Abstract
Various animal models of depression have been used to seek a greater understanding of stress-related disorders. However, there is still a great need for novel research in this area, as many individuals suffering from depression are resistant to current treatment methods. Women have a higher rate of depression, highlighting the need to investigate mechanisms of sex differences. Therefore, we employed a new animal model to assess symptoms of depression, known as intermittent swim stress (ISS). In this model, the animal experiences 100 trials of cold water swim stress. ISS has already been shown to cause signs of behavioral depression in males, but has yet to be assessed in females. Following ISS exposure, we looked at sex differences in the Morris water maze and forced swim test. The results indicated a spatial learning effect only in the hidden platform task between male and female controls, and stressed and control males. A consistent spatial memory effect was only seen for males exposed to ISS. In the forced swim test, both sexes exposed to ISS exhibited greater immobility, and the same males and females also showed attenuated climbing and swimming, respectively. The sex differences could be due to different neural substrates for males and females. The goal of this study was to provide the first behavioral examination of sex differences following ISS exposure, so the stage of estrous cycle was not assessed for the females. This is a necessary future direction for subsequent experiments. The current article highlights the importance of sex differences in response to stress.
Introduction
While the forms and severity may vary, stress is something that every person experiences. Stress is considered to be a common risk factor for a variety of psychological disorders, such as major depression (Wong & Licinio, Citation2001). Depression is a condition that millions of people suffer from every year (Knol et al., Citation2006), and is also one of the most prevalent and costly of brain diseases (Nemeroff & Owens, Citation2002). The prevalence and high cost for treatment are likely due to the fact that not everyone responds to the current treatment methods. In fact, <50% of depressed individuals fully recover following treatment (Berton & Nestler, Citation2006). Depression is also more likely to affect women than men, as the lifetime prevalence rate of depression is 21% for women and 13% for men (Nemeroff & Owens, Citation2002).
Pre-clinical models are an effective tool to evaluate the effects of depression, but novel models and approaches are necessary for drug discovery to provide better assistance to treatment-resistant individuals. Intermittent swim stress (ISS) is a recently developed model that involves exposure to a series of brief cold water swims that are both unpredictable and inescapable. Animal models that utilize inescapable tail shock or continuous swim stress are responsive to both serotonergic and noradrenergic antidepressants (Detke et al., Citation1995; Drugan et al., Citation2010; Maier & Watkins, Citation2005), while ISS is sensitive to noradrenergic, but resistant to serotonergic pharmacotherapy (Christianson et al., Citation2008, Drugan et al., Citation2010). This difference suggests distinct neural pathways that are unique to the ISS model that could provide new treatments for stress-related disorders, and ultimately depression. In males, ISS produces a number of behavioral changes, including immobility in the forced swim test (FST; Christianson et al., Citation2008; Drugan et al., Citation2010), instrumental learning differences in a swim escape test (Christianson & Drugan, Citation2005), as well as learning and memory effects in a hippocampally-dependent task known as the Morris water maze (MWM; Warner & Drugan, Citation2012). The behavioral impact of ISS has yet to be tested in females. The MWM also has clinical relevance as people suffering from depression have various comorbid cognitive deficits involving learning and episodic memory impairments (presumably related to impaired functional integrity of the hippocampus; Goodwin, Citation1997), as well as explicit verbal and visual memory deficiencies (Austin et al., Citation1999). Due to rates of depression affecting men and women differently, and the need for novel approaches to alleviate depression, the objective of the present study was to evaluate the effects of ISS exposure for both male and female rats.
Materials and methods
Subjects
16 male/16 female, 32 male/32 female, and 24 male/24 female Sprague–Dawley rats (SAS Derived, Charles River Labs, MI) were used for experiments 1, 2 and 3, respectively. Male and female rats were always housed separately from each other, and both weighed 180–200 g on arrival. All rats were housed four to a cage prior to behavioral testing and individually housed at the start of behavioral procedures with free access to food and water. The vivarium was maintained on a 12 h light/dark cycle with lights-on at 06:00 h. All behavioral testing occurred during the first 6 h of the light cycle. Rats were acclimated to the vivarium for at least 7 days prior to experimentation. The estrous cycle of the females was not taken into consideration for the current study. Because this was the first examination of sex differences following ISS exposure, we wanted to assess baseline behavioral measures prior to exploring the implications of the stage of estrous. All behavioral procedures were reviewed and approved by the University of New Hampshire Institutional Animal Care and Use Committee (IACUC).
Intermittent swim stress
ISS was administered in two Plexiglas cylinders (21 cm diameter and 42 cm height) with 0.64 cm wire mesh on the bottom, suspended above a black, plastic tank (28.6 cm height, 80.6 cm length and 45.7 cm width) filled with 15 ± 1 °C water [for photograph see Brown et al., Citation2001]. Rats received 100 trials in which the cylinders were lowered into the water to a depth of 20 cm for 5 s and then retracted to their original position (12.7 cm above the water). Space heaters blew warm air (≈36 °C) into the cylinders during inter-trial-intervals (10–100 s range, 55 s average). The swim stress apparatus was controlled by a computer with Med-PC hardware and software (Med-Associates, Georgia, VT). A confined control (CC) treatment was identical to ISS without exposure to water. Immediately after the respective procedures, ISS rats were warmed under incandescent lamps (75 W, 120 V just above the cage top) for 30 min. CC rats were placed under the same lamps to control for light exposure for 10 min, and then returned to the vivarium.
Morris water maze
Spatial learning and memory tests were conducted in a separate room from the ISS paradigm in a circular pool (122 cm in diameter) painted white and filled with water (24 ± 1 °C) to a depth of ∼48 cm. The water was made opaque with tempera paint (Vorhees & Williams, Citation2006) – ∼200 ml of white, nontoxic, liquid paint. Rats were placed at different cardinal directions of the pool (i.e. N, E, S, W). The platform was painted white and constructed out of PVC pipe and Plexiglas, with a diameter of 10 cm and a height of 50 cm. The platform was made visible by a flexible PVC cap (101 mm diameter and 41.3 mm height; PlumbQwik model: PQC-104) that would fit tightly on the platform. The visible portion of the platform was black and protruded 2 cm above the water’s surface (Mendez et al., Citation2008; Warner & Drugan, Citation2012). On each wall of the room, distinct symbols (e.g. black circle, cross, rectangle, and a yellow and black grid) were present for orientation during MWM acquisition and retention testing.
Twenty-four hours after stress, rats were trained in the MWM in pairs by an experimenter blind to group membership. This procedure was based on the format used by Healy & Drugan (Citation1996) and Warren et al. (Citation1991). The MWM was drained and fresh water was used for each group of 4 rats tested. Training consisted of 18 trials presented in 9 blocks of two trials each with a 5-min inter-block-interval. Before the training trials began, each rat was placed onto the platform for 10 s. If the rats left the platform during the 10 s, they were guided back to the platform by the experimenter. This process was repeated two times and immediately after the third placement, the training commenced with the first block of two trials. Rats were placed in different cardinal locations around the edge of the pool for each trial in a quasi-random order. Latency to find the platform was recorded with a stop watch for each trial. The platform remained in a fixed position located in the center of the northeast quadrant. If the platform was not located within 60 s, the rat was placed upon the platform and a latency of 60 s was recorded. All rats were given an additional 10 s to remain on the platform at the conclusion of each trial regardless of escape or placement. After a block of two trials was completed, rats were placed in an empty cage under a heating lamp in the same room with the MWM for 5 min. Each rat (of the two in the MWM room) experienced the block trials separately (Rudy & Paylor, Citation1988). The timing of the block trial and inter-block trial (5-min warming period) was alternated for each rat, such that when one rat was in the MWM, the other rat remained in his separate cage (i.e. the rats were never in the MWM at the same time). While potential ultrasonic vocalizations between the animals could be a concern, the researchers always ensured that a stressed animal was paired with a control during MWM testing. As a result, each pair of rats experienced similar environmental manipulations between treatments. Following the training session, all rats were hand dried with a towel and placed under the heating lamps for 10 min prior to being returned to the vivarium.
All memory trials were recorded and analyzed with an HVS tracking system (Model VP200 Software Version 10/96; Hampton, UK). By using contrast sensitivity, where the rat’s head and upper back were colored black with a permanent marker creating a contrast of the rat (black) with its surroundings (white), this system provides path analysis, time spent in all four quadrants, and swim speed of the rat during the probe trials in the MWM.
Forced swim test
Twenty-four hours after stress, rats were placed in clear Plexiglas cylinders (20 cm diameter and 46 cm height) where they were forced to swim in 24 ± 1 °C water at a depth of 30 cm. The water depth noted differs from the Porsolt et al. (Citation1977) procedure, which was 15 cm, but this alteration in the depth of the water was important to ensure that the rats were truly forced to swim. At 15 cm, the rats have the potential to touch the bottom of the cylinder, not being forced to swim, which can create a confounding variable upon interpretation of the results. In fact, numerous studies have used 30 cm for the water depth in the FST (Cryan et al., Citation2002; Detke et al., Citation1995; Drugan et al., Citation2010; Lucki, Citation1997).
The FST procedure occurred in a different room from either the ISS or MWM paradigms, and different rats were used for the FST and MWM tests. In the FST, the rats were forced to swim for 5 min, and were recorded with a video camera so their behaviors could be later analyzed. In terms of scoring the animals, the researchers incorporated a 5-s sampling procedure (Detke et al., Citation1995; Drugan et al., Citation2010; Lucki, Citation1997) where a countdown timer would emit a distinct auditory click every 5 s. Upon hearing the click, two independent raters would assess the type of behavior (i.e. immobility, swimming, and climbing) occurring for the rat at that given time point. The animal’s behaviors in the FST can be described as the following: immobility – floating and making only those movements necessary to keep the head above water, swimming – any active movements without the paws breaking the surface of the water and resulting in the animal to move in a circular fashion, and climbing – struggling against the walls with the forepaws breaking the surface of the water. Inter-rater reliability scores were obtained between the two observers for each of the three behaviors scored. The mean scores from the two raters were used for data analysis for each of the three behaviors previously mentioned (Christianson & Drugan, Citation2005; Drugan et al., Citation2010). Throughout the experimental and data analysis process, experimenters were blind to the group membership of the animals.
Experiments
Experiment 1 investigated the effects of ISS on the MWM using a visible platform. This experiment was conducted to determine if there were any residual, non-specific effects of stress on perceptual (e.g. context-specific interference) or motor (e.g. fatigue) function that might explain ISS-induced effects in the MWM. On the first day of experimentation, rats were randomly assigned into one of four groups: CC/Males (n = 8), ISS/Males (n = 8), CC/Females (n = 8), and ISS/Females (n = 8). On the second day, rats were trained in the visible platform MWM task. Spatial memory was assessed 1 h after the last training trial by placing rats in the MWM at the west quadrant with the platform removed. The rats’ swim paths were recorded for 60 s to determine if they searched in the target quadrant (i.e. where the platform was set during the training session; Healy & Drugan, Citation1996; Warren et al., Citation1991).
Experiment 2 investigated the effects of ISS on the MWM using a hidden platform. We wanted to assess the performance between males and females using a more difficult task (i.e. hidden platform) and evaluate the impact it would have during the memory trials. Rats were randomly assigned into one of four groups: CC/Males (n = 16), ISS/Males (n = 16), CC/Females (n = 16), and ISS/Females (n = 16). Each group of rats was exposed to the same stress pre-treatment and MWM learning protocol conditions described in the first experiment, with the exception that the platform was now hidden sitting 1 cm below the water surface (MWM filled with water to a depth of ∼51 cm). In addition, spatial memory was conducted in the same manner as experiment 1, 1 h post-learning trials.
Experiment 3 investigated the effects of ISS on the FST. The aim was to investigate the potential ISS-induced disparity between males and females using another behavioral endpoint (i.e. forced swim test). Rats were randomly assigned into one of four groups: CC/Males (n = 12), ISS/Males (n = 12), CC/Females (n = 12), and ISS/Females (n = 12).
Statistical analysis
All data were analyzed with the statistical package for the social sciences (SPSS) 21.0 software. A repeated measures analysis of variance (ANOVA) was used to analyze the learning trials, while a two-way ANOVA was used to analyze the memory measures (i.e. percentage of time in the target quadrant and average swimming speed) for all MWM experiments. Post hoc simple main effects were conducted to evaluate individual mean comparisons when there was a significant interaction effect. For the FST, the comparisons between the two independent rater’s scoring (inter-rater reliability) were achieved through bivariate correlations (Pearson’s r). For each behavior (immobility, swimming, climbing), a two-way ANOVA was conducted to determine statistical significance and a priori Bonferroni comparisons were conducted to evaluate individual mean comparisons. All analyses used an alpha level of 0.05 to determine statistical significance.
Results
Experiment 1: non-spatial learning with visible platform
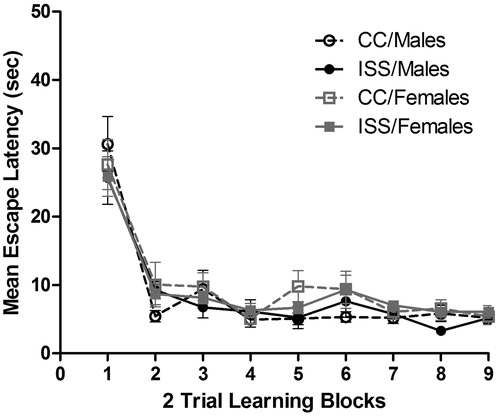
Prior exposure to ISS did not influence the mean latency to find the visible escape platform, as both ISS and CC treated rats appeared to acquire the escape response at the same rate. A repeated measures ANOVA was conducted with sex and treatment as the between subjects factor, and the learning block as the within subjects factor. A significant main effect was found for block [F(8,224) = 57.65, p<0.001], but non-significant effects for sex [F(1,28) = 2.72, p = 0.111], treatment [F(1,28) = 0.323, p = 0.575], sex × treatment interaction [F(1,28) = 0.072, p = 0.791], sex × block interaction [F(8,224) = 0.580, p = 0.794], treatment × block interaction [F(8,224) = 0.858, p = 0.552] and sex × treatment × block interaction [F(8,224) = 0.530, p = 0.833] (). Thus, escape performance improved with subsequent trials but no effects of sex or stress reached significance.
Experiment 1: spatial memory
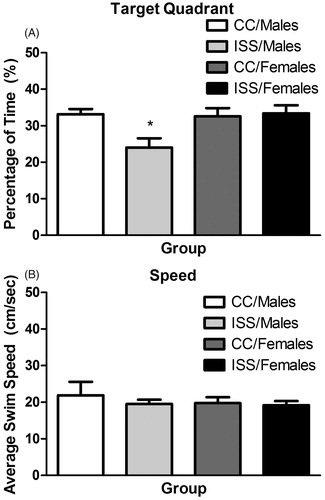
A 2 (sex: Males/Females) × 2 (treatment: CC/ISS) ANOVA was performed for all memory analyses. For percentage of time in the target quadrant, the ANOVA approached reliable significance for sex [F(1,28) = 4.17, p = 0.051] and treatment [F(1,28) = 3.71, p = 0.064] main effects, while the sex × treatment interaction [F(1,28) = 5.40, p = 0.028] was significant at the 0.05 level. Interpretation of the interaction, using simple main effects, indicated significant differences between ISS/Males and ISS/Females [F(1,28) = 9.53, p = 0.005] and CC/Males and ISS/Males [F(1,28) = 9.03, p = 0.006]. Thus, males exposed to ISS spent less time searching in the target quadrant compared to females and CC/Males (). For average swimming speed, the ANOVA revealed non-significant results for sex [F(1,28) = 0.322, p = 0.575], treatment [F(1,28) = 0.484, p = 0.492] and sex × treatment interaction [F(1,28) = 0.177, p = 0.677], so the performance differences noted above were not likely due to a locomotor effect ().
Figure 2. (A) Mean (±SEM) percentage of time to find the target quadrant for male and female rats 1 h after the last learning trial. *ISS/Males significantly differ (p < 0.05) from the CC/Males and ISS/Females groups. (B) Mean (±SEM) average swim speed (cm/s) for male and female rats 1 h after the last learning trial.
Experiment 2: spatial learning with hidden platform
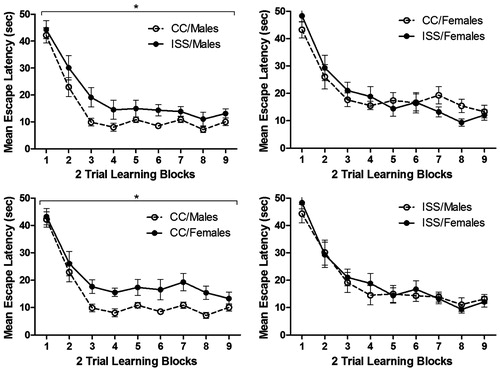
A repeated measures ANOVA was conducted with sex and treatment as the between subjects factor, and the learning block as the within subjects factor. A significant main effect was found for block [F(8,472) = 67.16, p < 0.001] and sex [F(1,59) = 4.06, p = 0.048], but a non-significant effect for treatment [F(1,59) = 1.55, p = 0.218]. There was a significant sex × treatment interaction [F(1,59) = 6.49, p = 0.014]. Interpretation of the interaction, using simple main effects, indicated significant differences between CC/Males and CC/Females [F(1,59) = 10.24, p = 0.002] and CC/Males and ISS/Males [F(1,59) = 7.07, p = 0.010]. Additionally, there were no significant differences for sex × block interaction [F(8,472) = 0.708, p = 0.684], treatment × block interaction [F(8,472) = 1.021, p = 0.419] and sex × treatment × block interaction [F(8, 472) = 0.255, p = 0.980] (). Thus, escape performance improved with subsequent trials, and CC/Males were comparatively different than CC/Females and ISS/Males.
Experiment 2: spatial memory
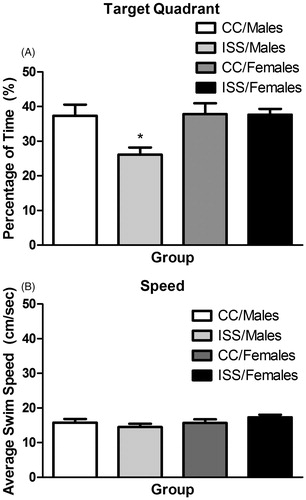
A 2 (sex: Males/Females) × 2 (treatment: CC/ISS) ANOVA was performed for all memory analyses. For percentage of time in the target quadrant, the ANOVA indicated significant sex [F(1,60) = 5.25, p = 0.026] and treatment [F(1,60) = 4.78, p = 0.033] main effects, as well as a sex × treatment interaction [F(1,60) = 4.48, p = 0.039]. Interpretation of the interaction, using simple main effects, indicated significant differences between ISS/Males and ISS/Females [F(1,60) = 9.71, p = 0.003] and CC/Males and ISS/Males [F(1,60) = 9.25, p = 0.003]. Thus, males exposed to ISS spent less time searching in the target quadrant compared to females and CC/Males (). For average swimming speed, the ANOVA revealed non-significant results for sex [F(1,60) = 2.06, p = 0.156], treatment [F(1,60) = 0.038, p = 0.847] and sex × treatment interaction [F(1,60) = 2.28, p = 0.136], and thus there were no apparent motor deficiencies between groups ().
Figure 4. (A) Mean (±SEM) percentage of time to find the target quadrant for male and female rats 1 hr after the last learning trial. *ISS/Males significantly differ (p < 0.05) from the CC/Males and ISS/Females groups. (B) Mean (±SEM) average swim speed (cm/s) for male and female rats 1 hr after the last learning trial.
Experiment 3: forced swim test
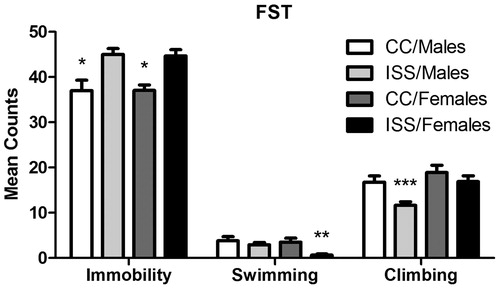
A Pearson’s r revealed that the inter-rater reliability correlations were high for all three behaviors measured: r(47) = 0.90 for immobility, r(47) = 0.93 for swimming, and r(47) = 0.87 for climbing (p < 0.001 for all behaviors). A 2 (sex: Males/Females) × 2 (treatment: CC/ISS) ANOVA was performed for all three behaviors. For immobility, the ANOVA indicated a non-significant sex main effect [F(1,41) = 0.007, p = 0.935], but a significant treatment main effect [F(1,41) = 22.00, p < 0.001]. The sex × treatment interaction [F(1,41) = 0.012, p = 0.913] was not significant. A priori Bonferroni contrasts (planned comparisons) indicated significant differences between CC/Males and ISS/Males (p = 0.001) and CC/Females and ISS/Females (p = 0.003). For swimming, the ANOVA revealed a non-significant main effect of sex [F(1,41) = 0.310, p = 0.086], but a significant treatment main effect [F(1,41) = 6.57, p = 0.014]. The sex × treatment interaction [F(1,41) = 1.85, p = 0.182] was not significant. A priori Bonferroni contrasts indicated significant differences between ISS/Males and ISS/Females (p = 0.035) and CC/Females and ISS/Females (p = 0.010). Finally, for climbing, the ANOVA revealed significant sex [F(1,41) = 8.03, p = 0.007] and treatment [F(1,41) = 7.40, p = 0.010] main effects, but a non-signifcant sex × treatment interaction [F(1,41) = 1.41, p = 0.242]. A priori Bonferroni comparisons revealed significant differences between ISS/Males and ISS/Females (p = 0.008) and CC/Males and ISS/Males (p = 0.010). These results demonstrated that both males and females displayed higher signs of behavioral depression (immobility) resulting from ISS exposure, where females and males also showed reduced swimming and climbing behavior, respectively ().
Figure 5. Mean (±SEM) counts of immobility, swimming and climbing, in a 5-min FST for male and female rats. *Significant difference (p < 0.05) from the ISS/Males and ISS/Females groups. **ISS/Females significantly differ (p < 0.05) from the CC/Females and ISS/Males groups. ***ISS/Males significantly differ (p < 0.05) from the CC/Males and ISS/Females groups.
Discussion
In the first experiment, there was no difference in learning trial performance between the groups when rats were exposed to a visible platform task. This was to be expected as we have seen a similar learning trial effect in the past (Warner & Drugan, Citation2012). Along with comparable swim speeds for all groups in all experiments during the memory trials, these points allay any concerns that prior ISS exposure could be resulting in motor or perceptual effects during MWM testing. Even though all rats behaved in a similar fashion during the learning trials, only the ISS/Males showed a memory effect, which was the same spatial memory effect observed in experiment 2. The consistent ISS-induced spatial memory deficit seen in males could be due to a greater hippocampal vulnerability resulting from acute stress that may not be present in females (Conrad et al., Citation2004). It is known that hippocampal cell proliferation following acute stress is suppressed for males, but not females, so females seem to be resilient to cognitive effects associated with memory (Falconer & Galea, Citation2003).
Additionally, in experiment 2, a hidden platform was used during the learning trials, rather than a visible platform. In this scenario, exposure to ISS had an adverse effect on the males, which provided support that stress affected MWM performance (Hölscher, Citation1999), and more specifically, ISS affected MWM performance (Warner & Drugan, Citation2012). However, the effects of ISS were not noted for the females during the learning trials.
Without prior ISS exposure, males performed differently than females in the spatial learning task. This was not a new phenomenon, as males contrasting females in a spatial learning task is a consistent trend in the literature (Coluccia & Louse, Citation2004 for review). The learning disparity may only be present in younger rats, because it is not apparent in older rats (i.e. 6 months), which could reflect a difference in maturation rate (Bucci et al., Citation1995). Also, the spatial learning effect for younger animals may not necessarily reflect poorer learning by the females, since they were quite proficient when tested for spatial memory in our study, but rather sex specific differences in search strategies (McCarthy & Konkle, Citation2005).
Several studies report that male and female rats do not use the same cues in spatial tasks. Males tend to rely on geographic markers, whereas females depend on landmark cues (Rodríguez et al., Citation2010; Roof & Stein, Citation1999). Upon further investigation, it has been suggested that males only rely on a single, geometric cue (Gaulin & FitzGerald, Citation1986; Williams & Meck, Citation1991). As long as the particular cue remained available, this resulted in a more efficient strategy for the males because they were not processing an abundant amount of information at once, and had a more direct route to accomplishing the spatial learning task. On the other hand, it has been mentioned that females rely on multiple, landmark cues. This strategy, while ultimately effective for spatial memory, resulted in slower learning as the females were processing more information at once and, thereby, were less efficient in their method (Roof & Stein, Citation1999). When the strategy was altered for males (forced to use a combination of two cue types), their spatial learning performance declined (Roof et al., Citation1993). The amount of information being processed seems to play a critical role for spatial learning, and this less effective strategy could result in a greater amount of thigmotaxis for females in the MWM as well (Beiko et al., Citation2004). This additional processing during spatial learning in females may be responsible for the protection against an ISS memory effect when tested 1 h post-training.
In experiment 3, CC/Males and CC/Females both showed reduced immobility in the forced swim test relative to the ISS groups. ISS/Females and ISS/Males differed from all other groups, for swimming and climbing behaviors, respectively. This further confirmed the ISS-induced immobility and climbing effects noted for males (Christianson et al., Citation2008; Drugan et al., Citation2010). The difference in performance between males and females for the swimming and climbing was an intriguing finding. In a study by Detke et al. (Citation1995), relating to the FST, swimming reflected serotonergic activity while climbing represented noradrenergic activity. More specifically, selective serotonin reuptake inhibitors (SSRIs) and norepinephrine selective reuptake inhibitors (NSRIs) replaced immobility with increased swimming and climbing behaviors, respectively. Others have also noticed that norepinephrine-selective drugs resulted in a reduction in immobility and elevation in climbing (Cryan et al., Citation2002). The reduction in swimming behavior for ISS/Females was consistent with the finding that females exposed to swim stress have a reduction in serotonergic activity, and these same neurochemical alterations are not noted in males (Dalla et al., Citation2010).
In expanding on the relation of serotonergic activity with ISS/Males, we have previously shown through pharmacological administration that ISS/Males were unresponsive to SSRIs in the FST (Christianson et al., Citation2008; Drugan et al., Citation2010). Our finding was in direct contrast with the typical FST paradigm (i.e. 15 min swim on day 1, 5 min swim on day 2), where SSRIs attenuate immobility (Detke et al., Citation1995; Drugan et al., Citation2010; Lucki, Citation1997). This could mean ISS exposure on day 1 could affect different neural systems. As such, ISS/Males who were given NSRIs reduced immobility and increased climbing behavior (Christianson et al., Citation2008; Drugan et al., Citation2010). These results provide further evidence that ISS could be partially mediated by norepinephrine for males (Warner & Drugan, Citation2012) and serotonin for females. Additionally, female gonadal hormones (e.g. estrogen and progesterone) modulate neurotransmitters such as serotonin (Bethea et al., Citation2002).
A limitation of the set of experiments was the fact that the estrous cycle (or role of gonadal hormones) of the females was not taken into consideration. There have been mixed results regarding the implications of the estrous cycle for performance in the water maze. Warren & Juraska (Citation1997) noted that females in estrus performed better than those in proestrus in the hippocampal task, while Berry et al. (Citation1997) revealed no differences between stages of the estrous cycle for females. In an appetitive spatial memory task (i.e. radial arm maze), learning and memory were enhanced in ovariectomized rats receiving estrogen supplementation (Daniel et al., Citation1997; Luine et al., Citation1998). Chesler & Juraska (Citation2000) extracted the ovaries from rats and assessed the significance of estrogen and progesterone in an aversive spatial memory task (i.e. MWM). Rats that were administered with either estrogen (emulating the proestrus stage) or progesterone (emulating the diestrus stage) alone did not differ in performance from controls. However, rats that received a combination of estrogen and progesterone revealed a MWM acquisition difference compared to controls. The disparity in performance for females could also be due to environmental manipulations in the MWM, as water temperature influences MWM performance. Warmer temperatures increased performance for proestrus rats and colder temperatures increased performance for estrus rats (Rubinow et al., Citation2004). In relation to the current study, Conrad et al. (Citation2004) investigated the role of the estrous cycle following acute stress (i.e. restraint stress). It was determined that acute stress enhanced levels of performance in the MWM for females, regardless of the stage of estrous. This mirrors the female acute stress effects noted in the present study for experiments 1 and 2.
In looking at the association of the estrous cycle for the forced swim test, females in the proestrus stage (higher levels of estrogen) exhibit lower immobility relative to those in diestrus (Frye & Walf, Citation2002), and the administration of estrogen attenuated depressive behaviors for rats (Rachman et al., Citation1998). In another study, immobility counts did not differ between estrus and diestrus, but the administration of a 5-HT3 antagonist reduced immobility in either of the two stages (Bravo & Maswood, Citation2006).
In terms of the validity of ISS as a newer animal model to assess symptoms of depression, prior work indicates that ISS causes higher levels of immobility in the FST (Brown et al., Citation2001; Christianson & Drugan, Citation2005; Drugan et al., Citation2010). The effects associated with ISS extend beyond immobility, as there have been instrumental (Christianson & Drugan, Citation2005) and spatial (Warner & Drugan, Citation2012) learning deficiencies as well as signs of stress-induced analgesia (Brown et al., Citation2001). Behavioral effects induced by ISS are reversed with the administration of norepinephrine-selective antidepressants in either the MWM (Warner & Drugan, Citation2012) or FST (Drugan et al., Citation2010).
Recently, ISS-induced effects have been revealed in the juvenile social exploration task demonstrated by a reduction in social exploration (unpublished results). These data support the trans-situational value of the model, as water did not serve as a contextual cue in the social exploration paradigm. The ISS model is an acute stressor that has resulted in physiological changes (i.e. elevated corticosterone levels; Drugan et al., Citation2005), and produced effects for up to 72 h – with the most robust effects occurring ∼24 h post-stress (Warner & Drugan, Citation2012). On the clinical level, cognitive impairments (Austin et al., Citation2001), decreased activity (Dantzer et al., Citation2008), stress-induced analgesia (Ford & Finn, Citation2008) and increased cortisol (Stetler & Miller, Citation2011) have also been noted for depressed patients, as well as symptoms of major depressive episodes persisting for longer periods of time (Spijker et al., Citation2002).
With the risk of depression being substantially higher for females compared to males (ratio of 2:1; Kessler, Citation2003), there is always a need to explore different research areas (such as those relating to ISS) to provide new insight into the field. Sex differences have not been previously investigated for ISS, but the current results of this paradigm appear to be dependent on the type of subsequent behavioral testing employed. While other behavioral tasks could have been investigated, the current paradigms were used to establish direct comparison to past ISS research. Both male and female rats exposed to ISS displayed similar behavioral differences in the FST, and female rats were more resilient in the MWM. Following exposure to acute stressors (other than inescapable swim or shock), memory is facilitated in females (Conrad et al., Citation2004), and estrogen appears to be a contributing factor for associative learning in female rats, as learning is improved by a stress-induced elevation of estrogen (Shors et al., Citation1999). Similar to ISS, inescapable shock causes escape learning deficiencies for males, but not females. In general, female rats are more active than male rats. This allows females to be less activity-restricted in response to shock, and thereby more resistant to stress-induced effects on escape learning (Kirk & Blampied, Citation1985) – which could be attributed to ISS as well. Contrary to shock models (Muller et al., Citation2011), the current results showed that females differed in time of immobility for the FST following ISS. The role of estrogen could also be a contributing factor for the FST differences in response to swim or shock stress, as estrogen levels rise to a higher degree following swim stress compared to shock – most notably during the diestrus stage (Shors et al., Citation1999).
Despite the large disparity in the rate of depression between men and women, the greater risk of depression for women appears to be due to an increased risk of first onset of the disorder and not necessarily to the degree of persistence or relapse (Kessler, Citation2003). With animal models, it is difficult to account for all of the variables that may trigger depression between men and women, but pre-clinical research attempts to provide greater clarity on the subject matter.
In summary, for spatial learning, there was no difference in performance between the males or females (regardless of the condition) for a visible platform task. When the platform was hidden, ISS exposure affected learning for the males, but not the females, and at baseline levels males responded differently to females. There was a consistent spatial memory effect (regardless of the learning procedure) as ISS/Males showed memory differences whereas females did not, and the swim speeds were comparable for all conditions. In the FST, there were ISS-induced effects for both males and females showing greater immobility. Additionally, males had reduced climbing and females had reduced swimming. While there are some baseline differences between males and females with regard to spatial learning, the effects of ISS exposure may affect different neural substrates for males (norepinephrine) and females (serotonin). Future work should investigate the implications of these neural mechanisms between males and females, as well as the implications of the estrous cycle.
Declaration of interest
Research was supported by the Undergraduate Research Opportunities Program, the Cole Neuroscience and Behavior Faculty Research Fund and the Department of Psychology Research Fund at the University of New Hampshire. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Acknowledgements
The behavioral protocols described were reviewed and approved by the University of New Hampshire Institutional Animal Care and Use Committee (IACUC). We have complied with the APA ethical standards in the treatment of the subjects in this study. Experiments 2 and 3 were conducted in partial fulfillment of honors in major for MKL. The authors would like to thank Edward J. O’Brien from the University of New Hampshire for his statistical assistance.
References
- Austin MP, Mitchell P, Goodwin GM. (2001). Cognitive deficits in depression: possible implications for functional neuropathology. Brit J Psychiatry 178:200–6
- Austin MP, Mitchell P, Wilhelm K, Parker G, Hickie I, Brodaty H, Chan J, et al. (1999). Cognitive function in depression: a distinct pattern of frontal impairment in melancholia? Psychol Med 29:73–85
- Beiko J, Lander R, Hampson E, Boon F, Cain DP. (2004). Contribution of sex differences in the acute stress response to sex differences in water maze performance in the rat. Behav Brain Res 151:239–53
- Bethea CN, Lu NZ, Gundlah C, Streicher JM. (2002). Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol 23:41–100
- Berry B, McMahan R, Gallagher M. (1997). Spatial learning and memory at defined points of the estrous cycle: effects on performance of a hippocampal-dependent task. Behav Neurosci 111:267–74
- Berton O, Nestler EJ. (2006). New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7:137–51
- Bravo G, Maswood S. (2006). Acute treatment with 5-HT3 receptor antagonist, tropisetron, reduces immobility in intact female rats exposed to the forced swim test. Pharmacol Biochem Behav 85:362–8
- Brown PL, Hurly C, Repucci N, Drugan RC. (2001). Behavioral analysis of stress controllability effects in a new swim stress paradigm. Pharmacol Biochem Behav 68:263–72
- Bucci DJ, Chiba AA, Gallagher M. (1995). Spatial learning in male and female Long-Evans rats. Behav Neurosci 109:180–3
- Chesler EJ, Juraska JM. (2000). Acute administration of estrogen and progesterone impairs the acquisition of the spatial Morris water maze in ovariectomized rats. Horm Behav 38:234–42
- Christianson JP, Drugan RC. (2005). Intermittent cold water swim stress increases immobility and interferes with escape performance in rat. Behav Brain Res 165:58–62
- Christianson JP, Rabbett S, Lyckland J, Drugan RC. (2008). The immobility produced by intermittent swim stress is not mediated by serotonin. Pharmacol Biochem Behav 89:412–23
- Coluccia E, Louse G. (2004). Gender differences in spatial orientation: a review. J Environ Psychol 24:329–40
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. (2004). Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Pharmacol Biochem Behav 78:569–79
- Cryan JF, Markou A, Lucki I. (2002). Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23:238–45
- Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. (2010). Sex differences in animal models of depression and antidepressant response. Basic Clin Pharmacol 106:226–33
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. (1997). Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav 32:217–25
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56
- Detke MJ, Rickels M, Lucki I. (1995). Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 121:66–72
- Drugan RC, Eren S, Hazi A, Silva J, Christianson JP, Kent S. (2005). Impact of water temperature and stressor controllability on swim stress-induced changes in body temperature, serum corticosterone, and immobility in rats. Pharmacol Biochem Behav 82:397–403
- Drugan RC, Macomber H, Warner TA. (2010). Intermittent and continuous swim stress-induced behavioral depression: sensitivity to norepinephrine- and serotonin-selective antidepressants. Psychopharmacology 212:85–92
- Falconer EM, Galea LA. (2003). Sex differences in cell proliferation, cell death and defensive behavior following acute predator odor stress in adult rats. Brain Res 975:22–36
- Ford GK, Finn DP. (2008). Clinical correlates of stress-induced analgesia: evidence from pharmacological studies. Pain 140:3–7
- Frye CA, Walf AA. (2002). Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav 41:306–15
- Gaulin SJ, FitzGerald RW. (1986). Sex differences in spatial ability: an evolutionary hypothesis and test. Am Nat 127:74–88
- Goodwin GM. (1997). Neuropsychological and neuroimaging evidence for the involvement of the frontal lobes in depression. J Psychopharmacol 11:115–22
- Healy DJ, Drugan RC. (1996). Escapable stress modulates retention of spatial learning in rats: preliminary evidence for involvement of neurosteroids. Psychobiology 24:110–17
- Hölscher C. (1999). Stress impairs performance in spatial water maze learning tasks. Behav Brain Res 100:225–35
- Kessler RC. (2003). Epidemiology of women and depression. J Affect Disorders 74:5–13
- Kirk RC, Blampied NM. (1985). Activity during inescapable shock and subsequent escape avoidance learning: female and male rats compared. New Zeal J Psychol 14:9–14
- Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. (2006). Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 49:837–45
- Lucki I. (1997). The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol 8:523–32
- Luine VN, Richards ST, Wu VY, Beck KD. (1998). Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav 34(2):149–62
- Maier SF, Watkins LR. (2005). Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev 29:829–41
- McCarthy MM, Konkle AT. (2005). When is a sex difference not a sex difference? Front Neuroendocrinol 26:85–102
- Mendez IA, Montgomery KS, LaSarge CL, Simon NW, Bizon JL, Setlow B. (2008). Long-term effects of prior cocaine exposure on Morris water maze performance. Neurobiol Learn Mem 89:185–91
- Muller JM, Morelli E, Ansorge M, Gingrich JA. (2011). Serotonin transporter deficient mice are vulnerable to escape deficits following inescapable shocks. Genes Brain Behav 10:166–75
- Nemeroff CB, Owens MJ. (2002). Treatment of mood disorders. Nat Neurosci 5:1068–70
- Porsolt RD, Le Pichon M, Jalfre M. (1977). Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–2
- Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. (1998). Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc Natl Acad Sci USA 95:13941–6
- Rodríguez CA, Torres A, Mackintosh NJ, Chamizo VD. (2010). Sex differences in the strategies used by rats to solve a navigation task. J Exp Psychol Anim B 36:395–401
- Roof RL, Stein DG. (1999). Gender differences in Morris water maze performance depend on task parameters. Physiol Behav 68:81–6
- Roof RL, Zhang Q, Glasier MM, Stein DG. (1993). Gender-specific impairment on Morris water maze task after entorhinal cortex lesion. Behav Brain Res 57:47–51
- Rubinow MJ, Arseneau LM, Beverly JL, Juraska JM. (2004). Effect of the estrous cycle on water maze acquisition depends on the temperature of the water. Behav Neurosci 118:863–8
- Rudy JW, Paylor R. (1988). Reducing the temporal demands of the Morris place-learning task fails to ameliorate the place-learning impairment of preweanling rats. Psychobiology 16:152–6
- Shors TJ, Pickett J, Wood G, Paczynski M. (1999). Acute stress persistently enhances estrogen levels in the female rat. Stress 3:163–71
- Spijker J, De Graaf R, Bijl RV, Beekman AT, Ormel J, Nolen WA. (2002). Duration of major depressive episodes in the general population: results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS). Br J Psychiatry 181:208–13
- Stetler C, Miller GE. (2011). Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 73:114–26
- Vorhees CV, Williams MT. (2006). Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–58
- Warner TA, Drugan RC. (2012). Morris water maze performance deficit produced by intermittent swim stress is partially mediated by norepinephrine. Pharmacol Biochem Behav 101:24–34
- Warren DA, Castro CA, Rudy JW, Maier SF. (1991). No spatial learning impairment following exposure to inescapable shock. Psychobiology 19:127–34
- Warren SG, Juraska JM. (1997). Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci 111:259–66
- Williams CL, Meck WH. (1991). The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology 16:155–76
- Wong ML, Licinio J. (2001). Research and treatment approaches to depression. Nat Rev Neurosci 2:343–51