Abstract
Findings suggest that stress-induced impaired learning and coping abilities may be attributed more to the psychological nature of the stressor, rather than its physical properties. It has been proposed that establishing controllability over stressors can ameliorate some of its effects on cognition and behavior. Gaining controllability was suggested to be associated with the development of stress resilience. Based on repeated exposure to the two-way shuttle avoidance task, we previously developed and validated a behavioral task that leads to a strict dissociation between gaining controllability (to the level that the associated fear is significantly reduced) and a fearful state of uncontrollability. Employing this protocol, we investigated here the impact of gaining or failing to gain emotional controllability on indices of anxiety and depression and on subsequent abilities to cope with positively or negatively reinforcing learning experiences. In agreement with previous studies, rats exposed to the uncontrollable protocol demonstrated high concentration of sera corticosterone, increased immobility, reduced duration of struggling in the forced swim test and impaired ability to acquire subsequent learning tasks. Achieving emotional controllability resulted in resilience to stress as was indicated by longer duration of struggling in the forced swim test, and enhanced learning abilities. Our prolonged training protocol, with the demonstrated ability of rats to gain emotional controllability, is proposed as a useful tool to study the neurobiological mechanisms of stress resilience.
Introduction
It has been proposed that establishing controllability over stressors can ameliorate some of their effects on cognition and behavior. This idea evolved from studies demonstrating that animals that were exposed to an aversive Pavlovian conditioning procedure, later failed to learn to escape and avoid footshocks in a shuttlebox (Overmier & Seligman, Citation1967). However, the effect of the stress experience was not evident following exposure to equivalent amounts of escapable shocks. It was argued that the controllability level an organism could exert over an aversive shock was a critical component, and that the lack of control is a key factor in further failure to learn (Maier et al., Citation1969; Seligman & Maier, Citation1967). These and similar findings suggested that stress-induced impaired learning is attributed to the psychological nature of the stressor, rather than to the physical properties associated with the shocks.
The level of controllability an organism can achieve over a stressor may determine the impact of that stressor and may play a critical role in the development of pathological behaviors following a traumatic event. Uncontrollable shock can result in a number of behavioral, physiological and immunological deficits, including learning deficits, reduced exploratory activity, reduced mobility in a swim test, reduced aggression, depressed immunocompetence, increased gastrointestinal lesions, stress-induced analgesia and reduced social interaction (for review see Brennan et al., Citation2003; Brown et al., Citation2001; Maier & Watkins, Citation2005). Additional outcomes include exaggerated contextual fear conditioning (Maier et al., Citation1995), increased neophobia (Minor et al., Citation1994), increased freezing duration (Warren & Rosellini, Citation1988), potentiation of morphine's rewarding properties (Will et al., Citation1998), impaired long-term potentiation in hippocampal slices (Shors et al., Citation1989) and a marked increase in noradrenaline release in the hypothalamus and amygdala 21-h post-training compared to rats exposed to controllable stress (Swenson & Vogel, Citation1983; Tanaka, Citation1999).
Many of the studies referring to controllable versus uncontrollable (aversive) experience in the two-way shuttle (TWS) avoidance test employed protocols of a single day exposure (Bland et al., Citation2006; Brennan et al., Citation2003; Drugan et al., Citation1985; Heinsbroek et al., Citation1991; Rozeske et al., Citation2009; Tanaka, Citation1999). We previously noticed that at the end of a single controllable-stress training day, even though rats have gained a level of operational controllability over the situation (i.e. the rats learned to avoid the shock with the presentation of the predictive cue), they nevertheless exhibited a high level of freezing to the context which was not different from that of the rats exposed to uncontrollable stress (Ilin & Richter-Levin, Citation2009). These results indicate that while the controllable stress group gained operational controllability, some aspects of “emotional” controllability (performing avoidance responses without fearful responses) have not yet been achieved (Ilin & Richter-Levin, Citation2009).
In order to put the emphasis on the development of emotional controllability (i.e. operational learning but with a reduced level of fear response) we developed and validated a training protocol, based on repeated exposure to the auditory TWS avoidance task (Ilin & Richter-Levin, Citation2009). This protocol consisted of six training sessions and designed in such a way that both the controllable and the uncontrollable stress groups were exposed to the same number and duration of tones and foot shocks, and in a similar pattern of presentation. While the performance of the controllable group determined the outcome of the sessions, the performance of the uncontrollable animals had no effect on the outcome. We previously found that with the prolonged training protocol, the conditioned stimulus (CS), which was fear-evoking for both training conditions at the end of the first day of training, turned to be an instructive cue for the controllable group (i.e. instruct them to shuttle to the other compartment) at the end of the prolonged training protocol, but remained fear-evoking for the uncontrollable group (Ilin & Richter-Levin, Citation2009).
In the present study, we make use of this behavioral protocol and investigate the impact of gaining or being unable to gain controllability on subsequent abilities to cope with positively or negatively reinforcing learning experiences and on indices of anxiety and depression.
Materials and methods
Subjects
Male Sprague–Dawley (SD) rats weighing 200–224 g (7–8 weeks old) were purchased from Harlan (Jerusalem, Israel) and habituated in the Brain and Behavior Research animal house facilities for four days. Five rats were housed per cage in 75 × 55×15 cm Plexiglas cages in temperature-controlled (23 ± 1 °C) animal quarters on a 12:12 light–dark cycle (lights on 07:00–19:00 h). They had ad libitum access to standard Purina rodent chow pellets and water. All behavioral tests were conducted during the light phase (08:00–17:00).
Ethical approval
All procedures and tests were approved by the Institutional Animal Care Committee and adhered to the guidelines of the US Institute of Laboratory Animal Research's Guide for the Care and Use of Laboratory Animals. The study was approved by the University of Haifa Ethics and Animal Care Committee.
General experimental procedure
Rats were first subjected to six days of training under either controllable or uncontrollable stress protocols, as described below. Separate cohorts of rats were then assigned to one of the following tests:
Trunk blood sample was collected from the tail 24 h after the last training session for later corticosterone immunoassay (Naïve: n = 7; uncontrollable: n = 7; controllable: n = 8).
Forced swim test (FST) 48 h after the last training session (Naïve: n = 12; uncontrollable: n = 9; controllable: n = 9).
Testing for exploratory behavior in a novel setting one month after the last training session (Naïve: n = 19; uncontrollable: n = 12; controllable: n = 14), followed by the visual TWS avoidance test.
The olfactory discrimination task (Naïve: n = 8; uncontrollable: n = 10; controllable: n = 11) was carried out 24 h after the last training session.
The controllable/uncontrollable training protocol
Apparatus
The TWS box, placed in a dimly-lit, ventilated, sound-attenuated cupboard, is a rectangular chamber (60 × 26×28 cm) divided by an opaque partition with a small passage (10 × 8 cm) that connects two equal sized, side-by-side, cube-shaped compartments. Both metal grid floors of the compartments are weight sensitive and electrifiable. Micro-switches transmit information about the location of the rat to a computer control and data collection program. This program controls both CS presentations (a tone (2 kHz; 85 dB) produced by loudspeakers located on the distal walls of the compartments) and electric shock deliveries (to the rats’ feet through the compartment floor, by a Solid State Shocker/Distributor, Coulbourn Instruments Inc., Lehigh Valley, PA).
Training
Rats were given a 10 min free exploration period in the TWS box followed immediately by a training session according to the group the rat belonged to, as described below. All the groups were subjected to 75, 50 or 25 conditioning trials each day for 6 days as described previously and below (Ilin & Richter-Levin, Citation2009).
The controllable conditioned group
The controllable stress group underwent a single daily training session of: 75 trials (on days 1 and 2); 50 trials (on days 3 and 4); and 25 trials (on days 5 and 6). Each trial consisted of a 10 s tone followed by a maximum of 10 s electrical foot shock (1 mA). The inter-trial interval (ITI) was 30 s ± 25%. Rats could exhibit one of the following behaviors: (1) Avoidance – shuttling to the adjacent chamber of the apparatus while only the tone was on, thus avoiding the shock altogether; (2) Escape – shuttling to the other compartment after the initiation of the shock, thus reducing the duration of exposure to the shock; (3) No Escape – not shuttling to the adjacent chamber, thus receiving the full length of the shock.
The uncontrollable conditioned group
This group was subjected to the same schedule as the controllable conditioned group, but the rats had no control over the stressor. The computer activated averaged protocols of tone/shock durations for each trial based on the performance of the controllable group and applied these averages to the uncontrollable group. In that way the uncontrollable group was subjected to a similar pattern of occurrences and durations of tones and foot shocks as the controllable group.
Naïve group
This group was habituated to the experimental environment in the same manner as the other groups. However, during the experimental trials, these rats remained in their home cage.
Corticosterone immunoassay
Trunk blood was collected following decapitation (under anesthesia, urethane 1.5 g/kg, i.p.), 24 h after the end of the training protocol (between 1200 and 1700 PM). Samples were centrifuged at 1000g for 20 min at 4 °C. Approximately 1 ml of serum from each rat was collected into 1.5 ml Eppendorf tubes and stored at −80 °C. Corticosterone concentration was assessed using DSL/10/81000 ELISA kits (DSL, Webster, TX). The sensitivity of the corticosterone assay was 12.5 µg/L.
Forced swim test
Forced swim test (FST) was conducted 48 h post-training in a cylindrical tank (40 cm high and 18 cm in diameter), which contained water (20–25 cm) at 25 °C (2 °C above room temperature) so that the rat could not touch the bottom with its hind paws. Rats were given a single 10-min exposure to the swim tank and were videotaped. Video films of each FST session were carefully analyzed by an observer blind to the treatment. The duration that subjects spent struggling or immobile was measured for 10 min. Struggling was defined as movement of the forelimbs and hindlimbs in which the front paws broke the surface of the water or scratched the sides of the container. Subjects were considered to be immobile when there was an absence of any movement other than that necessary to keep the head and nose above the water (when subjects were floating in a vertical position). After each session, the tank was cleaned and filled with clean water.
The olfactory discrimination task
Twenty four hours after the end of training under either controllable or uncontrollable stress protocols rats were assigned to the olfactory discrimination (OD) task. Before training rats were maintained on a 23.5 h water deprivation schedule, with food available ad libitum.
Apparatus and odors
Olfactory discrimination training protocol was performed in a four-arm radial maze, as described previously (Saar et al., Citation1999, Citation2001) with commercial odors that are regularly used in the cosmetics and food industry. An electronic “start” command randomly opens two of eight valves, releasing a positive-cue odor into one of the arms and a negative-cue odor into another; 8 s later, the two corresponding guillotine doors are lifted to allow the rat to enter the selected arms. After reaching the far end of an arm (90 cm long), the rat body interrupts an infrared beam, and a drop of drinking water is released from a water hose into a small drinking well (water is released only if the arm contains the positive-cue odor). A trial ends when the rat interrupts a beam, or in 10 s, if no beam is interrupted. A fan is operated for 15 s between trials, to remove odors.
Training
Each rat was trained to distinguish between a pair of odors in a 20 trial/day protocol. In each trial, the rat had to choose between the two odors (positive and negative cues) presented simultaneously. Rats were rewarded after choosing the positive-cue. Learning was considered as acquired after demonstration of at least 80% positive cue choices in the last 10 trials of the day (Saar et al., Citation1999; Staubli et al., Citation1987).
Exploratory behavior in a novel setting
This task was carried out 1 month after training in the auditory TWS. For this test a different TWS box was used from the one used in initial training. The boxes differed in appearance (different color and texture of side panels were used) and odor (the box was wiped with scented baby wipes).
Exploratory behavior in a novel setting was assessed by allowing the rats to explore both compartments for 10 min. An observing experimenter blind to the treatment recorded the rats' exploratory shuttles between compartments.
The visual TWS avoidance task
Rats were tested in the Visual TWS avoidance task in a single 100 trials session one month after the end of training under either controllable or uncontrollable protocols.
Apparatus
For this test a different TWS box was used from the one used in initial training. The boxes differed in appearance (different color and texture of side panels were used) and odor (the box was wiped with scented baby wipes).
The visual shuttle box was placed in a dimly-lit, ventilated, sound-attenuated cupboard. The visual shuttle box is a rectangular chamber (60 × 26×28 cm) divided by an opaque partition with a small passage (10 × 8 cm) that connects two equal sized, side-by-side, cube-shaped compartments. Both metal grid floors of the compartments are weight sensitive and electrifiable. Micro-switches transmit information about the location of the rat to a computer control and data collection program. This program controls both CS presentations (a light (40W)) and electric shock deliveries (to the rats' feet through the compartment floor, by a Solid State Shocker/Distributor, Coulbourn Instruments Inc., Lehigh Valley, PA).
Procedure
One session comprised of 100 trials. Each trial consisted of a 10 s light followed by a 10 s electrical foot shock (0.6 mA), which started on the last second of light presentation. The ITI was 30 s ± 25%. Rats could exhibit one of the following behaviors: (1) Avoidance, (2) Escape or (3) No Escape, as described above.
Statistical analysis
The results are expressed as means ± SEM. For statistical analysis, a one-way ANOVA test was applied. For post-hoc comparisons, the LSD contrast test was used with a confidence of 0.05.
Results
Effects of controllability on stress levels and on depression indices
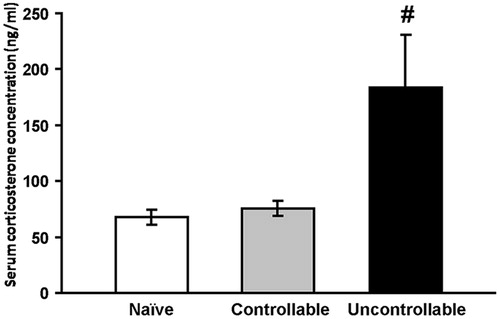
Corticosterone concentration in the circulating blood was measured 24 h after concluding six days of the prolonged TWS avoidance training. Corticosterone concentration of the controllable group was similar to that of the naïve group, both were significantly lower than that of the uncontrollable group [F(2,19) = 6.0, p < 0.01] ().
Figure 1. Concentration of corticosterone.
Basal circulating corticosterone concentration 24 h after the end of the prolonged training protocol (uncontrollable group: n = 7, naïve group: n = 7 and controllable group n = 8).
#Significantly different from naïve and controllable groups (one-way ANOVA, p < 0.01).
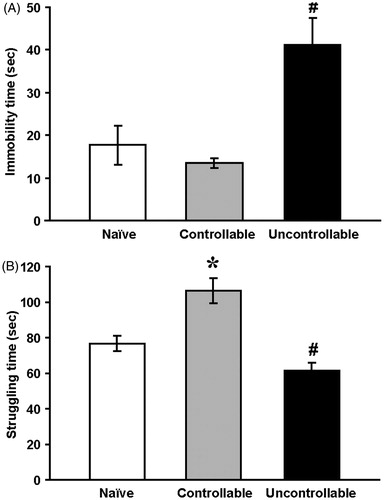
In accordance, immobility time duration in the FST was not different between the naïve and the controllable group but significantly higher in the uncontrollable group [F(2,27) = 9.9, p < 0.001] (). In contrast, struggling duration of the controllable group was significantly higher than that of the naïve and uncontrollable groups [F(2,27) = 17.7, p < 0.001], and struggling duration of the uncontrollable group was significantly lower than that of the naïves ().
Figure 2. Forced swim test.
Immobility time (a) and the amount of time spent struggling (b) of the uncontrollable group (n = 9), naïve group (n = 12) and controllable group (n = 9). One-way ANOVA was used.
*Significantly different from naïve and uncontrollable groups (p < 0.01); #Significantly different from naïve and controllable groups (p < 0.01).
The effect of controllability on learning a positively reinforcing task
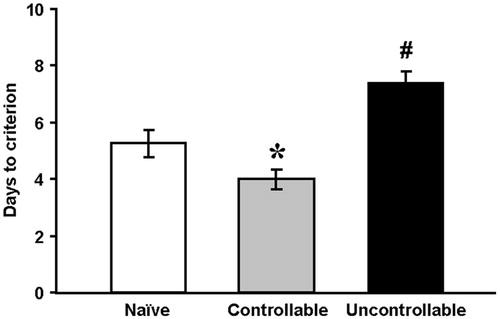
To assess whether controllability affected learning mechanisms we used the positively reinforcing OD task 24 h after the last training session in the TWS. The comparison variable for the OD task was the number of days to reach criterion (days to criterion). The one-way ANOVA revealed a significant effect of group on days to criterion [F(2,26) = 22.2, p < 0.001]. Post-hoc LSD testing indicated that discrimination learning in the controllable group was significantly faster than that of the naïve and the uncontrollable groups. The learning process of the uncontrollable group was significantly slower than that of the naïves and the controllable groups ().
Figure 3. Learning a positively reinforcing task: the olfactory discrimination task.
Performance on the olfactory discrimination task was determined by the number of days it took for the rats to reach criterion (controllable group: n = 11, naïve group: n = 8, and uncontrollable group: n = 10).
One-way ANOVA was used.
*Significantly different from naïve and uncontrollable groups (p < 0.01); #Significantly different from naïve and controllable groups (p < 0.01).
Longevity of the effects of controllability
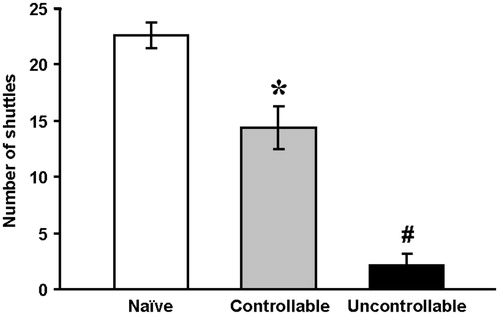
The exploratory behavior in a novel setting was measured one month after the end of the exposure to either the controllable or uncontrollable protocols. The one-way ANOVA revealed a significant effect of group on exploratory behavior in a novel setting 1 month after training. Exploratory behavior of the uncontrollable group was significantly lower than that of the naïve and controllable groups [F(2,42) = 53.7, p < 0.001]. Exploratory behavior of the controllable group was significantly higher than that of the uncontrollable, although significantly lower than that of the naïve group ().
Figure 4. Exploratory behavior in a novel setting one month after training.
Exploratory behavior of the uncontrollable (n = 12) group, naïve group (n = 19) and controllable group (n = 14) was determined by the number of shuttles performed in the novel shuttle boxes.
One-way ANOVA.
*Significantly different from naïve and uncontrollable groups (p < 0.01); #Significantly different from naïve and controllable groups (p < 0.01).
The visual TWS avoidance task
A contextually different shuttle box with a visual CS rather than auditory was used for this purpose. Rats were tested in the visual TWS one month after the last training session of the auditory TWS.
Avoidance responses
The one-way ANOVA revealed a significant effect of group on percent of avoidance responses during the visual TWS avoidance task (). The controllable group exhibited significantly more avoidance responses compared to the naïve and the uncontrollable groups [F(2,41) = 77.1, p < 0.001].
Figure 5. Learning under stressful conditions: the visual TWS avoidance task.
Avoidance responses (A), escape responses (B) and no escape responses (C) in the visual TWS avoidance task (controllable group: n = 14; naïve group: n = 18 and uncontrollable group: n = 12).
One-way ANOVAs were used for all statistical comparisons.
*Significantly different from naïve and uncontrollable groups (p < 0.01); &Significantly different from the naïve group (p < 0.01); #Significantly different from naïve and controllable groups (p < 0.01).
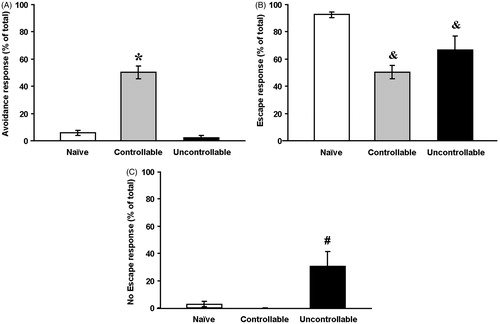
Escape responses
The one-way ANOVA revealed a significant effect of group on percent of escape responses [F(2,41) = 15.2, p < 0.001]. Both the controllable and the uncontrollable groups exhibited significantly less escape responses compared to the naïve group. No significant difference was found between the controllable and the uncontrollable groups ().
No Escape responses
The one-way ANOVA revealed a significant effect of group on percent of no escape responses [F(2,41) = 9.7, p < 0.001]. No Escape responses of the uncontrollable group exhibited significantly more of these responses compared to the naïve and the controllable groups ().
Discussion
The current findings confirm previous results we had obtained using the prolonged training protocol (Ilin & Richter-Levin, Citation2009). Rats that were exposed to the prolonged controllable training protocol demonstrated emotional controllability (i.e. reduced level of fear) as was reflected by concentration of blood circulating corticosterone and by higher levels of exploratory behavior compared with their counterpart uncontrollable group.
The impact of the prolonged uncontrollable training went beyond simply maintaining high level of fear. In the forced swim test these rats exhibited increased immobility and reduced level of struggling compared not only to the controllable group but also to the naïve rats. These results are in agreement with previous findings demonstrating depression of active behavior in uncontrollable rats relative to controllable rats (Weiss et al., Citation1981). Since immobility is interpreted as an index of depression (Arushanian & Makushkina, Citation1989, Porsolt et al., Citation1978) we propose that our uncontrollable protocol induces a form of learned helplessness and depressive symptoms.
The hypothalamic–pituitary–adrenal (HPA) axis and its final effectors, the glucocorticoids, are essential components of an individual's capacity to cope with stress. Hyperactivity of the HPA axis is a common finding in patients with major depression (reviewed by Arborelius et al., Citation1999; de Kloet et al., Citation2005). We found that 24 h post-training corticosterone concentration of the uncontrollable group still showed increased HPA axis responsiveness as compared to naïve and controllable rats, lending further support to the notion that these rats have developed symptoms relevant to depression. This group also exhibited long lasting (1 month) reduction of exploratory behavior, a behavior is a commonly used index of anxiety and depression (Campbell et al., Citation2003; Gregus et al., Citation2005; Kalynchuk et al., Citation1997; Ramos & Mormede, Citation1998).
In addition, the uncontrollable rats exhibited impaired ability to acquire subsequent learning tasks, whether these were positively or negatively reinforced. Not only did they perform worse than the controllable group but they were also impaired compared to the naïve rats. These findings are in agreement with previous studies that demonstrated that exposure of rats to uncontrollable stress hampered their ability to learn to escape from footshock in a shuttle box, effects that were found to be related to activation of the dorsal raphe nucleus (Maier & Watkins, Citation2005). Later, Amat et al. (Citation2006) extended these findings to show that these effects of uncontrollable stress on raphe activation and on behavior are mediated by the ventral medial prefrontal cortex.
Anxiety- and depression-related disorders are the most prevalent psychiatric conditions and frequently show co-morbidity (Krishnan, Citation2003; Rouillon, Citation1999). The interesting overlap between the emotional changes observed in depression and anxiety disorders suggest a common psychopathological pathway that may account for the high co-morbidity between these disorders (Hettema, Citation2008; Mergl et al., Citation2007). Less marked, but equally relevant, are the cognitive changes observed in depression and anxiety disorders (Castaneda et al., Citation2008), and the idea of a continuum between emotional changes and cognitive impairments has recently gained support (Sotiropoulos et al., Citation2008; Swaab et al., Citation2005). In agreement with that, the uncontrollable rats exhibited impaired learning abilities both in the OD and visual TWS tasks, 24 h and 1 month after training. Thus, the present results confirm and extend previously published work supporting the notion that uncontrollable stress could result in depressive-like behaviors (Bessa et al., Citation2009; Weiss et al., Citation1981). Much of the research made on animal models of depression employs chronic uncontrollable stress. In those models, chronic moderate stressors are applied over considerable time (3 weeks to 3 months) (Henn & Vollmayr, Citation2005; Strekalova et al., Citation2004; Willner, Citation1997; Willner et al., Citation1992). In our model, we used a much briefer stress paradigm (six days), and found it to be nevertheless effective in inducing long-lasting depressive-like symptoms. Furthermore, the findings that adverse consequences of our protocol could still easily be detected one month after the experience () indicate that this protocol produces long-lasting symptoms, and is thus an effective protocol to be used for testing the impact of prolonged pharmacological treatment.
Frequently, in studies referring to controllable versus uncontrollable (aversive) experience, the protocol used is of a single day exposure (Amat et al., Citation2006; Bland et al., Citation2006; Brennan et al., Citation2003; Drugan et al., Citation1985; Heinsbroek et al., Citation1991; Rozeske et al., Citation2009; Tanaka, Citation1999). At the end of that acute stress protocol, rats that were subjected to the controllable protocol might have gained a level of functional controllability over the situation, but the level of fear remains high (Ilin & Richter-Levin, Citation2009), indicating that while a level of functional controllability was achieved (i.e. the rats learned to avoid the shock by shuttling) an emotional controllability was not gained yet. Nevertheless, our prolonged (six days) training protocol enabled the animal to gain emotional controllability as was evident by reduced fear responses (Ilin & Richter-Levin, Citation2009; ). This group, that achieved emotional controllability exhibited longer duration of struggling behavior as compared to both naïve and uncontrollable groups, suggesting that these rats adopted a more active coping strategy than the other two groups. Furthermore, this controllable group showed enhanced odor discrimination learning () and better abilities for learning under stress compared not only to the uncontrollable rats but also to naïve rats (). It is important to note that superior learning abilities cannot be attributed to familiarity with the task, since these were observed not only in the visual two-way shuttle avoidance task, but also in the odor discrimination task, which is quite different from the avoidance tasks and is a positively reinforcing task.
Although with a different behavioral task (operant escape/avoidance task), Tsuda & Tanaka (Citation1985) did examine and compare the outcome of a single session training with that of a prolonged training protocol. They too describe two stages of acquiring the task: an initial stage, in which animals have not yet firmly acquired the behaviors necessary for effective coping, and a later stage, in which animals have mastered the coping task. They found that noradrenaline turnover normalized in the controllable animals only when they have reached that second level of acquiring the task, a stage we termed as emotional controllability, after five training days. Employing avoidance–escape training, in which the rat was able to control shock by performing a wheel-turning response, Coco & Weiss (Citation2005) also examined the effects of prolonged training (six training sessions followed by a test session were given over a period of four weeks). This study found that control over a stressor, as opposed to having no control, gave rise to augmented neural activation (measured by fos activation) mainly in the mesocorticolimbic dopaminergic system, suggesting that mastering controllability is associated with activation of “reward” signals.
Based on previous results (Ilin & Richter-Levin, Citation2009) we suggested a distinction between “operational controllability” and “emotional controllability”, since rats that were given a single training day, although already demonstrated operational learning (i.e. avoidance responses), still exhibited high levels of fear, indicating that emotionally, they had not yet gained control. Only following prolonged training a reduction in the fear response started to accompany the avoidance behavior, indicating the gaining of “emotional controllability”. In the present study, we have employed the prolonged exposure protocol that indeed led to the development of “emotional controllability”. Although not explicitly indicated, it is postulated that the development of only “operational controllability” would not be sufficient and “emotional controllability” is required for achieving resilience. There is no simple way of making a direct comparison of that sort, since developing “operational controllability” requires only two days of training while “emotional controllability” requires five or more days of training. Few studies that have indirectly addressed the question of cross-task transfer of learning in relation to shuttle avoidance tasks (e.g. Brush et al., 1963) suggest that a single training day is not sufficient for inducing “coping”-associated effects, but more studies are required in order to clarify this assumption.
Gaining controllability over an aversive situation has long been associated with beneficial effects on subsequent coping abilities of the animal (Mineka & Hendersen, Citation1985). More recently, such findings are starting to be discussed in the context of stress resilience and the potential implications of decreasing the likelihood of developing stress-induced psychopathology (Southwick et al., Citation2005). The recognition of the potential for positive outcomes of stressful experiences is one of the most interesting developments in the area of stress and coping (Moskowitz et al., Citation2003). Coping responses may be modified through experience with effort-based rewards – leading to an increased sense of control over one's environment, reduced fear and enhanced resilience in stressful situations (Lambert, Citation2006; Ursin & Olff, Citation1993; Weiss, Citation1968). Our prolonged training protocol with the demonstrated ability of animals to gain emotional controllability and to demonstrate enhanced ability for learning under stress is thus proposed as a useful tool to study the neurobiological and physiological mechanisms of stress resilience.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by a 2007research grant to G.R-L from the Institute for the study of affective Neuroscience (ISAN) at the University of Haifa endowed by the Hope for Depression Research Foundation (HDRF), by a 1403/07 grant of the Israel Science Foundation, and by a grant from the German Israeli Project Cooperation (DIP).
References
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. (2006). Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci 26(51):13264–72
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. (1999). The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160(1):1–12
- Arushanian EB, Makushkina EN. (1989). [Behavioral “despair” in female rats and gonadal function]. Zh Vyssh Nerv Deiat Im I P Pavlova 39(6):1129–33
- Bessa JM, Mesquita AR, Oliveira M, Pego JM, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. (2009). A trans-dimensional approach to the behavioral aspects of depression. Front Behav Neurosci 3:1
- Bland ST, Schmid MJ, Greenwood BN, Watkins LR, Maier SF. (2006). Behavioral control of the stressor modulates stress-induced changes in neurogenesis and fibroblast growth factor-2. Neuroreport 17(6):593–7
- Brennan FX, Grahn RE, Watkins LR, Maier SF. (2003). Serum cholesterol levels and stressor controllability in rats. Physiol Behav 79(4–5):757–60
- Brown PL, Hurley C, Repucci N, Drugan RC. (2001). Behavioral analysis of stress controllability effects in a new swim stress paradigm. Pharmacol Biochem Behav 68(2):263–72
- Brush FR, Myer JS, Palmer ME. (1963). The effects of kind of prior training and intersession interval upon subsequent avoidance learning. J Comp Physiol Phychol 56(3):539--45
- Campbell T, Lin S, DeVries C, Lambert K. (2003). Coping strategies in male and female rats exposed to multiple stressors. Physiol Behav 78(3):495–504
- Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lonnqvist J. (2008). A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord 106(1–2):1–27
- Coco ML, Weiss JM. (2005). Neural substrates of coping behavior in the rat: possible importance of mesocorticolimbic dopamine system. Behav Neurosci 119(2):429–45
- de Kloet ER, Joels M, Holsboer F. (2005). Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6(6):463–75
- Drugan RC, Ader DN, Maier SF. (1985). Shock controllability and the nature of stress-induced analgesia. Behav Neurosci 99(5):791–801
- Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. (2005). Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res 156(1):105–14
- Heinsbroek RP, van Haaren F, Feenstra MG, Boon P, van de Poll NE. (1991). Controllable and uncontrollable footshock and monoaminergic activity in the frontal cortex of male and female rats. Brain Res 551(1–2):247–55
- Henn FA, Vollmayr B. (2005). Stress models of depression: forming genetically vulnerable strains. Neurosci Biobehav Rev 29(4–5):799–804
- Hettema JM. (2008). The nosologic relationship between generalized anxiety disorder and major depression. Depress Anxiety 25(4):300–16
- Ilin Y, Richter-Levin G. (2009). ERK2 and CREB activation in the amygdala when an event is remembered as “fearful” and not when it is remembered as “instructive”. J Neurosci Res 87(8):1823–31
- Kalynchuk LE, Pinel JP, Treit D, Kippin TE. (1997). Changes in emotional behavior produced by long-term amygdala kindling in rats. Biol Psychiatry 41(4):438–51
- Krishnan KR. (2003). Comorbidity and depression treatment. Biol Psychiatry 53(8):701–6
- Lambert KG. (2006). Rising rates of depression in today's society: consideration of the roles of effort-based rewards and enhanced resilience in day-to-day functioning. Neurosci Biobehav Rev 30(4):497–510
- Maier SF, Grahn RE, Watkins LR. (1995). 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci 109(3):404–12
- Maier SF, Seligman ME, Solomon RL. (1969). Pavlovian fear conditioning and learned helplessness. In: Campbell BA, Church RM, editors. Punishment and aversive behavior. New York: Appleton-Century-Crofts. p 299--343
- Maier SF, Watkins LR. (2005). Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev 29(4–5):829–41
- Mergl R, Seidscheck I, Allgaier AK, Moller HJ, Hegerl U, Henkel V. (2007). Depressive, anxiety, and somatoform disorders in primary care: prevalence and recognition. Depress Anxiety 24(3):185–95
- Mineka S, Hendersen RW. (1985). Controllability and predictability in acquired motivation. Annu Rev Psychol 36:495–529
- Minor TR, Dess NK, Ben-David E, Chang WC. (1994). Individual differences in vulnerability to inescapable shock in rats. J Exp Psychol Anim Behav Process 20(4):402–12
- Moskowitz JT, Folkman S, Acree M. (2003). Do positive psychological states shed light on recovery from bereavement? Findings from a 3-year longitudinal study. Death Stud 27(6):471–500
- Overmier JB, Seligman ME. (1967). Effects of inescapable shock upon subsequent escape and avoidance responding. J Comp Physiol Psychol 63(1):28–33
- Porsolt RD, Anton G, Blavet N, Jalfre M. (1978). Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47(4):379–91
- Ramos A, Mormede P. (1998). Stress and emotionality: a multidimensional and genetic approach. Neurosci Biobehav Rev 22(1):33–57
- Rouillon F. (1999). Anxiety with depression: a treatment need. Eur Neuropsychopharmacol 9(Suppl 3):S87–92
- Rozeske RR, Der-Avakian A, Bland ST, Beckley JT, Watkins LR, Maier SF. (2009). The medial prefrontal cortex regulates the differential expression of morphine-conditioned place preference following a single exposure to controllable or uncontrollable stress. Neuropsychopharmacology 34(4):834–43
- Saar D, Grossman Y, Barkai E. (1999). Reduced synaptic facilitation between pyramidal neurons in the piriform cortex after odor learning. J Neurosci 19(19):8616–22
- Saar D, Grossman Y, Barkai E. (2001). Long-lasting cholinergic modulation underlies rule learning in rats. J Neurosci 21(4):1385–92
- Seligman ME, Maier SF. (1967). Failure to escape traumatic shock. J Exp Psychol 74(1):1–9
- Shors TJ, Seib TB, Levine S, Thompson RF. (1989). Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science 244(4901):224–6
- Sotiropoulos I, Cerqueira JJ, Catania C, Takashima A, Sousa N, Almeida OF. (2008). Stress and glucocorticoid footprints in the brain-the path from depression to Alzheimer's disease. Neurosci Biobehav Rev 32(6):1161–73
- Southwick SM, Vythilingam M, Charney DS. (2005). The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol 1:255–91
- Staubli U, Fraser D, Faraday R, Lynch G. (1987). Olfaction and the “data” memory system in rats. Behav Neurosci 101(6):757–65
- Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. (2004). Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 29(11):2007–17
- Swaab DF, Bao AM, Lucassen PJ. (2005). The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev 4(2):141–94
- Swenson RM, Vogel WH. (1983). Plasma catecholamine and corticosterone as well as brain catecholamine changes during coping in rats exposed to stressful footshock. Pharmacol Biochem Behav 18(5):689–93
- Tanaka M. (1999). Emotional stress and characteristics of brain noradrenaline release in the rat. Ind Health 37(2):143–56
- Tsuda A, Tanaka M. (1985). Differential changes in noradrenaline turnover in specific regions of rat brain produced by controllable and uncontrollable shocks. Behav Neurosci 99(5):802–17
- Ursin H, Olff M. (1993). Psychobiology of coping and defence strategies. Neuropsychobiology 28(1–2):66–71
- Warren DA, Rosellini RA. (1988). Effects of librium and shock controllability upon nociception and contextual fear. Pharmacol Biochem Behav 30(1):209–14
- Weiss JM. (1968). Effects of coping responses on stress. J Comp Physiol Psychol 65(2):251–60
- Weiss JM, Goodman PA, Losito BG, Corrigan S, Charry JM, Bailey WH. (1981). Behavioral depression produced by an uncontrollable stressor: relationship to norepinephrine, dopamine, and serotonin levels in various regions of the rat brain. Brain Res Rev 3:167–205
- Will MJ, Watkins LR, Maier SF. (1998). Uncontrollable stress potentiates morphine's rewarding properties. Pharmacol Biochem Behav 60(3):655–64
- Willner P. (1997). Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 134(4):319–29
- Willner P, Muscat R, Papp M. (1992). Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev 16(4):525–34
