Abstract
We previously observed that social instability stress (SS: daily 1 h isolation and change of cage partners for 16 days) in adolescence, but not in adulthood, decreased context and cue memory after fear conditioning in male rats. Effects of stress are typically sex-specific, and so here we investigated adolescent and adult SS effects in females on the strength of acquired contextual and cued fear conditioning, as well as extinction learning, beginning either the day after the stress procedure or four weeks later. For SS in adolescence, SS females spent more time freezing (fear measure) during extinction than did controls, whereas SS in adulthood had no effect on any measure of fear conditioning. The results also indicated an effect of age: females in late adolescence show more rapid extinction of cue and better memory of extinction of context compared to adult females, which may indicate resilience to acute footshock in adolescence. Thus fear circuitry continues to mature into late adolescence, which may underlie the heightened plasticity in response to chronic stressors of adolescents compared to adults.
Introduction
Anxiety, or pathological fear, is the most common and persistent psychiatric illness (Sheehan & Sheehan, Citation2007). Its onset is typically in adolescence, and stressful experiences are important precursors of anxiety and of other mood disorders (Anisman et al., Citation2008). Adolescents may have a heightened vulnerability for anxiety because of the ongoing maturation of the hypothalamic-pituitary-adrenal axis and of related neural circuitry that leads to the prolonged release of glucocorticoids in response to stressors in adolescence compared to adulthood (reviewed in McCormick & Green, 2013). Further, adaptations to early life stressors may increase the risk of psychopathology in adulthood (McCrory et al., Citation2012).
Fear conditioning, which is the formation of a Pavlovian association between a neutral, discrete stimulus (such as a tone) and an aversive unconditioned stimulus (such as a shock), is a useful model for investigating the neural circuitry underlying anxiety. In addition to an association between a cue and an unconditioned stimulus, a learned fear of the place in which the fear conditioning occurred (contextual conditioning) may be formed. Fear conditioning is dependent on the same brain regions that are susceptible to effects of stressors, including the amygdala, hippocampus, and the medial prefrontal cortex (Rodrigues et al., Citation2009), all of which continue to mature in adolescence. In adult rodents, chronic stress enhances cued and contextual memory of fear conditioning in some studies (e.g. Conrad et al., Citation1999; Sanders et al., Citation2010) or the extinction or renewal of fear in other studies (Miracle et al., Citation2006; Mitra & Sapolsky, Citation2009). Nevertheless, many of the effects of chronic stress experienced in adulthood dissipate within a short time of cessation of the stress exposures (e.g. Bloss et al., Citation2011; Hoffman et al., Citation2011; Lin et al., Citation2008; Sousa et al., Citation2000), which is in contrast to the long-lasting effects that have been reported for stressors experienced in adolescence (e.g. Avital & Richter-Levin, Citation2005; Isgor et al., Citation2004; McCormick et al., Citation2012).
In studies investigating fear conditioning in adulthood after exposure to stressors in adolescence in rodents, it is generally found that male rats stressed in adolescence exhibit more robust fear conditioning in adulthood (greater freezing to cue, context, or resistance to extinction relative to controls) (Toledo-Rodriguez & Sandi, Citation2007; Tsoory et al., Citation2010; Yee et al., Citation2012). In contrast, we found that social instability stress (change of cage partners after one hour isolation daily from postnatal day 30–45) reduced freezing to both the conditioning context and the cue in adult males compared to controls (Morrissey et al., Citation2011). The same stress procedure to adult animals had no effect on fear conditioning, providing direct evidence of the differential sensitivity of adolescents and adults to the stress procedure. Fewer studies of fear conditioning after adolescent stress have included females, although there is evidence that the effects of stress exposures differ for males and females whether experienced in adulthood or in adolescence (e.g. Sanders et al., Citation2010; Shors et al., Citation2001). In mice, however, exposure to chronic variable stressors from postnatal day 37 through 44 had no effect on memory for cue conditioning in adulthood in either males or females (Taylor et al., 2013). Consistent with our finding in male rats, administering stressors to adolescent female rats reduced freezing to a conditioned cue in adulthood (Toledo-Rodriguez & Sandi, Citation2007). Thus, in the present study, we predicted that females exposed to social instability stress (SS) during mid-adolescence (postnatal days 30–45) may show reduced memory for context after fear conditioning in adulthood, as we had found for males (Morrissey et al., Citation2011).
The experiment also provided us the opportunity to investigate age differences in fear conditioning in late adolescent females compared to adult females. Although cue and contextual conditioning and fear extinction is adult-like by about 24 days of age (Kim & Richardson, Citation2010; Li et al., Citation2012), neural processing during such tasks may differ for adolescents and adults (e.g. Sturman & Moghaddam, Citation2011). Further, compared to adults (> 70 days of age), adolescent male rats have enhanced contextual fear conditioning both pre-pubertally (28–31 days of age Esmoris-Arranz et al., Citation2008) and post-pubertally (47 days of age Morrissey et al., Citation2011). Sex differences in contextual fear conditioning are evident as early as postnatal days 28–31, but comparison with adults in the study was limited by the inclusion of late adolescents in the adult group (adult group involved postnatal days 50–70) (Esmoris-Arranz et al., Citation2008). Whether female rats differ in fear conditioning in late adolescence compared to in adulthood as do males is unknown. We predicted that late adolescent females will show increased memory of fear conditioning compared to adult females, based on our finding of heightened anxiety-like behavior at that age compared to adult females (McCormick et al., Citation2008).
Methods
Animals
Female Long-Evans rats were obtained at 22 (n = 66) or at 62 days of age (n = 48) from Charles River Laboratories (St. Constance, Québec, Canada) and housed in pairs in Plexiglas-walled cages (46 cm × 24 cm × 20 cm). Rats were kept on a 12 h light–dark cycle (lights on at 08:00 h) with access to rat chow and water ad libitum. Rats within a cage were identified by tail markings made with a felt marker. Use of animals was in adherence to the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85–23, revised, 1996), Canadian Council on Animal Care guidelines, and approved by the Brock University Animal Care and Use Committee. Every effort was made to minimize the number of animals used per group and to minimize the suffering of animals used throughout all experimental procedures.
Social instability stress procedure
Adolescent and adult rats were randomly assigned into the social instability stress (SS) group or to the no stress control (CTL) group. The SS procedure was as previously reported (reviewed in McCormick, Citation2010). In brief, on postnatal days (PND) 30–45 (adolescent stress group) or 70–85 (adult stress group), SS rats were kept for 1 hour in isolation in ventilated, round plastic containers (approximately 14 cm in diameter for adolescents and approximately 18 cm in diameter for adults) in a room separate from the colony. After isolation each day, the rats were returned to the colony to be housed with a new SS partner in a new cage. The daily isolation occurred at various times during the light cycle to decrease the predictability of the event. After the last isolation on PND 45 or 85, rats were returned to their original cage partner and stayed with that cage partner for the remainder of the experiment. CTL rats were not disturbed except for regular cage maintenance. CTL rats were always housed with another CTL rat, and SS rats were always housed with another SS rat.
Fear conditioning
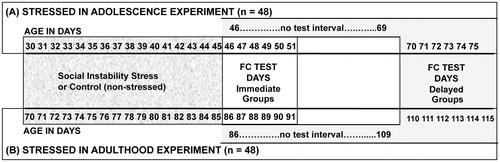
SS and CTL rats were randomly assigned to either the Immediate Test (testing began the day after the stress procedure on PND 46 or 86) or Delayed Test groups (testing began on PND 77 or 117, approximately 4 weeks after the stress procedure). The training and test procedures involved 6 consecutive days, with tests performed between 10:00 h and 16:00 h on each day to minimize circadian influences (see for experimental design and timing of procedures).
Figure 1. A timeline illustrating the experimental design and ages of rats in the adolescent stress and adult stressed experiments and the days of the fear conditioning sessions for the two time of testing groups (Immediate and Delayed).
Apparatus
Rats were trained in cohorts of four (two SS and two CTL rats), each in one of four identical cages (30 cm × 37 cm × 25 cm) located inside sound-attenuating chambers. Assignment to one of the four conditioning chambers was counterbalanced across groups, and cage partners were tested within the same cohort. A small fan located on an adjacent wall of the sound-attenuating chamber provided ventilation, and the chamber was illuminated with an LED light source during every session (all equipment from Panlab, Spain). The rear and sidewalls of the cages were made of black stainless steel and the door was made of clear Plexiglas. The ceiling contained a speaker providing background noise (68 dB) and the presentation of a tone (2000 Hz, 80 dB) as a conditioned stimulus (CS). Flooring consisted of 19 stainless steel rods wired to a shock source and a solid state scrambler to allow for the delivery of a 0.5 mA, 1 s foot shock as the unconditioned stimulus (US). The floor was situated on a weight transducer connected to a load cell coupler designed to detect fine movement. All chambers were connected to a central computer that controlled the experimental procedure for each chamber simultaneously. The computer software (Freezing) displayed the percentage of time spent freezing for each individual component of the protocols of each session. To record a freezing event (the measure of fear), the load cell coupler weight transducer required activity to be below the activity threshold for a minimum of 2000 ms. In a separate study, we found that the correlation between automated measure of freezing and manual recording of freezing from videotapes was r = 0.93, with the two measures not significantly different, t11 = 1.55, p = 0.15. Context A was the cage as described, and a new context (Context B) in the chambers was provided by altering both visual and tactile information through the use of white inserts to cover three walls, a blue steel plate to cover the steel rods on the floor, and a black silicone mat was placed on the plate.
Fear conditioning to context and cue (training session)
The protocol was similar to that used in our investigation of the effects of social instability stress on fear conditioning in male rats (Morrissey et al., Citation2011). On the first test day, rats were trained for context and cue conditioning within the same session. Rats were placed in Context A, given 5 min to explore the chamber, after which began five tone-shock pairings consisting of a 15 s (2000 Hz, 80 dB) tone co-terminating with a 1 s (0.5 mA) foot shock, with an inter-stimulus-interval (ISI) of 1 min. Rats were returned to their home cages 1 min after the last tone-shock pairing.
Context and cue retrieval tests
On the second test day, 24 h after the training session, rats were placed into their respective test cages within each chamber for the context retrieval test. Rats were placed in the same context (Context A) as in the conditioning session in the absence of both tone and shock for 5 min during which freezing to the context alone was measured. On the third day, 48 h after the training session, rats were placed in Context B and after 1 min, five 15 s tone were presented with an inter-tone interval of 1 min. Freezing to the tone in the absence of a co-terminating shock was measured. Our previous study with males presented the tone continuously for 5 min to equate the durations of the context and tone tests (Morrissey et al., Citation2011), which may be considered a test of cue generalization rather than memory for cue (Maren, Citation2008). In this experiment we kept tone presentations during memory tests the same as during fear conditioning.
Extinction
Two extinction sessions separated by 2 h were conducted on the fourth day. Extinction sessions involved the same procedure as that of fear conditioning, except in the absence of repeated foot-shocks. The only difference between sessions was that session 1 had a five minute exploration period in the chamber before the five tone presentations, and session 2 had only a one minute exploration period in the chamber before the five tone presentations.
Post-extinction context and cue tests
On the fifth day, rats were assessed for memory for extinction of the context (same procedure as the original context retrieval test conducted three days earlier). On the sixth and final day, 48 h after the first extinction session, memory for the extinction of the cue was assessed. The post-extinction cue test was the same procedure as the original cue retrieval test conducted three days earlier.
Footshock sensitivity test
A separate group of adolescent SS (n = 9) and CTL (n = 9) female rats were tested for sensitivity to footshock at PND 70 using the same equipment as for fear conditioning. Once inside the chamber, animals were given a three minute habituation period after which they received a series of five footshocks, with the first beginning at an amplitude of 0.1 mA, and thereafter increasing by 0.1 mA increments up to 0.5 mA. An inter-trial interval (ITI) of one minute was used for all trials. Behavioral responses to the foot shock stress were scored based on methods previously described (Beatty & Beatty, Citation1970; Kosten et al., Citation2005). Behaviors were defined as either: no response (the failure for the shock to elicit any observable change in behavior), flinch (a sudden change in posture such as a noticeable movement of the head, or a change from sniffing to the licking of the front paws), shuffle (a rapid movement involving the use of two to four legs and a change of location), jump (all four legs leaving the ground simultaneously), and sonic vocalization. The current amplitudes at which these behaviors first appeared were recorded and taken as the shock sensitivity thresholds for each animal. Because some rats did not flinch and some did not shuffle, but all jumped and vocalized, the behaviors of flinch and shuffle were combined, and when both appeared, the mean amplitude of the two behaviors was used.
Determination of estrous cycle phase
Because of evidence that the phase of the estrous cycle may influence fear conditioning (Milad et al., Citation2009), vaginal smears were obtained from rats as the last experimental procedure on test days (procedures based on Marcondes et al., Citation2002). Smears were collected using a pipette filled with 10 µl of saline and were placed on a slide to dry. The characteristics of cells in the smears (e.g. presence of leucocytes, cornified cells, and/or epithelial cells) under low magnification were the basis of classification as proestrus, estrus, diestrus I, or diestrus II. Because of the small proportion of rats in proestrus on any given day, the phases of proestrus and estrus were combined as were the phases of diestrus I and diestrus II.
Data analysis
Statistical analyses involved between group and mixed-factor ANOVA, with repeated dependent measures (percentage of time freezing during phases of fear conditioning; amplitude at which a specific behavior appeared) and between group factors of Stress Group (SS/CTL) and Time of Test (immediate or delayed) using the software package SPSS v. 20 (Chicago, IL) for MacIntosh computers. Adolescent social stress and adult social stress experiments were analyzed separately. Preliminary analyses indicated estrous cycle phase did not account for any of the difference observed between Stress Groups or between Time of Test groups, and thus this factor was not included in subsequent analyses to increase statistical power (specifically, analyses were conducted two ways: (1) analyses conducted using only those rats in diestrus on day of fear conditioning, and (2) analyses conducted using only those rats in diestrus on day of specific measure. These analyses allowed inclusion of a minimum of 75% of rats in each group, although it meant inclusion of different rats per analysis, with no difference in the proportion of SS and CTL rats removed from an analysis. The primary results of an effect in adolescent stressed rats on freezing during extinction and of the reduced freezing in adolescents compared to adults after fear conditioning remained in analyses with the smaller sample sizes). An alpha level of p < 0.05 was used to determine statistical significance. Post hoc analysis consisted of F tests for simple effects and Bonferroni corrected t-tests, where appropriate. Summaries of the main findings are provided in and .
Table 1. Symbols indicate whether freezing was the same as (=), greater than (↑), or less than (↓) for control rats after social instability stress (SS) administered in adolescence or in adulthood in females in the current study or in males in a previous study and tested either soon after or weeks after the stress procedure.
Table 2. Symbols indicate whether freezing was the same as (=), greater than (↑), or less than (↓) for adult rats in females in this study and in males in a previous study (Morrissey et al., Citation2011).
Results
Social instability stress in adolescence
Training phase
Adolescent social instability stress procedure did not affect the acquisition phase of fear conditioning. A mixed-factor ANOVA of freezing during the Training Phase (freezing during the 5 min exploration before shock, freezing during the five 15 s tone + shock presentations, and freezing during the post-shock inter-stimulus-intervals) found no effect of Stress Group freezing during any phase of the training session or interaction of the three factors (all p > 0.25, see ). The effect of Time of Test was not significant (F1,44 = 2.94, p = 0.09). Rats froze less during exploration than during either the tone/shock presentations or the post-shock inter-tone-intervals (main effect of Training Phase, F2,88 = 1378.03, p < 0.0001; post hoc comparisons, both p < 0.0001).
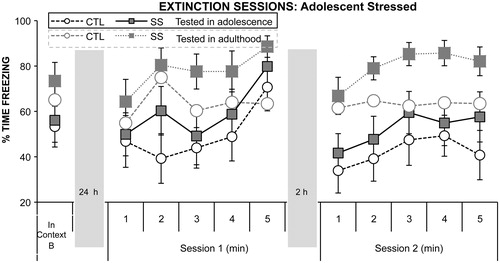
Figure 2. Mean (+/− SEM) time spent freezing in the stressed in adolescence experiment during (A) the first test day, in which fear was conditioned, (B) the memory test days (context on the second and cue on the third test day), (C) the two extinction sessions on the fourth day, and (D) the memory for extinction test days (context on the fifth and cue on the sixth test day). In (C), adolescent socially stressed (SS) rats froze more to the presentation of tones (*p = 0.03) and during the inter-tone intervals (#p = 0.04) than did control (CTL) rats. In the second session, rats in the Immediate test group (adolescent rats) froze less to the presentation of tones (@p = 0.006) and during the inter-tone intervals (p = 0.001) than did the Delayed test group (adult rats). In (D), rats in the Immediate group spent less time freezing than did rats in the Delayed group to the conditioning context (@p < 0.0001).
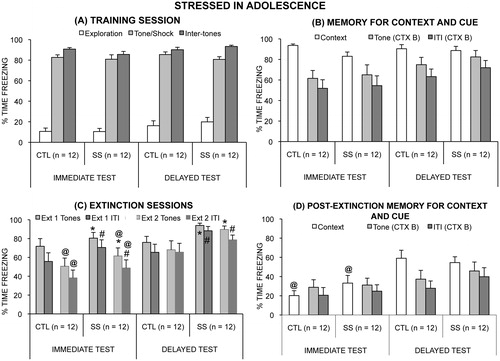
Context and cue retrieval tests: There was no effect of adolescent social instability stress on memory for context or for cue. A between group ANOVA found no main effect of Stress Group (p = 0.10) or of Time of Testing (p = 0.75), and no interaction of the two factors (p = 0.23) on freezing during the context test. When t-tests were performed for the Time of Testing groups separately to further explore the hypothesis of impaired memory after social instability stress, the SS rats froze less than did CTL rats (p = 0.025) when tested soon after the stress procedure, but did not differ from CTL rats (p = 0.77) when tested after a delay of several weeks (Delayed group) ().
For the Cue test, rats froze more during the tone presentations than during the inter-tone interval (F1,44 =45.60, p < 0.0001), but there was no main effect of Stress Group or interaction among the factors (all p > 0.45). Although rats in the Immediate group tended to freeze less than rats in the Delayed group during the tones and during the inter-tone interval, the difference did not meet statistical significance (p = 0.06 and p = 0.08) ().
Extinction
Adolescent social instability stress affected rats’ freezing to the tone during extinction. SS rats froze more to the presentation of tones and during the inter-tone intervals than did CTL rats (main effect of Stress Group, F1,44 = 5.29, p = 0.03 and F1,44 = 4.24, p = 0.05, respectively) (). Although the interaction of Stress Group and Time of Testing was not significant, the effect of Stress Group was largely driven by those rats tested as adults, because when the analyses were conducted for the Time of Test groups separately, the effect of adolescent stress remained significant in those tested as adults but not in those tested in adolescence (see for the main effect of Stress Group illustrated across time intervals in the session for those tested in adolescence and those tested as adults). The interaction of Session and Time of Testing was significant for both freezing to the tone presentations (F1,44 = 5.64, p = 0.02) and freezing during the inter-tone intervals (F1,44 = 7.31, p = 0.01). Post hoc tests indicated that whereas the two Time of Test groups did not differ in freezing to the tone presentations and during the inter-tone intervals in the first extinction session (p = 0.16 and p = 0.09), rats in the Immediate group (adolescents) spent less time freezing in the second session than did rats in the Delayed group (adults) (p = 0.006 and p = 0.001) ().
Memory for context and cue extinction
There was no effect of Stress Group (p = 0.55) or interaction of Stress Group and Time of Testing (p = 0.22) on freezing to the conditioning context after extinction. Rats in the Immediate group (adolescent rats) spent less time freezing than did rats in the Delayed group (adult rats) to the conditioning context (F1,44 = 18.36, p < 0.0001). Rats spent more time freezing to the tone than during the inter-tone interval (F1,44 = 20.23, p < 0.0001), however, no other main effect or interaction of effects were significant (all p > 0. 15) ().
Social instability stress in adulthood
Training phase
The adult social instability stress procedure did not affect the acquisition phase of fear conditioning. A mixed-factor ANOVA of freezing during the Training Phase (freezing during the 5 min exploration before shock, freezing during the five 15 s tone + shock presentations, and freezing during the post-shock inter-tone intervals) found no effect of Stress Group or of Time of Testing on freezing during any phase of the training session or interaction of the three factors (all p > 0.30; see ). Rats froze less during exploration than during either tone presentation or post-shock inter-tone intervals (main effect of Training Phase, F2,88 = 418.36, p < 0.0001; post hoc comparisons, both p < 0.0001).
Figure 4. Mean (+/− SEM) time spent freezing in the stressed in adulthood experiment during (A) the first test day, in which fear was conditioned, (B) the memory test days (context on the second and cue on the third test day), (C) the two extinction sessions on the fourth day, and (D) the memory for extinction test days (context on the fifth and cue on the sixth test day).
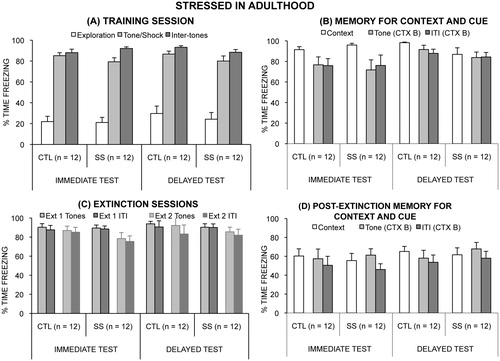
Context and cue retrieval tests: There was no main effect of either Stress Group (p = 0.35) or Time of Testing (p = 0.74), but their interaction was significant, F1,44 = 4.71, p = 0.035. Post hoc analysis for Time of Testing groups separately, however, found no significant difference between SS and CTL rats in either the Immediate (p = 0.19) or Delayed groups (p = 0.10). For the Cue test, there were no main effects or significant interactions of the factors on freezing (all p > 0.28, except main effect of Time of Testing: p = 0.08) ().
Extinction
There was no effect of Stress Group, of Time of Testing or interaction of Stress Group and Time of Testing on freezing to the conditioning context after extinction (all p > 0.44). There was no effect of Stress Group, of Time of Testing or interaction of Stress Group and Time of Testing on freezing to the tones or freezing during the inter-tone intervals after extinction (all p > 0.33) ().
Memory for context and cue extinction
There was no effect of Stress Group (p = 0.56), of Time of Testing (p = 0.45), or interaction of Stress Group and Time of Testing (p = 0.92) on freezing to the conditioning context after extinction. Rats spent more time freezing to the tone than during the inter-tone interval (F1,44 = 23.66, p < 0.0001), however, no other main effect or interaction of effects were significant (all p > 0.07) ().
Footshock sensitivity in adulthood after stress in adolescence
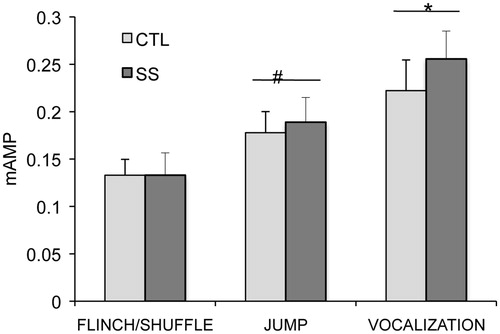
Social stress in adolescence did not affect sensitivity to footshock when tested as adults (p = 0.55), and Stress Group did not interact with the type of behavioral responses (p = 0.73). Behavioral responses appeared at different footshock amplitudes (F2,32 = 31.26, p < 0.0001), with flinches/shuffles occurring at a lower amplitude than jumps (p < 0.001), and jumps at a lower amplitude than vocalizations (p = 0.008) ().
Figure 5. Mean (+/− SEM) current amplitude that elicited behaviours of flinch/shuffle, jump, and sonic vocalization. There were no significant differences between CTL and SS female rats in current amplitude necessary to elicit each behavior. Jumps occurred at significantly higher current amplitudes than flinch/shuffle (#p < 0.0001), and sonic vocalizations occurred at significantly higher current amplitudes than jumps (*p = 0.008).
Discussion
Adolescent social instability stress in female rats produced modest alterations in behavior associated with fear conditioning compared to control, non-stressed female rats. The same social instability stress procedure administered to adult females was without immediate or lasting effect, consistent with our previous report of no effect of adult social instability stress in males (Morrissey et al., Citation2011). Together, these results suggest a heightened sensitivity to this stress procedure in adolescence compared to in adulthood. Most studies of stressors in adolescence did not investigate the stress procedures when administered in adulthood, thus limiting the extent to which it could be concluded that the stress effects were specific to the adolescent period. The effects of adolescent social instability, however, were not the same for females as were found previously for males (Morrissey et al., Citation2011). Whereas in males adolescent social instability stress decreased memory for context and cue after fear conditioning irrespective of whether they were tested soon after or several weeks after the stress exposures, a reduction in memory for context was found in female rats exposed to adolescent social instability stress only when tested soon after the stress exposures. In addition, adolescent stressed females exhibited heightened freezing during extinction sessions compared to controls irrespective of whether fear conditioning occurred soon after or long after the stress exposure, whereas there were no differences among groups during extinction in male rats. These results are consistent with reports that the lasting effects of adolescent social instability stress and of other adolescent stressors on various behavioral and neural measures often differ for males and females (as reviewed in Green & McCormick, 2013; McCormick & Green, 2013). Our results, however, contrast with the facilitation of extinction found in adolescent females that underwent different stress exposures then tested at PND 47 and compared to control females (Toledo-Rodriguez et al., 2012). Thus, some stress procedures may confer some resilience depending on when the stressors are administered and the sex of the animal (see reviews by Dalla & Shors, Citation2009; Luine et al., Citation2007).
In general, there was not much evidence of extinction during extinction trials, in that only the youngest groups showed a significant decline in freezing from the first to the second extinction session. All groups, however, froze less to the cue in the test session after extinction, indicating some effectiveness of the extinction sessions. Although females exposed to adolescent social instability stress froze more than did control females during cue extinction trials, they did not differ on the memory for extinction of cue when tested two days later. A similar dissociation between fear expression during extinction and retention of extinction has been reported in adult male rats that underwent a partial, but not a full, single prolonged stress procedure a week before fear conditioning compared to control male rats (Knox et al., Citation2012). Thus, consistent with the interpretation provided by Knox and colleagues for their results, it appears that adolescent social instability stress in females does not prevent the acquisition of extinction, rather it enhances the expression of conditioned fear memory during extinction. Evidence from a conditional cell ablation of neurogenesis in mice suggests that neurogenesis in the dentate gyrus in the adolescent period has a sex-specific role in fear memories when tested in adulthood (Cushman et al., Citation2012). Social instability stress was found to alter neurogenesis in adolescence in both female (McCormick et al., Citation2010) and male (McCormick et al., Citation2012) rats, which thus may contribute to some of the behavioural effects observed here.
The effect of adolescent social instability stress in extinction sessions did not interact with time of testing. However, when post hoc analyses were conducted for the two time of tests separately, the effect of adolescent social instability stress remained significant among those tested weeks after the stress exposure as adults and was not significant (though in the same direction) for those tested soon after the stress exposures in adolescence. Other studies have observed greater delayed than immediate effects of adolescent stress on measures of spatial performance (e.g. Isgor et al., Citation2004; McCormick et al., Citation2010, Citation2012). The differences between the SS and CTL groups are unlikely due to differences in pain sensitivity; the footshock amplitudes at which flinching, shuffling, jumping, and sonic vocalization were displayed did not differ between SS and CTL females. Likewise, there were no differences between SS and CTL females in the order of onset of these behaviours, with flinches occurring before shuffles, shuffles occurring before jumps, and jumps occurring before sonic vocalizations, and the mean amplitude at which these behaviours were displayed were similar to that reported in the literature (e.g. Baran et al., Citation2009; Kosten et al., Citation2005).
Differences among the groups may have been obscured by variability in estrous cycle phase at time of testing. Higher concentrations of estradiol, as during the proestrous phase, have been found to either facilitate extinction and the consolidation of extinction memories (Milad et al., Citation2009; Zeidan et al., Citation2011) or enhance fear acquisition (Chang et al., Citation2009). Further, shock thresholds are higher during proestrus than during metestrus (Leer, Citation1988). Although the sample sizes were too small to rule out systematic estrous cycle differences between the groups, stress in adolescence has been found to disrupt the reproductive axis into adulthood in some studies (e.g. Laroche et al., Citation2009a; Raap et al., Citation2000). We found, however, no difference in the ability to conceive, or in size and weight of litter, between control and SS female rats (unpublished observations); thus any differences in gonadal function between the groups are likely subtle. Nevertheless, the lack of control over estrous cycle phase may have masked group differences.
The differences between adolescent SS and CTL rats likely do not reflect differences in hypothalamic–pituitary–adrenal responses to the footshock as adults. Although the SS procedure does result in marked elevations in corticosterone concentrations in plasma during the course of the exposures (McCormick et al., Citation2007), no differences in stress-induced release between SS and control female rats were found using either restraint (McCormick et al., Citation2005), an elevated platform (McCormick et al., Citation2008), or forced swim (Mathews et al., Citation2008) as the stressor. In contrast, several weeks after exposure to the social instability procedure in adulthood, SS females had heightened corticosterone release after restraint compared to controls (McCormick et al., Citation2005). Thus, there may be differential sensitivity to chronic stress over the lifespan rather than heightened sensitivity to chronic stress in adolescence in females.
Shipping stress is a concern in developmental studies (Wiley & Evans, Citation2009), particularly when attempting to gauge differential sensitivity of adolescents and adults. To minimize the effects of shipping in our studies with the social instability stress in adolescence procedure, we have animals shipped at the time of weaning when they would be undergoing the stress of change in housing that occurs at weaning [e.g. movement of cage to another housing room is sufficient to elevate HPA function for at least 24 h in adults, (Olfe et al., Citation2010)]. Further, there is evidence that lasting effects of shipping stress on reproductive function in mice occurred when shipped at 42 or 56 days of age (post-pubertal, late-adolescence), but not younger (21 or 28 days of age) or later (70 days, adults) (Ismail et al., Citation2011; Laroche et al., Citation2009a,b). In rats, there is some evidence that weaning at 30 days of age has a bigger impact on adult HPA function than does weaning at 21 days of age (the most commonly used age of weaning) (Cook, Citation1999). Although we have not directly compared our social instability stress procedure in rats reared in our colony to rats obtained from a breeder, we found similar behavioral responses to amphetamine in mid-adolescent rats reared in our colony to those obtained from a breeder (Mathews et al., Citation2011). Nevertheless, it is possible that a background of shipping stress in either age group obscured any effects of social instability stress.
The adolescent stress groups also provided the opportunity to examine age differences in fear conditioning, because immediate groups were tested in late adolescence whereas delayed groups were tested as adults. In our previous study, we showed that in late adolescence (conditioning on PND 46), there was enhanced contextual fear conditioning compared to adults (conditioning on PND 86) (Morrissey et al., Citation2011), consistent with the age difference reported for earlier in adolescence in rats (PND 28–31) and in mice (PND 28–35) compared to adults (Esmoris-Arranz et al., Citation2008; Ito et al., Citation2009). No age difference in contextual fear conditioning, however, was reported in a study of male mice (McDermott et al., Citation2012). Our results for females are like those for males during the conditioning phase, with no age difference in freezing during the cue and shock pairings. In contrast to the results for males, and in contrast to our hypothesis that adolescent females would show enhanced memory for fear conditioning compared to adult females, there was no age difference in freezing to context in females. Freezing to the cue, though not statistically significant (p = 0.06), was in the opposite direction than hypothesized, with adolescent females freezing less to the cue than did adult females. Further, late adolescent females spent less time freezing during the extinction sessions than did adult females, which suggests less resistance to extinction in adolescence in females. Nevertheless, the enhanced extinction of fear of the conditioned stimulus in adolescent compared to adult females was not accompanied by enhanced memory for extinction of cue; the age groups did not differ in freezing to the tone after extinction. Adolescents froze less to the context after extinction than did adults, which suggests better memory for contextual extinction in the younger animals. The differences observed are not readily explained by age differences in pain sensitivity (shock thresholds are lower in female rats 46–50 days of age than in females 71–75 days of age and are the result of differences in body size; Beatty & Fessler, Citation1976; Pare, Citation1969). Memory for conditioning typically increases with higher shock intensities (e.g. Cordero et al., Citation1998), yet adolescent females, for which pain is expected at lower intensities than for adults, did not differ in freezing during conditioning, had reduced rather than increased memory for cue, and did not differ in freezing to the context compared to adults. Furthermore, the same age difference in pain sensitivity is also observed in males (Beatty & Fessler, Citation1976; Pare, Citation1969), and yet the age difference in fear conditioning was opposite to that observed for males. Thus, adolescent females may be more resilient to fear conditioning than both adolescent males and adult females.
It is unlikely that the age difference in freezing during extinction involves differential gonadal function between late-adolescent and adult females; serum luteinizing hormone and estradiol concentrations peak in female rats at about PND 35 (Zapatero-Caballero et al., 2004), which is around the time of vaginal opening and first ovulation (Ojeda & Urbanski, 1994), and regular estrous cycles begin approximately a week later (see review by McCormick & Mathews, Citation2010). Furthermore, a study in male mice found that adolescent males extinguished fear more quickly than adults, and the age difference was not attenuated by gonadectomy, which was without effect on extinction (McDermott et al., Citation2012). Nor is it likely that the age difference in extinction is related to maturation of adrenal function; adult-like concentrations of circulating corticosterone are found in females by about PND 45 (Pignatelli et al., 2006). Instead, the age difference most likely reflects ongoing maturation of the neural systems mediating extinction. The infralimbic prefrontal cortex is critical for acquisition, consolidation, and retrieval of extinction memory, and both the hippocampus and prefrontal cortex are important for the retention of extinction (reviewed in Milad & Quirk, Citation2012; Tronson et al., Citation2012). Although there is evidence that the neural control of extinction of conditioned fear is adult-like by 24 days of age (Kim & Richardson, Citation2010; Li et al., Citation2012), the amygdala, hippocampal formation, and prefrontal cortex continue to develop well into late adolescence (see reviews by Brenhouse & Andersen, Citation2011; Crews et al., Citation2007; Spear, Citation2000).
In sum, although the results are consistent with the hypothesis of heightened sensitivity to chronic stressors in adolescence than in adulthood, the impact of social instability stress on fear conditioning was modest, evident only during the extinction session. The more pronounced differences in extinction and in memory of extinction in rats tested in late adolescence compared to in adulthood, however, highlight the prolonged developmental trajectory of fear learning systems. These results also show that adolescent females may be more resilient than adults when encountering some acute stressors such as footshock in that they showed reduced freezing to context and cue after fear conditioning compared to adults. The challenge for future studies will be to link such functional differences to specific neural mechanisms at each stage of ontogeny.
Declaration of interest
The authors report no conflicts of interest. CMM holds a Canada Research Chair in Behavioural Neuroscience and the research was supported by a Natural Sciences and Engineering Research Council (NSERC) Discovery Grant.
Acknowledgements
We thank Mark Cummings and Bianca Anderson for assistance in data collection.
References
- Anisman H, Merali Z, Stead JDH. (2008). Experiential and genetic contributions to depressive- and anxiety-like disorders: clinical and experimental studies. Neurosci Biobehav Rev 6:1185–206
- Avital A, Richter-Levin G. (2005). Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int J Neuropsychopharmacol 8:163–73
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. (2009). Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem 91:323–32
- Beatty W, Beatty P. (1970). Hormonal determinants of sex differences in avoidance behavior and reactivity to electric shock in the rat. J Comp Physiol Psychol 73:446–5
- Beatty WW, Fessler RB. (1976). Ontogeny of sex differences in open-field testing and sensitivity to electric shock in the rat. Physiol Behav 16:413–17
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, McEwen BS, Morrison JH. (2011). Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J Neurosci 31:7831–9
- Brenhouse HC, Andersen SL. (2011). Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev 35:1687–703
- Chang YJ, Yang CH, Liang YC, Yeh CM, Hung CC, Hsu KS. (2009). Estrogen modulates sexually dimorphic contextual fea extinction in rats through estrogen receptor b. Hippocampus 19:1142–50
- Conrad CD, Magariños AM, LeDoux JE, McEwen BS. (1999). Repeated restraint stress facilitates fear conditioning independently of causing hippocampal ca3 dendritic atrophy. Behav Neurosci 113:902–13
- Cook CJ. (1999). Patterns of weaning and adult responses to stress. Physiol Behav 67:803–8
- Crews F, He J, Hodge C. (2007). Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav 86:189–99
- Cordero MI, Merino JJ, Sandi C. (1998). Correlational relationship between shock intensity and corticosterone secretion on the establishment and subsequent expression of contextial fear conditioning. Behav Neurosci 112:885–91
- Cushman JD, Maldonado J, Kwon EE, Garcia AD, Fan G, Imura T, Sofroniew MV, Fanselow MS. (2012). Juvenile neurogenesis makes essential contributions to adult brain structure and plays a sex-dependent role in fear memories. Front Behav Neurosci 6:3 . doi: 10.3389/fnbeh.2012.00003
- Dalla C, Shors TJ. (2009). Sex differences in learning processes of classical and operant conditioning. Physiol Behav 97:229–38
- Esmoris-Arranz FJ, Mendez C, Spear NE. (2008). Contextual fear conditioning differs for infant, adolescent, and adult rats. Behav Processes 78:340–50
- Green MR, McCormick CM. (2013). Effects of stressors in adolescence on learning and memory in rodent models. Horm Behav 64:364--79
- Hoffman AN, Krigbaum A, Ortiz JB, Mika A, Hutchinson KM, Bimonte-Nelson HA, Conrad CD. (2011). Recovery after chronic stress within spatial reference and working memory domains: correspondence with hippocampal morphology. Eur J Neurosci 34:1023–30
- Isgor C, Kabbaj M, Akil H, Watson SJ. (2004). Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus 14:636–48
- Ismail N, Garas P, Blaustein JD. (2011). Long-term effects of pubertal stressors on female sexual receptivity and estrogen receptor-α expression in cd-1 female mice. Horm Behav 59:565–71
- Ito W, Pan BX, Yang C, Thakur S, Morozov A. (2009). Enhanced generalization of auditory conditioned fear in juvenile mice. Learn Mem 16:187–92
- Kim JH, Richardson R. (2010). New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biol Psychiat 67:297–303
- Knox D, Nault T, Henderson C, Liberzon I. (2012). Glucocorticoid receptors anad extinction retention deficits in the single prolonged stress model. Neuroscience 223:163–73
- Kosten TA, Miserendino MJ, Bonbace JC, Lee HJ, Kim JJ. (2005). Sex-selective effects of neonatal isolation on fear conditioning and foot shock sensitivity. Behav Brain Res 157:235–44
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. (2009a). Enduring influences of peripubertal/adolescent stressors on behavioral response to estradiol and progesterone in adult female mice. Neuroendocrinology 150:3717–25
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. (2009b). Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology 150:2351–8
- Leer MN. (1988). Elevated shock threshold in sexually receptive female rats. Physiol Behav 42:617–20
- Li S, Kim JH, Richardson R. (2012). Differential involvement of the medial prefrontal cortex in the expression of learned fear across development. Behav Neurosci 126:217–25
- Lin YH, Westenbroek C, Bakker P, Termeer J, Liu AH, Li XJ, Ter Horst GJ. (2008). Effects of long-term stress and recovery on the prefrontal cortex and dentate gyrus in male and female rats. Cereb Cortex 18:2762–74
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. (2007). Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol 19:743–51
- Marcondes FK, Bianchi FJ, Tanno AP. (2002). Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62:609–14
- Maren S. (2008). Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci 8:1661–6
- Mathews IZ, Kelly H, McCormick CM. (2011). Low doses of amphetamine lead to immediate and lasting locomotor sensitization in adolescent, not adult, male rats. Pharmacol Biochem Behav 97:640–7
- Mathews IZ, Wilton A, Styles A, McCormick CM. (2008). Heightened neuroendocrine function in males to a heterotypic stressor and increased depressive behaviour in females after adolescent social stress in rats. Behav Brain Res 190:33–40
- McCormick CM, Green MR. (2013). From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience 249:242--57
- McCormick CM, Merrick A, Secen J, Helmreich DL. (2007). Social instability in adolescence alters the central and peripheral hypothalamic–pituitary–adrenal responses to a repeated homotypic stressor in male and female rats. J Neuroendocrinol 19:116–26
- McCormick CM, Nixon F, Thomas C, Lowie B, Dyck J. (2010). Hippocampal cell proliferation and spatial memory performance after social instability stress in adolescence in female rats. Behav Brain Res 208:23–9
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE. (2005). Long-lasting, sex- and age-specific effects of social stress on corticosterone responses to restraint and locomotor responses to psychostimulants in rats. Horm Behav 48:64–74
- McCormick CM, Smith C, Mathews IZ. (2008). Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res 187:228–38
- McCormick CM, Thomas CM, Sheridan CS, Nixon F, Flynn JA, Mathews IZ. (2012). Social instability stress in adolescent male rats alters hippocampal neurogenesis and produces a deficit in spatial location memory in adulthood. Hippocampus 22:1300–12
- McCrory E, De Brito SA, Viding E. (2012). The link between child abuse and psychopathology: a review of neurobiological and genetic research. J R Soc Med 105:151–6
- McDermott CM, Liu D, Schrader LA. (2012). Role of gonadal hormones in anxiety and fear memory formation and inhibition in male mice. Physiol Behav 105:1168–74
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. (2009). Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 164:887–95
- Milad MR, Quirk GJ. (2012). Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 63:129–51
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. (2006). Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem 85:213–18
- Mitra R, Sapolsky RM. (2009). Effects of enrichment predominate over those of chronic stress on fear-related behavior in male rats. Stress 12:305–12
- Morrissey MD, Mathews IZ, McCormick CM. (2011). Enduring deficits in contextual and auditory fear conditioning after adolescent, not adult, social instability stress in male rats. Neurobiol Learn Mem 95:46–56
- Ojeda SR, Urbanski HF. (1994). Puberty in the rat. In: Knobil E, Neill JD, editors. The physiology of reproduction. Vol. 99. New York: Raven Press. p 363--410
- Olfe J, Domanska G, Schuett C, Kiank C. (2010). Different stress-related phenotypes of balb/c mice from in-house or vendor: alterations of the sympathetic and HPA axis responsiveness. BMC Physiol 10:2
- Pare WP. (1969). Age, sex, and strain differences in the aversive threshold to grid shock in the rat. J Comp Physiol Psychol 69: 214–18
- Pignatelli D, Xiao F, Gouveia AM, Ferreira JG, Vinson GP. (2006). Adrenarche in the rat. J Endocrinol 191:301--8
- Raap DK, Morin B, Medici CN, Smith RF. (2000). Adolescent cocaine and injection stress effects on the estrous cycle. Physiol Behav 70:417–24
- Rodrigues SM, LeDoux JE, Sapolsky RM. (2009). The influence of stress hormones on fear circuitry. Annu Rev Neurosci 32:289–313
- Sanders MJ, Stevens S, Boeh H. (2010). Stress enhancement of fear learning in mice is dependent upon stressor type: effects of sex and ovarian hormones. Neurobiol Learn Mem 94:254–62
- Sheehan DV, Sheehan KH. (2007). Current approaches to the pharmacologic treatment of anxiety disorders. Psychopharm Bull 40:98–109
- Shors TJ, Chua C, Falduto J. (2001). Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci 21:6292–7
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OFX, Paula-Barbosa MM. (2000). Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience 97:253–66
- Spear LP. (2000). Neurobehavioral changes in adolescence. Curr Dir Psychol Sci 9:111–14
- Sturman DA, Moghaddam B. (2011). Reduced neuronal inhibition and coordination of adolescent prefrontal cortex during motivated behavior. J Neurosci 31:1471–8
- Taylor SB, Taylor AR, Koenig JI. (2013). The interaction of disrupted type II neuregulin 1 and chronic adolescent stress on adult anxiety- and fear-related behaviors. Neuroscience 249:31--42
- Toledo-Rodriguez M, Pitiot A, Paus T, Sandi C. (2012). Stress during puberty boosts metabolic activation associated with fear-extinction learning in hippocampus, basal amygdala and cingulate cortex. Neurobiol Learn Mem 98:93–101
- Toledo-Rodriguez M, Sandi C. (2007). Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat. Neur Plast 2007:71203
- Tronson NC, Corcoran KA, Jovaseic V, Radulovic J. (2012). Fear conditoning and extinction: emotional states encoded by distinct signaling pathways. Trends Neurosci 35:145–55
- Tsoory MM, Guterman A, Richter-Levin G. (2010). “Juvenile stress” alters maturation-related changes in expression of the neural cell adhesion moleecule l1 in the limbic system: Relevance for stress-related psychopathologies. J Neurosci Res 88:369–80
- Wiley JL, Evans RL. (2009). To breed or not to breed? Empirical evaluation of drug effects in adolescent rats. Int J Dev Neurosci 27:9–20
- Yee N, Schwarting RKW, Fuchs E, Wohr M. (2012). Juvenile stress potentiates aversive 22-khz ultrasonic vocalizations and freezing during auditory fear conditioning in adult male rats. Stress 15:533–44
- Zapatero-Caballero H, Sanchez-Franco F, Fernandez-Mendez C, Frutos MGS, Botella-Cubells LM, Fernandez-Vazquez G. (2004). Gonadotropin-releasing hormone receptor gene expression during pubertal development of female rats. Biol Reprod 70:348–55
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. (2011). Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiat 70:920–7