Abstract
Considering its pervasive and uncontrollable influence in drug addicts, understanding the neurobiological processes through which stress contributes to drug use is a critical goal for addiction researchers and will likely be important for the development of effective medications aimed at relapse prevention. In this paper, we review work from our laboratory and others focused on determining the neurobiological mechanisms that underlie and contribute to stress-induced relapse of cocaine use with an emphasis on the actions of corticotropin-releasing factor in the ventral tegmental area (VTA) and a key pathway from the bed nucleus of the stria terminalis to the VTA that is regulated by norepinephrine and beta adrenergic receptors. Additionally, we discuss work suggesting that the influence of stress in cocaine addiction changes and intensifies with repeated cocaine use in an intake-dependent manner and examine the potential role of glucocorticoid hormones in the underlying drug-induced neuroadaptations. It is our hope that research in this area will inform clinical practice and medication development aimed at minimizing the contribution of stress to the addiction cycle, thereby improving treatment outcomes and reducing the societal costs of addiction.
Introduction
Treatment of drug addiction – an unmet medical need
The impact of drug addiction on society is often underestimated. While the costs of enforcement, incarceration and treatment are certainly sizable, these costs are overshadowed by the toll that drug addiction takes on its victims, their families and the community. Statistics on substance abusers indicate that they are more likely to be incarcerated or on parole, more likely to be homeless, less likely to be employed, more likely to require emergency room treatment, and more likely to engage in domestic violence. In 2010, an estimated 22.5 million Americans were abusing illicit substances (SAMHSA, Citation2012). Despite the prevalence of abuse, the estimated number of individuals receiving treatment is disturbingly low: approximately 4.1 million individuals. For those who do enter treatment, outcome indicators remain disappointingly poor, with consistently high relapse rates, between 70% and 90% within one year, across all drug classes (Brandon et al., Citation2007). For this reason, the development of new and more effective approaches aimed at relapse prevention is a top priority for addiction researchers. This review will focus primarily on how stress contributes to relapse to drug use in cocaine addicts. Understanding these contributions in cocaine addicts is particularly important, considering that there are currently no FDA-approved medications for the management of cocaine addiction, making it a critical unmet medical need. Additionally, while there are certainly differences in the mechanisms that underlie addiction across illicit drug classes, many of the processes that will be described also apply to other classes of abused drugs (e.g. opiates, alcohol and nicotine).
Relapse of drug use
Numerous clinical and anecdotal reports have suggested that common triggers for drug relapse include (1) “slipping up” and re-engaging in drug use; (2) encountering cues previously associated with drug use; and (3) the onset of a stressful life event. Unlike other triggers for drug relapse, stress is typically unavoidable in daily life and thus remains an important target for the development of new relapse prevention strategies. Current treatment practice aimed at relapse prevention consists primarily of counseling/behavioral therapy and, while it is true that these strategies have efficacy and may indeed be sufficient for sustained abstinence in some addicts, it is clear that, for many addicts, their best chance to remain drug abstinent requires pharmacotherapy. The development of medications aimed at preventing relapse requires identification of the triggers/circumstances that contribute to relapse and the underlying neurobiological mechanisms through which they work.
Stress-associated drug use and relapse – evidence from human studies
It has become evident that there is a strong interaction between stress and drug use in human addicts (Sinha, Citation2009; Sinha et al., Citation2011). There is a high degree of co-morbidity between substance abuse and stress-associated disorders such as depression, anxiety, personality disorder and post-traumatic stress disorder (PTSD). This prevalence is especially pronounced in cocaine addicts, with epidemiological studies citing 50–70% of addicts as also suffering from stress-associated psychological disorders (Chen et al., Citation2011; Rounsaville et al., Citation1991). In many cases, stress precedes the development of addiction, as early life stress and childhood trauma serve as strong predictors for later development of alcohol and drug dependence (Brown et al., Citation1995; Enoch, Citation2011; Hyman et al., Citation2008; Khoury et al., Citation2010). There is also a strong relationship between drug use and PTSD. The severity of PTSD symptoms can predict cue-elicited drug craving (Saladin et al., Citation2003). Between 20% and 40% of those suffering from PTSD use alcohol and illicit drugs to self-medicate their symptoms (Back et al., Citation2006; Leeies et al., Citation2010). More specifically, in one study, 95% of those with both a cocaine dependence and PTSD reported a functional relationship between their PTSD symptoms and their cocaine use with 86% reporting that a worsening of symptoms resulted in an increase in drug use (Back et al., Citation2006). These numbers highlight the impact that stress can have on drug use and why this relationship remains an important area of study to identify at-risk populations for the development of substance abuse disorders.
In clinical laboratory settings, the ability of triggers to provoke relapse is often measured by the occurrence of drug craving, defined as an intense desire to take the drug. In addicts, drug use is often preceded by drug craving (Sinha, Citation2009). The same stimuli that have been suggested to trigger relapse – drug re-exposure, drug-associated cues and stress – also tend to evoke craving under controlled laboratory conditions. A number of laboratory stressors, such as guided imagery and stress scripts are associated with increases in craving for alcohol (Fox et al., Citation2008; Sinha et al., Citation2009), marijuana (McRae-Clark et al., Citation2011), opiates (Constantinou et al., Citation2010) and cocaine (Sinha et al., Citation1999, Citation2006). Furthermore, in cocaine and heroin users who record daily stress levels as well as drug craving and mood, a strong positive correlation between real life stress and drug craving is observed (Preston & Epstein, Citation2011). While it has been difficult to establish a direct cause–effect relationship between stress and relapse to the use of illicit drugs in humans, stress or reactivity to stressors, is predictive of relapse (Back et al., Citation2010; Breese et al., Citation2005; Sinha et al., Citation2006).
Overview
This review will summarize research investigating the interaction between stress and drug addiction, with a focus on proposed neurobiological mechanisms underlying stress-associated relapse to drug use. Although the emphasis of this review is on cocaine, the processes and mechanisms described likely pertain to all abused drugs. Accordingly, the overarching goal of the research that is reviewed is to develop intervention strategies aimed at minimizing the role of stress in drug addiction, regardless of the drug class.
Preclinical approaches for studying relapse
Although progress in the field of functional imaging has reached the point where such approaches have begun to reveal the processes in the human brain that are associated with drug craving and use, to date most of our understanding of the pathways underlying relapse has stemmed from the use of preclinical rodent models and has involved the use of reinstatement-based approaches in which relapse is defined according to the ability of stimuli to re-establish, or reinstate, extinguished drug-seeking behavior. For the most part, reinstatement studies have utilized variations of two protocols for studying drug seeking: (a) drug self-administration (SA) and (b) drug-induced conditioned place preference (CPP).
Self-administration/reinstatement
The SA approach, in which rodents are trained, through operant conditioning, to emit responses that result in systemic drug delivery, has long been used for the preclinical study of drug abuse and addiction. Most, but not all, of the work examining the reinstatement of cocaine seeking following SA has involved the use of rats. Typically in these studies, rats are trained to lever press or nose poke in order to give themselves (i.e. self-administer) intravenous drug infusions through a jugular catheter. The validity of this approach stems from the observation that drugs such as cocaine that are commonly abused by humans are typically self-administered by rats, while drugs that have no abuse liability in humans are not. Once SA is established, the study of relapse requires that drug-seeking behavior be suppressed. This is most commonly accomplished by replacing the drug solution with an inert substance, such as saline, resulting in behavioral extinction. Once extinguished, the ability of various manipulations to reinstate drug seeking (e.g. extinguished lever pressing previously reinforced by cocaine) can be examined. There have been a number of reports suggesting that many of the same types of stimuli that trigger relapse in addicts (drug re-exposure, drug-associated cues, and stressors) can induce reinstatement in rats. The most common stressor that is used in the reinstatement approach is the delivery of intermittent mild electric footshock through the grid floors of the SA chamber immediately prior to the reinstatement test (Ahmed & Koob, Citation1997; Erb et al., Citation1996).
CPP/reinstatement
A second commonly used approach for the preclinical examination of relapse involves testing for the reinstatement of extinguished CPP. Although this approach can be applied in rats, its use in mice has had greater impact due to difficulties associated with the use of the intravenous SA method in mice along with the availability of powerful genetic mouse approaches that are currently not widely developed in rats. With this approach, the rewarding effects of drugs become associated with a distinct context through classical conditioning with repeated pairing such that, when given a choice, mice will develop a preference for the drug-paired environment over a second environment not paired with drug administration. Once this CPP is observed, mice undergo extinction by providing them with access to the drug environment until the preference (usually measured as time spent in the paired environment) dissipates, at which time reinstatement testing is conducted. In this model, reinstatement is defined according to the ability of various stimuli to restore preference for the drug-paired environment. As is the case with the SA-based approach, stimuli that trigger relapse in addicts tend to induce reinstatement of extinguished CPP in mice. The most common stressor used in the reinstatement of CPP is a brief forced swim immediately prior to being placed in the apparatus (Kreibich & Blendy, Citation2004; Mantsch et al., Citation2010).
Although both CPP- and SA-based approaches are used to study relapse, it should be recognized that the two approaches are different in a number of important ways, including the contingency of drug administration, the involvement of operant (SA) versus Pavlovian (CPP) conditioning, the pattern and net amount of drug exposure and the types of stressors used to evoke reinstatement of drug seeking. Thus, drug seeking measured using the two paradigms very likely reflects similar, but not identical, processes that contribute to drug use in humans.
Neurobiological mediators of stress-induced cocaine use
Overview of anatomy underlying stress-induced relapse
Research in our laboratory and others has focused on understanding the neurobiological mechanisms that underlie stress-induced relapse. Before discussing these mechanisms, it is first necessary to provide a brief overview of the neurocircuitry that has been implicated in stress-induced reinstatement (). As is the case with other stimuli (e.g. priming injections of cocaine and exposure to cocaine-associated cues), stressor-induced reinstatement requires activation of a key glutamatergic corticostriatal pathway from the medial prefrontal cortex (mPFC) to the nucleus accumbens (NAc) core that regulates output to the ventral pallidum to evoke cocaine-seeking behavior (Kalivas & McFarland, Citation2003). Reversible inactivation of each of these regions has been reported to prevent footshock-induced reinstatement (McFarland et al., Citation2004). During stress, this corticostriatal pathway appears to be regulated by dopamine (DA) actions at D1 receptors, likely on pyramidal cells, in the mPFC, as local delivery of D1 receptor antagonists has been reported to prevent stress-induced reinstatement (Capriles et al., Citation2003; McFarland et al., Citation2004; Sanchez et al., Citation2003). Stress-induced increases in cortical DA appear to increase pyramidal cell excitability via multiple mechanisms, include post-synaptic effects of excitatory neurotransmission and effects on GABAergic interneuron populations (Seamans et al., Citation2001; Xu & Yao, Citation2010). The source of dopaminergic projections to the mPFC is the VTA, which receives inputs from a number of brain regions implicated in the integration of stress responses, including the NAc shell, the central nucleus of the amygdala and the bed nucleus of the stria terminalis (BNST) – collectively referred to as the “extended amygdala”. Inactivation of each of these regions interferes with stress-induced reinstatement (Erb & Stewart Citation1999; McFarland et al., Citation2004). Although efferent projections from each of these regions to the VTA, as well as connections between other stress-responsive regions and components of the corticostriatal pathway, contribute to stress-induced relapse, recent research has focused on the role of an important pathway from the BNST to the VTA in stress-induced cocaine seeking. Key elements of this pathway include norepinephrine (NE) and the neuropeptide, corticotropin-releasing factor (CRF). Thus, while it has become clear that other neurobiological processes, most notably the neuropeptide dynorphin, via its actions at kappa opioid receptors (Graziane et al., Citation2013) and substance P, via actions at the neurokinin 1 (NK1) receptor (Schank et al., Citation2013), are important mediators of stress-related drug use, the remainder of this review will focus on the actions of CRF and NE in this pathway from the BNST to the VTA.
Figure 1. Neurocircuitry that contributes to stress-induced relapse. (1) During stress, ascending noradrenergic projections from the locus coeruleus (LC) and A1 region release norepinephrine (NE) into the bed nucleus of the stria terminalis (BNST) and central (Ce) amydgala. (2) Beta-2 adrenergic receptor activation by NE in the BNST activates a pathway that releases CRF into the ventral tegmental area (VTA). (3) CRF, largely through actions at CRF-R1 receptors in the VTA, activates a dopaminergic (DA) projection to the medial prefrontal cortex (mPFC). (4) DA activation of D1 receptors in the mPFC increase the activity of pyramidal cells that comprise a glutamatergic (GLU) projection to the nucleus accumbens (NAc) core. GLU release into the NAc core regulates outputs to the ventral pallidum to induce drug craving/use.
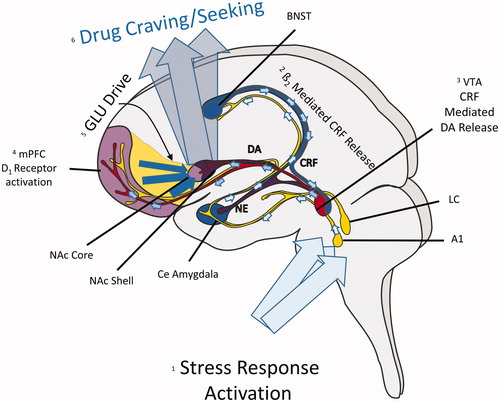
When examining the pathways that promote drug use, it is important to recognize that the widespread effects of stressors in the brain include pathways through which drug-associated cues and context contribute to drug seeking, including those regulated by the hippocampus and the basolateral amygdala (Fuchs et al., Citation2005; Meil & See, Citation1997). Although a detailed overview of these pathways is beyond the scope of this review, understanding how they are influenced during stress is clearly important for a complete understanding of how stressors promote relapse.
Stressor regulation of the mesocorticolimbic DA system
The ability of stressful stimuli to reinstate cocaine seeking requires activation of the VTA. Inhibition of the VTA prevents footshock-induced reinstatement following cocaine SA in rats (McFarland et al., Citation2004) and stress-induced drug seeking appears to require glutamatergic neurotransmission in the region, as demonstrated by the ability of intra-VTA kynurenic acid to block footshock-induced reinstatement (Wang et al., Citation2005; Wise & Morales, Citation2010). Furthermore, the activity of VTA afferents and glutamatergic neurotransmission in the VTA are increased in response to acute stress (Briand et al., Citation2010; Saal et al., Citation2003; Ungless et al., Citation2001).
As the source of dopaminergic cells that comprise the mesocorticolimbic system, the VTA has long been considered to serve as the central regulator of appetitive reward-related behaviors (Wise & Rompre, Citation1989) and related processes, including incentive salience (Robinson & Berridge, Citation1993) and reward prediction (Schultz, Citation2007). However, the responsiveness of the VTA to aversive/stressful stimuli and its role in coordinating stress-related behavioral responses is less clear. Aversive stimuli and stressors have been reported to produce both increases and decreases in VTA DA cell firing (Brischoux et al., Citation2009; Ungless et al., Citation2004, Citation2010; Valenti et al., Citation2011). The observed variation in responses of DA cells in the VTA to these stimuli is likely attributable to a number of factors, including the target cell population in the VTA, the environmental context at the time of stressor delivery, the pharmacological and environmental history prior to testing, qualitative attributes of the aversive/stressful stimuli and the time-course over which DA responses are measured.
The probability that distinct subgroups of DA cells in the VTA are differentially regulated by stressors is likely important in terms of the mechanism through which stressors promote drug use. It has become clear that discrete populations of VTA DA cells are responsible for DA release in different terminal domains and that the responses of these cell populations to various stimuli, including stressors (Abercrombie et al., Citation1989; Deutch et al., Citation1991; Lammel et al., Citation2011), and their role in determining behavioral reactivity during stress (Chaudhury et al., Citation2013) is not uniform. For example, it has been demonstrated that footshock-induced increases in Fos expression in the VTA are selective for DA cells that project to the mPFC (Deutch et al., Citation1991). Considering that stressors reliably increase mesocortical DA neurotransmission (Abercrombie et al., Citation1989; Deutch et al., Citation1991; Horger & Roth, Citation1996) and the established role for DA D1 receptor activation in the mPFC in stress-induced cocaine seeking (Capriles et al., Citation2003; McFarland et al., Citation2004; Sanchez et al., Citation2003), these findings strongly suggest that stressors regulate relapse by increasing DA levels in the VTA and promoting excitation of pyramidal cells that project to the NAc core.
In contrast to the reliable stressor-induced effects on mesocortical DA neurotransmission, the effects of stressors/aversive stimuli on DA in the NAc are less clear and a well-defined role for NAc DA in stress-induced cocaine seeking has not been established. Both stress-induced increases and decreases in NAc DA have been reported (Abercrombie et al., Citation1989; Anstrom et al., Citation2009; Deutch et al., Citation1991; Di Chiara et al., Citation1999; Kalivas & Duffy, Citation1995; Oleson et al., Citation2012; Roitman et al., Citation2008; Tidey & Miczek, Citation1996). As is the case with VTA DA neuronal reactivity, the observed variation in the NAc DA response to stressful/aversive stimuli observed across studies are likely attributable to a number of factors, including the context associated with stressor delivery, the pharmacological and environmental history prior to testing, qualitative attributes of the aversive/stressful stimuli, and the time-course over which DA responses are measured, as well as the subregion of the NAc (e.g. core versus shell) in which DA is measured (Budygin et al., Citation2012). It is notable that the NAc shell is thought to play an important role in hedonic processing, and reductions in DA levels in response to unambiguously aversive stimuli have been observed in the shell (McCutcheon et al., Citation2012; Roitman et al., Citation2008), in part through the inhibitory influence of GABAergic projections from the rostromedial tegmentum (RMTg; Lammel et al., Citation2012). Interestingly, rodent studies have placed the NAc shell upstream from the VTA in the pathway responsible for stress-induced cocaine seeking (Briand et al., Citation2010; McFarland et al., Citation2004). To the extent that stressor regulation of the VTA-shell and VTA-mPFC DA projections represents activation of distinct circuits that subserve different stress-regulated functions (i.e. hedonic state versus motivated behavior), it may be that stress-induced reductions in DA in the NAc shell, through effects on projections to the mPFC that regulate the mesocortical pathway could promote cocaine seeking. While somewhat speculative, this possibility is consistent with self-reports from cocaine addicts who often cite self-medication of a dysphoric state as their primary motivation for use. Thus, DA levels in the shell may relay information regarding hedonic state back to the VTA to guide behavior via regulation of motivational neurocircuitry and therefore may also represent a mechanism through which stressors influence drug use.
Role of CRF in the VTA in stress-induced cocaine-seeking
CRF
CRF is a 41 amino acid neuropeptide (Vale et al., Citation1981) that is involved in the integration of the endocrine, autonomic, emotional, immune and behavioral responses of an organism to stress (Bale & Vale, Citation2004; Dunn & Berridge, 1990). In addition to its essential role in the activation of the HPA axis, CRF has widespread actions throughout the brain, where it functions as a neuromodulator and contributes to a number of stress-related behavioral responses. CRF produces its effects through two receptors, CRF-R1 and CRF-R2 (Dautzenberg & Hauger, Citation2002), both of which primarily couple to and activate Gs-proteins, thereby stimulating intracellular cAMP levels (although a number of other CRF-activated signaling pathways have been noted) (Grammatopoulos, Citation2012). The CRF-R1 receptor has a higher affinity for CRF and its relatively widespread expression overlaps with CRF immunoreactivity in the brain (Charlton et al., Citation1987; Koob, Citation2010; Swanson et al., Citation1983). By contrast, the CRF-R2 receptor has a lower affinity for CRF and its expression tends to be more restricted (Lovenberg et al., Citation1995). The CRF-R2 receptor preferentially binds to members of another family of neuropeptides, the urocortins, and may bind differentially to CRF in the presence of the CRF binding protein (Ungless et al., Citation2003; Wang et al., Citation2007). Among the many targets for CRF are brain regions implicated in stress-induced drug seeking, including the NAc, mPFC, VTA and BNST (Charlton et al., Citation1987; Swanson et al., Citation1983), all of which express CRF receptors (Van Pett et al., Citation2000).
CRF and stress-induced reinstatement
A number of preclinical studies suggest that CRF is required for stress-induced relapse to cocaine use. Following cocaine SA by rats, intracerebroventricular (icv) administration of CRF reinstates extinguished cocaine seeking (Buffalari et al., Citation2012; Erb et al., Citation2006; Mantsch et al., Citation2008), while central administration of CRF receptor antagonists prevents reinstatement by footshock stress (Erb et al., Citation1998; Graf et al., Citation2011; Shaham et al., Citation1998). The involvement of CRF in stress-induced reinstatement appears to be largely independent of its actions on the HPA axis. We and others have found that surgical adrenalectomy along with basal corticosterone replacement shortly prior to testing fails to alter reinstatement in response to footshock or central administration of CRF (Erb et al., Citation1998; Graf et al., Citation2011).
Sites of CRF regulation of cocaine seeking
CRF is known to regulate the activity of a number of brain regions that have been implicated in stress-induced reinstatement, including the BNST (Kash et al., Citation2008; Nobis et al., Citation2011; Silberman et al., Citation2013), VTA (Korotkova et al., Citation2006; Refojo et al., Citation2011), mPFC (Tan et al., Citation2009), central nucleus of the amygdala (Erb et al., Citation2001; Pollandt et al., Citation2006; Zorrilla et al., Citation2012) and NAc (Lemos et al., Citation2012). Research examining CRF mediation of stress-induced reinstatement of cocaine seeking has primarily focused on two of these regions: the BNST and the VTA. Since research in our laboratory has recently focused on the latter region, this review will emphasize the role of CRF in the VTA in stress-induced relapse.
Role of CRF in the VTA in stress-induced cocaine seeking
The VTA receives CRF-positive projections from a number of brain regions, including the BNST and central nucleus of the amygdala (Rodaros et al., Citation2007) and expresses both CRF-R1 and CRF-R2 receptors (Goeders et al., Citation1990; Korotkova et al., Citation2006; Van Pett et al., Citation2000) as well as the CRF binding protein (Wang & Morales, Citation2008). Increases in extracellular CRF levels in the VTA during footshock-induced reinstatement in rats, as measured using radioimmunoassay in samples acquired via in-vivo microdialysis, have been reported (Wang et al., Citation2005). Moreover, intra-VTA delivery of CRF is sufficient to reinstate extinguished cocaine seeking following SA in rats, while the delivery of CRF receptor antagonists into the VTA prevents footshock-induced reinstatement (Blacktop et al., Citation2011; Wang et al., Citation2005, Citation2007).
Role for VTA CRF-R1 versus CRF-R2 receptors stress-induced cocaine seeking
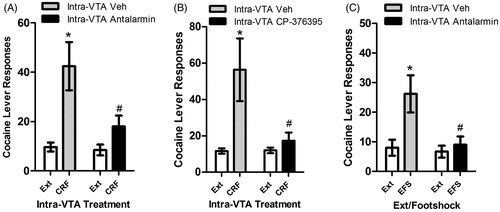
Both CRF-R1 and CRF-R2 receptors in the VTA have been implicated in stress-induced cocaine seeking. We have found that intra-VTA pretreatment with the CRF-R1 receptor-selective antagonists antalarmin or CP-376395 blocks reinstatement of extinguished cocaine seeking in response to intra-VTA CRF () or footshock (), while intra-VTA pretreatment with the CRF-R2 receptor-selective antagonists astressin2B or antisauvagine-30 has no effect (Blacktop et al., Citation2011). Similarly, intra-VTA delivery of the CRF-R1 receptor-selective agonist, cortagine, reinstates extinguished cocaine seeking, while local administration of the CRF-R2 receptor antagonist, rat urocortin 2, does not (Blacktop et al., Citation2011). These findings are consistent with reports that systemic administration of the CRF-R1 receptor antagonists also prevent stress-induced reinstatement following SA of cocaine (Shaham et al., Citation1998), heroin (Shaham et al., Citation1998), alcohol (Lê et al., Citation2000) and nicotine (Bruijnzeel et al., Citation2009), suggesting that activation CRF-R1 receptors in the VTA is both necessary for stress-induced cocaine use and sufficient to induce cocaine seeking.
Figure 2. Effects of intra-VTA injections of CRF-R1 receptor antagonists on reinstatement by intra-VTA CRF delivery and footshock stress in LgA rats. CRF-R1 activation is necessary in the VTA for stress-induced reinstatement as bilateral injections of the CRF-R1 antagonist antalarmin (500 ng/side; 2A) or CP-376395 (500 ng/side; 2B) blocks reinstatement induced by bilateral intra-VTA delivery of CRF (500 ng/side). Bilateral injections of antalarmin (500 ng/side) also block reinstatement induced by electric footshock (EFS; 2C). In all cases, significant reinstatement was observed in rats pretreated with vehicle (*p < 0.05 versus Ext) but not CRF-R1 receptor antagonists and responding during reinstatement was significantly lower following CRF-R1 receptor antagonists compared to vehicle (#p < 0.05 versus Veh). This figure has been reproduced from Blacktop et al. (Citation2011) with permission.
Interestingly, a role for VTA CRF-R2 receptors in stress-induced relapse has also been reported (Wang et al., Citation2005, Citation2007). In these experiments, which used prolonged delivery into the VTA via reverse microdialysis, it was found that intra-VTA delivery of antagonists at CRF-R2 but not CRF-R1 receptors prevented reinstatement by either footshock or local CRF administration. The putative mechanism through which CRF interacts with CRF-R2 receptors in the VTA to induce cocaine seeking involves the CRF-binding protein, consistent with a report that CRF can potentiate N-methyl-d-aspartate (NMDA) receptor-mediated synaptic transmission in the VTA through a similar interaction (Ungless et al., Citation2003). It was reported that antagonists that also displace CRF from its binding protein prevented reinstatement and the associated effects on neurotransmission in the VTA. Only CRF-R2 receptor agonists that also associate with the CRF binding protein induce cocaine seeking (Wang et al., Citation2007). While it is likely that differences in the mode and duration of intra-VTA CRF/antagonist delivery and the SA history contributed to discrepancies between studies, the implication of both receptors in stress-induced cocaine seeking is not particularly surprising, considering the apparent expression profile of both receptors as well as the complexity of CRF’s cellular actions in the VTA.
CRF regulation of VTA function
Compatible with its apparent role as a mediator of the complex regulation of VTA function during stress, actions of CRF in the VTA are widespread and consist of both direct excitatory (Korotkova et al., Citation2006; Wanat et al., Citation2008) and inhibitory (Beckstead et al., Citation2009; Wanat et al., Citation2013) effects on dopaminergic neuronal activity. Furthermore, it has been found that CRF indirectly facilitates excitatory synaptic transmission through post-synaptic trafficking of NMDA and α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Hahn et al., Citation2009; Ungless et al., Citation2003) and promotion of glutamate release (Hahn et al., Citation2009; Wang et al., Citation2005). These latter findings have led to the hypothesis that, during stress, CRF release into the VTA excites DA cells, thereby activating mesocorticolimbic neurotransmission and promoting drug use (Wise & Morales, Citation2010). Consistent with this hypothesis, CRF delivery into the VTA increases local DA release measured during reinstatement testing and that glutamate receptor antagonism in the VTA using kynurenic acid prevents both increases in local DA levels and stressor- and CRF-induced reinstatement (Wang et al., Citation2005).
However, an exclusively excitatory mechanism is incompatible with the reported inhibitory effects of stress and CRF on dopaminergic neurotransmission that is observed under many conditions. One possible explanation that could account for these inhibitory actions of CRF in the VTA is that the effects of CRF on DA neuronal activity vary with subpopulations of DA cells. For example, CRF may selectively activate DA neurons that project to the mPFC, where DA receptor antagonism has been reported to prevent stress-induced reinstatement of cocaine seeking (Capriles et al., Citation2003; McFarland et al., Citation2004; Sanchez et al., Citation2003), while inhibiting or producing no effects on other DA cell populations, such as those that project to the NAc shell. Consistent with this possibility, deletion of CRF-R1 receptors in midbrain DA neurons selectively decreases stress-induced prefrontal cortical DA release (Refojo et al., Citation2011).
An alternative hypothesis is that, rather than producing global inhibitory or excitatory effects in the VTA, CRF functions as a coordinator of neurotransmission in the VTA, generally silencing DA cellular activity in the absence of drug-associated stimuli while opportunistically promoting signaling via key excitatory inputs that relay drug-related/appetitive information to the VTA. This possibility is supported by reports that administration of a CRF receptor antagonist increased basal population activity of DA neurons in the VTA but attenuates evoked increases in NAc DA (Lodge & Grace, Citation2005). As a coordinator of VTA function, CRF may serve as a critical mediator not only of stress-induced drug use, but also of response selection and coping behaviors during periods of stress. This proposed role for CRF may have implications for understanding a range of conditions associated with pathological stress responses.
Role of the BNST in stress-induced cocaine seeking
CRF-releasing afferents to the VTA
CRF-releasing afferents into the VTA co-release both glutamate and GABA (Tagliaferro & Morales, Citation2008) and include projections that originate in several regions known to be critical regulators of stress-related hormonal and behavioral responses, including the central nucleus of the amygdala (CeA), the paraventricular nucleus of the hypothalamus, and the BNST (Rodaros et al., Citation2007). Of these regions, the BNST is of particular interest due to demonstrated involvement in stress-induced cocaine seeking (Briand et al., Citation2010; McFarland et al., Citation2004).
Role of the BNST in cocaine seeking behavior
The BNST consists of groups of anatomically and functionally inter-related subnuclei that collectively serve as an important integration complex for stress-related information and as an interface between stress and reward/motivational systems (Egli & Winder, Citation2003; Jennings et al., Citation2013; Park et al., Citation2012; Silberman & Winder, Citation2013). Subregions of the BNST are activated during stress-induced cocaine seeking (Briand et al., Citation2010; Brown et al., Citation2011) and inactivation of the BNST prevents stress-induced reinstatement in both mouse and rat models (Briand & Blendy, Citation2010; McFarland et al., Citation2004). One or more pathways from the BNST to the VTA appear to be particularly important for its regulation of drug use, as disconnection of the BNST from the VTA prevents context-dependent drug seeking (Sartor & Aston-Jones, Citation2012). Both glutamatergic and GABAergic projections from the BNST to the VTA have been characterized (Georges & Aston-Jones, Citation2001; Kudo et al., Citation2012), and a recent optogenetic study identified distinct roles for the GABAergic and glutamatergic projections to the VTA resulting in rewarding and aversive phenotypes, respectively (Jennings et al., Citation2013). In addition to direct projections to the VTA, the BNST also appears to indirectly regulate DA cells in the VTA via projections to the RMTg, which inhibits VTA function through GABAergic neurons that are stress-responsive. However, the exact contribution of these pathways to stress-induced cocaine seeking is unclear.
In the BNST, CRF appears to be an important regulator of projections to the VTA. It has recently been reported that CRF released from a local population of cells within the BNST activates CRF-R1 receptors on the terminals of glutamatergic afferents and promotes excitatory regulation of neurons that project directly to the VTA (Silberman et al., Citation2013). Key regions that send projections to the BNST include the CeA (Zahm et al., Citation1999). The projection from the CeA regulates CRF release in the BNST and appears to be critical for stress-induced relapse (Erb & Stewart, Citation1999). Disconnection of this pathway by tetrodotoxin (TTX) administration in the CeA in one hemisphere and contralateral CRF receptor antagonism in the BNST of the other prevents footshock-induced reinstatement (Erb et al., Citation2001). Interestingly, many of the projections from the CeA have been reported to also release CRF (Beckerman et al., Citation2013). However, the relative contributions of CRF released from CeA afferent versus local cell populations to stress-induced cocaine seeking is unclear.
To summarize, the BNST is a key site for the convergence between stress and reward systems. The distinct CRF-releasing projection from the BNST to the VTA is an important pathway regulating stress-induced reinstatement. Another key system that regulates neurotransmission in the BNST and therefore appears to be critical for stress-induced relapse is the noradrenergic system.
Noradrenergic involvement in stress-induced cocaine use
Evidence for noradrenergic involvement in stress-induced relapse
A role for central noradrenergic signaling in stress-induced relapse has been established. Noradrenergic innervation arising from both the locus coeruleus and other mesencephalic/medullary cell groups is widespread in the CNS and its targets include regions implicated in stress-induced drug use such as the NAc shell, the mPFC, the CeA and the BNST, all of which show abundant adrenergic receptor expression (Berridge, Citation2008). In cocaine addicts, stress- and cue-induced craving is associated with elevated NE (Sinha et al., Citation2003). Moreover, yohimbine, an α-2 adrenoceptor antagonist that promotes NE release, can induce drug craving in users of opiates and alcohol (Stine et al., Citation2002). Conversely, α-2 adrenoceptor agonist drugs, such as clonidine, which suppress central noradrenergic neurotransmission, attenuate stress-induced craving in cocaine users (Jobes et al., Citation2011). These findings are paralleled by reports that the α-2 adrenoceptor antagonist, yohimbine, or the more selective α-2A adrenoceptor antagonist BRL-44,408, induces reinstatement of cocaine seeking in both mouse CPP (Mantsch et al., Citation2010; Vranjkovic et al., Citation2012) and rat SA models (Brown et al., Citation2009; Feltenstein & See, Citation2006). Moreover, α-2 adrenoceptor agonists block stress-induced reinstatement in both rats (Erb et al., Citation2000) and mice (Mantsch et al., Citation2010). Notably, the sufficiency of noradrenergic signaling for cocaine seeking is further supported by the finding that central (icv) administration of NE itself can induce reinstatement following SA in rats (Brown et al., Citation2011). Altogether, these data suggest medications aimed at suppressing stress-induced increases in noradrenergic transmission may have utility for relapse prevention in cocaine addiction.
Role of adrenergic receptors in stress-induced relapse
Using the mouse CPP/reinstatement approach, our laboratory has investigated the role of different adrenergic receptor subtypes in stress-induced relapse. We have found that reinstatement by a stressor, forced swim, is prevented by pre-treatment with the β-adrenoceptor antagonist, propranolol, but not by the α-1 adrenoceptor antagonist prazosin, suggesting that the β-adrenoceptor is the key target for stress-induced reinstatement (Mantsch et al., Citation2010). Furthermore, β-adrenoceptor activation is not only necessary for stress-induced reinstatement but is sufficient to induce reinstatement on its own, as administration of the β-adrenoceptor agonist isoproterenol induces reinstatement of extinguished CPP in mice (Vranjkovic et al., Citation2012). These findings are supported by studies showing that β-adrenoceptor deficient mice do not exhibit forced swim-induced reinstatement (Vranjkovic et al., Citation2012). Specifically, the β-2 adrenoceptor appears to be required for stress-induced reinstatement. Administration of the β-2 adrenoceptor selective antagonist ICI-188,551, but not the β-1 adrenoceptor antagonist betaxolol, blocks forced swim-induced reinstatement in mice (Vranjkovic et al., Citation2012), while administration of the β-2 adrenoceptor receptor-selective agonist, clenbuterol, is sufficient to reinstate (Vranjkovic et al., Citation2012). summarizes research on the contribution of NE and adrenergic receptors to reinstatement of cocaine seeking behavior and the involvement of the specific subtypes of adrenergic receptors to stress-induced reinstatement.
Table 1. Involvement of norepinephrine and adrenergic receptors in stress-induced reinstatement of cocaine seeking behavior.
Role of beta adrenergic signaling in the BNST in stress-induced cocaine seeking
Beta adrenergic receptors are widely expressed throughout the brain, including many regions implicated in stress-induced relapse, such as the CeA, PFC and BNST (Asanuma et al., Citation1991; Rainbow et al., Citation1984). The BNST, however, has emerged as a prime region of interest for involvement in stress-induced reinstatement. The BNST receives denser noradrenergic innervation from A1 and A2 adrenergic neurons, components of the ventral noradrenergic bundle (VNAB), than the locus coeruleus (Aston-Jones et al., Citation1999; Shin et al., Citation2008). Consistent with this, lesions or disruption of the LC do not have an effect on stress-induced reinstatement of either extinguished opiate SA in rats (Shaham et al., Citation2000) or extinguished opiate CPP in mice (Wang et al., Citation2001). However, lesions of the VNAB or blockade of noradrenergic signaling in the BNST prevent footshock-induced reinstatement of extinguished morphine CPP (Wang et al., Citation2001). Within the BNST, β-adrenoceptors are critical for stress-induced reinstatement as blockade of β-adrenoceptors, with a cocktail of β-1 and β-2 receptor antagonists, prevents footshock-induced reinstatement of extinguished cocaine seeking in rats (Leri et al., Citation2002). More specifically, we have recently found that blockade of β-2 adrenoceptors with the specific antagonist ICI-118,551 prevents footshock-induced reinstatement of cocaine seeking in rats (unpublished findings). These findings are consistent with results from mouse studies demonstrating a critical role of β-2 adrenoceptors in stress-induced reinstatement of drug-seeking behavior. In the BNST, β-adrenoceptors have been reported to facilitate both GABAergic (Dumont & Williams, Citation2004) and glutamatergic (Egli et al., Citation2005; Nobis et al., Citation2011) neurotransmission. In the latter case, the effect of β-adrenoceptors appears to involve regulation of CRF release from local neuron populations within the BNST and activation of CRF-R1 receptors on glutamatergic terminals that regulate neurons that project directly to the VTA (Silberman et al., Citation2013).
Beta adrenergic receptor regulation of a BNST to VTA pathway
Both GABAergic (Kudo et al., Citation2012) and glutamatergic (Georges & Aston-Jones, Citation2002) pathways from the BNST to the VTA have been identified, some of which likely co-release CRF (Rodaros et al., Citation2007). Connectivity between the two regions is functional for cocaine seeking behavior, as disconnection of the pathway by inhibition of the VTA in one hemisphere and inhibition of the BNST in the other attenuates the expression of cocaine-induced CPP (Sartor & Aston-Jones, Citation2012). In light of findings that β-adrenergic receptors in the BNST regulate excitatory processes that control projections to the VTA (Silberman et al., Citation2013) and that antagonism of β-adrenergic receptors in the BNST prevents stress-induced reinstatement (Leri et al., Citation2002), we hypothesize that, during stress NE release in the BNST and activation of β-2 adrenergic receptors stimulates a pathway that releases CRF into the VTA and activates CRF-R1 receptors to induce reinstatement. Consistent with this hypothesis, reinstatement of cocaine seeking by central NE administration can be blocked by CRF receptor antagonism, suggesting that CRF is downstream from NE in the pathway that mediates stress-induced drug seeking (Brown et al., Citation2009).
To summarize, NE, via actions at β-2 adrenergic receptors, is an important mediator of stress-induced reinstatement. A key site of action for NE is the BNST where β-adrenergic receptors regulate CRF-mediated excitation of a critical pathway to the VTA that appears to release CRF. Considering that NE is also released into other regions implicated in stress-induced cocaine seeking, including the mPFC (Gresch et al., Citation1995; Mitrano et al., Citation2014; Tassin et al., Citation1986), NAc (Mitrano et al., Citation2012) (where NE can regulate DA signaling) and CeA (Leri et al., Citation2002), noradrenergic signaling may represent an important target for medications aimed at relapse prevention (see Weinshenker & Schroeder, Citation2007 for review).
Drug-induced neuroplasticity and stress-induced drug use
Given the pharmacological profile of cocaine, which includes the blockade of three primary monoamine transporters, it is not surprising that the neurobiology of cocaine addiction is complex and includes numerous adaptations and counter-adaptations that emerge with repeated drug use. Consequences of these adaptations appear to include persistent alterations in the tonic activity of a number of stress-responsive systems (Koob, Citation2008) and heightened stressor reactivity, both of which can contribute to persistent use. For instance, in human addicts, those that maintained a higher frequency of drug use later demonstrated greater drug craving, anxiety and HPA responsiveness to laboratory stressors and drug-related cues (Fox et al., Citation2005). This suggests that higher frequency of repeated drug use can induce drug intake-dependent neuroplasticity that impacts susceptibility to stress-induced relapse.
Long-access cocaine SA
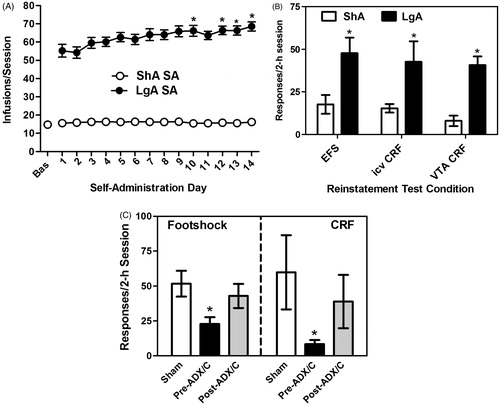
The excessive and dysregulated drug use that defines addiction can be modeled by providing rats with chronic, daily prolonged access to cocaine by SA (Ahmed & Koob, Citation1998). Rats given limited daily access to cocaine (short access; ShA; 2 h) show consistently stable lever responding and drug intake with repeated testing. However, rats that are permitted to self-administer under conditions of prolonged daily access to cocaine (long-access; LgA; 6 + h) progressively escalate their cocaine intake over a 14-d period (; Ahmed & Koob, Citation1998; Mantsch et al., Citation2004). The consequences of repeated drug use during the prolonged daily sessions can be observed weeks after the cessation of SA testing as a heightened susceptibility to reinstatement in response to cocaine priming (Mantsch et al., Citation2004) and drug-associated cues (Kippin et al., Citation2006). Research in our lab has examined how a history of excessive drug use can influence later susceptibility to stress-induced relapse. We have found that rats given prolonged daily LgA to cocaine over 14 d later show robust reinstatement to intermittent electric footshock stress following extinction, whereas animals that are given daily ShA to cocaine do not (; Blacktop et al., Citation2011; Mantsch et al., Citation2008), suggesting that repeated drug use can produce neuroplastic changes that establish stress as a trigger for relapse.
Figure 3. Glucocorticoid-dependent increases in stress- and CRF-induced reinstatement following long-access (LgA) cocaine self-administration (SA). Graphs have been reproduced with permission from Mantsch et al. (Citation2008) (panels A and B), Blacktop et al., Citation2011 (panel B) and Graf et al. (Citation2011) (panel C). Rats provided access to cocaine under LgA conditions (14 × 6 h/d; 1 mg/kg/inf cocaine) show a progressive escalation of cocaine SA (*p < 0.05 versus SA day 1), whereas rats provided short-access to cocaine (ShA rats; 14 × 2 h/d) do not (Panel A). Compared to ShA rats, LgA rats show heightened susceptibility to reinstatement in response to a stressor (electric footshock), icv CRF delivery, or administration of CRF directly into the VTA (*p < 0.05; LgA versus ShA; Panel B). The establishment of heightened stress- and CRF-induced reinstatement is reliant on elevated corticosterone at the time of LgA SA testing (Panel C). Elimination of the glucocorticoid response via surgical adrenalectomy and basal corticosterone replacement prior to repeated LgA SA testing (pre-SA ADX/C) prevented later reinstatement by footshock or icv CRF (*p < 0.05 versus Sham). However, when rats underwent ADX/C after repeated LgA cocaine SA but prior to reinstatement testing (post-SA ADX/C), effects on reinstatement were not observed.
Drug-induced neuroplasticity and relapse
The finding that the contribution of stress to drug relapse varies depending on the prior history of drug use implies that the neurobiological processes that underlie stress-related drug use are fundamentally altered as a result of drug-induced neuroplasticity that emerges in an intake-dependent manner. Defining these processes and understanding how they are affected over time with repeated drug exposure should reveal new targets for medication development aimed at minimizing the contribution of stress to the addiction process and preventing relapse. Towards this end, addiction researchers have begun to investigate what changes occur as a result of excessive drug use in pathways that underlie stress-induced reinstatement. A number of cocaine-induced neuroplastic changes, including increases in synaptic strength, potentiation of excitatory synapses, activation of cAMP-response element binding protein (CREB) and extracellular-regulated kinase (ERK), increases in AMPAR/NMDAR ratios, and dendritic remodeling, have been identified in pathways that underlie reinstatement of drug-seeking behavior (Koob & Volkow, Citation2010; Mameli et al., Citation2011). However, in this review we will focus primarily on CRF systems and their regulation by glucocorticoids.
Role for glucocorticoids in relapse-related neuroplasticity
In cocaine addicts, the cyclic pattern of cocaine use and withdrawal is associated with profound effects on the stress-responsive hypothalamic-pituitary-adrenal (HPA) axis. In humans, both cocaine use (Ward et al., Citation1999) and withdrawal (Koob & Kreek, Citation2007) result in elevated adrenocorticotropic hormone (ACTH) and cortisol levels and the emergence of addiction is associated with dysfunction of the HPA axis that includes heightened responsiveness to both stressors and drug-associated cues (Sinha et al., Citation2006). Similar changes can be observed in animal models. For example, self-administered cocaine increases glucocorticoid levels in rats and monkeys (Broadbear et al., Citation1999; Rivier & Vale, Citation1987), while withdrawal from chronic cocaine produces pronounced HPA activation (Zhou et al., Citation2003). Likewise, we have demonstrated that repeated SA results in a heightened HPA response to stress (Mantsch et al., Citation2007). Notably, when rats self-administer cocaine under LgA conditions, the resulting elevation of glucocorticoids is prolonged and more pronounced (Mantsch et al., Citation2003). Thus, elevated glucocorticoids are positioned as potential contributors to addiction-related neuroplasticity that emerges with repeated cocaine use. Consistent with this possibility, alterations in HPA function appear to be predictive of stress-evoked craving, which is heightened in a use-dependent manner (Sinha et al., Citation2006).
Glucocorticoids and stress-induced reinstatement following long-access SA
Using the SA/reinstatement approach, our laboratory has recently provided evidence that the elevation of glucocorticoids at the time of cocaine use is an important determinant of later susceptibility to stress-induced relapse. When rats undergo surgical adrenalectomy, accompanied by basal corticosterone replacement, prior to repeated LgA SA, later reinstatement by footshock is prevented (Graf et al., Citation2011). By contrast, when rats are adrenalectomized after SA, but prior to reinstatement testing, footshock-induced reinstatement is unaffected (), suggesting that elevated glucocorticoids are critical for the establishment of neuroplasticity that determines susceptibility to stress-induced relapse of cocaine use but are not necessary for the expression of this behavior.
The mechanisms through which glucocorticoids regulate stress reactivity likely involve glucocorticoid receptor (GR) activation, although non-genomic, non-GR mechanisms may also be involved. Notably, there is some evidence that glucocorticoids promote addiction-related neuroplasticity through a concerted effort with peripheral and central noradrenergic signaling. For example, it has been reported that cocaine-induce behavioral sensitization requires both adrenal secretion of corticosterone and epinephrine (de Jong et al., Citation2009). Moreover, appetitive learning-related neuroplasticity requires both elevated glucocorticoid levels and noradrenergic neurotransmission in the NAc shell (Wichmann et al., Citation2012).
Glucocorticoids and the effects of chronic stress on drug use
The apparent contribution of glucocorticoids to addiction-related neuroplasticity also establishes them as potential neurobiological substrates through which chronic stress can influence the addiction process. Consistent with reports that stress at the time of conditioning can promote cocaine-induced CPP (McLaughlin et al., Citation2003), exposing rats to stress at the time of daily SA testing can escalate cocaine SA (Mantsch & Katz, Citation2007). When rats were tested for SA during daily sessions in which a 5-min exposure to electric footshock alternated with 30 min of access to cocaine (repeated four times), cocaine intake increased over time, whereas stable patterns of SA were observed in control rats. This escalation of cocaine SA was prevented by surgical adrenalectomy and basal corticosterone replacement. Interestingly, in these studies, corticosterone delivery only restored escalated SA in adrenalectomized rats that also received footshock during testing, suggesting that glucocorticoids are likely interacting with other stress-responsive mechanisms to promote cocaine use.
Cocaine-induced neuroplasticity within the CRF system
The augmented stress responses and heightened susceptibility to stress-induced relapse that emerges in cocaine addicts with repeated use are likely attributable in part to the emergence of heightened CRF reactivity. Indeed, Koob and colleagues (see Koob & Kreek, Citation2007 for review) have identified the CRF systems as one of several key allostatic opponent process systems, along with dynorphin, noradrenergic and endocannabinoid systems, that are recruited with repeated drug use and contribute to escalating cocaine use patterns (Specio et al., Citation2008), as well as anxiety-related cocaine withdrawal symptoms (Basso et al., Citation1999). Moreover, the activity of the CRF system (Richter & Weiss, Citation1999; Zhou et al., Citation1996; Zorrilla et al., Citation2001, Citation2012) and CRF sensitivity (Erb et al., Citation2005; Hahn et al., Citation2009; Nobis et al., Citation2011; Orozco-Cabal et al., Citation2006) are increased upon repeated cocaine exposure in brain regions implicated in stress-induced relapse. For example, repeated cocaine appears to recruit CRF regulation of neurotransmission in the VTA (Wang et al., Citation2005, Citation2007), increase VTA CRF receptor binding (Goeders et al., Citation1990) and establish CRF-R1 regulation of excitatory transmission in VTA (Hahn et al., Citation2009) while diminishing inhibitory effects of G protein-gated inwardly rectifying potassium (GIRK) (Beckstead et al., Citation2009). The contribution of augmented activity of the CRF system to the heightened susceptibility to cocaine seeking observed following LgA SA has recently been examined. The Koob laboratory has demonstrated that the ability of a CRF-R1 receptor antagonist to attenuate SA is only observed in LgA rats displaying escalated patterns of intake (Specio et al., Citation2008). Our laboratory has found that the ability of icv (Mantsch et al., Citation2008) or intra-VTA (Blacktop et al., Citation2011) delivery of CRF to reinstate extinguished cocaine seeking is also recruited in an intake-dependent manner after LgA SA, and is only observed in LgA rats ().
Glucocorticoid regulation of CRF-related neuroplasticity
The establishment of neuroplastic changes in the CRF system that promote stress-induced cocaine use likely involves glucocorticoids. In contrast to the well-characterized negative feedback that glucocorticoids exert on CRF regulation of the HPA axis (Ratka et al., Citation1989), glucocorticoids augment the activity of the CRF systems implicated in relapse, as observed, for example, by positive regulation of CRF mRNA expression in the extended amygdala (Schulkin et al., Citation1998). Consistent with this role for glucocorticoids, eliminating the glucocorticoid response during repeated long-access cocaine SA prevents later reinstatement in response to icv CRF delivery (; Graf et al., Citation2011).
Cocaine-induced neuroplasticity in noradrenergic systems
A number of reports suggest that repeated cocaine exposure produces long-lasting adaptations within central noradrenergic systems that may lead to enhanced reactivity to stress (Baumann et al., Citation2004; Belej et al., Citation1996; Beveridge et al., Citation2005; Lanteri et al., Citation2008; Macey et al., Citation2003; Wee et al., Citation2008). These adaptations likely have consequences for cocaine use. For example, it has been reported that cocaine SA is attenuated by the α-1 adrenergic receptor antagonist drug, prazosin, after repeated long-access, but not short-access, testing (Wee et al., Citation2008). Repeated cocaine has also been shown to alter β-adrenergic regulation of CRF and excitatory neurotransmission in the BNST (Nobis et al., Citation2011), suggesting that changes in this key BNST to VTA pathway may contribute to the heightened susceptibility to stress-induced relapse that emerges with repeated excessive cocaine SA.
There is also evidence that glucocorticoids can regulate the function of the noradrenergic system, thereby contributing to addiction-related changes in noradrenergic neurotransmission. For example, changes in glucocorticoid levels have been reported to alter the expression of adrenergic receptors (Jhanwar-Uniyal & Leibowitz, Citation1986), the NE transporter (Fan et al., Citation2013), and the synthetic enzyme dopamine beta hydroxylase (Fan et al., Citation2013). Glucocorticoids also alter the effects of NE on synaptic transmission (Joëls et al., Citation1991) and cellular signaling (Mobley et al., Citation1983). The extent to which these glucocorticoid-dependent alterations contribute to addiction remains to be determined.
Stage-setting effects of stress
“Stage-setting” effects of stress
The inability of stress to induce reinstatement under ShA conditions should not be interpreted to suggest that stress only plays a role in reinstatement in animals with a history of LgA SA. Recent work in our lab has demonstrated that, while stress does not trigger reinstatement after animals self-administer cocaine under ShA conditions, it can serve as a stage setter for reinstatement in these rats, promoting drug seeking upon exposure to other stimuli (Graf et al., Citation2013). Following SA under ShA conditions, neither footshock nor a sub-threshold cocaine priming injection (2.5 mg/kg) alone is sufficient to produced reinstatement. However, when the two stimuli are combined, robust reinstatement is observed. Our findings are consistent with reports that the magnitude of reinstatement triggered by cocaine-related cues is also increased by exposure to acute stressors (Buffalari & See, Citation2009; Feltenstein & See, Citation2006) and, importantly, parallel reports that in human addicts triggers for drug use are more powerful when they are encountered during periods of stress (Coffey et al., Citation2002; De La Garza et al., Citation2009; Duncan et al., Citation2007). Notably, we have found that the stage-setting and relapse-triggering effects of stress may involve distinct neurobiological mechanisms. While relapse-triggering effects of stress rely heavily on CRF-dependent activation of the VTA, the stage-setting effects of stress seem to heavily rely on corticosterone inhibition of the secondary monoamine transporter, organic cation transporter 3 (OCT3). Recent work from our laboratory has demonstrated that blockade of OCT3 results in stress-potentiated reinstatement of cocaine-seeking behavior likely through decreased clearance of DA in the NAc (Graf et al., Citation2013). Better understanding these mechanisms and identifying situations or subpopulations of addicts in which stress can set the stage for versus trigger use will likely inform the development of more effective treatment approaches.
Summary
To summarize, while stressful life events clearly contribute to cocaine use, the effects of stress appear to vary depending on the prior history of drug use. Our data suggest that stress is most problematic in individuals with a history of excessive drug consumption in whom it can directly trigger relapse. However, our findings also imply that, in all users, stress can serve as a risk factor for relapse by promoting relapse in response to other triggers through its stage-setting effects. The contribution of stress to the addiction process appears to be largely attributable to glucocorticoid-dependent neuroplasticity that emerges with prior use and likely involves neurobiological mediators that connect stress-responsive and motivational systems in the brain.
Similarities and differences between cocaine and other classes of abused drugs
This review has focused on the neurobiological processes through which stressful stimuli can promote relapse to cocaine use. While there are certainly differences in how stress contributes to drug seeking across various classes of illicit drugs (e.g. opiates and ethanol), the assumption is that many of the processes are overlapping. For example, both CRF-R1 receptor activation (Bruijnzeel et al., Citation2009; Lê et al., Citation2000; Shaham et al., Citation1997) and central noradrenergic signaling (Erb et al., Citation2000; Lê et al., Citation2005; Shaham et al., Citation2000; Yamada & Bruijnzeel, Citation2011) also block stress-induced reinstatement of heroin-, nicotine- and alcohol-seeking following SA in rats. Likewise, elevated glucocorticoid levels have been implicated in neuroplasticity that promotes alcohol consumption (Vendruscolo et al., Citation2012) and opiate responsiveness (Deroche et al., Citation1995), although glucocorticoid regulation of stress reactivity has not been reported. Moreover, both heroin and cocaine SA establish the ability of footshock to increase VTA glutamate release (Wang et al., Citation2005, Citation2012) and extended access heroin SA augments later stress-induced reinstatement (Ahmed et al., Citation2000) in a manner comparable to cocaine (Mantsch et al., Citation2008). This is not to suggest that distinctions between drugs classes in the contribution of stress to relapse or the underlying processes do not exist. Considering the variation in pharmacological mechanisms of action among illicit drugs, it should not be surprising that differences in how relapse-related circuitry is subsequently engaged during periods of stress also varies. For example, although adrenergic signaling appears to be necessary for stress-induced drug seeking across a range of abused drugs, the adrenergic receptor involved may vary. Alpha-1 adrenergic receptor antagonists have been reported to block stress-induced drug seeking following alcohol SA in rats (Lê et al., Citation2011) but not following cocaine-induced CPP in mice (Mantsch et al., Citation2010). Understanding the differences and similarities between drugs in terms of how their use is regulated by stress is important for the guidance of development efforts aimed at establishing new approaches for relapse prevention.
Future directions
As the mechanisms that contribute to stress-induced relapse are highly complex and numerous and are not yet fully defined, there is still much that needs to be understood about how stress regulates drug use. Indeed, although it is well established that stress-responsive regions such as the BNST are highly connected with the VTA and therefore can regulate motivated behavior, the phenotypes of these connections, where they synapse, and how precisely they regulate distinct neuronal subpopulations and microcircuits within the VTA is unknown. For example, efferents from the BNST appear to directly innervate DA cells in the VTA and to indirectly regulate VTA DA neurons via projections to the RMTg, likely in an opposing manner. The relative contribution of these pathways to drug seeking is not known. The picture is not much clearer within the BNST, where recent work has revealed a highly complex microcircuitry involving NE, CRF and inputs from regions such as the amygdala that controls efferent pathways to the VTA.
Another unanswered question relates to the complex regulation of DA by stress and CRF. Clearly stress-induced increases in mesocortical DA release are critical for relapse. However, the regulation of NAc DA by stressors is less clear, particularly in the shell, which likely regulates VTA projections responsible for drug-seeking behavior. Understanding the relationship between NAc DA and stress-induced drug seeking may provide important insight into how negative mood states contribute to drug use.
Additionally, while this review has focused primarily on CRF and NE, a number of other stress mediators, including opioids, endocannabinoids, substance P and serotonin have been implicated in stress-induced drug seeking. Understanding how and where these mediators regulate the pathways responsible for drug seeking, as well the actions of CRF, NE and glucocorticoids beyond what has been described in this review, will be important for identifying new targets for managing stress-related drug use.
Last, it has been established that the induction of addiction-related neuroplasticity, including that which increases susceptibility to stress-induced relapse, requires elevated glucorticoid levels at the time of drug use. However, the precise nature of these glucocorticoid-dependent neuroadaptations is unknown and the processes through which glucocorticoids put these adaptations in place are poorly understood. Determination of these processes could provide insight into how to slow down or halt the progression of addiction.
Conclusions
In contrast to other classes of abused drugs (e.g. opiates, alcohol, nicotine), there are currently no FDA-approved medications for the management of cocaine addiction, making it a critical unmet medical need. Stress is a powerful, pervasive and unavoidable contributor to cocaine addiction. For this reason, understanding the neurobiological mechanisms through which stress promotes drug use in cocaine addicts will serve as a particularly important step in the development of new and more effective therapeutic strategies for managing addiction, especially in subpopulations of individuals whose cocaine use is stress-related. While research in our laboratory and others strongly suggests that medications targeting CRF and noradrenergic systems should hold some promise as potential treatments, the hope is that continued research in this area will reveal new therapeutic targets for medication development with the goal of minimizing the contribution of stress to the addiction cycle, thereby improving treatment outcomes and reducing the societal costs of addiction.
Declaration of interest
J.R.M. is supported by National Institute on Drug Abuse (NIDA; grant number DA15758). J.R.M., D.F.P., J.M.B. and J.R.M.R. are all employees of Marquette University. J.R.M. is also a co-founder of, shareholder in, consultant for Promentis Pharmaceuticals. The authors declare no conflicts of interest related to contents of this article.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. (1989). Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem 52:1655–8
- Ahmed SH, Koob GF. (1997). Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berl) 132:289–95
- Ahmed SH, Koob GF. (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300
- Ahmed SH, Walker JR, Koob GF. (2000). Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22:413–21
- Anstrom KK, Miczek KA, Budygin EA. (2009). Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience 161:3–12
- Asanuma M, Ogawa N, Mizukawa K, Haba K, Hirata H, Mori A. (1991). Distribution of the beta-2 adrenergic receptor messenger RNA in the rat brain by in situ hybridization histochemistry: effects of chronic reserpine treatment. Neurochem Res 16:1253–6
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. (1999). The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci 877:486–98
- Back SE, Brady KT, Jaanimagi U, Jackson JL. (2006). Cocaine dependence and PTSD: a pilot study of symptom interplay and treatment preferences. Addict Behav 31:351–4
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, et al. (2010). Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend 106:21–7
- Bale TL, Vale WW. (2004). CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44:525–57
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. (1999). Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 145:21–30
- Baumann MH, Milchanowski AB, Rothman RB. (2004). Evidence for alterations in alpha2-adrenergic receptor sensitivity in rats exposed to repeated cocaine administration. Neuroscience 125:683–90
- Beckerman MA, Van Kempen TA, Justic NJ, Milner TA, Glass MJ. (2013). Corticotropin-releasing factor in the mouse central nucleus of the amygdala: ultrastructural distribution in NMDA-NR1 receptor subunit expressing neurons as well as projection neurons to the bed nucleus of the stria terminalis. Exp Neurol 239:120--32
- Beckstead MJ, Gantz SC, Ford CP, Stenzel-Poore MP, Phillips PE, Mark GP, Williams JT. (2009). CRF enhancement of GIRK channel-mediated transmission in dopamine neurons. Neuropsychopharmacology 34:1926–35
- Belej T, Manji D, Sioutis S, Barros HM, Nobrega JN. (1996). Changes in serotonin and norepinephrine uptake sites after chronic cocaine: pre- vs. post-withdrawal effects. Brain Res 736:287–96
- Berridge CW. (2008). Noradrenergic modulation of arousal. Brain Res Rev 58:1–17
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. (2005). Effects of chronic cocaine self-administration on norepinephrine transporters in the nonhuman primate brain. Psychopharmacology (Berl) 180:781–8
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR. (2011). Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J Neurosci 31:11396–403
- Brandon TH, Vidrine JI, Litvin EB. (2007). Relapse and relapse prevention. Annu Rev Clin Psychol 3:257–84
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Le DA, et al. (2005). Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res 29:185–95
- Briand LA, Blendy JA. (2010). Molecular and genetic substrates linking stress and addiction. Brain Res 1314:219–34
- Briand LA, Vassoler FM, Pierce RC, Valentino RJ, Blendy JA. (2010). Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein. J Neurosci 30:16149–59
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. (2009). Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A 106:4894–9
- Broadbear JH, Winger G, Cicero TJ, Woods JH. (1999). Effects of self-administered cocaine on plasma adrenocorticotropic hormone and cortisol in male rhesus monkeys. J Pharmacol Exp Ther 289:1641–7
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. (1995). Stress, vulnerability and adult alcohol relapse. J Stud Alcohol 56:538–45
- Brown ZJ, Tribe E, D'Souza NA, Erb S. (2009). Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 203:121–30
- Brown ZJ, Nobrega JN, Erb S. (2011). Central injections of noradrenaline induce reinstatement of cocaine seeking and increase c-fos mRNA expression in the extended amygdala. Behav Brain Res 217:472–6
- Bruijnzeel AW, Prado M, Isaac S. (2009). Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry 66:110–7
- Budygin EA, Park J, Bass CE, Grinevich VP, Bonin KD, Wightman RM. (2012). Aversive stimulus differentially triggers subsecond dopamine release in reward regions. Neuroscience 201:331–7
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE. (2012). Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol Behav 105:209–14
- Buffalari DM, See RE. (2009). Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiol Behav 98:614–7
- Capriles N, Rodaros D, Sorge RE, Stewart J. (2003). A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 168:66–74
- Charlton BG, Ferrier IN, Perry RH. (1987). Distribution of corticotropin-releasing factor-like immunoreactivity in human brain. Neuropeptides 10:329–34
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, et al. (2013). Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493:532–6
- Chen KW, Banducci AN, Guller L, Macatee RJ, Lavelle A, Daughters SB, Lejuez CW. (2011). An examination of psychiatric comorbidities as a function of gender and substance type within an inpatient substance use treatment program. Drug Alcohol Depend 118:92–9
- Coffey SF, Saladin ME, Drobes DJ, Brady KT, Dansky BS, Kilpatrick DG. (2002). Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug Alcohol Depend 65:115–27
- Constantinou N, Morgan CJ, Battistella S, O'Ryan D, Davis P, Curran HV. (2010). Attentional bias, inhibitory control and acute stress in current and former opiate addicts. Drug Alcohol Depend 109:220–5
- Dautzenberg FM, Hauger RL. (2002). The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci 23:71–7
- de Jong IE, Steenbergen PJ, de Kloet ER. (2009). Behavioral sensitization to cocaine: cooperation between glucocorticoids and epinephrine. Psychopharmacology 204:693–703
- De La Garza R 2nd, Ashbrook LH, Evans SE, Jacobsen CA, Kalechstein AD, Newton TF. (2009). Influence of verbal recall of a recent stress experience on anxiety and desire for cocaine in non-treatment seeking, cocaine-addicted volunteers. Am J Addict 18:481–7
- Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, Piazza PV. (1995). Stress-induced sensitization and glucocorticoids. I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci 15:7181–8
- Deutch AY, Lee MC, Gillham MH, Cameron DA, Goldstein M, Iadarola MJ. (1991). Stress selectively increases fos protein in dopamine neurons innervating the prefrontal cortex. Cereb Cortex 1:273–92
- Di Chiara G, Loddo P, Tanda G. (1999). Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry 46:1624–33
- Dumont EC, Williams JT. (2004). Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci 24:8198–204
- Duncan E, Boshoven W, Harenski K, Fiallos A, Tracy H, Jovanovic T, Hu X, et al. (2007). An fMRI study of the interaction of stress and cocaine cues on cocaine craving in cocaine-dependent men. Am J Addict 16:174–82
- Dunn AJ, Berridge CW. (1990). Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 15:71–100
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. (2005). Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology 30:657–68
- Egli RE, Winder DG. (2003). Dorsal and ventral distribution of excitable and synaptic properties of neurons of the bed nucleus of the stria terminalis. J Neurophysiol 90:405–14
- Enoch MA. (2011). The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 214:17–31
- Erb S, Funk D, Le AD. (2005). Cocaine pre-exposure enhances CRF-induced expression of c-fos mRNA in the central nucleus of the amygdala: an effect that parallels the effects of cocaine pre-exposure on CRF-induced locomotor activity. Neurosci Lett 383:209–14
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. (2000). Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology 23:138–50
- Erb S, Petrovic A, Yi D, Kayyali H. (2006). Central injections of CRF reinstate cocaine seeking in rats after postinjection delays of up to 3 h: an influence of time and environmental context. Psychopharmacology (Berl) 187:112–20
- Erb S, Salmaso N, Rodaros D, Stewart J. (2001). A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 158:360–5
- Erb S, Shaham Y, Stewart J. (1996). Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 128:408–12
- Erb S, Shaham Y, Stewart J. (1998). The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci 18:5529–36
- Erb S, Stewart J. (1999). A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci 19:RC35
- Fan Y, Chen P, Li Y, Cui K, Noel DM, Cummins ED, Peterson DJ, et al. (2013). Corticosterone administration up-regulated expression of norepinephrine transporter and dopamine β-hydroxylase in rat locus coeruleus and its terminal regions. J Neurochem. [Epub ahead of print]. doi: 10.1111/jnc.12459
- Feltenstein MW, See RE. (2006). Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res 174:1–8
- Fox HC, Hong KI, Siedlarz K, Sinha R. (2008). Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology 33:796–805
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. (2005). Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology 30:880–91
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. (2005). The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30:296–309
- Georges F, Aston-Jones G. (2001). Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci 21:RC160
- Georges F, Aston-Jones G. (2002). Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci 22:5173–87
- Goeders NE, Bienvenu OJ, De Souza EB. (1990). Chronic cocaine administration alters corticotropin-releasing factor receptors in the rat brain. Brain Res 531:322–8
- Graf EN, Hoks MA, Baumgardner J, Sierra J, Vranjkovic O, Bohr C, Baker DA, Mantsch JR. (2011). Adrenal activity during repeated long-access cocaine self-administration is required for later CRF-Induced and CRF-dependent stressor-induced reinstatement in rats. Neuropsychopharmacology 36:1444–54
- Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, Robble MA, et al. (2013). Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci 33:11800–10
- Grammatopoulos DK. (2012). Insights into mechanisms of corticotropin-releasing hormone receptor signal transduction. Br J Pharmacol 166:85–97
- Graziane NM, Polter AM, Briand LA, Pierce RC, Kauer JA. (2013). Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron 77:942–54
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. (1995). Local influence of endogenous norepinephrine on extracellular dopamine in rat medial prefrontal cortex. J Neurochem 65:111–16
- Hahn J, Hopf FW, Bonci A. (2009). Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J Neurosci 29:6535–44
- Horger BA, Roth RH. (1996). The role of mesoprefrontal dopamine neurons in stress. Crit Rev Neurobiol 10:395–418
- Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R. (2008). Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend 92:208–16
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. (2013). Distinct extended amygdala circuits for divergent motivational states. Nature 496:224–8
- Jhanwar-Uniyal M, Leibowitz SF. (1986). Impact of circulating corticosterone on alpha 1- and alpha 2-noradrenergic receptors in discrete brain areas. Brain Res 368:404–8
- Jobes ML, Ghitza UE, Epstein DH, Phillips KA, Heishman SJ, Preston KL. (2011). Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology (Berl) 218:83–8
- Joëls M, Bouma G, Hesen W, Zegers Y. (1991). Increased effect of noradrenaline on synaptic responses in rat CA1 hippocampal area after adrenalectomy. Brain Res 550:347–52
- Kalivas PW, Duffy P. (1995). Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res 675:325–8
- Kalivas PW, McFarland K. (2003). Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 168:44–56
- Kash TL, Nobis WP, Matthews RT, Winder DG. (2008). Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci 28:13856–65
- Khoury L, Tang YL, Bradley B, Cubells JF, Ressler KJ. (2010). Substance use, childhood traumatic experience, and posttraumatic stress disorder in an urban civilian population. Depress Anxiety 27:1077–86
- Kippin TE, Fuchs RA, See RE. (2006). Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 187:60–7
- Koob G, Kreek MJ. (2007). Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164:1149–59
- Koob GF. (2008). A role for brain stress systems in addiction. Neuron 59:11–34
- Koob GF. (2010). The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res 1314:3–14
- Koob GF, Volkow ND. (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35:217–38
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. (2006). Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci 23:2677–85
- Kreibich AS, Blendy JA. (2004). cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J Neurosci 24:6686–92
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, Minami M, Watanabe M. (2012). Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J Neurosci 32:18035–46
- Lammel S, Ion DI, Roeper J, Malenka RC. (2011). Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70:855–62
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. (2012). Input-specific control of reward and aversion in the ventral tegmental area. Nature 491:212–17
- Lanteri C, Salomon L, Torrens Y, Glowinski J, Tassin JP. (2008). Drugs of abuse specifically sensitize noradrenergic and serotonergic neurons via a non-dopaminergic mechanism. Neuropsychopharmacology 33:1724–34
- Lê AD, Harding S, Juzytsch W, Funk D, Shaham Y. (2005). Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 179:366–73
- Lê AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. (2000). The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 150:317–24
- Lê AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. (2011). Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl) 218:89–99
- Leeies M, Pagura J, Sareen J, Bolton JM. (2010). The use of alcohol and drugs to self-medicate symptoms of posttraumatic stress disorder. Depress Anxiety 27:731–6
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE. (2012). Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature 490:402–6
- Leri F, Flores J, Rodaros D, Stewart J. (2002). Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci 22:5713–18
- Lodge DJ, Grace AA. (2005). Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J Pharmacol Exp Ther 314:201–6
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. (1995). Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA 92:836–40
- Macey DJ, Smith HR, Nader MA, Porrino LJ. (2003). Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. J Neurosci 23:12–6
- Mameli M, Bellone C, Brown MT, Luscher C. (2011). Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nat Neurosci 14:414–6
- Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP. (2008). Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology (Berl) 195:591–603
- Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, Ziegler DR. (2007). Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res 1167:101–11
- Mantsch JR, Katz ES. (2007). Elevation of glucocorticoids is necessary but not sufficient for the escalation of cocaine self-administration by chronic electric footshock stress in rats. Neuropsychopharmacology 32:367–76
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. (2010). Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology 35:2165–78
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. (2003). Neuroendocrine alterations in a high-dose, extended-access rat self-administration model of escalating cocaine use. Psychoneuroendocrinology 28:836–62
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. (2004). Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 175:26–36
- McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF. (2012). Encoding of aversion by dopamine and the nucleus accumbens. Front Neurosci 6:137
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. (2004). Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci 24:1551–60
- McLaughlin JP, Marton-Popovici M, Chavkin C. (2003). Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci 23:5674–83
- McRae-Clark AL, Carter RE, Price KL, Baker NL, Thomas S, Saladin ME, Giarla K, et al. (2011). Stress- and cue-elicited craving and reactivity in marijuana-dependent individuals. Psychopharmacology (Berl) 218:49–58
- Meil WM, See RE. (1997). Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res 87:139–48
- Mitrano DA, Pare JF, Smith Y, Weinshenker D. (2014). D1-dopamine and α1-adrenergic receptors co-localize in dendrites of the rat prefrontal cortex. Neuroscience 258:90--100
- Mitrano DA, Schroeder JP, Smith Y, Cortright JJ, Bubula N, Vezina P, Weinshenker D. (2012). α-1 Adrenergic receptors are localized on presynaptic elements in the nucleus accumbens and regulate mesolimbic dopamine transmission. Neuropsychopharmacology 37:2161–72
- Mobley PL, Manier DH, Sulser F. (1983). Norepinephrine-sensitive adenylate cyclase system in rat brain: role of adrenal corticosteroids. J Pharmacol Exp Ther 226:71–7
- Nobis WP, Kash TL, Silberman Y, Winder DG. (2011). Beta-adrenergic receptors enhance excitatory transmission in the bed nucleus of the stria terminalis through a corticotrophin-releasing factor receptor-dependent and cocaine-regulated mechanism. Biol Psychiatry 69:1083–90
- Oleson EB, Gentry RN, Chioma VC, Cheer JF. (2012). Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. J Neurosci 32:14804–8
- Orozco-Cabal L, Pollandt S, Liu J, Shinnick-Gallagher P, Gallagher JP. (2006). Regulation of synaptic transmission by CRF receptors. Rev Neurosci 17:279–307
- Park J, Wheeler RA, Fontillas K, Keithley RB, Carelli RM, Wightman RM. (2012). Catecholamines in the bed nucleus of the stria terminalis reciprocally respond to reward and aversion. Biol Psychiatry 71:327–34
- Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallagher P. (2006). Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci 24:1733–43
- Preston KL, Epstein DH. (2011). Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology (Berl) 218:29–37
- Rainbow TC, Parsons B, Wolfe BB. (1984). Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci U S A 81:1585–9
- Ratka A, Sutanto W, Bloemers M, de Kloet ER. (1989). On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation. Neuroendocrinology 50:117–23
- Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, et al. (2011). Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science 333:1903–7
- Richter RM, Weiss F. (1999). In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse 32:254–61
- Rivier C, Vale W. (1987). Cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin-releasing factor (CRF)-mediated mechanism. Brain Res 422:403–6
- Robinson TE, Berridge KC. (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–91
- Rodaros D, Caruana DA, Amir S, Stewart J. (2007). Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience 150:8–13
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. (2008). Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci 11:1376–7
- Rounsaville BJ, Anton SF, Carroll K, Budde D, Prusoff BA, Gawin F. (1991). Psychiatric diagnoses of treatment-seeking cocaine abusers. Arch Gen Psychiatry 48:43–51
- Saal D, Dong Y, Bonci A, Malenka RC. (2003). Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37:577–82
- Saladin ME, Drobes DJ, Coffey SF, Dansky BS, Brady KT, Kilpatrick DG. (2003). PTSD symptom severity as a predictor of cue-elicited drug craving in victims of violent crime. Addict Behav 28:1611–29
- Sanchez CJ, Bailie TM, Wu WR, Li N, Sorg BA. (2003). Manipulation of dopamine d1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience 119:497–505
- Sartor GC, Aston-Jones G. (2012). Regulation of the ventral tegmental area by the bed nucleus of the stria terminalis is required for expression of cocaine preference. Eur J Neurosci 36:3549–58
- Schank JR, King CE, Sun H, Cheng K, Rice KC, Heilig M, Weinshenker D, Schroeder JP. (2013). The role of the neurokinin-1 receptor in stress-induced reinstatement of alcohol and cocaine seeking. Neuropsychopharmacology. [Epub ahead of print]. doi: 10.1038/npp.2013.309
- Schulkin J, Gold PW, McEwen BS. (1998). Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology 23:219–43
- Schultz W. (2007). Multiple dopamine functions at different time courses. Annu Rev Neurosci 30:259–88
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. (2001). Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci 21:3628–38
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. (1998). CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor 1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 137:184–90
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. (1997). Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci 17:2605–14
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. (2000). Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci 12:292–302
- Shin JW, Geerling JC, Loewy AD. (2008). Inputs to the ventrolateral bed nucleus of the stria terminalis. J Comp Neurol 511:628–57
- Silberman Y, Matthews RT, Winder DG. (2013). A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J Neurosci 33:950–60
- Silberman Y, Winder DG. (2013). Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. Front Psychiatry 4:42
- Sinha R. (2009). Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol 14:84–98
- Sinha R, Catapano D, O'Malley S. (1999). Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 142:343–51
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. (2006). Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 63:324–31
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. (2009). Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology 34:1198–208
- Sinha R, Shaham Y, Heilig M. (2011). Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 218:69–82
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. (2003). Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 170:62–72
- Specio SE, Wee S, O'Dell LE, Boutrel B, Zorrilla EP, Koob GF. (2008). CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 196:473–82
- Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. (2002). Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry 51:642–51
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2012). Results from the 2011 National Survey on Drug Use and Health: Mental Health Findings, NSDUH Series H-45, HHS Publication No. (SMA) 12-4725. Rockville, MD: Substance Abuse and Mental Health Services Administration
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. (1983). Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36:165–86
- Tagliaferro P, Morales M. (2008). Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J Comp Neurol 506:616–26
- Tan LA, Xu K, Vaccarino FJ, Lovejoy DA, Rotzinger S. (2009). Teneurin C-terminal associated peptide (TCAP)-1 attenuates corticotropin-releasing factor (CRF)-induced c-Fos expression in the limbic system and modulates anxiety behavior in male Wistar rats. Behav Brain Res 201:198–206
- Tassin JP, Studler JM, Hervé D, Blanc G, Glowinski J. (1986). Contribution of noradrenergic neurons to the regulation of dopaminergic (D1) receptor denervation supersensitivity in rat prefrontal cortex. J Neurochem 46:243–8
- Tidey JW, Miczek KA. (1996). Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res 721:140–9
- Ungless MA, Argilli E, Bonci A. (2010). Effects of stress and aversion on dopamine neurons: implications for addiction. Neurosci Biobehav Rev 35:151–6
- Ungless MA, Magill PJ, Bolam JP. (2004). Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303:2040–2
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. (2003). Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron 39:401–7
- Ungless MA, Whistler JL, Malenka RC, Bonci A. (2001). Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411:583–7
- Vale W, Spiess J, Rivier C, Rivier J. (1981). Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213:1394–7
- Valenti O, Lodge DJ, Grace AA. (2011). Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci 31:4280--9
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, et al. (2000). Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428:191–212
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW Jr., Logrip ML, Rivier C, et al. (2012). Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci 32:7563–71
- Vranjkovic O, Hang S, Baker DA, Mantsch JR. (2012). beta-adrenergic receptor mediation of stress-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: roles for beta1 and beta2 adrenergic receptors. J Pharmacol Exp Ther 342:541–51
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. (2008). Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol 586:2157–70
- Wanat MJ, Bonci A, Phillips PE. (2013). CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat Neurosci 16:383–5
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. (2005). Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci 25:5389–96
- Wang B, You ZB, Rice KC, Wise RA. (2007). Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 193:283–94
- Wang B, You ZB, Wise RA. (2012). Heroin self-administration experience establishes control of ventral tegmental glutamate release by stress and environmental stimuli. Neuropsychopharmacology 37:2863–9
- Wang HL, Morales M. (2008). Corticotropin-releasing factor binding protein within the ventral tegmental area is expressed in a subset of dopaminergic neurons. J Comp Neurol 509:302–18
- Wang X, Cen X, Lu L. (2001). Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur J Pharmacol 432:153–61
- Ward AS, Collins ED, Haney M, Foltin RW, Fischman MW. (1999). Blockade of cocaine-induced increases in adrenocorticotrophic hormone and cortisol does not attenuate the subjective effects of smoked cocaine in humans. Behav Pharmacol 10:523–9
- Wee S, Mandyam CD, Lekic DM, Koob GF. (2008). Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol 18:303–11
- Weinshenker D, Schroeder JP. (2007). There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology 32:1433–51
- Wichmann R, Fornari RV, Roozendaal B. (2012). Glucocorticoids interact with the noradrenergic arousal system in the nucleus accumbens shell to enhance memory consolidation of both appetitive and aversive taste learning. Neurobiol Learn Mem 98:197–205
- Wise RA, Rompre PP. (1989). Brain dopamine and reward. Annu Rev Psychol 40:191–225
- Wise RA, Morales M. (2010). A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Res 1314:38–43
- Xu TX, Yao WD. (2010). D1 and D2 dopamine receptors in separate circuits cooperate to drive associative long-term potentiation in the prefrontal cortex. Proc Natl Acad Sci U S A 107:16366–71
- Yamada H, Bruijnzeel AW. (2011). Stimulation of α2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropharmacology 60:303–11
- Zahm DS, Jensen SL, Williams ES, Martin JR, 3rd. (1999). Direct comparison of projections from the central amygdaloid region and nucleus accumbens shell. Eur J Neurosci 11:1119–26
- Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ. (1996). Corticotropin-releasing factor and type 1 corticotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during “binge”-pattern cocaine administration and chronic withdrawal. J Pharmacol Exp Ther 279:351–8
- Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ. (2003). Alterations in hypothalamic-pituitary-adrenal axis activity and in levels of proopiomelanocortin and corticotropin-releasing hormone-receptor 1 mRNAs in the pituitary and hypothalamus of the rat during chronic ‘binge' cocaine and withdrawal. Brain Res 964:187–99
- Zorrilla EP, Valdez GR, Weiss F. (2001). Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 158:374–81
- Zorrilla EP, Wee S, Zhao Y, Specio S, Boutrel B, Koob GF, Weiss F. (2012). Extended access cocaine self-administration differentially activates dorsal raphe and amygdala corticotropin-releasing factor systems in rats. Addict Biol 17:300–8
