Abstract
Hypoxia, the deprivation of adequate oxygen supply, constitutes a direct threat to survival by disrupting cardiovascular or respiratory homeostasis and eliciting a respiratory distress. Although hypoxia has been shown to increase brain vulnerability and impair basic cognitive functions, only one study has examined its effect on decision-making. The present study examined the effect of mild hypoxia on individual’s loss aversion, that is, the tendency to be more affected by losses than equal sized gains. A sample of 26 participants were asked to either accept or reject a series of mixed gambles once in an oxygen-depleted environment (14.1% oxygen concentration) and once in a normoxic environment (20.9% oxygen concentration). Each gamble involved a 50–50 chance of winning or losing specified amounts of money. Mild hypoxia decreased loss aversion: on average in the normoxic condition participants accepted gambles if the gain was at least 2.4 times as large as the loss, whereas in the oxygen-depleted condition participants accepted gambles if the gain was at least 1.7 times as large as the loss. Mild hypoxia may push individuals to be less cautious in daily decisions that involve a trade-off between a gain and a loss.
Introduction
Following a minor car accident, is it worthwhile to call the police? When one is behind in studying for an exam, is it worthwhile to spend the night out drinking and chatting with friends? What makes decisions difficult is that there is rarely a dominant option, so one needs to consider trade-offs. A factor that might increase the difficulty and/or the quality of decisions is that they are frequently made under stress. Interestingly, a recent review by Starcke & Brand (Citation2012) highlights that only a handful of studies have investigated the impact of stress on decision-making. The review suggests that stress prompts more automatic responding with little cognitive reflection. In the handful of studies that addressed directly the impact of stress in decision-making, its effects were predominantly negative. For example, Jones et al. (Citation2011) showed that a subjective feeling of time pressure increased the number of bets on disadvantageous gambles (i.e. bets with negative expected value) and decreased the number of bets on advantageous gambles (i.e. bets with positive expected value). The objective of the present study is to address the effect of a stressor, hypoxia, on decision-making. Before we proceed with the study, we describe the specific effect that we examine (loss aversion) and discuss theoretical and empirical distinctions between different types of stress.
Loss aversion
A basic phenomenon of choice under risk is that losses loom larger than gains (Kahneman & Tversky, Citation1984; Tversky & Kahneman, Citation1991). For example, the negative impact of losing €20 is greater than the positive impact of gaining €20. This asymmetry in sensitivity to losses and gains is known as loss aversion and has been used to explain people’s tendency to reject bets that offer an equal chance of winning and losing a certain amount of money (e.g. a bet with 50–50 chance of winning or losing €20). Loss aversion is a robust phenomenon. It has been documented in a wide range of laboratory and field studies (Chen et al., Citation2006; Haigh & List, Citation2005; Johnson & Goldstein, Citation2003; Mercer, Citation2005; Post et al., Citation2008; Tovar, Citation2009). The experimental evidence suggests that losses loom about twice as large as equal-sized gains. The predominant account for explaining loss aversion is Prospect Theory (Kahneman & Tversky, Citation1979), which describes risky choice using a value function, which is convex in the domain of losses and concave in the domain of gains. To account for loss aversion, the value curve is steeper for losses than it is for gains.
Stress types
The present research addresses the effect of a stressor (mild hypoxia) on loss aversion. Different stressors have been shown to activate different stress-regulatory circuits (for a review, see Herman & Cullinan, Citation1997; Ulrich-Lai & Herman, Citation2009). Because of that, stressors have been frequently distinguished into processive (also known as neurogenic, phychogenic, and exteroceptive) and systemic (also known as interoceptive) (Dayas et al., Citation2001; Herman & Cullinan, Citation1997; Li & Sawchenko, Citation1998; Sawchenko et al., Citation2000). According to supporters of this distinction, processive stressors require the assembly and processing of signals from multiple sensory modalities to initiate a stress response (Herman & Cullinan, Citation1997). Time pressure, giving a public speech, and exposure to a novel environment, are all examples of processive stressors. Processive stressors do not usually represent an immediate threat to the organism, but rather are recognized, interpreted and anticipated as possible threats by higher order brain structures. In contrast, systemic stressors have an immediate survival value, representing a “real” threat for the genuine homeostatic challenge. They are not interpreted by higher-order brain structures, but rather are directly relayed to the hypothalamus by visceral efferent pathways. Examples of systemic stressors include ether and hypoxia, which involve a reduced availability of oxygen at cellular level. Specifically, hypoxia threatens survival by disrupting cardiovascular or respiratory homeostasis and eliciting a respiratory distress. Anatomical evidence suggests that information on blood oxygenation is directly relayed from sensory receptors in the carotid body to the hypothalamus by way of a single synapse with neurons in the nucleus of the solitary tract or ventrolateral medulla (Kalia & Welles, Citation1980; Swanson & Sawchenko, Citation1983).
The processive – systemic distinction has been challenged as an oversimplification (Pacak et al., Citation1998; Pacak & Palkovits, Citation2001). The two physiological systems have been shown to work together, both in terms of overlap in their underlying neural circuitry and their physiological functions (for a review see, Ulrich-Lai & Herman, Citation2009). Although no conclusive data are currently available, it is generally recognized that stressful information that is purely psychological in nature is elementally different from information from systemic pathways. Studies on how stress impacts decision-making have used processive stressors (Bechara & Damasio, Citation2005; Janis & Mann, Citation1977; Jones et al., Citation2009; Mather et al., Citation2009; Porcelli & Delgado, Citation2009; Preston et al., Citation2007; Starcke et al., Citation2008). In contrast, in the present research we used a systemic stressor, mild hypoxia. Hypoxia has been shown to increase brain vulnerability and impair basic cognitive functions (Lieberman et al., Citation1994, Citation1995; Nelson et al., Citation1990; Townes et al., Citation1984). However, studies have yet to examine its effect on complex cognitive tasks such as decision-making. One exception is a study by Pighin et al. (Citation2012) which showed that individuals under mild hypoxia exhibit an exacerbation of the reflection effect, people’s tendency to be risk averse in the domain of gains and risk-seeking in the domain of losses. Specifically, hypoxia increased risk-seeking in the domain of losses, but left risk preferences unaffected in the domain of gains. No study has examined if losses loom larger than gains when people are under a systemic stress, such as hypoxia. The aim of the present research was to fill this gap.
Methods
Participants
A sample of 26 right-handed university students (12 males and 14 females; mean age 23 years ± 6.8, ranging from 19 to 28) volunteered to participate. The study and the informed consent procedure were approved by the Research Ethics Committee of the University of Verona (Verona, Italy, Prot. N. 58). There are no documented adverse health symptoms in healthy individuals due to normobaric mild hypoxia with oxygen concentration between 15 and 13% for exposures shorter than 6 h (Angerer & Nowak, Citation2003). Nevertheless, because mild hypoxia may be detrimental to individuals with heart failure (Ward et al., Citation2000), we excluded participants that used a cardiac pacemaker or had a heart condition. Participants were all enrolled after written informed consent. Before entering in the hypoxic room, each participant was visited by an experienced sport medical doctor of the Research Center “Sport, Mountain and Health”, to test for the presence of possible health problems or adverse symptoms (such as headache, breathlessness, and autonomic disorders).
Stress induction and manipulation checks
All participants were tested in three research sessions, separated by a 7-d interval: a familiarization session in normoxic condition (oxygen concentration of 20.9%, which corresponds to an altitude of 0 m above sea level); a control session in normoxic condition (identical to the familiarization session); and an experimental session in hypoxic condition (oxygen concentration of 14.1%, which corresponds to an altitude of 3000 m above sea level)Footnote1. The familiarization session was always first and aimed to familiarize participants with the experimental setting in the hypoxic room.Footnote2 The order of the normoxic and hypoxic sessions was counterbalanced: half of the participants received the normoxic session followed by the hypoxic one, while the other half received the reverse order. Both participants and experimenter were blind as to the order of the sessions. Participants were tested one at a time. The experimenter applied to them technical equipment to measure physiological parameters, and asked them to watch a nature documentary video clip for 20 min.Footnote3 Subsequently, participants had to perform the loss aversion task, which we describe below. At the end of the task, they were asked to indicate which session they believed they were in (i.e. normal oxygen session or partially decreased oxygen session), and to fill a questionnaire concerning their feelings ().
Table 1. The subjective feelings questionnaire.
Two physiological parameters were monitored: heart rate and oxygen arterial saturation (SaO2). Heart rate was recorded in 5 s intervals (Polar Electro Oy, Kempele, Finland) and was expected to be higher in the hypoxic versus the normoxic session. SaO2 was measured by a portable pulsoximeter (Intermed SAT-500) on the index finger of the right hand at three points during a session: at the end of the video clip (about 25 min after entrance in the hypoxic room), midway through the loss aversion task (about 35 min after entrance in the hypoxic room), and at the end of the loss aversion task (about 60 min after entrance in the hypoxic room). SaO2 level decreased during the first 3–5 min after the entrance in the hypoxic room and remained fairly constant thereafter. A mean SaO2 value in each session was computed by averaging the three SaO2 measures from each participant. SaO2 values were expected to be lower in the hypoxic than the normoxic session. In order to prevent a possible health risk for the participants, SaO2 was also monitored by a staff member who was present during the experiment; the staff member was instructed to stop the experiment if SaO2 levels were lower than 80% (this never happened). An appropriate medical ambulatory near the hypoxic room and an experienced medical doctor were also available in the Research Center building in case of emergency.
Decision-making task
The loss aversion task was a computer based task adapted from Tom et al. (Citation2007) and De Martino et al. (Citation2010). Participants were presented on a computer screen with a series of mixed gambles. For each gamble, participants were asked to either accept or reject it (). At the beginning of the task, participants received a cash endowment of €32.
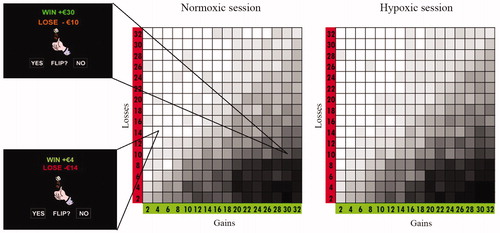
Figure 1. Choice frequencies distribution in the normoxic session (left graph) and in the hypoxic session (right graph). Each cell represents one of the 256 gambles that participants had to decide on. (Two examples of such gambles are given on the left of the figure.) The darker a cell’s color, the higher the percentage of participants that accepted that specific gamble. The diagonal represents gambles which have an expected value of €0. It can be seen, that in both sessions most participants accepted gambles that were below the diagonal (had a positive expected value), and rejected ones that were above the diagonal (had a negative expected value). It can also be seen that the acceptance rate generally increased as one moves toward the right bottom corner of these figures (these cells have progressively higher expected values).
The task began with an instruction phase followed by 10 practice trials. Then, a series of 256 mixed gambles (split in four 64-trials rounds) followed each giving a 50–50 chance of winning or losing a specified amount of money. The gambles were constructed as follows. The sizes of the potential gain and loss were manipulated in increments of €2, with gains ranging from +€2 to +€32 (16 steps), and losses ranging from −€2 to −€32 (16 steps). Each gamble involved a single one of the resulting 16 × 16 possible gain–loss combinations (). Participants had to respond using a two-key keyboard. They were instructed to be fast and accurate. As outcome feedback has been shown to influence cognitive processing (Coricelli et al., Citation2005; Tom et al., Citation2007; Tversky & Kahneman, Citation1992), we withheld giving feedback. Instead, we informed participants that at the end of the experiment one trial from the ones they had accepted in one of the three sessions would be extracted at random and played for real money. The result of the extracted gamble was added to the initial endowment of €32 and determined the participant’s final payment.Footnote4,Footnote5
Results
Manipulation checks
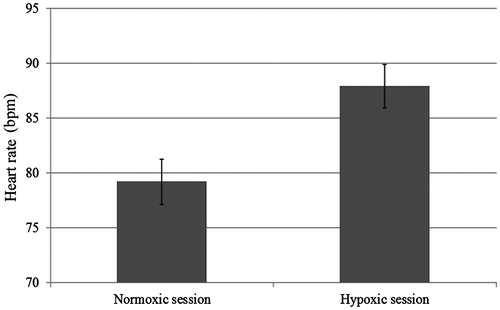
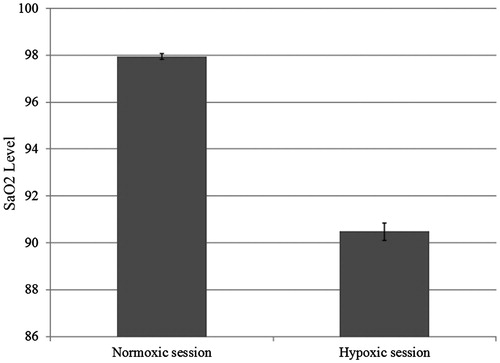
As predicted, the oxygen depletion manipulation altered physiological responses. On average, participants in the hypoxic versus the normoxic session showed an 11.8% increase in heart rate [means = 87.9 versus 79.2; t(25) = 5.62, p < 0.001; see ] and a 7.6% decrease in SaO2 levels [means = 90.5 versus 97.9; t(25) = 18.54, p < 0.001; see ]. Furthermore, participants seemed to be unaware of the oxygen manipulation: of the 26 participants, 4 guessed correctly both the hypoxic and the normoxic condition, 3 guessed correctly the hypoxic condition only, 7 the normoxic condition only, and 12 always guessed wrong. A McNemar test revealed that participants were unable to correctly identify which session they were in (p = 0.34). Moreover, as reported in , participants’ self-reported feelings did not differ across sessions: applying the Bonferroni correction (p < 0.004), no significant difference between the normoxic and the hypoxic sessions was obtained. Our oxygen manipulation seemed to have worked and was not detected by participants.
Decision-making task
visually represents the choice frequencies distribution in the normoxic and in the hypoxic conditions. Light cells represent the gambles that were accepted by few participants, while darker cells those that were accepted by many participants. As can be seen in , overall participants accepted gambles with positive expected values (those under the diagonal) and rejected ones with negative expected values (those up from the diagonal); this is evidence that participants understood the task. Following Tom et al. (Citation2007), we examined behavioral sensitivity to gains and losses by fitting a logistic regression with the size of the potential gain and loss as independent variables, and the response of participants to either accept or reject the gamble as the dependent variable. This analysis was performed separately for each participant and for each session. Then, a behavioral loss aversion index (λ) was computed for each participant and for each session:
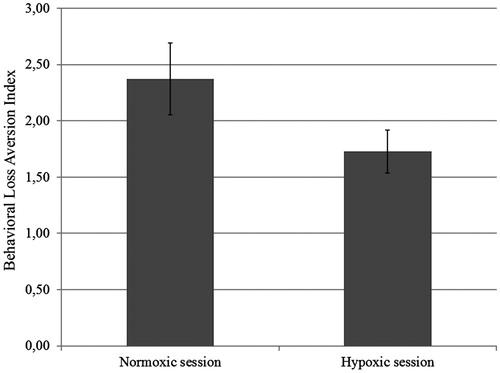
where βloss and βgain are, respectively, the unstandardized regression coefficients for the loss and gain variables. This index gives a reliable measure of loss aversion and has a straightforward interpretation: a λ value of 1 means that, on average, for even chances to win and lose, a gamble is acceptable if the gain is at least as large as the loss (e.g. Win €16–Lose €16); an λ of 2 means that, on average, for even chances to win and lose, a gamble is acceptable if the gain is at least twice as large as the loss (e.g. Win €32–Lose €16). Overall participants in the control condition were loss averse (mean λ = 2.37 ± 1.64), accepting gambles in which the potential gain was at least 2.37 times the amount of the potential loss. This result accords with previous findings (De Martino et al., Citation2010; Tversky & Kahneman, Citation1992). The same participants in the hypoxic condition continued to be loss averse (mean λ = 1.73 ± 1.01), but to a lesser degree: F(1, 24) = 9.42, p = 0.005, partial η2 = 0.28. A total of 21 of the 26 participants (81%) showed a decrease of loss aversion in the hypoxic condition (). This effect was not influenced by session order: F(1, 24) = 0.40, p = 0.53, partial η2 = 0.016.
Figure 4. Participants’ behavioral loss aversion indexes (λ) in the normoxic and in the hypoxic session.
As a further means of analyzing participants’ behavioral sensitivity to gains and losses, we examined risk premiums, that is, the minimum expected monetary value of a gamble at which a participant is indifferent between accepting or rejecting it. Following De Martino et al. (Citation2010), for each participant and condition, we first performed a logistic regression using as a dependent variable the choice of a participant (to accept or reject a given gamble) and as a predictor the net expected value of that gamble (EV = Pr[Win] [€|Win] + Pr[Loss] [€|Loss] = 0.5 [€|Win] + 0.5 [€|Loss]). The resulting regression coefficients were used to estimate the expected value at which a given participant was indifferent between accepting or rejecting a gamble; we used the following formula:
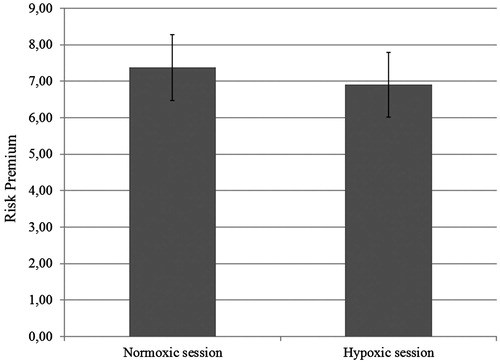
where β stands for the unstandardized regression coefficient and a for the intercept of the logistic regression. Given that participants exhibited smaller loss aversion in the hypoxic than in the normoxic session, we expected smaller risk premiums in the hypoxic session. This prediction was confirmed: the mean risk premium in the hypoxic session was €6.90 (±€4.5) compared to €7.37 (±€4.6) in the normoxic session, t(25) = 1.90, p = 0.034, one-tailed (). Unsurprisingly, loss aversion indexes and risk premiums were significantly correlated both in the normoxic session (r = 0.40, p = 0.041) and the hypoxic session (r = 0.64, p < 0.001).
Discussion
The present study examined the impact of a systemic stressor, mild hypoxia, on loss aversion (Tversky & Kahneman, Citation1991, Citation1992). Physiological measures suggest that our manipulation worked: compared to when they participated in the normoxic session, when in the hypoxic session participants exhibited an increase in heart rate and a decrease in SaO2 levels. Behavioral data suggest that mild hypoxia decreases loss aversion: in the normoxic condition participants accepted gambles if the gain was at least 2.4 times as large as the loss, whereas in the oxygen-depleted condition when the gain was at least 1.7 times as large as the loss.
The present study supports the idea that stress modulates decision-making and risk taking, potentially by influencing how the positive and negative aspects involved in decisional processes are weighted. The present findings dovetail with those obtained by Porcelli & Delgado (Citation2009) and Pighin et al. (Citation2012), which showed that acute stress exacerbates people’s tendency to make conservative choices in the domain of gains and risky choices in the domain of losses.
Although the present results do not define specific mechanisms underlying the effect of hypoxia on loss aversion, they seem to implicate automatic, bottom-up processes (Bechara & Damasio, Citation2005; Damasio, Citation1994; Damasio et al., Citation1991), given that participants were unable to differentiate between the normoxic and hypoxic sessions. This idea is also coherent with previous data suggesting that systemic stimuli can potentially affect psychogenic processes even if they are not interpreted by higher-order brain structures, but only processed in multiple limbic forebrain structures, which are implicated in automatic processing (e.g. in the amygdala, see Sawchenko et al., 1996; Sawchenko et al., Citation2000; Xu et al., 1999).
According to dual-process approaches (Evans, Citation2003; Kahneman & Frederick, Citation2007; Reyna, Citation2004), stressful environments should exacerbate behavioral biases by privileging automatic responding over cognitive reflection. On the assumption that loss aversion is an automatic response, stressful environments should increase it, the opposite of our findings. However, the assumption that loss aversion is a purely automatic response has not been empirically established. Indeed, the available data on how stress affects loss aversion are mixed: studies using time pressure (a processive stressor) suggested that stress increases motivation to avoid losses (Wright & Weitz, Citation1977), while others suggested the opposite (Carnevale et al., Citation1993; Smith et al., Citation1982). In a more recent study on time pressure, Jones et al. (Citation2011) examined its effect on betting decisions about mixed gambles. They found that subjective feelings of urgency interacted with the expected value of a gamble to determine choice: with respect to a control condition, time pressure decreased bets on gambles with positive expected values, and increased bets on gambles with negative expected values. This finding is consistent with the pattern of choices observed in the present research. In both studies, when under stress, participants accept gambles with lower expected value.
Recently, Mather & Lighthall (Citation2012) proposed the STARS model (Stress Triggers Additional Reward Salience), which postulates that stress can affect dopaminergic reward-processing brain regions, which largely determine reward values (Rangel et al., Citation2008). Although this approach could represent a promising route for future investigation, the present findings do not singularly support such a mechanism. The documented decrease in loss aversion could indicate that mild hypoxia reduces the aversion to losses, increases the attractiveness of gains, or does both. Consider a gamble that involves a 50–50 chance of winning or losing €10. Under mild hypoxia, it could be that the prospect of losing €10 is felt less, the prospect of gaining €10 is felt more, or both. Future research can examine the precise underpinnings of the present effect.
The present study investigated, in a systematic and controlled way, the effect of mild hypoxia on individuals’ behavioral sensitivity to gains and losses involved in a decision, adding experimental support to the idea that decisional processes are altered by adverse environmental conditions (McCammon, Citation2002). The effect of mild hypoxia can be dangerous because it often remains undetected. The cognitive appraisal of hypoxia is not as fast or direct as is its physical appraisal, and so as it takes effect, individuals get no warning. Pilots’ anecdotal reports about pressurization system failure, and alpinists’ reports concerning accidents at high altitude, support this: no signs of distress or illness, but, on the contrary, a greater feeling of confidence and relaxation. Future research could investigate the possible relation between the present results and the greater feeling of confidence previously reported under hypoxia. It is plausible that a greater feeling of confidence might induce an increase in risk taking behavior through a decrease in loss aversion.
The altitudes that we studied (3000 m) are slightly higher than those experienced by crew members and passengers in a modern jet airliner flying at usual cruising altitudes, where cabin altitude is kept between 1830 and 2133 m, according to FAR Code of US Federal Regulations released by the Federal Aviation Administration (Citation2004). Although experimental evidence identifies the threshold for significant physiological alterations to be between 2438 and 3048 m (see, e.g. Liberman et al., 1994; Townes et al., Citation1984), the minimum altitude at which cognitive performance becomes significantly impaired has been, and remains, a controversial issue with important implications for flight safety.
Although further research is necessary to qualify the effect of adverse environmental factors on decision-making and risk-taking, and to investigate whether the reported small but significant changes in loss aversion are behaviorally meaningful to an individual, the present research provides a first step. Exposure to mildly oxygen-depleted air might alter individuals’ tradeoff between positive and negative aspects of choices, and because of this it has implications for the safety of alpinists, pilots, militaries, and scientists who frequently work under mild hypoxia. Mild hypoxia may push individuals to be less cautious without even being aware of it.
Declaration of interest
The authors report no conflicts of interest.
Acknowledgements
We thank Benedetto De Martino for useful comments on an earlier draft of this article. We are thankful to the Research Center for Sport, Mountain, and Health (University of Verona – Italy) for financially supporting the present research and for letting us use the hypoxic room. We are especially thankful to Alessandro Leonardi for the hypoxic room settings, to Massimo Vescovi for programming the necessary E-Prime softwares, and to Anesa Bahtic for helping with data collection.
Notes
1The present study adopted the same levels of the independent variable (20.9% versus 14.1%) as in Pighin et al. (Citation2012), because it was found that such oxygen concentrations produced significant physiological alterations while keeping the oxygen manipulation undetected. The present study and Pighin et al. (Citation2012) study do share the same experimental procedure, but they do not share neither participants nor data.
2The hypoxic room is a chamber where a hypoxic environment can be created via an air separation unit that pumps oxygen-depleted air into the room: whereas the total pressure stays the same, the oxygen content (%) is reduced in order to decrease the partial pressure of oxygen in the body. The room temperature (21 °C) and air dampness (32%) were kept constant across sessions.
3Time required for the physiological alterations induced by hypoxia to take place.
4Average earning was €37.
5Due to a misinterpretation, we told participants that at the end of the experiment one trial from the ones they had accepted would be selected at random and honored for real money, whereas, De Martino et al. (Citation2010; see also, Tom et al., Citation2007) told participants that one trial (from all the trials) would be randomly selected and honored for real money according to their actual decision (we guess that the participants understood this to mean that if the selected trial is one that the they declined, then they would get €0). This difference in incentive scheme, however, did not affect participants’ behavior as the mean loss aversion coefficient found in the control session is in full accordance with previous findings (De Martino et al., Citation2010; Tversky & Kahneman, Citation1992).
References
- Angerer P, Nowak D. (2003). Working in permanent hypoxia for fire protection-impact on health. Int Arch Occup Environ Health 76:87–102
- Bechara A, Damasio AR. (2005). The somatic marker hypothesis: a neural theory of economic decision. Games Econ Behav 52:336–72
- Carnevale PJ, Gentile S, de Dreu CKW. (1993). Frame and time pressure in bilateral negotiation. Unpublished manuscript, University of Illinois at Urbana-Champaign
- Chen M, Lakshminarayanan V, Santos L. (2006). How basic are behavioral biases? Evidence from capuchin monkey trading behavior. J Polit Econ 114:517–37
- Coricelli G, Critchley HD, Joffily M, O’Doherty JP, Sirigu A, et al. (2005). Regret and its avoidance: a neuroimaging study of choice behavior. Nat Neurosci 8:1255–62
- Damasio AR. (1994). Descartes’ error: emotion, reason, and the human brain. New York: Grosset/Putnam
- Damasio AR, Tranel D, Damasio H. (1991). Somatic markers and the guidance of behavior: theory and preliminary testing. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal lobe function and dysfunction. New York: Oxford University Press. p 217–29
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. (2001). Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdale and in medullary noradrenergic cell groups. Eur J Neurosci 14:1143–1152
- De Martino B, Camerer CF, Adolphs R. (2010). Amygdala damage eliminates monetary loss aversion. Pnas 107:3788–92
- Evans JSBT. (2003). In two minds: dual-process accounts of reasoning. Trends Cogn Sci 7:454–9
- Federal Aviation Administration. (2004). FAR Code of US Federal Regulations. Parts 25, 121 and 125. Washington, DC: US Department of Transportation
- Haigh M, List J. (2005). Do professional traders exhibit myopic loss aversion? An experimental analysis. J Finance 60:523–34
- Herman JP, Cullinan WE. (1997). Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20:78–84
- Janis IL, Mann L. (1977). Decision making: a psychological analysis of conflict, choice, and commitment. New York, NY: Free Press
- Johnson EJ, Goldstein D. (2003). Medicine. Do defaults save lives? Science 302:1338–9
- Jones CL, Minati L, Harrison NA, Ward J, Critchley HD. (2011). Under pressure: response urgency modulates striatal and insula activity during decision-making under risk. PLoS ONE 6(6):e20942
- Jones LK, Yoon T, Kim JJ. (2009). Stress impairs decision-making in rats. Available from Nature Proceedings
- Kahneman D, Frederick S. (2007). Frames and brains: elicitation and control of response tendencies. Trends Cogn Sci 11:45–6
- Kahneman D, Tversky A. (1979). Prospect theory: an analysis of decisions under risk. Econometrica 47:263–91
- Kahneman D, Tversky A. (1984). Choices, values and frames. Am Psychol 39:341–50
- Kalia M, Welles RV. (1980). Brain stem projections of the aortic nerve in the cat: a study using tetramethyl benzidine as the substrate for horseradish peroxidase. Brain Res 188:23–32
- Li HY, Sawchenko PE. (1998). Hypothalamic effector neurons and extended circuitries activated in “neurogenic” stress: a comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J Comp Neurol 393:244–66
- Lieberman P, Protopapas A, Kanki BG. (1995). Speech production and cognitive deficits on Mt. Everest. Aviat Space Environ Med 66:857–64
- Lieberman P, Protopapas A, Reed E, Youngs W, Kanki BG. (1994). Cognitive defects at altitude. Nature 372:325
- McCammon I. (2002). Evidence of heuristics traps in recreational avalanche accidents. Proceedings of the International Snow Science Workshop, Penticton, British Columbia, September 30–October 4
- Mather M, Gorlick M, Kryla-Lighthall N. (2009). To brake or accelerate when the light turns yellow? Stress reduces older adults’ risk taking in a driving game. Psychol Sci 20:174–6
- Mather M, Lighthall NR. (2012). Risk and reward are processed differently in decisions made under stress. Curr Dir Psychol Sci 21:36–41
- Mercer J. (2005). Prospect theory and political science. Annu Rev Polit Sci 8:1–21
- Nelson TO, Dunlowsky J, White DM, Steinberg J, Townes BD, Anderson D. (1990). Cognition and metacognition at extreme altitudes on Mount Everest. J Exp Psychol Gen 119:367–74
- Pacak K, Palkovits M. (2001). Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 22:502–48
- Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. (1998). Heterogeneous neurochemical responses to different stressors: a test of Sely’s doctrine of nonspecificity. Am J Physiol 275:1247–55
- Pighin S, Bonini N, Savadori L, Hadjichristidis C, Antonetti T, Schena F. (2012). Decision making under hypoxia: oxygen depletion increases risk seeking for losses but not for gains. Judgm Decis Mak 7:472–7
- Porcelli AJ, Delgado MR. (2009). Acute stress modulates risk taking in financial decision making. Psychol Sci 20:278–83
- Post T, Van den Assem M, Baltussen G, Thaler R. (2008). Deal or no deal? Decision making under risk in a large-payoff game show. Am Econ Rev 98:38–71
- Preston SD, Stansfield RBS, Buchanan TW, Bechara A. (2007). Effects of anticipatory stress on decision making in a gambling task. Behav Neurosci 121:257–63
- Rangel A, Camerer C, Montague PR. (2008). A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci 9:545–56
- Reyna V. (2004). How people make decisions that involve risk: a dual process approach. Psychol Sci 13:60–6
- Sawchenko PE, Brown ER, Chan RK, Ericsson A, Li HY, Roland BL, Kovࢳ KJ. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog Brain Res 107:201–22
- Sawchenko PE, Li HY, Ericsson A. (2000). Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res 122:61–78
- Smith DL, Pruitt D, Carnevale PJ. (1982). Matching and mismatching: the effect of own limit, other’s toughness, and time pressure on concession rate in negotiation. J Pers Soc Psychol 42:876–83
- Starcke K, Brand M. (2012). Decision making under stress: a selective review. Neurosci Biobehav R 36:1228–48
- Starcke K, Wolf OT, Markowitsch HJ, Brand M. (2008). Anticipatory stress influences decision making under explicit risk conditions. Behav Neurosci 122:1352–60
- Swanson LW, Sawchenko PE. (1983). Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6:269–324
- Tom SM, Fox CR, Trepel C, Poldrack RA. (2007). The neural basis of loss aversion in decision-making under risk. Science 315:515–18
- Tovar P. (2009). The effects of loss aversion on trade policy: theory and evidence. J Int Econ 78:154–67
- Townes B, Hornbein T, Schoene R, Sarnquist F, Grant I. (1984). High altitude and man. In: West JB, Lahiri S, editors. Human cerebral function at extreme altitude. Bethesda, DC: American Physiological Society. p 31–6
- Tversky A, Kahneman D. (1991). Loss aversion in riskless choice: a reference dependent model. Q J Econ 106:1039–61
- Tversky A, Kahneman D. (1992). Advances in prospect theory: cumulative representation of uncertainty. J Risk Uncertain 5:297–323
- Ulrich-Lai YM, Herman JP. (2009). Neural regulation of endocrine and autonomic stress responses. Nat Rev: Neurosci 10:397–409
- Ward PW, Milledge JS, West JB. (2000). High altitude medicine and physiology. London: Arnold
- Wright P, Weitz B. (1977). Time horizon effects on product evaluation strategies. J Market Res 14:429–43
- Xu Y, Day TA, Buller KM. (1999). The central amygdala modulates hypothalamic-pituitary-adrenal axis responses to systemic interleukin-1 β administration. Neuroscience 94:175–83
- Zigmond AS, Snaith RP. (1983). The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361–70