been larger if the sample had included a larger portion of inactive students. Moreover, our investigation generates new knowledge in that we distinguished between the time spent in MPA versus VPA, whereas Skoluda et al. (Citation2012) only assessed total training time. This distinction seems important as even elite endurance athletes only spend moderate amounts of time in VPA (Seiler & Kjerland, Citation2006).
According to Stalder and Kirschbaum (Citation2012) findings from direct and indirect validation studies showed that hair cortisol levels constitute a valid index of long-term systemic cortisol concentrations. Nevertheless, they showed that hair cortisol concentrations can also be due to local synthesis and release of cortisol because different skin compartments such as the hair follicle provide a functional equivalent of the HPA axis (Ito et al., Citation2005; Slominski et al., Citation2005). While the current findings indicate that the elevated hair cortisol concentrations are associated with physical activity-based systemic cortisol secretion, it cannot be completely ruled out that increased sweating has contributed to heightened hair cortisol levels.
In the present study, no significant correlation existed between VPA or MPA and subjectively perceived stress. This is at odds with previous research, in which significant negative associations were reported between perceived stress and self-report measures of physical activity (Aldana et al., Citation1996; Schnohr et al., Citation2005). Nevertheless, the findings of the present study add to the existing literature as, to the best of our knowledge, this is the first study that examined the relationship between objectively assessed physical activity and subjectively perceived stress (Gerber & Pühse, Citation2009). In contrast, the finding that hair cortisol levels were not significantly associated with self-perceived stress is in line with most previous research (Dowlati et al., Citation2010; Karlen et al., Citation2011; Skoluda et al., Citation2012).
Moreover, the association between hair cortisol concentrations and VPA remained nearly unchanged after taking into account participants’ age and gender. A previous study showed that women and younger individuals have higher hair cortisol concentrations (Dettenborn et al., Citation2012b). In our sample, however, neither gender nor age was significantly associated with hair cortisol levels. Nevertheless, in the present study, the age range was too low to reliably assess the relation between hair cortisol and age.
Because VPA was significantly associated with increased hair cortisol concentrations, but not with the PSS, one might question whether hair cortisol can be used as a biopsychological measure of chronic stress in physically active young adults and athlete populations. The behavioral pattern for this population (performing several bouts of intense physical activity each week) makes it difficult to evaluate the source of cortisol (Gerber et al., Citation2012). Thus, high levels of cortisol measured in regular recreational exercisers and athlete populations should, presumably, be considered differently with respect to both metabolic consequences during exercise as well as the chronic adverse health effects that are associated with chronically high levels of cortisol. Activating the neuroendocrinological stress response during exercise is considered beneficial over time, mainly due to the adaptation of the metabolic system, including increased cortisol metabolism and simultaneous reduction of the target tissue glucocorticoid sensitivity (Holmes et al., Citation2010).
Important limitations of the present study were its relatively small sample size, its cross-sectional design, and its focus on university students. Nevertheless, compared to a previous study with young adults (M = 19.9 pg/mg, SD = 33.35; range: 1.45 pg/mg to 212.0 pg/mg; Karlen et al., Citation2011), comparable cortisol levels were found in our sample. Moreover, the statistical analyses were not adjusted for other possible confounds such as use of hair products, sunlight or heat (Kirschbaum et al., Citation2009; Li et al., Citation2011). We also acknowledge that accelerometer data may not be suited to assess all forms of physical activity such as muscle strengthening activities (e.g. resistance exercise), although they might increase cortisol in the circulation (Ahtiainen et al., Citation2004; Cardinale et al., Citation2010). Furthermore, no information was available about which specific activities students engaged in. In the present study, every activity with >5724 cpm (>6 MET) was classified as vigorous although activities within this category vary in terms of intensity (Ainsworth et al., Citation2000). Additionally, hair cortisol concentrations were related to the last three months while accelerometer data was only available for a 1-week period. It is possible that a longer assessment period would have increased the precision of the physical activity estimate, but it is difficult to say whether this would have resulted in increased or decreased relationships between the study variables. Nevertheless, all participants confirmed that the last seven days reflected their typical activity levels. Moreover, Karlen et al. (Citation2011) showed that serious life events predicted increased hair cortisol, but general perceived stress did not. Thus, adjusting for critical life events instead of general perceived stress might have provided different results. Furthermore, no information was available about participants’ physical fitness, although fitness levels might influence the relationship between VPA and hair cortisol concentration. Fitness status is related to stress reactivity during exercise; thus compared to sedentary individuals, aerobically fit persons show less cardiovascular and neuroendocrinological activation in response to exercise (Wittert et al., Citation1996). In contrast, it is unclear if physically active persons have attenuated HPA-axis activation in response to psychological stress (Jackson & Dishman, Citation2006).
Conclusions
Research about the relationship between physical activity and hair cortisol is in an early stage, and this is only the second study regarding this issue. This study shows that objectively assessed levels of VPA are significantly associated with increased levels of cortisol in human hair. This finding raises the question regarding the use of hair cortisol as a biopsychological measure of chronic stress among young adults. In particular, among participants with high VPA it may be difficult to evaluate the different sources of cortisol. Even with detailed information regarding training history and fitness, it will be a challenge for the researcher to estimate the presumed impact of psychosocial stress on the cortisol level measured in hair due to ceiling effects associated with exercise-based long-term cortisol elevation. Future studies should work towards tests of causality; e.g. whether hair cortisol concentrations change when participants engage in exercise programs with moderate or high intensity or whether stopping exercise training due to an injury is associated with declining hair cortisol concentrations. Given the challenges regarding the interpretation of hair cortisol level in athletes, researchers must use caution when inferring from high hair cortisol levels associations with health and disease among young exercisers and athletes.
Declaration of interest
The entire study was conducted without external funding. The authors alone are responsible for the content and writing of the paper. All authors declare that there is no conflict of interest.
Acknowledgements
We thank Lucia Bühlmayer, Andrea Glantschnig, Ramona Graf, Stefanie Häfeli, Christian Herrmann, Annemie Kostezer, and Elvis Maccarone for their contribution regarding recruitment of participants, data collection and data processing. Finally, we thank all participants for their valuable time and effort in this study. Last, we are particularly grateful to the hair cortisol laboratory of Prof. Clemens Kirschbaum (Technische Universität Dresden, Germany) for hair cortisol analysis.
References
- Ahtiainen JP, Pakarinen A, Kraemer WJ, Hakkinen K. (2004). Acute hormonal responses to heavy resistance exercise in strength athletes versus nonathletes. Can J Appl Physiol 29:527–43
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, et al. (2000). Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc 32:S498–516
- Aldana SG, Sutton LD, Jacobson BH. (1996). Relationship between leisure time physical activity and perceived stress. Percept Mot Skills 82:315–21
- Borer K. (2003). Exercise endocrinology. Champaign, IL: Human Kinetics
- Cardinale M, Soiza RL, Leiper JB, Gibson A, Primrose WR. (2010). Hormonal responses to a single session of whole-body vibration exercise in older individuals. Br J Sports Med 44:284–8
- Cirimele V, Kintz P, Dumestre V, Goulle JP, Ludes B. (2000). Identification of ten corticosteroids in human hair by liquid chromatography-ionspray mass spectromety. Forensic Sci Int 107:381–8
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. Mahwah, NJ: Erlbaum
- Cohen S, Kamarck T, Mermelstein R. (1983). A global measure of perceived stress. J Health Soc Behav 24:385–96
- Cohen S, Williamson GM. (1988). Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health. Newbury Park: Sage. p 31–67
- Delcorral P, Mahon AD, Duncan GE, Howe CA, Craig BW. (1994). The effect of exercise on serum and salivary cortisol in male-children. Med Sci Sports Exerc 26:1297–301
- Dettenborn L, Muhtz C, Skoluda N, Stalder T, Steudte S, Hinkelmann K, Kirschbaum C, Otte C. (2012a). Introducing a novel method to assess cumulative steroid concentrations: Increased hair cortisol concentrations over 6 months in medicated patients with depression. Stress 15:348–53
- Dettenborn L, Tietze A, Kirschbaum C, Stalder T. (2012b). The assessment of cortisol in human hair: Associations with sociodemographic variables and potential confounders. Stress 15:578–88
- Dowlati Y, Herrmann N, Swardfager W, Thomson S, Oh PI, van Uum S, Koren G, Lactôt K. (2010). Relationship between hair cortisol concentrations and depressive symptoms in patients with coronary artery disease. Neuropsychiatr Dis Treat 6:393–400
- Freedson PS, Melanson E, Sirard J. (1998). Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 30:777–81
- Gerber M, Brand S, Lindwall M, Elliot C, Kalak N, Herrmann C, Pühse U, Jonsdottir IH. (2012). Concerns regarding hair cortisol as a biomarker of chronic stress in exercise and sport science. J Sports Sci Med 11:571–81
- Gerber M, Pühse U. (2009). Do exercise and fitness protect against stress-induced health complaints? A review of the literature. Scand J Public Health 37:801–19
- Gow R, Thomson S, Rieder M, van Uum S, Koren G. (2010). An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int 196:32–7
- Holmes ME, Ekkekakis P, Eisenmann JC. (2010). The physical activity, stress and metabolic syndrome triangle: a guide to unfamiliar territory for the obesity researcher. Obes Rev 11:492–507
- Holsboer F, Ising M. (2010). Stress hormone regulation: Biological role and translation into therapy. Annu Rev Psychol 61:81–109
- Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. (2005). Human hair follicles display a functional equivalent of the hypthalamic-pituitary-adrenal (HPA) axis and synthesize cortisol. FASEB J 19:1332–4
- Jackson EM, Dishman RK. (2006). Cardiorespiratory fitness and laboratory stress: a meta-regression analysis. Psychophysiology 43:57–72
- Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. (2007). The relationship between stress and hair cortisol in healthy pregnant women. Clin Invest Med 30:E103–7
- Karlen J, Ludvidsson J, Frostell A, Theodorsson E, Faresjo T. (2011). Cortisol in hair measured in young adults – A biomarker of major life stressors? BMC Clin Pathol 11:12. doi:10.1186/1472-6890-11–12
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. (2009). Hair as a retrospective calendar of cortisol production – increased cortisol incorportation into hair in the third trimester of pregnancy. Psychoneuroendocrinology 34:32–7
- Lazarus RS, Folkman S. (1984). Stress, appraisal, and coping. New York: Springer
- Leenders NY, Nelson TE, Sherman WM. (2003). Ability of different physical activity monitors to detect movement during treadmill walking. Int J Sports Med 24:43–50
- Li J, Xie Q, Gao W, Xu Y, Wang S, Deng H, Lu Z. (2011). Time course of cortisol loss in hair segments under immersion in hot water. Clin Chim Acta 413:434–40
- Luger A, Deuster PA, Gold PW, Loriaux DL, Chrousos GP. (1988). Hormonal responses to the stress of exercise. Adv Exp Med Biol 245:273–780
- Manenschijn L, Koper JW, Lamberts SWJ, van Rossum EFC. (2011). Evaluation of a method to measure long term cortisol levels. Steroids 76:1032–6
- Miller G, Chen E, Zhou E. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull 133:25–45
- Negrao AB. (2000). HPA axis response to graded exercise in atypical depression. Biol Psychiatry 47:75S
- Raul JS, Cirimele V, Ludes B, Kintz P. (2004). Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem 37:1105–11
- Rothney MP, Apker GA, Song Y, Chen KY. (2008). Comparing the performance of three generation of ActiGraph accelerometers. J Appl Physiol 105:1091–7
- Rowlands AV. (2007). Accelerometer assessment of physical activity in children: an update. Pediatr Exerc Sci 19:252–66
- Schnohr P, Kristensen TS, Prescott E, Scharling H. (2005). Stress and life dissatisfaction are inversely associated with jogging and other types of physical activity in leisure time – the Copenhagen City Heart Study. Scand J Med Sci Sports 15:107–12
- Seiler S, Kjerland GØ. (2006). Quantifying training intensity distribution in elite endurance athletes: is there evidence for an ‘‘optimal’’ distribution? Scand J Med Sci Sports 16:49–56
- Shephard RJ, Sidney KH. (1975). Effects of physical exercise on plasma growth hormone and cortisol levels in human subjects. Exerc Sport Sci Rev 3:1–30
- Silva P, Mota J, Esliger D, Welk G. (2010). Technical reliability assessment of the Actigraph GT1M accelerometer. Meas Phys Educ Exerc Sci 14:79–91
- Skoluda N, Dettenborn L, Stalder T, Kirschbaum C. (2012). Elevated hair cortisol concentrations in endurance athletes. Psychoneuroendocrinology 37:611–7
- Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminiski J, Sweatman T, Wortsman J. (2005). CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metabol 288:E4701–6
- Stalder T, Kirschbaum C. (2012). Analysis of cortisol in hair – state of the art and future directions. Brain Behav Immun 26:1019–29
- Steptoe A, Wardle J, Fuller R, Holte A, Justo J, Sanderman R, Wichstrom L. (1997). Leisure-time physical exercise: prevalence, attitudinal correlates, and behavioral correlates among young Europeans from 21 countries. Prev Med 26:845–54
- Steudte S, Stalder T, Dettenborn L, Klumbies E, Foley P, Beesdo-Baum K, Kirschbaum C. (2011). Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Res 186:310-4
- Thomson S, Koren G, Fraser LA, Rieder M, Friedman TC, Van Uum SH. (2010). Hair analysis provides a historical record of cortisol levels in Cushing's syndrome. Exp Clin Endocr Diab 118:133–8
- Trost SG, Pate RR, Freedson PS, Sallis JF, Taylor WC. (2000). Using objective physical activity measures with youth: how many days of monitoring are needed? Med Sci Sports Exerc 25:426–31
- van Uum S, Sauvé B, Fraser LA, Morley-Forster P, Paul TL, Koren G. (2008). Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress 11:483–8
- Wittert GA, Livesey JH, Espiner EA, Donald RA. (1996). Adaptation of the hypothalamopituitary adrenal axis to chronic exercise stress in humans. Med Sci Sports Exerc 28:1015–19
- Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, Newman C, Koren G. (2007). Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology 92:42–9