Abstract
Spanish medical graduates who apply for a medical specialty training position (MIR) must take an examination that will shape their future personal and professional lives. Preparation for the test represents an important stressor that persists for several months. The aim of this study was to elucidate the stress pattern of this group and evaluate possible changes in the circadian rhythm of cortisol release in medical graduates preparing for this test. A repeated-measures longitudinal study was performed, measuring the salivary cortisol concentrations in 36 medical graduates (13 males and 23 females; mean age of 24.2 years) on five sampling days. Five cortisol samples were collected from 07:00 to 21:00 h in order to monitor changes in the circadian rhythm. On all sampling days (except on the day of the official examination), anxiety and psychological stress were evaluated with the Spanish versions of the State-Trait Anxiety Inventory (STAI) and the Perceived Stress Scale (PSS). During the study period, participants showed higher levels of anxiety than the Spanish reference population as well as a progressive increase in self-perceived stress. A significant increase in salivary cortisol concentration was observed in both chronic (study and examination preparation) and acute (examinations) situations. Our results suggest that the cortisol awakening response (CAR) may be a good indicator of anticipatory stress but is unaffected by long-term examination preparation. Comparison of results between the official examination day and the mock examination days yielded evidence that learning may modulate the behavior of the hypothalamic–pituitary–adrenal axis.
Introduction
Stress is a major health problem due to its involvement in the aetiology of multiple organic and psychological pathologies (Arnetz & Ekman, Citation2006; Chrousos, Citation2009). When a situation is perceived as stressful, a series of physiological mechanisms are set in motion, notably activation of the sympathetic and adrenomedullary systems and of the hypothalamic–pituitary–adrenal (HPA) axis, which leads to increased cortisol release (Nader et al., Citation2010).
Salivary cortisol has been widely used as a biochemical marker in stress research because saliva can be readily collected under different conditions and repeatedly throughout the day (Hellhammer et al., Citation2009; Kudielka et al., Citation2009; Miller et al., Citation2013). The linear relationship between blood cortisol (free and bound to cortisol-binding globulin) concentrations and salivary cortisol concentrations is well established (Hellhammer et al., Citation2009; Nater et al., Citation2008). Cortisol release follows a circadian rhythm, peaking at 30–60 min after awakening and then gradually tapering off over the day (Nader et al., Citation2010; Ranjit et al., Citation2005).
Studies investigating stress and cortisol secretion have generally been conducted under laboratory conditions. The most widely used instrument has been the Trier Social Stress Test (TSST), which induces a standardized form of psychobiological stress under laboratory conditions (Kirschbaum et al., Citation1993; Kudielka et al., Citation2007; Stauble et al., Citation2013). Other similar strategies have been adopted to evaluate the effects of stress (Kidd et al., Citation2011), but fewer studies have evaluated stress under real-life conditions due to design and sampling difficulties (Berndt et al., Citation2012; González-Cabrera et al., Citation2012; Strahler et al., Citation2010; Taylor et al., Citation2008).
A meta-analysis of 208 studies on acute stress under laboratory conditions reported that the maximum cortisol response is observed at 20–40 min after the onset of the stressful event (Dickerson & Kemeny, Citation2004). Variable findings have been published on cortisol release during acute stress situations under natural conditions (Michaud et al., Citation2008), including academic examinations (Duan et al., Citation2013; Stowell, Citation2003). Increased cortisol release during examination periods has been observed by some authors (Lacey et al., Citation2000; Murphy et al., Citation2010; Weekes et al., Citation2006) but not by others (Hulme et al., Citation2011; Takatsuji et al., Citation2008), and some researchers even reported a reduction in salivary cortisol release (Vedhara et al., Citation2000). Most of these studies have been performed under conditions that are simulated or of minor importance to the participant, when examination results have a variable impact on the personal or professional future of the participants, which may explain the medium-to-low reactivity of the hypothalamic–pituitary–adrenal (HPA) axis observed (Stowell, Citation2003; Weekes et al., Citation2006).
In contrast, chronic stress has mainly been evaluated under real-life conditions, when cortisol release appears to follow a diurnal curve that is flattened in comparison to controls (Nater et al., Citation2008; Ranjit et al., Citation2005; Vachon-Presseau et al., Citation2013). Other parameters used to assess chronic stress, such as the area under the curve (AUC) for repeated cortisol measurements (Nater et al., Citation2008; Pruessner et al., Citation2003a) and the cortisol awakening response (CAR), have not yielded consistent results. The CAR (Clow et al., Citation2010) is the increase in cortisol concentrations in the first 30 min after awakening and has been proposed as an accurate indicator of chronic stress (Fries et al., Citation2009; Walker et al., Citation2011). However, CAR results have been controversial (Law et al., Citation2013; Lovell et al., Citation2012), especially those obtained during academic examinations (Duan et al., Citation2013; Gaab et al., Citation2006; Hewig et al., Citation2008).
The present study was conducted in a population of medical graduates preparing for the national professional examination (MIR examinationFootnote1) to enter the only medical specialty training system in Spain. In 2012, there were nearly 14,000 graduates for 6707 training positions (residencies). Candidates are rank-ordered based on their test scores, and the rank order obtained determines the possibility of choosing a particular medical specialty, hospital and city; those ranked below the number of available appointments will not be able to train as specialists and will not have access to a position for at least 1 year before retaking the examination. The vital importance of this examination for the personal and professional futures of the candidates can be expected to generate high levels of acute and chronic stress. Elucidation of the possible stress pattern of this group may be useful for the development of tools to manage stress during this period and improve the graduates’ performance. We hypothesized that an extended chronic stressor plus periodic acute stressor events would produce higher levels of chronic and acute stress and result in changes in the circadian rhythm of cortisol release in medical graduates preparing for the MIR test. The objectives of this study were to test these hypotheses and simultaneously evaluate the anxiety and perceived stress levels of the graduates by means of self-report psychological questionnaires.
Method
Participants: sample and selection
The study population was recruited from among 120 medical graduates preparing intensively for the MIR examination from July 2011 to January 2012 at an academy (AulaMir®) on the premises of the College of Physicians of Granada Province (Spain) through an informative presentation during one of the course sessions. Non-probability incidental sampling was used to enroll the largest possible number of volunteers. Out of the 87 volunteers initially recruited, 51 were excluded after applying the study inclusion/exclusion criteria, leaving a final sample of 36 participants (13 males and 23 females) with a mean ± SD age of 24.2 ± 0.7 years. The mean duration of sleep was 408 ± 22 min/day and the mean study time was 510 ± 74 min/day.
Study inclusion criteria were: (1) enrolment in the AulaMir academy during the academic year 2011–2012 to prepare for the MIR examination in January 2012; (2) signing of informed consent to participate; (3) commitment to maintaining a stable circadian rhythm of sleep/wake during the MIR examination preparation period (from July 2011), arising between 07:00 and 07:20 h and going to bed at 23:00–24:00 h; (4) agreement to abstain from medication for 24 h before the samplings at 07:00 h and (5) performance of all tests and evaluations ordered by the academy, ensuring that all participants experienced the same stress events. Exclusion criteria were: (1) a traumatic psychological event in the previous 6 months; (2) pregnancy or receipt of hormone replacement therapy, use of oral contraceptives, drugs or any other chronic treatment that might affect the HPA axis; (3) a smoking habit and (4) performance of physical exercise >2 h/day (Hellhammer et al., Citation2009).
Research design
A longitudinal study with repeated measures was undertaken, assessing the salivary cortisol concentrations at different time points on 4 days in all participants and on a fifth day in a proportion of them. Each participant served as his/her own control, thereby reducing the variability of experimental error. During the MIR preparation process, all students experienced the same events. Every weekday, all participating students studied in the morning and attended classes in the afternoon. On Fridays, the students took a mock examination in their usual classroom. The psychological anxiety and perceived stress levels of the students were also evaluated on day 1, at 2 days before the official examination and at 10–15 days after the results were published. summarizes the activities of the graduates on the sampling days and the variables measured.
Table 1. Sampling days with activities and measured variables.
Samples were gathered at the following five time points (see ) to determine the circadian rhythm of cortisol release: (1) at ∼07:00 h (immediately after waking); (2) at ∼07:30 h (30 min after waking, i.e. the time of peak hormone secretion, for obtaining CAR values; Clow et al., Citation2010); (3) at 11:00 h; (4) at 16:00 h and (5) at 21:00 h. The 16:00 h sample alone was taken on the day of the official examination in order to minimize interference with the students’ activities.
On each sampling day, data were gathered from each participant to check compliance with the study inclusion criteria and protocol, including any drug intake in the previous 24 h, any traumatic psychological event since the previous evaluation, maintenance of regular sleep–wake rhythm during the previous week, any moderate–intense physical exercise in the previous 24 h, mean study hours/day and, in the females, the date of the last menstruation in order to detect any possible changes in their menstrual cycle.
Psychological evaluation
All participants completed two self-report questionnaires on day 1, day 62 (2 days before the official examination) and between days 106 and 111. One was the validated Spanish version of the State-Trait Anxiety Inventory (STAI) (Spielberger et al., Citation1982) and the other was the Spanish adaptation of the Perceived Stress Scale (PSS) (Trujillo & González-Cabrera, Citation2007). The STAI questionnaire has two 20-item sub-scales that can be used independently: STAI-State to evaluate the current level of anxiety, and STAI-Trait to assess stable aspects of anxiety. In this version, the score for each item ranges from 1 to 3, with a maximum score of 60. The second questionnaire used was the Spanish adapted version of the Perceived Stress Scale (PSS/EEP), including 14 items, with each item scoring 1–4, and a maximum score of 56 (Trujillo & González-Cabrera, Citation2007). This questionnaire is validated for the Spanish population (Cronbach alpha = 0.79), but specific reference values have not been established.
Salivary cortisol
The saliva samples were collected in Salivette® tubes (Sarstedt International, Nümbrecht, Germany) and then centrifuged at 1459 × g for 10 min and stored at −22 °C until further analysis. Salivary cortisol concentrations were measured in µg/dl using an electrochemiluminescence immunoassay (Elecsys Cortisol® kit, Roche Diagnostics, Rotkreuz, Switzerland) in a Cobas® c8000 analyser (Roche Diagnostics, Rotkreuz, Switzerland). The sensitivity limit for the cortisol assays was 0.014 μg/dl. The intra-assay and inter-assay coefficients of variance were 6.23% and 9.19%, respectively.
Procedure
Each participant was individually followed up. Two days before the sampling day, each participant was given a salivary cortisol tube with written instructions, included frequently asked questions (FAQs), as recommended for correct cortisol sampling (Pruessner et al., Citation2003b). On the first two sampling days (days 1 and 49), samples for the first four time points were kept refrigerated and given to the researchers at 16:00 h in the academy, and the fifth sample was taken at 21:00 h at the academy, after the mock examination. For the third sample on day 62 (relaxation day), all samples were kept refrigerated until given to the researchers on the following day. On day 65, samples were taken between 15:00 and 16:00 h at the official examination site, using mobile equipment to preserve the samples. The final sample was taken 10–15 days after the participants had been given their national examination score and rank order, i.e. 40–45 days after completing the examination; many participants had left Granada by this time, in part explaining the reduced number (n = 8) who gave samples on this day. All samples were stored at 4 °C from the time of collection until their centrifugation.
Ethical considerations
Participation in the study was voluntary, anonymous and disinterested, and the informed consent form signed by participants included the option to withdraw at any stage. The study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of the University of Granada (Spain).
Data analysis
SPSS 15.0 software (IBM®, Chicago, IL) was used for the statistical analyses and SigmaPlot 11.0 for graphical representations. After applying the Shapiro–Wilks test to determine the normality of the data distribution, the cortisol data were logarithmically transformed (ln [x + 1]), because values were close to zero and non-normally distributed. Psychological data were typified. Frequency analysis and measures of central tendency and dispersion were performed, followed by one-way analysis of variance (ANOVA) and then a repeated-measures ANOVA, adjusting the degrees of freedom with the Greenhouse–Geisser correction when Mauchly’s sphericity assumption was violated. Greenhouse–Geisser test results were expressed as GG-ε. The within-subject factor was each cortisol sample or the AUC or CAR, and the levels of each factor were the four sampling days (excluding the optional sampling on days 106–111). The between-subject factor was the sex of the participants. Effect sizes were calculated for the significant results by means of partial eta-squared (η2) analysis, which expressed the degree of variance. Pairwise comparisons between factor levels were performed with Bonferroni correction. Student’s t-tests for dependent samples and for one sample were applied, linear regressions were performed, the AUC of cortisol was calculated with the trapezoidal method (Pruessner et al., Citation2003a) and the CAR was determined according to Clow et al. (Citation2010). An alpha value of p < 0.05 was considered for all analyses.
Results
The STAI questionnaire results (see ) showed higher levels of anxiety in both male and female participants on days 1 and 62 in comparison to the reference population. When the day 1 and day 61 results were compared, only the STAI-Trait score for the males showed a significant increase (t = −5.125; p < 0.001). Results (means ± SD) for the Spanish version of PSS showed a higher level of perceived stress (t = −27.61, p < 0.001) in the participants on day 62 (32.53 ± 10.42) than on day 1 (27.86 ± 10.85). At 10–15 days after receiving their examination results, the mean STAI score was below the reference population level (see ) and the PSS score was below the value on day 1 (17.24 ± 6.25; p < 0.001).
Table 2. Results (mean ± SD) obtained with the validated Spanish version of the State-Trait Anxiety Inventory (STAI). These values were compared with the reference values for the Spanish population using a one-sample Student’s t-test.
depicts the mean cortisol concentration ± SD at each time point on each sampling day, and significant differences (Bonferroni tests). One-way repeated-measure ANOVAs2 showed no differences in cortisol concentrations among sampling days in the samples taken at ∼07:00 h (GG-ε1.942 = 1.681; p = 0.19; η2 = 0.047), significant differences in the samples at ∼07:30 h, i.e. 30 min after awakening (GG-ε1.842 = 1.681; p < 0.005; η2 = 0.152), no differences in sample at 11:00 h (GG-ε1.645 = 2.190; p = 0.13; η2 = 0.061), and significant differences among sampling days in the samples taken at 16:00 h, i.e. immediately before the mock examination on days 1 and 49 (GG-ε2.210 = 12.242; p < 0.001 η2 = 0.313), and in those taken at 21:00 h, i.e. immediately after the examination on days 1 and 49 (GG-ε1.952 = 19.397; p < 0.001; η2 = 0.370). The sample taken on the official examination day (day 65), showed significant differences only when compared to the sample on the relaxation day (day 62) (p < 0.001).
Figure 1. Salivary cortisol profile (mean ± SD) between 07:00 and 21:00 h on each sampling day. Cortisol concentrations are expressed in μg/dl; n = 36 for sampling days 1, 49, 62 and 65, n = 8 for sampling days 106–111. Statistically significant comparisons for same time of day: *p < 0.007 versus day 49; +p < 0.012 versus day 1; **p < 0.002 versus day 1; ***p < 0.001 versus day 49, day 65; +++p < 0.001 versus day 1, day 49. All possible pairwise comparisons, using the Bonferroni test, between the within-subject factor (salivary cortisol concentration) and the levels of each factor (sampling day).
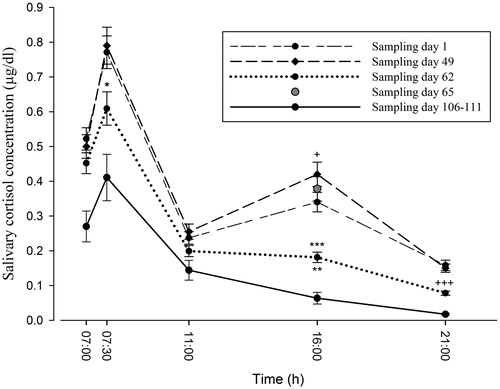
Significant differences in CAR values () were found among sampling days (GG-ε1.785 = 3.624; p = 0.037; η2 = 0.096). Pairwise comparisons with the Bonferroni correction showed that CAR values only significantly differed between sampling days 49 and 62 (p < 0.05). Significant pairwise differences in AUC values (GG-ε1.519 = 35.648; p < 0.001; η2 = 0.519) were found between all sampling days (p < 0.009). No significant interactions were found between the study variables and sex or age in these comparisons.
Table 3. Cortisol Awakening Response (CAR) and Area Under the Curve (AUC) from salivary cortisol concentration measurements.
Cortisol values at the different time points were also compared between sampling days 106–111 and the other sampling days in the eight participants tested on days 106–111, using the Student’s t-test for dependent samples. The values in these eight students on days 106–111 significantly differed (p = 0.023) from their own cortisol values measured on previous sampling days with the exception of the 11:00 h sample (p = 0.81).
Finally, in order to establish whether concentrations at 11:00 h (4 h after awakening) could be a marker of anticipatory stress, preparing the body for a stressful event (mock examination), we performed linear regressions in which the logarithmic value of the cortisol concentration at 11:00 h on the first, third and fifth sampling days (days 1, 49, 62 and 106–111, respectively) was the independent variable and the logarithmic value of the concentration at 16:00 h on the same days was the dependent variable. The salivary cortisol concentration at 16:00 h was predicted by the concentration at 11:00 h on day 1 (r2 = 0.400; β = 0.632; t = 4.761, p < 0.001 [0.483–1.202]) and day 49 (r2 = 0.251; β = 0.501; (t = 3.374, p < 0.002 [0.285–1.147]), when there was a mock examination, but not on day 62 [r2 = 0.117; p = 0.051] or days 106–111 [r2 = 0.001; p < 0.946] sampling days, when there was no mock examination.
Discussion
In the present study, medical graduates preparing for the national professional examination (MIR examination) showed high levels of anxiety and a progressive increase in self-perceived stress over the 7-month preparation period, evidenced by psychological test results and a significant increase in salivary cortisol concentration in both chronic (study and examination preparation) and acute (examination) situations.
These medical graduates were preparing for an examination that would determine their future career, and a poor result might preclude them from any specialist training position or even any post in the profession. Self-reported anxiety during the examination preparation showed higher levels in this group than in the reference population (Spielberger et al., Citation1982), particularly among the males (Duan et al., Citation2013). Perceived stress (Trujillo & González-Cabrera, Citation2007) also significantly increased as the examination day approached. These increases were correlated with a rise in cortisol release, both under the long term stressors and under acute events (mock examinations).
This situation allowed us to evaluate the effects of real-life acute and chronic stressful situations on the salivary cortisol concentrations of healthy subjects in a real-life setting of critical importance for their future personal and professional lives (Duan et al., Citation2013; Felmingham et al., Citation2012; Goldman-Mellor et al., Citation2012; González-Cabrera et al., Citation2012). Strict inclusion and exclusion criteria were rigorously applied, and considerable care was taken to ensure that the study participants underwent the same stressful conditions (Nakajima et al., Citation2012; Taverniers et al., Citation2011), strengthening the internal validity of this longitudinal study (Hewig et al., Citation2008; McFarlane et al., Citation2011). The circadian rhythm of cortisol release was represented graphically with five points (Nater et al., Citation2008), including the two time points (awakening and 30 min later) required to calculate the CAR (Clow et al., Citation2010; Law et al., Citation2013; Ranjit et al., Citation2005). This MIR preparation-examination appears to be a good model to study stress under chronic and acute real landmark circumstances. This aspect has been controversial in less critical academic situations (Stowell, Citation2003; Weekes et al., Citation2006).
The cortisol release measured at 16:00 h, immediately before a mock examination and therefore a situation of possible acute stress, was significantly higher on day 49 than on day 1. This was not an unexpected finding, because the mock examination on day 1 was one of a regular series of similar examinations that students took in the classroom on the same day every week, whereas the examination on day 49 was held at the same site in which they would take the real examination in 2 weeks time under identical conditions (). AUC values were higher on day 49 than on day 1; however, it should be noted that the AUC would have been influenced by the acute examination episode at 16:00 h, which would have altered the circadian rhythm of cortisol release. Thus, unlike the findings of other investigations (Vachon-Presseau et al., Citation2013), the increase in the AUC may have been caused more by acute than chronic stress (Nater et al., Citation2008; Pruessner et al., Citation2003b). CAR values were not significantly different between days 49 and 1 (). Besides the chronic stress generated during the 7-month preparation period, acute stress appears to have been increased (see ) by the proximity of the official examination and by the realistic simulation of the actual examination conditions (Rohleder et al., Citation2007).
An unexpected finding was the lower cortisol release immediately before the official examination (16:00 h on day 65), than immediately before the mock examination in the official examination hall (16.00 h on day 49). This may suggest that individuals learned from their experience of realistic examination-like conditions to offset their stress levels on the day of the actual examination. Other authors have described the benefits of training programmes to reduce stress and modulate the HPA axis response to an acute stress event (Burbeck et al., Citation2002; Iglesias et al., Citation2012).
The AUC and the CAR have been proposed as chronic stress markers (Clow et al., Citation2010; Nater et al., Citation2008). Our CAR results for chronic stress on days 1, 49 and 62 are consistent with previous findings of no significant changes in CAR values in the context of degree examinations (Gaab et al., Citation2006; Hewig et al., Citation2008), although they were reported to be increased in relation to oral examinations, which are more emotionally charged (Hewig et al., Citation2008). As noted above, given the combination of chronic and acute emotional stress evidenced in our participants by the questionnaire data, the CAR may not be sensitive to chronic stress in academic stress situations. Our findings are in agreement with results obtained in athletes under psychologically demanding conditions (Strahler et al., Citation2010). However, the differences in CAR values between the day with the highly-realistic mock examination (day 49) and the day of relaxation (day 62) suggest that the CAR may be a good indicator of anticipatory stress (Fries et al., Citation2009; Hewig et al., Citation2008; Kunz-Ebrecht et al., Citation2004). It has also been proposed that a physiological response to stress in the absence of actual sensory input, as observed on day 62, may be conditioned by the memory of previous experiences that left an emotional “footprint” (Gaab et al., Citation2006; Taverniers et al., Citation2011).
Our findings shed some light on the influence of the time interval between the CAR and the stressful event, i.e. the “CAR time window” (Fries et al., Citation2009). Rohleder et al. (Citation2007) studied participants in a ballroom dancing competition and reported that the interval between the CAR change and a stressful event could be longer than 24 h, as observed in the CAR values of our participants on day 62 (3 days before the official examination). Nevertheless, our study demonstrates that the increase in the CAR was greater when the stressful event was due on the same day, as on days 1 and 49 (window of 10–12 h) than when it was 3 days away (day 62; window of >24 h), and this difference was significant between days 1 and 62 (). On days 106–111, the participants (though reduced in number) returned to a baseline situation, given that they already knew their examination results and their final ranking order. As expected, the cortisol release values were lower than on the first three sampling days, suggesting a decrease in acute and chronic stress levels after the disappearance of the stressor (Nater et al., Citation2008; Strahler et al., Citation2010). The lower values on days 106–111 than on day 62, which were both characterized by an absence of acute stress, may be attributable not only to the difference in chronic stress (before versus after the official examination) but also to the presence of anticipatory stress on day 62 (Fries et al., Citation2009). However, the results for days 106–111 should be interpreted with caution, due to the reduced sample size (n = 8).
Our sample reflected the significant preponderance of females in medical training in Europe (Delgado et al., Citation2011; Elston, Citation2009). We observed no gender differences, although other authors have reported a greater cortisol release by males than females during acute stress situations in both real-life and laboratory settings (Lacey et al., Citation2000; Stroud et al., Citation2002; Weekes et al., Citation2006).
Despite our demanding exclusion criteria, which required participants to accept strict behavioral rules for 7 months, the sample size was similar to that in other studies on the issue addressed here (Berndt et al., Citation2012; Campisi et al., Citation2012; De Andrés-García et al., Citation2012; Gaab et al., Citation2006; McFarlane et al., Citation2011). Nevertheless, a larger number of participants would have increased the quality of our evidence. A strength of our study is that we obtained a cortisol profile based on five saliva samples/day (Nater et al., Citation2008; Strahler et al., Citation2010), although a more frequent sampling protocol would further elucidate the daily pattern. Besides the small sample size on the last sampling day (days 106–111), a further study weakness is that we did not control for the influence of the menstrual cycle on the release of cortisol in the women (Hellhammer et al., Citation2009). As well as the application of robust statistical procedures, a major study strength that supports the internal validity of our investigation was the rigorous study design. The extraordinary degree of cooperation offered by these young graduates allowed numerous potential confounders to be ruled out.
The main conclusions that can be drawn from our findings are: (1) the 7-month long preparation for a real examination of vital importance to the examinee produces a significant increase in anxiety levels, which is associated with changes in the circadian rhythm of cortisol release, in response not only to the preparation period but also to mock examinations, which act as acute stressors; (2) the AUC and CAR do not appear to be useful indicators of chronic stress in subjects simultaneously experiencing acute stressor effects; (3) the CAR may be a good indicator of anticipatory stress when the stressful event is on the same day as its measurement; (4) the return to baseline concentrations in a limited sample of participants at 10–15 days after learning their examination results may possibly reflect the end of stressful circumstances and (5) simulations may induce a learning process that can modulate the behavior of the HPA axis in real-life challenging situations.
Declaration of interest
The authors report no conflicts of interest. This study was supported by the Department of Biochemistry, Molecular Biology and Immunology III and Institute of Neuroscience, University of Granada, Spain.
Acknowledgements
The authors are grateful to the participating students for their generous cooperation and to Richard Davies for assistance with the English version.
Notes
1Medical Intern Resident (Médico Interno Residente).
References
- Arnetz BB, Ekman R. (2006). Stress in health and disease. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA
- Berndt S, Strahler J, Kirschbaum C, Rohleder N. (2012). Lower stress system activity and higher peripheral inflammation in competitive ballroom dancers. Biol Psychol 91:357–64
- Burbeck R, Coomber S, Robinson M, Tood C. (2002). Occupational stress in consultants in accident and emergency medicine: a national survey of levels of stress at work. Emerg Med J 9:234–8
- Campisi J, Bravo Y, Cole J, Gobeil K. (2012). Acute psychosocial stress differentially influences salivary endocrine and immune measures in undergraduate students. Physiol Behav 107:317–21
- Chrousos GP. (2009). Stress and disorders of the stress system. Nat Rev Endocrinol 5:374–81
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. (2010). The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev 35:97–103
- De Andrés-García S, Moya-Albiol L, González-Bono E. (2012). Salivary cortisol and immunoglobulin A: responses to stress as predictors of health complaints reported by caregivers of offspring with autistic spectrum disorder. Horm Behav 62:464–74
- Delgado A, Saletti-Cuesta L, Lopez-Fernandez LA, Luna JD, Mateo-Rodriguez, I. (2011). Gender and the professional career of primary care physicians in Andalucia (Spain). BMC Health Services Res 11:51–7
- Dickerson S, Kemeny M. (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 130:355–91
- Duan H, Yuan Y, Zhang L, Qin S, Zhang K, Buchanan TW, Wu J. (2013). Chronic stress exposure decreases the cortisol awakening response in healthy young men. Stress 16:630–7
- Elston MA. (2009). Women and medicine. London: Royal College of Physicians
- Felmingham K, Tranb T, Fongb W, Bryantb R. (2012). Sex differences in emotional memory consolidation: the effect of stress-induced salivary alpha-amylase and cortisol. Biol Psychol 89:539–44
- Fries F, Dettenborn L, Kirschbaum C. (2009). The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol 72:67–73
- Gaab J, Sonderegger L, Scherrer S, Ehlert U. (2006). Psychoneuroendocrine effects of cognitive-behavioral stress management in a naturalistic setting – a randomised controlled trial. Psychoneuroendocrinology 31:428–38
- Goldman-Mellor S, Hamer M, Steptoe A. (2012). Early-life stress and recurrent psychological distress over the lifecourse predict divergent cortisol reactivity patterns in adulthood. Psychoneuroendocrinology 37:1755–68
- González-Cabrera J, Fernández-Prada M, Molina-Ruano R, Blázquez A, Guillén-Solvas J, Peinado JM. (2012). Psychosocial risk at work, self-perceived stress and cortisol in saliva in a sample of emergency physicians in Granada. Emergencias 24:101–6
- Hellhammer D, Wüst S, Kudielka B. (2009). Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 34:163–71
- Hewig J, Schlotzd W, Gerhardsb F, Breitensteine C, Lürkenb A, Naumannc E. (2008). Associations of the cortisol awakening response (CAR) with cortical activation asymmetry during the course of an exam stress period. Psychoneuroendocrinology 33:83–91
- Hulme P, French J, Agrawal S. (2011). Changes in diurnal salivary cortisol levels in response to an acute stressor in healthy young adults. J Am Psychiatr Nurses Assoc 17:339–49
- Iglesias S, Azzara S, Argibay JC, Arnaiz M, Carpineta M, Granchetti H, Lagomarsino E. (2012). Psychological and physiological response of students to different types of stress management programs. Am J Health Promot 26:e149–57
- Kidd T, Hamer M, Steptoe A. (2011). Examining the association between adult attachment style and cortisol responses to acute stress. Psychoneuroendocrinology 36:771–9
- Kirschbaum C, Pirke KM, Hellhammer DH. (1993). The ‘Trier Social Stress Test’ -- a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81
- Kudielka B, Hellhammer DH, Würt S. (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology 34:2–18
- Kudielka BM, Hellhammer DH, Kirschbaum C. (2007). Ten years of research with the Trier Social Stress Test – Revisited. In: Harmon-Jones E, Winkielman P, editors. Social neuroscience: integrating biological and psychological explanations. New York: Guilford Press. p 56--83
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. (2004). Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology 29:516–28
- Lacey K, Zaharia MD, Griffiths J, Ravindran AV, Merali Z, Anisman H. (2000). A prospective study of neuroendocrine and immune alterations associated with the stress of an oral academic examination among graduate students. Psychoneuroendocrinology 25:339–56
- Law R, Hucklebridge F, Thorn L, Evans P, Clow A. (2013). State variation in the cortisol awakening response. Stress 16:483–92
- Lovell B, Moss M, Wetherell MA. (2012). With a little help from my friends: psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil 33:682–7
- McFarlane A, Barton C, Yehuda R, Wittert G. (2011). Cortisol response to acute trauma and risk of posttraumatic stress disorder. Psychoneuroendocrinology 36:720–7
- Michaud K, Matheson K, Kelly O, Anisman H. (2008). Impact of stressors in a natural context on release of cortisol in healthy adult humans: a meta-analysis. Stress 11:177–97
- Miller R, Plessow F, Rauh M, GrÂschl M. Kirschbaum C. (2013). Comparison of salivary cortisol as measured by different immunoassays and tandem mass spectrometry. Psychoneuroendocrinology 38:50–7
- Murphy L, Denis R, Ward CP, Tartar JL. (2010). Academic stress differentially influences perceived stress, salivary cortisol, and immunoglobulin-A in undergraduate students. Stress 13:365–70
- Nader N, Chrousos G, Kino T. (2010). Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab 21(5):277–86
- Nakajima Y, Takahashi T, Shetty V, Yamaguchi M. (2012). Patters of salivary cortisol levels can manifest work stress in emergency care providers. J Psysiol Sci 62:191–7
- Nater UM, Youngblood LS, Jones JF, Unger ER, Millerm AH, Reeves WC, Heim C. (2008). Alterations in diurnal salivary cortisol rhythm in a population-based sample of cases with chronic fatigue syndrome. Psychosom Med 70:298–305
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. (2003a). Two formulas for the computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–31
- Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. (2003b). Self-reported depressive symptoms and stress levels in healthy young men: associations with the cortisol response to awakening. Psychosom Med 65:92–9
- Ranjit N, Young E, Kaplan G. (2005). Material hardship alters the diurnal rhythm of salivary cortisol. Int J Epidemiol 34:1138–43
- Rohleder R, Beulen S, Chen E, Wolf J, Kirschbaum C. (2007). Stress on the dance floor: the cortisol stress response to social-evaluative threat in competitive ballroom dancers. Pers Soc Psychol Bull 33:69–85
- Spielberger CD, Gorsuch RL, Lushene R. (1982). Manual del Cuestionario de Ansiedad Estado/Rasgo (STAI). Madrid, España: TEA Ediciones
- Stauble M, Thompson L, Morgan G. (2013). Increases in cortisol are positively associated with gains in encoding and maintenance working memory performance in young men. Stress 16(4):402–10
- Stowell J. (2003). Use and abuse of academic examinations in stress research. Psychosom Med 65:1055–7
- Strahler K, Ehrlenspiel F, Heene M, Brand R. (2010). Competitive anxiety and cortisol awakening response in the week leading up to a competition. J Sport Exerc Psychol 11:148–54
- Stroud LR, Salovey P, Epel ES. (2002). Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry 52:318–27
- Takatsuji K, Sugimoto Y, Ishizaki S, Ozaki Y, Matsuyama E, Yamaguchi Y. (2008). The effects of examination stress on salivary cortisol, immunoglobulin A, and chromogranina in nursing students. Biomed Res 29:221–4
- Taverniers J, Smeets T, Lo Bue S, Syroit J, Van Ruysseveldt J, Pattyn N, Von Grumbkow J. (2011). Visuo-spatial path learning, stress, and cortisol secretion following military cadets’ first parachute jump: the effect of increasing task complexity. Cogn Affect Behav Neurosci 11:332–43
- Taylor MK, Reis JP, Sausen KP, Padilla GA, Marham AE, Potterat EG, Drummond SPA. (2008). Trait anxiety and salivary cortisol during free living and military stress. Aviat Space Env Med 79:129–35
- Trujillo HM, González-Cabrera J. (2007). Propiedades psicométricas de la versión española de la “Escala de estrés percibido” (EEP). Psicología Conductual 15(3):457–77
- Vachon-Presseau E, Roy M, Martel MO, Caron E, Marin MF, Chen J, Albouy G, et al. (2013). The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain 136:815–27
- Vedhara K, Hyde J, Gilchrist D, Tytherleigh M, Plummer S. (2000). Acute stress, memory, attention and cortisol. Psychoneuroendocrinology 25:535–49
- Walker S, O’Connor D, Schaefer A, Talbot D, Hendrickx H. (2011). The cortisol awakening response: associations with trait anxiety and stress reactivity. Pers Individ Dif 51:123–7
- Weekes N, Lewis R, Patel F, Garrison-Jakel J, Berger D, Lupien S. (2006). Examination stress as an ecological inducer of cortisol and psychological responses to stress in undergraduate students. Stress 9:199–206
