Abstract
Acute exposure to severe stressors causes marked activation of the hypothalamic–pituitary–adrenal (HPA) axis that is reflected on the day after higher resting levels of HPA hormones and sensitization of the HPA response to novel (heterotypic) stressors. However, whether a single exposure to a severe stressor or daily repeated exposure to the same (homotypic) stressor modifies these responses to the same extent has not been studied. In this experiment, we studied this issue in adult male Sprague-Dawley rats daily exposed for seven days to a severe stressor such as immobilization on boards (IMO). A first exposure to 1 h IMO resulted in a marked activation of the HPA axis as reflected in plasma levels of adrenocorticotropic hormone (ACTH) and corticosterone, and such activation was significantly reduced after the seventh IMO. On the day after the first IMO, higher resting levels of ACTH and corticosterone and sensitization of their responses to a short exposure to an open-field (OF) were observed, together with a marked hypoactivity in this environment. Repeated exposure to IMO partially reduced hypoactivity, the increase in resting levels of HPA hormones and the ACTH responsiveness to the OF on the day after the last exposure to IMO. In contrast, corticosterone response was gradually increased, suggesting partial dissociation from ACTH. These results indicate that daily repeated exposure to the same stressor partially reduced the HPA response to the homotypic stressor as well as the sensitization of HPA axis activity observed the day after chronic stress cessation.
Introduction
Exposure to acute stressors triggers a myriad of immediate physiological and behavioral changes, including the activation of the hypothalamic–pituitary–adrenal (HPA) and sympathetic-medullo-adrenal axes, the two prototypical stress systems. In addition to these immediate effects, acute severe stressors result in longer-lasting effects that persist for at least some days after stress (Armario et al., Citation2008). More specifically, high-intensity electric shocks (either foot-shocks or tail-shocks) or different types of restraint or immobilization (IMO) procedures caused marked and prolonged activation of the HPA axis (Fleshner et al., Citation1995; García et al., Citation2000; Márquez et al., Citation2002) and inhibition of active behavior in novel environments for the next hours (i.e. Armario et al., Citation1991; Berridge & Dunn, Citation1989; Lehnert et al., Citation1984; Plaznik et al., Citation1988). Importantly, some residual effects persist for 24 h or a few days and are reflected in higher resting levels of corticosterone (Fleshner et al., Citation1995; Martí et al., Citation1993; Ottenweller et al., Citation1992, Citation1994; Servatius et al., Citation1994), hypo-activity in novel environments (Armario et al., Citation1991; Kennett et al., Citation1985) and in some studies, enhanced anxiety (Calvo et al., Citation1998; Cancela et al., Citation1995; Steenbergen et al., Citation1990).
Moreover, a single exposure to severe stressors has two other important long-lasting consequences. First, when confronted again with the same stressor, a reduced HPA response is observed that is positively related to the intensity of the stressor (Armario et al., Citation2004; Martí et al., Citation2001). Second, the reduction of the HPA response to the homotypic stressor appears to be a learning-like process as the HPA response to novel (heterotypic) stressors, including the exposure to novel environments or the administration of a low dose of endotoxin, is exacerbated (Belda et al., Citation2008, Citation2012; Gagliano et al., Citation2008; Johnson et al., Citation2002; Martí et al., Citation2001; O’Connor et al., Citation2003, Citation2004). This sensitized HPA responsiveness is observed despite the prolonged release of corticosterone on the preceding day and the presence of high resting levels of corticosterone before the challenging stressor, which should result in higher glucocorticoid negative feedback. Interestingly, long-lasting HPA sensitization caused by tail-shock stress appears to be associated to impaired negative glucocorticoid feedback (O’Connor et al., Citation2003). This scenario is reminiscent of the theory of stress-induced facilitation of the HPA axis, elaborated some decades ago by Dallman & Jones (Citation1973), which posed that stress exposure would induce a facilitation of the HPA response to further stressors that would overcome negative glucocorticoid feedback. In conclusion, a single exposure to some severe stressors can result in a reduced HPA response to the homotypic stressor, whereas the response to novel stressors becomes sensitized.
How all these changes reflecting the impact of a single exposure to severe stressors are affected by repeated exposure to the same situation? It is well-known that repeated confrontation with a homotypic stressor usually leads to a reduction of the response of the HPA axis and other physiological variables that has been termed adaptation or habituation (Armario, Citation2006; Grissom & Bhatnagar, Citation2009; Kvetnansky et al., Citation1984, Citation2009; Martí & Armario, Citation1998). Hence, repeated exposure to the same stressor appears to act like a single exposure regarding the capacity to reduce the HPA response. Similarly, this reduced response to a daily repeated stressor is related to the repeated experience with the particular stressor, as the response to heterotypic stressors is normal or enhanced (Armario, Citation2006; Bhatnagar & Vining, Citation2003; Grissom & Bhatnagar, Citation2009; Kvetnansky et al., Citation2009; Martí & Armario, Citation1998; Weinberg et al., Citation2009). Studies exploring effects of chronic unpredictable stress on the HPA response to novel stressors suggest varying degrees of habituation or facilitation (Armario et al., Citation1985; Marin et al., Citation2007; Ostrander et al., Citation2006; Ulrich-Lai et al., Citation2007).
The precise reasons for the inconsistent effects of chronic stress on the HPA response to heterotypic stressors (Armario et al., Citation1988; Cox et al., Citation1985; Hashimoto et al., Citation1988; Hauger et al., Citation1990; Rabasa et al., Citation2011) remain elusive, as a single exposure to high intensity stressors consistently induces heterotypic HPA sensitization (see above). It is possible that the expression of chronic stress-induced sensitization would depend on the characteristics of the challenging heterotypic stressor (Chen et al., Citation2008; Spiga et al., Citation2009). Alternatively, chronic stress-induced HPA sensitization might be only observed with chronic stressors having certain particular characteristics in terms of quality, intensity or duration of daily exposure. Given these caveats, it is critical to determine whether repeated experience with a particular stressor can alter not only the response to the same stressor but also the other typical consequences described previously (i.e. altered resting levels of HPA hormones and heterotypic sensitization). In other words, whether repeated exposure to the same stressor result not only in a reduced HPA response to the same stressor but also in a lower impact on all other consequences of the stressor, including behavioral inhibition. This is an important question for two main reasons. First, from a theoretical perspective, repeated exposure to severe stressors could result in progressively higher allostatic load or, on the contrary, on lower impact because of successful development of adaptive mechanisms. Second, in translational medicine, it is critical to determine the impact of an isolated stressful event relative to repeated exposure to traumatic situations on psychiatric diseases, particularly with respect to post-traumatic stress disorder. It is still unclear whether a prior history of trauma exposure can result in vulnerability or resilience (Breslau et al., Citation1999, Citation2008; Mollica et al., Citation1998; Yehuda et al., Citation1995).
The aims of this work was to study how daily repeated exposure to a severe stressor such as IMO can alter some of their neuroendocrine and behavioral consequences measured on the day after exposure. More precisely, we studied changes in resting levels of HPA hormones, as well as behavioral and hormonal (HPA axis) responsiveness to an open-field (OF) as a heterotypic emotional stressor.
Material and methods
Animals
We used male Sprague-Dawley rats, 80 d old (506 ± 42 g), obtained from the breeding centre of the Universitat Autònoma de Barcelona. After arrival at the laboratory, they were pair-housed in standard conditions of temperature (21 ± 1 °C) on a 12-h light/12-h dark schedule (lights on at 0800 h). Food and water were available ad libitum. The experimental protocol was approved by the Committee of Ethics of the Universitat Autònoma de Barcelona, followed the “Principles of laboratory animal care” and was carried out in accordance with the European Communities Council Directives (86/609/EEC).
General procedure
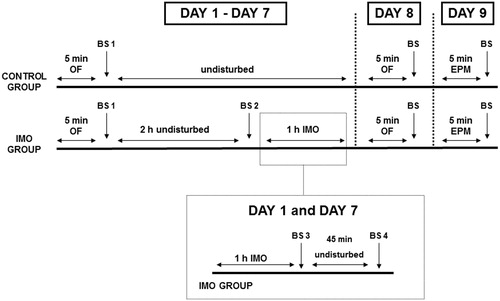
Animals were habituated to handling for five non-consecutive days for approximately 2 min a day, starting one day after their arrival. Blood samples were taken the day after the last handling, to habituate animals to the tail nick procedure. Two days before starting the chronic stress procedure, a blood sample in resting conditions was taken. Afterwards, animals were assigned at random into control (n = 10) and chronic IMO (n = 10) groups. Chronic IMO animals were stressed by taping their four limbs to metal mounts attached to a board (Gagliano et al., Citation2008). Head movements were restricted with two plastic pieces (7 cm × 6 cm) placed in each side of the head, and the body was secured to the board by means of a piece of plastic cloth (10 cm wide) that spanned the trunk region, maintained in place with Velcro. All the experimental procedures, including behavioral testing, stress and blood sampling, were performed in the morning.
The chronic IMO procedure (see ) lasted for seven consecutive days during which all animals were exposed daily to a rectangular OF for 5 min. Immediately after the OF exposure, blood samples were taken. Then, both control and IMO rats were returned to the vivarium, but IMO rats were taken again 2 h later to be blood sampled under resting conditions, just before being immobilized for 1 h. Resting blood samples were not taken prior to OF exposure in order to prevent any interference with behavioral and hormonal responses to the OF. Animals were transported from the vivarium to experimental situation in transparent transportation cages (not in their regular home-cage). In the chronic IMO group, blood samples were also taken on day 1 and day 7, immediately after the end of IMO and at 45 min post-IMO (R45) in order to evaluate the effect of repeated exposure to IMO on the response to the homotypic stressor. On the day after the last exposure to IMO, all animals were again tested in the OF for 5 min, and the following day (that is, 2 d after the last IMO), all animals were tested in the elevated plus-maze (EPM) for 5 min. Immediately after that, a blood sample was taken to measure adrenocorticotropic hormone (ACTH) and corticosterone responses to the EPM.
Blood sampling
The tail-nick consisted of gently wrapping the animals with a cloth, making a 2 mm incision at the end of one of the tail veins and then massaging the tail while collecting, within 2 min, 300 µl of blood into ice-cold ethylenediaminetetraacetic acid capillary tubes (Sarsted, Granollers, Spain). The cage-mates were sampled simultaneously (two experimenters were sampling at the same time and a third was gently holding the two rats). This procedure is extensively used in our laboratory because low resting levels of hormones are obtained (e.g. Belda et al., Citation2004; Vahl et al., Citation2005).
Exposure to the OF and the EPM
The OFs were rectangular plastic cages (56 cm × 36.5 cm × 31 cm) opened at the top. A white curtain surrounded the cages to minimize exposure to external stimuli and a red 25 W bulb was placed 1.5 m above the centre of the floor of the OF. Animals were transported from the vivarium to the room were the OFs were placed in small black cages. The two animals in the same home-cage were simultaneously exposed in the same room to two different OFs. Behavior was videotaped from the top for subsequent manual analysis of horizontal activity (areas crossed) by an experimenter unaware of the treatment. The floor of the OF was divided into 12 areas (four central and eight peripheral), and it was considered that an area was crossed when the animal cross it with the four paws. The apparatus was cleaned carefully between animals with a water solution containing ethanol (5%, v/v).
The EPM, adapted from Pellow & File (Citation1986), consisted of four black wooden arms at right angles to each other, connected to a central square (10 cm2) to form the shape of a plus sign and elevated 50 cm above the floor. Each arm was 46 cm long and 10 cm wide. Two of opposite arms had high walls (enclosed arms, 42 cm high), whereas the other two were the open arms that had a 0.5 cm ridge to provide an additional grip. The rat was placed facing a closed arm, and the subject was considered to be in a given arm when all the four paws were inside. The procedure lasted 5 min. A black curtain surrounded the EPM to minimize exposure to external stimuli, and a red bulb (25 W) was placed 1.5 m above the centre of the floor of the apparatus. The cage-mates were simultaneously tested in two separate rooms under the same conditions. Behavior was videotaped from the top for subsequent manual analysis by an experimenter unaware of the treatment. Number of entries into closed and open arms and time spent in each arm were measured. The apparatus was cleaned carefully between animals with a water solution containing ethanol (5%, v/v).
Biochemical analysis
Plasma ACTH and corticosterone levels were determined by double-antibody radioimmunoassay (RIA). In brief, ACTH RIA used 125I-ACTH (PerkinElmer Life Science, Boston, MA) as the tracer, rat synthetic ACTH 1–39 (Sigma, Barcelona, Spain) as the standard and an antibody raised against rat ACTH (rb7) kindly provided by Dr. W.C. Engeland (Department of Surgery, University of Minnesota, Minneapolis, MN). The characteristics of the primary antibody have been described previously (Engeland et al., Citation1989) and we followed a non-equilibrium procedure. First antibody was added on day 1 and 125I-ACTH on day 2. After one additional 18 h incubation period, the second antibody (goat anti-rabbit) was added. Corticosterone RIA used 125I-corticosterone-carboximethyloxime-tyrosine-methylester (ICN-Biolink 2000, Barcelona, Spain), synthetic corticosterone (Sigma) as the standard and an antibody raised in rabbits against corticosterone–carboximethyloxime–bovine serum albumin (BSA) kindly provided by Dr. G. Makara (Inst Exp Med, Budapest, Hungary). After an 18 h incubation period, the second antibody (goat anti-rabbit) was added. The characteristics of the primary antibody and the basic RIA procedure have been described previously (Zelena et al., Citation2003). All samples to be statistically compared were run in the same assay to avoid inter-assay variability. The intra-assay coefficient of variation was less than 8% for ACTH and corticosterone. The sensitivity of the assays was 12.5 pg/ml for ACTH and 1 ng/ml for corticosterone.
Statistical analysis
The Statistical Package for Social Science program version 17 for Windows was used (IBM Corporation, Armonk, NY). To study behavioral and hormonal data in the OF, a generalized estimated equations model (GEE) (Hardin & Hilbe, Citation2003) was used, with one between-subjects factor (GROUP, two levels) and one within-subjects factor (DAY, eight levels). To analyze behavioral and hormonal data in the EPM, the Student t-test was used. To study hormonal responses to IMO, a GEE analyses were performed with two within-subjects factors: DAY (two levels: day 1 and day 7) and TIME (two levels: post-IMO and R45 min). To study resting hormonal levels during the chronic IMO, a GEE was performed with DAY (seven levels) as the one within-subjects factor. In all cases, if statistically significant interactions were found, additional pairwise comparisons were made. Instead of the general linear model (analysis of variance, ANOVA), we used the generalized linear model for repeated measures (GEE) because this tool is more flexible than the general linear model for the following reasons: (1) the method can be used with several multiple types of data distribution (normal, binomial, Poisson, gamma or inverse-Gaussian), (2) the analysis can be run with missing data in the repeated-measures data and (3) homogeneity of variance is not required. In this analysis, to study the significance of the effects, we use the Wald statistic instead of the “F”. Notably, analysis by ANOVA revealed similar results.
Results
shows the effect of chronic IMO on resting levels of ACTH () and corticosterone () over the days. The analysis of ACTH revealed a significant effect for day (Wald X2(6) = 122.43, p < 0.001). Further comparisons showed that the increase in ACTH levels was always significant with respect to day 1, except on day 7. At this time, ACTH response was lower than that observed on day 2 (p < 0.05). The analysis of resting corticosterone levels revealed a significant effect for day (Wald X2(6) = 160.72, p < 0.001), but the pattern was more erratic than ACTH (see particular comparisons in ). Plasma levels obtained on day 1 2 h after exposure to the OF appears to be true resting levels as in controls, and these levels were similar to those observed before starting the experimental procedure (mean and standard error are shown): 14 ± 3 ng/ml before chronic stress versus 12 ± 3 ng/ml the first day of the chronic stress protocol.
Figure 2. Effect of daily exposure to IMO on plasma levels of ACTH and corticosterone taken daily under resting conditions (A and B) or after 5 min of exposure to the open-field (C and D). Means and SEM are shown (n = 10 per group). Resting levels were only obtained in chronic IMO rats: #p = 0.06, $p < 0.05, $$p < 0.001, $$$p < 0.001 vs day 1; +p < 0.05, ++p < 0.01 vs day 2. The response to the open-field was obtained in both stress-naïve (control) and chronic IMO rats. *p < 0.05, **p < 0.01, *** p < 0.001 vs control group; +p < 0.05, ++p < 0.01 vs corresponding values on day 2.
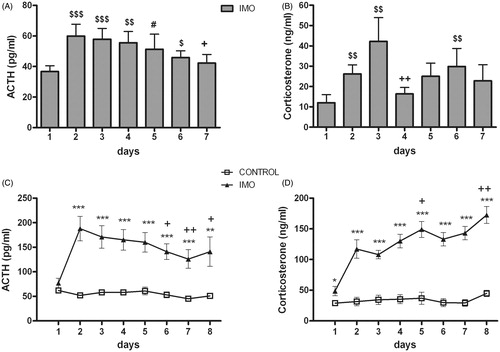
Exposure to IMO produced an endocrine sensitization in response to the OF, which acted as a mild stressor. The analysis of ACTH () levels showed a significant effect for group (Wald X2(1) = 24.27, p < 0.001); day (Wald X2(7) = 198.35, p < 0.001) and the group x day interaction (Wald X2(7) = 168.48, p < 0.001). Decomposition of the interaction indicated that IMO animals showed higher ACTH levels than controls during all exposures (day 2 from day 7, p < 0.001 and day 8, p < 0.01). Further comparisons revealed that ACTH response of IMO group on day 6 (p < 0.05), day 7 (p < 0.01) and day 8 (p < 0.05) was statistically different from day 2, indicating that endocrine sensitization attenuated over the days. Regarding corticosterone response to the OF, the statistical analysis showed significant effects for group (Wald X2(1) = 67.84, p < 0.001), day (Wald X2(7) = 225.60, p < 0.001) and the group x day interaction (Wald X2(7) = 166.23, p < 0.001). The decomposition of the interaction () showed that IMO animals presented higher corticosterone levels than controls on all days (day 1, p < 0.05; day 2 to day 8, p < 0.001). In contrast to the ACTH response, the corticosterone response of the chronic IMO group increased over the days: corticosterone levels were higher on day 5 (p < 0.05) and day 8 (p < 0.001) than on day 2.
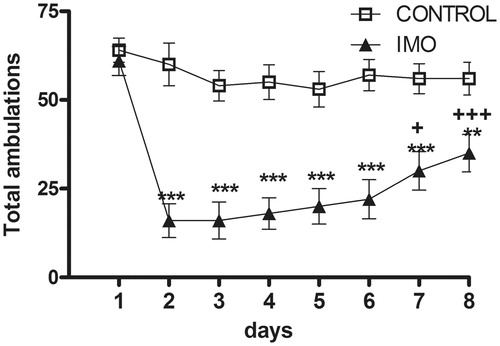
As shows, chronic stress decreased horizontal activity (total number of areas crossed, including central and peripheral). Statistical analysis revealed a significant effect for group (Wald X2(1) = 31.07, p < 0.001); day (Wald X2(7) = 113.03, p < 0.001); and the group x day interaction (Wald X2(7) = 46.05, p < 0.001). The decomposition of the interaction revealed that IMO animals showed hypo-activity compared to controls from day 2 to day 8 (day 2 to 7, p < 0.001 and day 8, p < 0.01). IMO-induced hypo-activity was attenuated across days (day 2 vs day 7, p < 0.05, and vs day 8, p < 0.001) although never reached control levels. Finally, when separately analyzing central and peripheral ambulations, the data showed the same general pattern of results (data not shown).
Figure 3. Total number of areas crossed (central and peripheral) during the 5 min exposure to the open-field in control and chronic IMO groups. Means and SEM are shown (n = 10 per group). The open-field exposure occurred each day 2 h prior to IMO. On day 1, animals had not been stressed prior to the open-field exposure and therefore the values represent the baseline activity. **p < 0.01, ***p < 0.001 vs control group; +p < 0.05, +++p < 0.001 vs corresponding values on day 2.
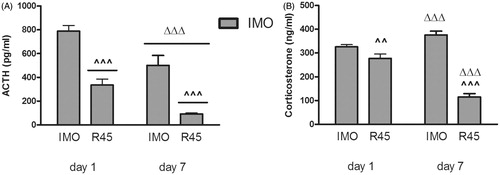
When the effect of prior chronic IMO on the HPA response to the homotypic stressor was studied (), the statistical analysis of plasma levels of ACTH revealed significant effects for day (Wald X2(1) = 23.70, p < 0.001) and time (Wald X2(1) = 72.08, p < 0.001), whereas the day × time interaction was not significant. After repeated exposure to IMO, ACTH levels decreased not only immediately after IMO but also at 45 min post-IMO (, p < 0.001 in the two cases). Regarding plasma corticosterone (), the statistical analysis showed significant effects for day (Wald X2(1) = 70.57, p < 0.001), time (Wald X2(1) = 108.45, p < 0.001) and the day × time interaction (Wald X2(1) = 92.78, p < 0.001). The decomposition of the interaction showed that corticosterone levels increased on day 7 compared with day 1 immediately after IMO (p < 0.001), whereas a significant decrease was observed at 45 min post-IMO (p < 0.001).
Figure 4. Plasma levels of ACTH (A) and corticosterone (B) immediately after 1 h of IMO and at 45 min post-IMO (R45) on day 1 (first exposure) and day 7 (last exposure). Means and SEM are shown (n = 10 per group). ^^p < 0.01, ^^^p < 0.001 vs post-IMO; ΔΔΔp < 0.001 vs day 1.
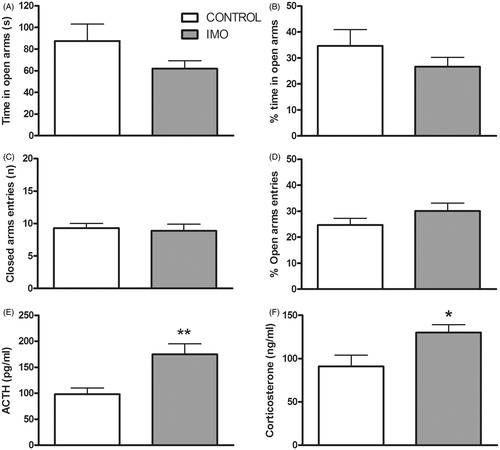
The statistical analysis of EPM behavior 48 h after the last stress exposure revealed no statistical difference between control and IMO groups in any variable (). The statistical analysis of ACTH () and corticosterone () responses to the EPM indicated significant mean differences for the two hormones, with a greater response in chronic IMO animals (t(20) = −3.21, p < 0.01 and t(20) = −2.59, p < 0.05, respectively).
Figure 5. Effects of prior exposure to chronic IMO on behavior and endocrine response in the EPM. Animals were exposed for 5 min to the EPM 48 h after the last IMO. Means and SEM are shown (n = 10 per group). Time spent in open arms (A), percent time spent in open arms (B), percent open arm entries (C), number of closed arm entries (D) and plasma levels of ACTH (E) and corticosterone (F). *p < 0.05, **p < 0.01 vs control group.
Discussion
The aims of this work were to characterize how IMO-induced changes in HPA function and behavior are modified over repeated exposure to the same stressor. To this end, a control group and a chronic IMO group were included. The results showed that the first exposure to IMO caused increased resting levels of HPA hormones, inhibition of activity in an OF and enhanced HPA responsiveness to this environment. After repeated experience with IMO, all the above changes were partially reduced, except plasma corticosterone responsiveness to the OF that progressively increased.
The first exposure to IMO resulted in higher resting levels of ACTH and corticosterone on the next morning, together with greater ACTH and corticosterone responses to a heterotypic stressor (exposure to an OF). Moreover, inhibition of OF activity was also found. All these changes are in complete agreement with previously reported data. Changes in resting levels of corticosterone in the morning of the first days after acute exposure to IMO and other severe stressors have been consistently found (Belda et al., Citation2012; Fleshner et al., Citation1995; Martí et al., Citation1993; Ottenweller et al., Citation1992, Citation1994; Servatius et al., Citation1994). Moreover, a single exposure to IMO or tail-shocks induced a sensitization of the HPA response to additional acute stressors (Belda et al., Citation2008; Citation2012; Gagliano et al., Citation2008; Johnson et al., Citation2002; O’Connor et al., Citation2003, Citation2004). Acute exposure to severe stressors has also been found to induce marked hypoactivity that can be still observed on the day after the stressor (Armario et al., Citation1991; Berridge & Dunn, Citation1989; Lehnert et al., Citation1984; Plaznik et al., Citation1988).
Although the above pattern of effects of acute exposure to severe stressors is well-characterized, it is still unclear how this pattern can change after repeated exposure to the same stressor. Our results demonstrate that upon repeated exposure to IMO, most of the changes observed after the first exposure to the stressor were partially maintained, although the magnitude of some of the changes decreased. Animals exposed to IMO for the first time showed a strong activation of the HPA axis, but this activation was markedly reduced after the seventh IMO, confirming adaptation/habituation to the repeated stressor (Armario et al., Citation1988; García et al., Citation2000; Irwin & Hauger, Citation1988; Márquez et al., Citation2004; Rabasa et al., Citation2011). Although the post-IMO (R45) levels of corticosterone did reflect the adaptation of ACTH observed immediately after the termination of IMO, not surprisingly (Armario et al., Citation1988; García et al., Citation2000; Márquez et al., Citation2004; Rabasa et al., Citation2011), corticosterone levels just after IMO were higher on day 7 than day 1. This repeatedly observed finding of higher plasma levels of corticosterone just after the stressor in chronic IMO rats is explained by the fact that plasma corticosterone just after exposure to a severe stressor does not reflect actual ACTH levels. Under these conditions, the capability of the adrenal to respond to ACTH is completely saturated even with the levels of ACTH observed after partial adaptation. Thus, plasma corticosterone is mainly a reflection of the increase in adrenal mass typically observed after chronic stress (Márquez et al., Citation2004) and the enhanced maximum response to circulating ACTH (i.e. Armario et al., Citation1988; Ulrich-Lai et al., Citation2006).
In chronic IMO rats, high resting levels of ACTH were observed in the morning that progressively returned ensuing days to those of stress-naïve rats. Corticosterone followed the same pattern, although the changes were more erratic. This is likely to be due to the clearly pulsatile secretion of corticosterone (Atkinson et al., Citation2006) that makes it difficult to reliably evaluate the actual corticosterone secretion in individual rats or in small size samples. Only Natelson’s group has studied resting corticosterone levels after chronic intermittent exposure to another severe stressor (high intensity tail-shocks), finding maintenance of high resting levels after repeated stress (Ottenweller et al., Citation1992) or return to pre-stress levels (Servatius et al., Citation1994). It is unclear whether the inconsistencies are due to pulsatile secretion or differences in sensitivity to stress between different cohorts of rats.
Sensitization of both ACTH and corticosterone responses to the heterotypic stressor (OF) persisted over days, but a small decline was observed in ACTH, in striking contrast to increased plasma corticosterone. As plasma corticosterone usually reaches maximum level at 15 min after initial exposure to brief stressors (Le Mevel et al., Citation1979), the fact that stress-induced sensitization was also noted in plasma corticosterone as soon as 5 min after initial exposure to the OF strongly suggests that sensitization could be also reflected in faster corticosterone synthesis and release after stress (Sakellaris & Vernikos-Danellis, Citation1975; Vernikos et al., Citation1982). This effect may specifically affect the adrenal cortex and increase over days, in contrast to the more central sensitization reflected in plasma ACTH. Although humoral factors other than ACTH may contribute to adrenocortical sensitization, this could be mediated by the neural innervation of the adrenal (Ulrich-Lai et al., Citation2006). Further studies are needed to precisely characterize the dissociation between the two HPA axis hormones.
To our knowledge, this is the first study directly comparing how single and daily repeated exposure to the same stressor affects the sensitization of the HPA response to a novel heterotypic emotional stressor. These results are in contrast to those reported by Hauger et al. (Citation1990) who reported a progressively greater sensitization of the ACTH response to ether in animals repeatedly exposed to restraint, whereas the corticosterone response gradually decreased. As ether is a very particular systemic stressor, the discrepancies may be related to the type of challenging stressor used, a topic that remains to be further explored.
It is of note that sensitization of the HPA axis after a single exposure to stress has been always observed using low intensity and short duration heterotypic stressors, mainly exposure to novel environments or a low dose of endotoxin (Belda et al., Citation2008, Citation2012; Gagliano et al., Citation2008; Johnson et al., Citation2002; O’Connor et al., Citation2003, Citation2004). The intensity and duration of the heterotypic stressors may be important when studying how a history of chronic stress can alter the response to novel stressors. This fact could contribute to explain, at least in part, the controversial results in the literature regarding HPA response to heterotypic stressors after chronic repeated stress (see Dallman et al., Citation1992; Martí & Armario, Citation1998).
The precise mechanisms involved in stress-induced heterotypic HPA sensitization are not well-known, although the evidence clearly point to supra-pituitary processes. Acute stress-induced sensitization is at least, in part, explained by impaired glucocorticoid negative feedback (O’Connor et al., Citation2003) and is paralleled by a greater activation of the paraventricular nucleus of the hypothalamus (O’Connor et al., Citation2004), the key nucleus in the regulation of the HPA axis. Other recent studies have demonstrated that neither glucocorticoids nor type 1 corticotropin-releasing-hormone (CRH) receptors appears to be involved in acute IMO-induced HPA sensitization (Belda et al., Citation2012). Nevertheless, acute and chronic restraint increased ACTH response to acute noise, and this effect is blocked by a specific antagonist of the vasopressin AV1b (or AV3) receptors (Spiga et al., Citation2009). As the ACTH response to exogenous vasopressin administration was normal, it appears that sensitization is associated to enhanced vasopressin release into the pituitary portal blood. However, the role of vasopressin in chronic stress-induced HPA sensitization is still controversial (Chen et al., Citation2008). Chronic stress-induced HPA sensitization may involve the posterior, but not the anterior, paraventricular nucleus of the thalamus (Bhatnagar & Dallman, Citation1998; Fernandes et al., Citation2002), whereas orexin projections participate in the induction, but not the expression, of the phenomenon (Heydendael et al., Citation2011).
The typical inhibition of activity in the OF caused by a first exposure to IMO on the day after was partially reduced by repeated exposure to the stressor. This is in accordance with our own previous results with chronic IMO (Pol et al., Citation1992) and also with some results from other laboratories that used different types of stressors and/or different behavioral consequences, including changes in anxiety-like behavior (Cancela et al., Citation1995; Kennett et al., Citation1985, Citation1986; Netto et al., Citation2002). Although there is evidence that long-lasting (days) IMO-induced inhibition of activity in novel environments and HPA sensitization follow a different time-course (Gagliano et al., Citation2008), these data show a parallelism in the consequences of repeated exposure to IMO on ACTH (not corticosterone) and behavioral inhibition, suggesting a reduced impact of repeated versus single exposure. Regarding the possible neurochemical mechanisms involved in IMO-induced behavioral inhibition, there is some evidence that inhibition of activity caused by some severe stressors is likely to be related to noradrenaline depletion in some brain areas. Both behavioral inhibition noradrenaline depletions can be prevented by supplementing the diet with tyrosine, the precursor of noradrenaline synthesis (Lehnert et al., Citation1984; Reinstein et al., Citation1984). Similarly, a previous history of chronic stress protects from acute-stress induced catecholamine depletion because of the increased biosynthetic capability of the adrenal medulla and central noradrenergic areas (Anisman & Larry, Citation1985; Kvetnansky et al., Citation2009; Stone & McCarty, Citation1983; Weiss et al., Citation1975) and also protects from behavioral inhibition (i.e. Weiss et al., Citation1975). The issue of the non-specific protection offered by a prior history of chronic stress and the types of behavioral and physiological responses affected has been scarcely studied in the past decades despite its critical role in the vulnerability-resilience dilemma.
To further confirm heterotypic sensitization after chronic stress, control and chronic IMO rats were exposed to the EPM 2 days after the last exposure to IMO. Although HPA sensitization was again observed in chronic IMO rats, neither hypoactivity nor any other alteration in EPM behavior was observed in those animals. These data may suggest that after chronic IMO, HPA sensitization is longer-lasting than hypoactivity. Moreover, no evidence for enhanced anxiety after chronic IMO was obtained. This is not particularly surprising as the literature regarding the influence of daily repeated exposure to the same stressor on anxiety-like behavior is controversial (i.e. Cancela et al., Citation1995; Kim & Han, Citation2006; Pawlak et al., Citation2003; Rabasa et al., Citation2011; Vyas et al., Citation2004).
In order to study resting levels of HPA hormones and their responsiveness to novel stressors over the days in the same subjects, we used a within-subjects experimental design that involves exposure of the animals daily to the OF and sampling blood every day under resting conditions and after stress. To avoid the interference of blood sampling just before exposure to the OF with the behavioral and endocrine response, we took samples just after the OF and then 2 h later to obtain presumably resting levels. This time was chosen because there is ample evidence that after exposure to mild and short-duration stressors, this time is enough to return plasma levels of HPA hormones to resting conditions (i.e. García et al., Citation2000; Ma & Lightman, Citation1998; Wotjak et al., Citation1998). In fact, plasma levels of corticosterone obtained 2 h after OF exposure in control animals did not differ from those obtained under strictly resting conditions 2 d before starting the experimental procedures, thus supporting the assumption. Another caveat is the extent to which the combination of two stressors (OF exposure and IMO) may interfere with the process of adaptation/habituation to each one. We consider this unlikely for several reasons. First, no change over the days in behavioral and HPA responsiveness to the OF was found in control animals, thus indicating that no habituation to the OF occurred in controls. This is in accordance with our previous results (Gagliano et al., Citation2008) and other reports (Hennessy et al., Citation1977; Osborne & Seggie, Citation1980) suggesting that habituation to mild stressors is not easy to achieve under certain conditions. Second, prior exposure to a heterotypic stressor (pedestal) has been reported to accentuate rather than reduce apparent habituation of the HPA axis to the homotypic stressor, likely because of enhanced negative glucocorticoid feedback due to pedestal-induced corticosterone release (Pace et al., Citation2001). Finally, we have unpublished data demonstrating that the reduction of the HPA response to IMO after a single previous experience with the same stressor is not affected by exposure to an OF just before IMO.
In summary, these results demonstrate that repeated exposure to IMO resulted in reduced response of the HPA axis to the homotypic stressor and lower behavioral impact on the day after. The higher resting levels of ACTH and corticosterone typically observed on the day after as well as the sensitization of the ACTH response to emotional heterotypic stressors partially decreased over the days of IMO exposure, but corticosterone response to heterotypic stressors increased, suggesting a partial dissociation from ACTH. It thus appears that from a pituitary view, reduced responsiveness to the homotypic stressor after daily repeated stress is paralleled by reduced heterotypic sensitization, suggesting that repeated exposure to a stressor both the response to the homotypic stressor and heterotypic sensitization partially habituate. The mechanisms involved in such a habituation process as well as in the dissociation between ACTH and corticosterone remain to be further explored.
Declaration of interest
The authors report no conflicts of interest.
The laboratory was supported by grants from the Spanish Ministry of Science and Innovation (SAF2011-28313), Instituto de Salud Carlos III (RD12/0028/0014, Redes Temáticas de Investigación Cooperativa en Salud, Ministerio de Sanidad y Consumo) and Plan Nacional sobre Drogas and Generalitat de Catalunya (SGR2009-16). Núria Daviu and Cristina Rabasa were recipients of MEC Fellowships.
References
- Anisman H, Larry SS. (1985). Psychological and physiological interactions. In: Burchfield SR, editor. Neurochemical consequences of stress: contribution of adaptative processes. New York: Hemisphere Publishing Corp. p 68–98
- Armario A. (2006). The hypothalamic-pituitary-adrenal axis: what can it tell us about stressors? CNS & Neurol Disord Drug Targets 5:485–501
- Armario A, Escorihuela RM, Nadal R. (2008). Long-term neuroendocrine and behavioural effects of a single exposure to stress in adult animals. Neurosci Biobehav Rev 32:1121–35
- Armario A, Gil M, Martí J, Pol O, Balasch J. (1991). Influence of various acute stressors on the activity of adult male rats in a holeboard and in the forced swim test. Pharmacol Biochem Behav 39:373–7
- Armario A, Hidalgo J, Giralt M. (1988). Evidence that the pituitary-adrenal axis does not cross-adapt to stressors: comparison to other physiological variables. Neuroendocrinology 47:263–7
- Armario A, Restrepo C, Castellanos JM, Balasch J. (1985). Dissociation between adrenocorticotropin and corticosterone responses to restraint after previous chronic exposure to stress. Life Sci 36:2085–92
- Armario A, Vallès A, Dal-Zotto S, Márquez C, Belda X. (2004). A single exposure to severe stressors causes long-term desensitisation of the physiological response to the homotypic stressor. Stress 7:157–72
- Atkinson HC, Wood SA, Kershaw YM, Bate E, Lightman SL. (2006). Diurnal variation in the responsiveness of the hypothalamic-pituitary-adrenal axis of the male rat to noise stress. J Neuroendocrinol 18:526–33
- Belda X, Daviu N, Nadal R, Armario A. (2012). Acute stress-induced sensitization of the pituitary-adrenal response to heterotypic stressors: independence of glucocorticoid release and activation of CRH1 receptors. Horm Behav 62:515–24
- Belda X, Fuentes S, Nadal R, Armario A. (2008). A single exposure to immobilization causes long-lasting pituitary-adrenal and behavioral sensitization to mild stressors. Horm Behav 54:654–61
- Belda X, Márquez C, Armario A. (2004). Long-term effects of a single exposure to stress in adult rats on behavior and hypothalamic-pituitary-adrenal responsiveness: comparison of two outbred rat strains. Behav Brain Res 154:399–408
- Berridge CW, Dunn AJ. (1989). Restraint-stress-induced changes in exploratory behavior appear to be mediated by norepinephrine-stimulated release of CRF. J Neurosci 9:3513–21
- Bhatnagar S, Dallman M. (1998). Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84:1025–39
- Bhatnagar S, Vining C. (2003). Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav 43:158–65
- Breslau N, Chilcoat HD, Kessler RC, Davis GC. (1999). Previous exposure to trauma and PTSD effects of subsequent trauma: results from the Detroit Area Survey of Trauma. Am J Psychiatry 156:902–7
- Breslau N, Peterson EL, Schultz LR. (2008). A second look at prior trauma and the posttraumatic stress disorder effects of subsequent trauma: a prospective epidemiological study. Arch Gen Psychiatry 65:431–7
- Calvo N, Martijena ID, Molina VA, Volosin M. (1998). Metyrapone pretreatment prevents the behavioral and neurochemical sequelae induced by stress. Brain Res 800:227–35
- Cancela LM, Bregonzio C, Molina VA. (1995). Anxiolytic-like effect induced by chronic stress is reversed by naloxone pretreatment. Brain Res Bull 36:209–13
- Chen J, Young S, Subburaju S, Sheppard J, Kiss A, Atkinson H, Wood S, et al. (2008). Vasopressin does not mediate hypersensitivity of the hypothalamic pituitary adrenal axis during chronic stress. Ann N Y Acad Sci 1148:349–59
- Cox RH, Hubbard JW, Lawler JE, Sanders BJ, Mitchell VP. (1985). Cardiovascular and sympathoadrenal responses to stress in swim-trained rats. J Appl Physiol 58:1207–14
- Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker CD, Strack AM, Cascio CS. (1992). Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol 4:517–26
- Dallman MF, Jones MT. (1973). Corticosteroid feedback control of ACTH secretion: effect of stress-induced corticosterone secretion on subsequent stress responses in the rat. Endocrinology 92:1367–75
- Engeland WC, Miller P, Gann DS. (1989). Dissociation between changes in plasma bioactive and immunoreactive adrenocorticotropin after hemorrhage in awake dogs. Endocrinology 124:2978–85
- Fernandes GA, Perks P, Cox NK, Lightman SL, Ingram CD, Shanks N. (2002). Habituation and cross-sensitization of stress-induced hypothalamic-pituitary-adrenal activity: effect of lesions in the paraventricular nucleus of the thalamus or bed nuclei of the stria terminalis. J Neuroendocrinol 14:593–602
- Fleshner M, Deak T, Spencer RL, Laudenslager ML, Watkins LR, Maier SF. (1995). A long-term increase in basal levels of corticosterone and a decrease in corticosteroid-binding globulin after acute stressor exposure. Endocrinology 136:5336–42
- Gagliano H, Fuentes S, Nadal R, Armario A. (2008). Previous exposure to immobilisation and repeated exposure to a novel environment demonstrate a marked dissociation between behavioral and pituitary-adrenal responses. Behav Brain Res 187:239–45
- García A, Martí O, Vallès A, Dal-Zotto S, Armario A. (2000). Recovery of the hypothalamic-pituitary-adrenal response to stress. Effect of stress intensity, stress duration and previous stress exposure. Neuroendocrinology 72:114–25
- Grissom N, Bhatnagar S. (2009). Habituation to repeated stress: get used to it. Neurobiol Learn Mem 92:215–24
- Hardin JM, Hilbe JM. (2003). Generalized estimating equations. Boca Raton: Champan and Hall/CRC
- Hashimoto K, Suemaru S, Takao T, Sugawara M, Makino S, Ota Z. (1988). Corticotropin-releasing hormone and pituitary-adrenocortical responses in chronically stressed rats. Regul Pept 23:117–26
- Hauger RL, Lorang M, Irwin M, Aguilera G. (1990). CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Res 532:34–40
- Hennessy JW, Levin R, Levine S. (1977). Influence of experiential factors and gonadal hormones on pituitary-adrenal response of the mouse to novelty and electric shock. J Comp Physiol Psychol 91:770–7
- Heydendael W, Sharma K, Iyer V, Luz S, Piel D, Beck S, Bhatnagar S. (2011). Orexins/hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology 152:4738–52
- Irwin MR, Hauger RL. (1988). Adaptation to chronic stress. Temporal pattern of immune and neuroendocrine correlates. Neuropsychopharmacology 1:239–42
- Johnson JD, O’Connor KA, Deak T, Spencer RL, Watkins LR, Maier SF. (2002). Prior stressor exposure primes the HPA axis. Psychoneuroendocrinology 27:353–65
- Kennett GA, Chaouloff F, Marcou M, Curzon G. (1986). Female rats are more vulnerable than males in an animal model of depression: the possible role of serotonin. Brain Res 382:416–21
- Kennett GA, Dickinson SL, Curzon G. (1985). Central serotonergic responses and behavioural adaptation to repeated immobilisation: the effect of the corticosterone synthesis inhibitor metyrapone. Eur J Pharmacol 119:143–52
- Kim KS, Han PL. (2006). Optimization of chronic stress paradigms using anxiety- and depression-like behavioral parameters. J Neurosci Res 83:497–507
- Kvetnansky R, Németh S, Vigas M, Oprsalova Z, Jurcovicova J. (1984). Stress. The role of catecholamines and other neurotransmitters. In: Usdin E, Kvetnansky R, Axelrod J, editors. Plasma catecholamines in rats during adaptation to intermittent exposure to dfferent stressors. New York: Gordon and Breach Science Publishers. p 537–62
- Kvetnansky R, Sabban EL, Palkovits M. (2009). Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev 89:535–606
- Le Mevel JC, Abitbol S, Beraud G, Maniey J. (1979). Temporal changes in plasma adrenocorticotropin concentration after repeated neurotropic stress in male and female rats. Endocrinology 105:812–7
- Lehnert H, Reinstein DK, Strowbridge BW, Wurtman RJ. (1984). Neurochemical and behavioral consequences of acute, uncontrollable stress: effects of dietary tyrosine. Brain Res 303:215–23
- Ma XM, Lightman SL. (1998). The arginine vasopressin and corticotrophin-releasing hormone gene transcription responses to varied frequencies of repeated stress in rats. J Physiol 510:605–14
- Marin MT, Cruz FC, Planeta CS. (2007). Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav 90:29–35
- Márquez C, Belda X, Armario A. (2002). Post-stress recovery of pituitary-adrenal hormones and glucose, but not the response during exposure to the stressor, is a marker of stress intensity in highly stressful situations. Brain Res 926:181–5
- Márquez C, Nadal R, Armario A. (2004). The hypothalamic-pituitary-adrenal and glucose responses to daily repeated immobilisation stress in rats: individual differences. Neuroscience 123:601–12
- Martí O, Armario A. (1998). Anterior pituitary response to stress: time-related changes and adaptation. Int J Dev Neurosci 16:241–60
- Martí O, García A, Vallès A, Harbuz MS, Armario A. (2001). Evidence that a single exposure to aversive stimuli triggers long-lasting effects in the hypothalamus-pituitary-adrenal axis that consolidate with time. Eur J Neurosci 13:129–36
- Martí O, Gavaldà A, Jolín T, Armario A. (1993). Effect of regularity of exposure to chronic immobilization stress on the circadian pattern of pituitary adrenal hormones, growth hormone, and thyroid stimulating hormone in the adult male rat. Psychoneuroendocrinology 18:67–77
- Mollica RF, McInnes K, Poole C, Tor S. (1998). Dose-effect relationships of trauma to symptoms of depression and post-traumatic stress disorder among Cambodian survivors of mass violence. Br J Psychiatry 173:482–8
- Netto SM, Silveira R, Coimbra NC, Joca SR, Guimarães FS. (2002). Anxiogenic effect of median raphe nucleus lesion in stressed rats. Prog Neuropsychopharmacol Biol Psychiatry 26:1135–41
- O’Connor KA, Ginsberg AB, Maksimova E, Wieseler Frank JL, Johnson JD, Spencer RL, Campeau S, et al. (2004). Stress-induced sensitization of the hypothalamic-pituitary adrenal axis is associated with alterations of hypothalamic and pituitary gene expression. Neuroendocrinology 80:252–63
- O’Connor KA, Johnson JD, Hammack SE, Brooks LM, Spencer RL, Watkins LR, Maier SF. (2003). Inescapable shock induces resistance to the effects of dexamethasone. Psychoneuroendocrinology 28:481–500
- Osborne B, Seggie J. (1980). Behavioral, corticosterone, and prolactin responses to novel environment in rats with fornix transections. J Comp Physiol Psychol 94:536–46
- Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. (2006). Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology 147:2008–17
- Ottenweller JE, Servatius RJ, Natelson BH. (1994). Repeated stress persistently elevates morning, but not evening, plasma corticosterone levels in male rats. Physiol Behav 55:337–40
- Ottenweller JE, Servatius RJ, Tapp WN, Drastal SD, Bergen MT, Natelson BH. (1992). A chronic stress state in rats: effects of repeated stress on basal corticosterone and behavior. Physiol Behav 51:689–98
- Pace TW, Cole MA, Ward G, Kalman BA, Spencer RL. (2001). Acute exposure to a novel stressor further reduces the habituated corticosterone response to restraint in rats. Stress 4:319–31
- Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. (2003). Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci 6:168–74
- Pellow S, File SE. (1986). Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav 24:525–9
- Plaznik A, Tamborska E, Hauptmann M, Bidzinski A, Kostowski W. (1988). Brain neurotransmitter systems mediating behavioral deficits produced by inescapable shock treatment in rats. Brain Res 447:122–32
- Pol O, Campmany L, Gil M, Armario A. (1992). Behavioral and neurochemical changes in response to acute stressors: influence of previous chronic exposure to immobilization. Pharmacol Biochem Behav 42:407–12
- Rabasa C, Munoz-Abellán C, Daviu N, Nadal R, Armario A. (2011). Repeated exposure to immobilization or two different footshock intensities reveals differential adaptation of the hypothalamic-pituitary-adrenal axis. Physiol Behav 103:125–33
- Reinstein DK, Lehnert H, Scott NA, Wurtman RJ. (1984). Tyrosine prevents behavioral and neurochemical correlates of an acute stress in rats. Life Sci 34:2225–31
- Sakellaris PC, Vernikos-Danellis J. (1975). Increased rate of response of the pituitary-adrenal system in rats adapted to chronic stress. Endocrinology 97:597–602
- Servatius RJ, Ottenweller JE, Bergen MT, Soldan S, Natelson BH. (1994). Persistent stress-induced sensitization of adrenocortical and startle responses. Physiol Behav 56:945–54
- Spiga F, Harrison LR, MacSweeney CP, Thomson FJ, Craighead M, Lightman SL. (2009). Effect of vasopressin 1b receptor blockade on the hypothalamic-pituitary-adrenal response of chronically stressed rats to a heterotypic stressor. J Endocrinol 200:285–91
- Steenbergen HL, Heinsbroek RP, Van Hest A, Van de Poll NE. (1990). Sex-dependent effects of inescapable shock administration on shuttlebox-escape performance and elevated plus-maze behavior. Physiol Behav 48:571–6
- Stone EA, McCarty R. (1983). Adaptation to stress: tyrosine hydroxylase activity and catecholamine release. Neurosci Biobehav Rev 7:29–34
- Ulrich-Lai YM, Arnhold MM, Engeland WC. (2006). Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol 290:1128–35
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, et al. (2007). Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology 148:1823–34
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA, Herman JP. (2005). Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 289:823–8
- Vernikos J, Dallman MF, Bonner C, Katzen A, Shinsako J. (1982). Pituitary-adrenal function in rats chronically exposed to cold. Endocrinology 110:413–20
- Vyas A, Pillai AG, Chattarji S. (2004). Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience 128:667–73
- Weinberg MS, Bhatt AP, Girotti M, Masini CV, Day HE, Campeau S, Spencer RL. (2009). Repeated ferret odor exposure induces different temporal patterns of same-stressor habituation and novel-stressor sensitization in both hypothalamic-pituitary-adrenal axis activity and forebrain c-fos expression in the rat. Endocrinology 150:749–61
- Weiss JM, Glazer HI, Pohorecky LA, Brick J, Miller NE. (1975). Effects of chronic exposure to stressors on avoidance-escape behavior and on brain norepinephrine. Psychosom Med 37:522–34
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. (1998). Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience 85:1209–22
- Yehuda R, Kahana B, Schmeidler J, Southwick SM, Wilson S, Giller EL. (1995). Impact of cumulative lifetime trauma and recent stress on current posttraumatic stress disorder symptoms in holocaust survivors. Am J Psychiatry 152:1815–8
- Zelena D, Mergl Z, Foldes A, Kovacs KJ, Toth Z, Makara GB. (2003). Role of hypothalamic inputs in maintaining pituitary-adrenal responsiveness in repeated restraint. Am J Physiol Endocrinol Metab 285:E1110–17