Abstract
Behavioral modifications for the treatment of obesity, including caloric restriction, have notoriously low long-term success rates relative to bariatric weight-loss surgery. The reasons for the difference in sustained weight loss are not clear. One possibility is that caloric restriction alone activates the stress-responsive hypothalamo-pituitary-adrenocortical (HPA) axis, undermining the long-term maintenance of weight loss, and that this is abrogated after bariatric surgery. Accordingly, we compared the HPA response to weight loss in five groups of male rats: (1) high-fat diet-induced obese (DIO) rats treated with Roux-en-Y gastric bypass surgery (RYGB, n = 7), (2) DIO rats treated with vertical sleeve gastrectomy (VSG, n = 11), (3) DIO rats given sham surgery and subsequently restricted to the food intake of the VSG/RYGB groups (Pair-fed, n = 11), (4) ad libitum-fed DIO rats given sham surgery (Obese, n = 11) and (5) ad libitum chow-fed rats given sham surgery (Lean, n = 12). Compared with Lean controls, food-restricted rats exhibited elevated morning (nadir) non-stress plasma corticosterone concentration and increased hypothalamic corticotropin-releasing hormone and vasopressin mRNA expression, indicative of basal HPA activation. This was largely prevented when weight loss was achieved by bariatric surgery. DIO increased HPA activation by acute (novel environment) stress and this was diminished by bariatric surgery-, but not pair-feeding-, induced weight loss. These results indicate that the HPA axis is differentially affected by weight loss from caloric restriction versus bariatric surgery, and this may contribute to the differing long-term effectiveness of these two weight-loss approaches.
Introduction
Weight loss through caloric restriction and exercise is the first-line recommendation for obesity and its associated disorders (i.e. type-2 diabetes, hyperlipidemia, hypertension, sleep apnea and polycystic ovarian syndrome). Even though in the clinic a 10% body weight loss positively impacts metabolic disease processes (Wing et al., Citation2011), long-term weight loss is difficult to achieve and there is a high rate of recidivism to lifestyle modification (Pories et al., Citation1995). Although some pharmacologic therapies have proven useful for eliciting weight loss, maintaining the reduced body weight is often not possible following discontinuation of the pharmacologic agent. Overall, reductions in body weight by either behavior modification or pharmacotherapy last about 1–2 years (Pories et al., Citation1995). In the last decade, bariatric surgery has been increasingly used to achieve long-term durable weight loss with sustained body weight loss beyond 15 years following surgery (Pories et al., Citation1995). Bariatric surgery consists of procedures which manipulate the gut through invasive removal or rerouting of parts of the gastrointestinal tract to produce body weight reduction (Stefater et al., Citation2012). The mechanisms by which bariatric surgery produces sustained weight loss are not clear. One possibility is that weight loss via behavioral modifications may activate counter-regulatory systems, such as the hypothalamo-pituitary-adrenocortical (HPA) axis, whereas weight loss via bariatric surgery may not.
Activation of the HPA axis is one of the primary physiological responses to stress. This system has a low basal level of activity that varies with time of day, with enhanced activation occurring in response to stress (Herman et al., Citation2003). During stressor exposure, hypophysiotropic neurons in the paraventricular nucleus of the hypothalamus (PVH) are activated and secrete corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP), each of which acts on the anterior pituitary to promote the secretion of adrenocorticotropic hormone (ACTH) into the systemic circulation. ACTH, in turn, stimulates the zona fasciculata of the adrenal cortex to evoke the production and release of glucocorticoid hormones (i.e. corticosterone in rats and cortisol in humans). Glucocorticoids have numerous effects throughout the body, including mobilizing stored energy to promote survival during stress, and exerting negative feedback on the brain and anterior pituitary to limit further HPA activity. During chronic stress, adaptations of the HPA axis include elevated basal glucocorticoids at the nadir of the circadian rhythm, maintained or elevated HPA responses to a novel stressor that occur despite a history of glucocorticoid negative feedback, and increased expression of CRH and AVP mRNA in the PVH (Ulrich-Lai & Herman, Citation2009). Collectively, these changes result in increased cumulative glucocorticoid exposure during chronic stress, resulting in a number of negative side-effects, including diminished cognitive function (McEwen, Citation2001; Sapolsky et al., Citation1985), immune dysfunction (e.g. thymic atrophy) (Dimitrijevic et al., Citation2012; Elenkov & Chrousos, Citation2002), osteoporosis (Mitchell & Lyles, Citation1990; Patschan et al., Citation2001), and increased risk for mood and anxiety disorders (Tafet & Bernardini, Citation2003).
Importantly, such increases in HPA tone could undermine long-term weight-loss success, as caloric restriction can increase circulating glucocorticoids (Flak et al., Citation2011; Pankevich et al., Citation2010) and glucocorticoids are associated with increased food intake and body weight (Dallman, Citation2010; Green et al., Citation1992; Ulrich-Lai & Ryan, Citation2014). Moreover, glucocorticoids are linked to reduced behavioral flexibility promoting retention of “habits” (Cerqueira et al., Citation2005; Schwabe & Wolf, Citation2009; Soares et al., Citation2012). As such, the present work addresses the hypothesis that behavior-based weight-loss methods such as “dieting” or food restriction activate the HPA axis, whereas weight loss following bariatric surgery does not. In the present study, we directly compared HPA axis function in calorie-restricted rats to those losing comparable body weight following two commonly performed bariatric surgical procedures (Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG)), as well as to sham-operated chow and high-fat diet (HFD)-fed controls. We assayed plasma corticosterone and ACTH under unstressed conditions and during novel environment exposure as an index of HPA axis function. We also assessed the mRNA expression of stress-related genes in the hypothalamus.
Methods
Experimental animals
All procedures for animal use were approved by the University of Cincinnati Institutional Animal Care and Use Committee. Adult male Long-Evan rats (N = 60; 250–300 g; Harlan Laboratories, Indianapolis, IN) were individually housed and maintained in a room on a 12/12-h (lights on at 06:00 h and off at 18:00 h) light/dark cycle at 25 °C and 50–60% humidity. Following seven days acclimatization to the facilities, rats were given ad libitum access to water and palatable HFD (#D03082706, Research Diets, New Brunswick, NJ, 4.54 kcal/g; 41% fat), or were maintained on low-fat chow (#7012, Harlan Teklad, 3.41 kcal/g; 5.67% fat) for seven weeks prior to surgery. Rats were matched for body weight and assigned to 1 of 5 treatment groups: (a) maintained on chow and receiving sham-VSG (Lean), (b) maintained on HFD and receiving sham-VSG (Obese), (c) maintained on HFD and receiving VSG surgery (VSG), (d) maintained on HFD and receiving RYGB (RYGB) or (e) restricted to the average daily HFD intake of the VSG and RYGB groups and receiving Sham-VSG (Pair-fed). Pair-fed rats received their allotment of food once daily at a variable time of day (typically 4–8 h after lights on) to prevent anticipation of the food allotment and HPA axis entrainment (with potential metabolic consequences) to a scheduled meal (Honma et al., Citation1986). It should be noted that typically if an animal loses weight via food restriction, once they are returned to ad libitum feeding they will overeat to compensate for the prior period of reduced intake, thereby returning to their original (defended) body weight (Campfield & Smith, Citation1986; Heiderstadt et al., Citation2000). Notably, VSG and RYGB act, at least in part, by preventing this compensatory overeating (Chambers et al., Citation2011; Stefater et al., Citation2010). For instance, typically VSG and RYGB markedly reduce food intake and body weight during approximately days 3–14 postoperative, with food intake thereafter increasing to that of sham-operated controls. However, the initial weight loss is maintained despite receiving food ad libitum because VSG- and RYGB-treated rats do not compensate by overeating (Grayson et al., Citation2014).
Pre-operative care
One week prior to surgery, rats were given access to Osmolite OneCal liquid diet (Abbott, Columbus, OH) in addition to their normal solid food for two 24-h periods to familiarize them with this liquid diet. Four days prior to surgery, body composition was assessed using an Echo magnetic resonance image (MRI) analyzer (Houston, TX) in unanesthetized rats. Rats were solid-food restricted for 24 h prior to surgery and instead given free access to Osmolite.
Vertical sleeve gastrectomy
VSG was performed as previously described (Chambers et al., Citation2011; Grayson et al., Citation2014) using isoflurane anesthesia. Briefly, the surgery consisted of a midline abdominal laparotomy with exteriorization of the stomach. The lateral 80% of the stomach was excised using an Ethicon ETS 35-mm cutter/stapler gun, leaving a tubular gastric remnant in continuity with the esophagus. This gastric sleeve was then reintegrated into the abdominal cavity and the abdominal wall was closed in layers.
Sham-VSG
An abdominal laparotomy was performed as above. Manual pressure was applied with forceps to the exteriorized stomach and then the stomach was returned to the abdomen and the abdominal wall was closed in layers.
Roux-en-Y gastric bypass
RYGB was performed using isoflurane anesthesia as previously described (Chambers et al., Citation2011). After a midline laparotomy, the jejunum was exposed and transected 30 cm from the ligament of Treitz. A jejuna-jejunal anastomosis attached the proximal end of the jejunum to the distal cut jejunum allowing for a side-to-end “Y”. The exteriorized stomach was transected using an Ethicon ETS 35-mm cutter/stapler, and the distal gastric remnant was replaced. Another vertical staple line was made from the angle of His to remove most of the fundus. An anastomosis was made between the distal jejunal loop and the gastric pouch opening. The abdominal wall was closed in layers.
Post-operative care
Following surgery, rats received post-operative care for three days, consisting of injections of saline (5 ml, s.c., twice daily), Bupronex® (0.05 mg/kg, s.c., twice daily), and Metacam® (0.5 mg/kg, s.c., once daily). Rats were maintained on ad libitum Osmolite until their respective solid food was returned at three days following surgery. Body weight and food intake were monitored regularly through 30 days post-surgery.
Morning and evening non-stress blood collections
On post-operative day 45, tail blood was collected at approximately 45 min before the onset of dark (∼17:15 h, ∼circadian peak). On post-operative day 52, tail blood was taken within 1 hour after light onset (∼07:00 h, ∼circadian nadir). In both cases, rats were not disturbed for the preceding 18 h, and tail clip blood samples (∼200 μl) were collected quickly and quietly by trained personnel with the sampling completed within 3 min of first disturbing the rat to ensure assessment of pre-stress plasma corticosterone and ACTH concentrations, as described previously (Vahl et al., Citation2005). In all cases, blood samples were collected into EDTA-coated plastic tubes that were chilled on ice. Plasma was separated by centrifugation (6000 × g, 15 min, 4 °C) and stored at −80 °C until hormones were assayed (see below).
Novel environment challenge
A novel environment challenge was performed on post-operative days 60–61 to assess HPA activation by stress. At 2 h after the onset of the light phase, each rat was brought from the housing room into the procedure room. Pre-stress tail-blood samples (∼200 μl) were quickly collected by tail clip as described above to ensure assessment of pre-stress plasma corticosterone and ACTH (Vahl et al., Citation2005). Rats were then immediately placed into the bottom of a large, unfamiliar Plexiglas cylinder (approximately 25 cm in diameter) for 15 min. A second tail-blood sample (∼200 μl) was taken at the conclusion of the novel environment exposure and the rats were returned to their home cage. Additional tail-blood samples (∼200 μl) were collected in the home cage at 30, 60 and 120 min after the challenge onset. After collection of the 120-min sample, rats were euthanized by overdose with pentobarbital delivered intraperitoneally (1 mg/g body weight). The thymus and adrenal glands were removed, cleaned and weighed as general indices of HPA axis tone. Increased adrenal weight is often considered an index of greater prior cumulative ACTH exposure, as ACTH is an adrenal growth factor (Ulrich-Lai et al., Citation2006a). Similarly, reduced thymus weight is often considered an index of greater prior cumulative glucocorticoid exposure, as elevated glucocorticoids can induce thymic atrophy (Dimitrijevic et al., Citation2012; Ulrich-Lai et al., Citation2006b).
Plasma hormone assays: Corticosterone was measured by 125I radioimmunoassay kits from ICN Biochemicals (Cleveland, OH), which have an intra-assay coefficient of variation (CV) of 8.6%, an inter-assay CV of 13.6% and a minimum sensitivity of 12.5 ng/ml. Plasma ACTH concentrations were determined by RIA using a specific antiserum generously donated by Dr William C. Engeland (University of Minnesota, Minneapolis, MN) with [125I] ACTH (Amersham Biosciences, Piscataway, NJ) as the labeled tracer (Figueiredo et al., Citation2003); this assay has an intra-assay CV of 7.6%, an inter-assay CV of 13.3% and a minimum sensitivity of 15.6 pg/ml.
Semi-quantitative polymerase chain reaction
A second cohort of rats (n = 7–8/group) was prepared as described above; a second cohort of rats was used to avoid the possibility that the prior cohort's acute stress exposure could alter their hypothalamic mRNA expression. At 27–29 days post-surgery, rats were euthanized via CO2 asphyxiation, and brains were rapidly removed, frozen and stored at −80 °C until processing. Hand-dissection using gross anatomical landmarks (e.g. optic tract, pineal gland and fourth ventricle) was performed to isolate a block of tissue corresponding to the hypothalamus. Total RNA from microdissected tissue was isolated with Trizol reagent (Invitrogen, Carlsbad, CA) and purified using the RNeasy Mini Kit (Qiagen, Valencia, CA) with column DNAse treatment according to manufacturer's instructions. cDNA was then retrotranscribed from 1 to 2 μg of total RNA using the SuperScript III First Strand Synthesis Kit (Invitrogen, Carlsbad, CA). The cDNA was diluted and 100 ng of template cDNA from each sample was used to measure mRNA expression of selected genes by real-time quantitative PCR using pre-designed and validated Taqman PCR primer/probes sets (Applied Biosystems, Life Technologies, Grand Island, NY) on an ABI 7900HT Real-Time PCR System (Applied Biosystems, Inc.). We calculated mRNA expression relative to the housekeeping gene L32 (ABI) using the ΔΔ CT method. The following primer/probes were used: pomc (pro-opiomelanocortin, Rn00595020_m1), agrp (agouti-related transcript, Rn01431703_g1), crh CRH, Rn01462137_m1), avp (AVP, Rn00566449_m1), GR (nr3c1) (glucocorticoid receptor, Rn00561369_m1) and MR (nr3c2) (mineralocorticoid receptor, Rn00565562_m1).
Statistical analyses
For plasma hormone time course data, area-under-the-curve (AUC) was calculated using the trapezoidal rule. All statistical analyses were performed using GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA). Differences between two treatments or groups were assessed by two-tailed Student's t-test (e.g. EchoMRI body composition data). Differences between three or more treatments were analyzed using one-way analysis of variance (ANOVA) followed by a protected Tukey post-hoc test (e.g. hormone AUC, terminal organ weights and mRNA expression). To observe time-wise differences, two-way ANOVA with repeated measures was performed using Treatment Group and Time as factors (e.g. body weight, food intake and time course of plasma hormones). When there were significant main or interactive effects of Treatment Group, then specific differences between the various treatment groups were determined using a Bonferroni post-hoc test. All results are given as means ± SEM. Results were considered statistically significant when p < 0.05.
Results
Pair-feeding, VSG and RYGB resulted in comparable body weight loss
Prior to surgery, rats were given ad libitum access to HFD (or chow as controls) for seven weeks in order to produce diet-induced obesity. After seven weeks on these diets (), HFD-fed rats had significantly increased body weight [T53 = 4.34; p < 0.001] (). The majority of this increased body weight was due to increased fat mass [T53 = 5.13; p < 0.001] () with no change in lean body mass (), as determined by EchoMRI.
Figure 1. Metabolic parameters pre- and post-surgery. (A) Total, Lean and fat mass of male rats fed either chow (n = 12) or high-fat diet (HFD (n = 43) for seven weeks to induce obesity prior to surgical treatments. Student's t-test, *p < 0.05. (B) Body weights of Lean (chow-fed) and HFD-fed Obese, vertical sleeve gastrectomy (VSG), Roux-en-Y gastric bypass (RYGB) and Pair-fed (calorie-restricted) rats during the 30 days following surgical treatment. Two-way ANOVA with repeated measures followed by a Bonferroni post-hoc test, *p < 0.05 for Obese versus all other groups. (C) Daily average caloric intake during the 30 days post-surgery. Two-way ANOVA with repeated measures followed by a Bonferroni post-hoc test: *p < 0.05 for both Obese and Lean versus all other groups. (D) Daily average caloric intake normalized to body weight during the 30 days post-surgery. Two-way ANOVA with repeated measures followed by a Bonferroni post-hoc test: *p < 0.05 for both Obese and Lean versus all other groups. n = 7–13/group for panels B–D. Data are presented as mean ±SEM.
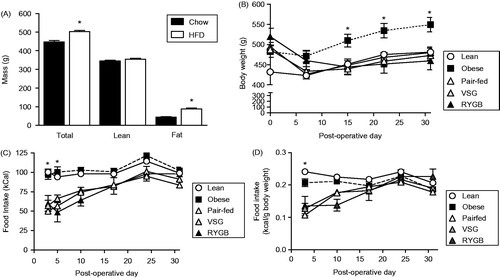
Rats then received their respective surgical treatment, and body weight and food intake were monitored. Post-surgical body weight () showed significant main effects of treatment group [F(4,196) = 3.29; p < 0.05], time [F(4,196) = 84.53; p < 0.001], and a significant treatment × time interaction [F(16,196) = 22.71; p < 0.001]. Post-hoc analysis showed that at 15–30 days post-surgery, the body weight of Obese rats was significantly greater than all other treatment groups. Moreover, VSG- and RYGB-treated rats lost > 10% of their body weight, as is typically seen after these procedures (Chambers et al., Citation2011; Grayson et al., Citation2014). These results indicate that (1) VSG and RYGB effectively reduced body weight to that of lean controls, and (2) caloric restriction (in the form of pair-feeding to the VSG and RYGB groups) produced the same extent of weight loss in Pair-fed rats as in VSG- and RYGB-treated rats.
Post-surgical total caloric intake () showed main effects of treatment group [F(4,215) = 13.45; p < 0.001] and time [F(5,215) = 28.48; p < 0.0001], with a treatment × time interaction [F(20,215) = 3.18; p < 0.001]). Post-hoc analysis showed that VSG, RYGB and Pair-fed groups all had decreased caloric intake during the first five post-operative days relative to both Lean and Obese controls. This indicates that VSG and RYGB significantly reduced initial post-operative calorie intake, with food intake then returning to that of the control groups, as is typically seen following these procedures (Grayson et al., Citation2014; Stefater et al., Citation2010). In addition, Pair-fed rats had a similar markedly reduced caloric intake initially after surgery, and thereafter consumed amounts comparable to the other groups (as would be expected, since these rats were given the same amount of food as that consumed by the VSG and RYGB groups). However, it is important to note that even at these later time points the Pair-fed group remained calorically restricted. That is, if these rats were allowed to consume ad libitum, they would actually overeat to compensate for the initial period of reduced intake in order to reach their original (defended) body weight (Stefater et al., Citation2010). Ongoing caloric restriction is required to constrain the food intake of the Pair-fed group to that of the VSG and RYGB groups in order to ensure that the Pair-fed group maintains reduced body weight.
Post-surgical food intake normalized to body weight () showed a similar pattern as seen in , with main effects of treatment [F(4,200) = 11.04; p < 0.001], time [F(4,200) = 33.53; p < 0.001] and a treatment × time interaction [F(16,200) = 9.08; p < 0.001]. Post-hoc analysis showed that the normalized caloric intake of the VSG, RYGB and Pair-fed groups was reduced when compared with both Lean and Obese controls at post-operative day 3, and this effect was normalized by post-operative day 10.
When the post-surgical body weight and food intake data are taken together, they indicate that bariatric surgery produced a significant and sustained weight loss that is accompanied by a transient reduction in caloric intake, as seen previously (Chambers et al., Citation2011; Grayson et al., Citation2014). Also, the pair-feeding (food restriction) procedure effectively matched the food intake and body weight loss of the bariatric surgery groups.
Non-stress HPA activity was differentially affected by pair-feeding versus bariatric surgery
Non-stress plasma corticosterone concentrations (; measured in the evening on post-operative day 45 and in the morning on post-operative day 52) indicated main effects of treatment [F(4,42) = 2.77; p < 0.05] and time [F(1,42) = 38.47; p < 0.001], with evening concentrations overall greater than the morning levels. There was no significant treatment × time interaction. Post-hoc analysis showed that plasma corticosterone concentration in the morning was elevated in Pair-fed rats relative to both Lean and Obese controls. In contrast, VSG- and RYGB-treated rats (of which both groups lost an equivalent amount of body weight to the Pair-fed group) did not have increased morning plasma corticosterone concentrations. Moreover, VSG-treated rats had significantly lower plasma corticosterone than Pair-fed rats, while RYGB-treated rats showed only a partial, non-significant reduction. These data indicate that caloric restriction-induced weight loss (pair-feeding) increased basal plasma corticosterone concentration near the circadian nadir, and that this increase was either completely or partially prevented by weight loss following VSG or RYGB, respectively.
Figure 2. Unstressed plasma corticosterone and adrenocorticotropic hormone (ACTH) concentrations in Lean (chow-fed) and high-fat diet (HFD)-fed Obese, vertical sleeve gastrectomy (VSG), Roux-en-Y gastric bypass (RYGB) and Pair-fed (calorie-restricted) rats. (A) Plasma corticosterone at 2 h after lights-on (morning) and at 1 hour prior to lights-off (evening). Differing letters denote significant differences between treatment groups (p < 0.05); two-way ANOVA with repeated measures followed by a Bonferroni post-hoc test. (B) Plasma ACTH at corresponding times to those in (A). n = 8–13/group. Data are shown as mean ± SEM.
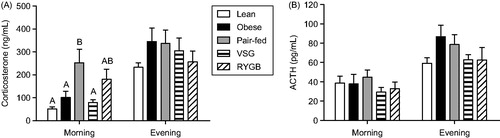
Non-stress plasma ACTH concentrations (; measured at same time points as plasma corticosterone) showed a main effect of time [F(1,44) = 53.38; p < 0.001], but no main effect of treatment, and no treatment × time interaction. These data indicate that there was a general increase in plasma ACTH concentration from morning to evening (as expected based on the circadian rhythm of HPA axis activity), and that this was unaltered by surgical treatment.
Post-stress HPA activity was differentially affected by pair-feeding versus bariatric surgery
The time course of the plasma corticosterone response to a 15-min novel environment stress was assessed on post-operative days 60–61 (). There was a main effect of treatment [F(4,184) = 4,68; p < 0.01] and time [F(4,184) = 124.3; p < 0.001], as well as a treatment × time interaction [F(16,184) = 1.86; p < 0.05]. Post-hoc analysis indicated that RYGB-treated rats had significantly lower plasma corticosterone compared to all other groups at 60 min after the onset of stress. The integrated (AUC) plasma corticosterone response to stress () varied with treatment [F(4,46) = 4.04; p < 0.01]. Post-hoc analysis showed that the integrated plasma corticosterone response was reduced by RYGB compared with Lean, Obese and Pair-fed groups. When the time course and integrated plasma corticosterone data are taken together, they indicate that the peak plasma corticosterone response to acute stress (at ∼30 min) was unaffected by treatment group. In addition, RYGB reduced the overall corticosterone response, in particular accelerating the recovery to baseline.
Figure 3. Acute post-stress plasma corticosterone and adrenocorticotropic hormone (ACTH) concentrations in Lean (chow-fed) and high-fat diet (HFD)-fed Obese, vertical sleeve gastrectomy (VSG), Roux-en-Y gastric bypass (RYGB) and Pair-fed (calorie-restricted) rats. (A) Plasma corticosterone response to a 15-min novel environment test (gray region in graph). Two-way ANOVA with repeated measures followed by a Bonferroni post-hoc test: *p < 0.05 for RYGB versus all other groups. (B) Area-under-the-curve (AUC) of the plasma corticosterone response shown in (A). One-way ANOVA followed by a Tukey post-hoc test: differing letters denote significant differences between treatment groups (p < 0.05). (C) Corresponding plasma ACTH concentrations from the acute novel environment test in A. Two-way ANOVA with repeated measures followed by a Bonferroni post-hoc test: *p < 0.05 versus Lean group; #p < 0.05 for Pair-fed versus Obese. (D) AUC of the plasma ACTH response shown in (C). One-way ANOVA followed by a Tukey post-hoc test: differing letters denote significant differences between treatment groups (p < 0.05). n = 7–13/group. Data are presented as mean ± SEM.
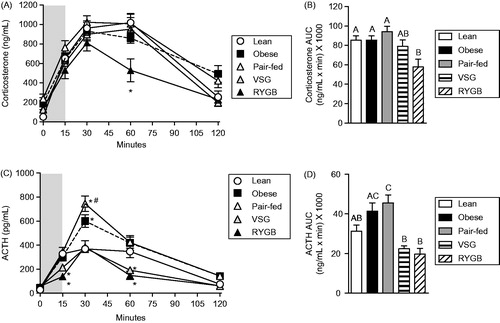
The time course of the plasma ACTH response to the 15-min novel environment stress () revealed main effects of treatment [F(4,188) = 13.8; p < 0.001] and time [F(4,188) = 199.8; p < 0.001], with a significant interaction of treatment and time [F(16,188) = 7.79; p < 0.001] (). Post-hoc analysis indicated that at 30 min after the onset of stress, Obese rats had increased plasma ACTH concentrations compared with Lean controls, and that this was further elevated in the Pair-fed group. In contrast, VSG and RYGB rats had reduced plasma ACTH concentrations at 15 and 60 min post-stress compared with Lean controls. Consistent with this, the integrated (AUC) plasma ACTH response to stress () varied with treatment group [F(4,47) = 11.97; p < 0.001]. More specifically, post-hoc analysis showed that both Obese and Pair-fed rats had increased integrated ACTH responses compared with Lean controls, whereas VSG- and RYGB-treated rats had lower integrated ACTH responses compared with Lean controls. When the time course and integrated plasma ACTH data are taken together, they indicate that obesity increased the peak ACTH response to stress, and that this was further increased by caloric restriction-induced weight loss (Pair-feeding). In contrast, VSG- and RYGB-induced weight loss not only reversed the potentiating effects of obesity, but also further reduced the ACTH response relative to Lean controls.
Adrenal and thymus weights
Terminal organ weights () were assessed immediately after the completion of the novel environment stress test (at 120 min post-stress on post-operative days 60–61). Thymus weight () differed between treatment groups [F(4,49) = 6.82; p < 0.001], with RYGB decreasing thymus weight relative to all other groups. Thymus weight normalized to body weight () was similarly affected by treatment [F(4,49) = 4.22; p < 0.01], with RYGB decreasing normalized thymus weight relative to the Lean and Pair-fed groups.
Figure 4. Terminal organ weights of Lean (chow-fed) and high-fat diet (HFD)-fed Obese, vertical sleeve gastrectomy (VSG), Roux-en-Y gastric bypass (RYGB) and Pair-fed (calorie-restricted) rats at 60 days after surgical treatments. (A) Thymus weight, (B) thymus weight normalized to body weight, (C) total adrenal weight (sum of left and right adrenals), and (D) total adrenal weight normalized to body weight. For all panels, differing letters denote statistically significant differences between treatment groups (p < 0.05): one-way ANOVA followed by a Tukey post-hoc test. n = 7–13/group. Data are presented as mean ± SEM.
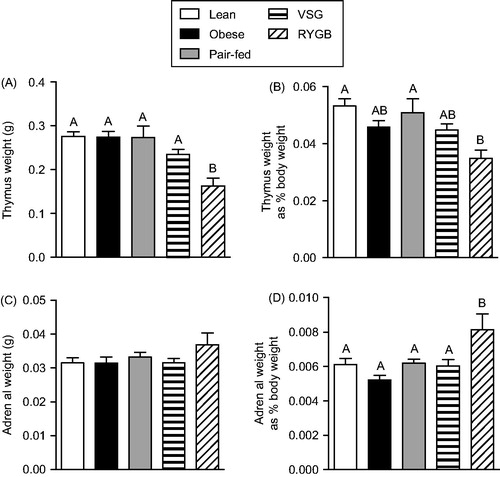
Total adrenal weight () was not altered by treatment group. However, adrenal weight normalized to body weight () was affected by treatment [F(4,49) = 5.3; p < 0.01], with RYGB increasing normalized adrenal weight relative to all other groups. Increased normalized adrenal weight coupled with reduced thymus weight in the RYGB-treated rats indicates a history of elevated HPA tone.
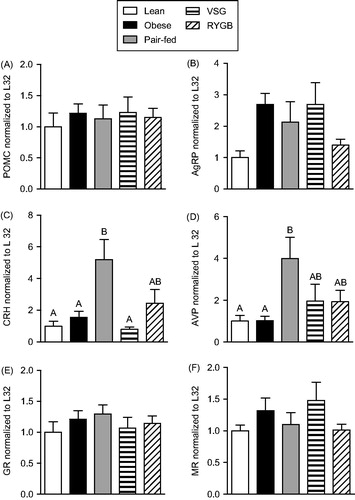
Hypothalamic mRNA expression of stress-related genes
The mRNA expression of each stress-related gene was normalized to L32 mRNA expression levels (there were no expression differences in this internal control (data not shown)). The hypothalamic expression of POMC (), GR () and MR () mRNAs was all unaffected by treatment group. AgRP mRNA expression () varied with treatment group [F(4,33) = 3.01; p < 0.05]. However, post-hoc analysis did not identify any specific group differences. CRH mRNA expression () varied with treatment group [F(4,33) = 5.65; p < 0.01], such that Pair-fed rats had greater CRH mRNA than Lean and Obese controls, with partial or complete prevention of this difference by RYGB or VSG, respectively. Similarly, AVP mRNA levels () varied with treatment group [F(4,34) = 3.35; p < 0.05], such that Pair-fed-, but not VSG- and RYGB-treated, rats had greater AVP mRNA than Lean and Obese controls. Taken together, these data indicate that caloric restriction-induced weight loss is associated with increased CRH and AVP mRNA expression in the hypothalamus, and that this effect is largely prevented in rats losing weight following bariatric surgery.
Figure 5. Hypothalamic mRNA expression in Lean (chow-fed) and high fat diet (HFD)-fed Obese, vertical sleeve gastrectomy (VSG), Roux-en-Y gastric bypass (RYGB) and Pair-fed (calorie-restricted) rats at 27–29 days after surgical treatments. (A) Pro-opiomelanocortin (POMC), (B) agouti-related peptide (AgRP), (C) Corticotropin-releasing hormone (CRH), (D) arginine-vasopressin (AVP), (E) glucocorticoid receptor (GR) and (F) mineralocorticoid receptor (MR). For all panels, differing letters denote statistically significant differences between treatment groups (p < 0.05); one-way ANOVA followed by a Tukey post-hoc test. Note that ANOVA identified an overall treatment effect for AgRP mRNA (p = 0.032), but no specific group differences were identified on subsequent post-hoc analysis. n = 7–8/group. Data are presented as mean ± SEM.
Discussion
The present work assessed whether weight loss via food restriction (by pair-feeding to the bariatric surgery groups) versus bariatric surgery differentially affects the HPA axis. Compared with Lean controls, weight loss by food restriction (Pair-fed) increased basal plasma corticosterone specifically in the morning, and also increased CRH and AVP mRNA expression in the hypothalamus. Together these results are indicative of increased basal HPA axis activity as a result of weight loss by caloric restriction. In contrast, bariatric surgery, which produced an equivalent amount of body weight loss, largely prevented these indices of basal HPA activation. Additionally, HFD-induced obesity increased the HPA axis (plasma ACTH) response to acute stress, and this was averted by weight loss following bariatric surgery, but not by food restriction.
The food restriction-induced increase in basal HPA tone resembles many of the HPA adaptations that occur in response to chronic stress, which similarly elevates morning non-stress plasma corticosterone concentration and hypothalamic CRH and AVP mRNA expression (Herman & Watson, Citation1995; Ulrich-Lai & Herman, Citation2009; Ulrich-Lai et al., Citation2007). Interestingly, food restriction-induced weight loss did not increase the HPA response to acute stress, perhaps because the elevated morning plasma corticosterone exerted more negative feedback, thereby attenuating HPA activation. Again, this resembles chronic stress facilitation, in which the HPA response to a novel acute stressor is maintained or enhanced despite a history of increased glucocorticoid negative feedback (Bhatnagar & Dallman, Citation1998). This indicates that caloric restriction-induced weight loss is sufficient to replicate many key features of chronic stress. Moreover, these findings may translate to people who are “dieting” to lose weight. Limited clinical data indicate that food restriction-induced weight loss in people is associated with increased plasma cortisol concentrations (Goodwin et al., Citation1988; Tomiyama et al., Citation2010; Witbracht et al., Citation2012). Glucocorticoids increase food intake, particularly driving the consumption of highly palatable, calorically dense foods (Dallman, Citation2010; Ulrich-Lai & Ryan, Citation2014) and undermine behavioral flexibility to promote continuation of past behavioral “habits” (Cerqueira et al., Citation2005; Schwabe & Wolf, Citation2009; Soares et al., Citation2012). Taken together, these findings indicate that increased HPA tone during weight loss by food restriction may undermine behavioral modifications to limit caloric intake, making it more difficult for people to maintain adherence to their “diet”, and resulting in poor long-term maintenance of weight loss.
Another important finding is that long-term intake of HFD, in addition to inducing obesity, was associated with increased HPA activation by acute stress, without altering indices of basal HPA tone. In particular, HFD-induced obesity increased the peak plasma ACTH, but not plasma corticosterone, response to acute stress. Such dissociations commonly occur and can result from many factors, including altered adrenal responsivity to ACTH, choice of sampling time points, and discrepancies between bioactive and immunoreactive ACTH (Bornstein et al., Citation2008; Engeland et al., Citation1989; Ulrich-Lai & Engeland, Citation2002). Collectively, these results are similar to other reports in which rodents consuming HFD (without other food options) for varying amounts of time have increased HPA activity (Kamara et al., Citation1998; Sharma et al., Citation2013; Tannenbaum et al., Citation1997), and are consistent with some clinical evidence suggesting that increased adiposity is associated with an exaggerated plasma cortisol response to psychological stress (Francis et al., Citation2013; Lu et al., Citation2014).
Importantly, bariatric surgery reduced much of the HPA activation that occurs with HFD-induced obesity or food restriction. For instance, the increased HPA response to acute stress that occurred with HFD-induced obesity (i.e. increased peak ACTH) was diminished by both bariatric surgical procedures, but not by food restriction, despite equivalent weight loss for these groups. This indicates that HPA axis activity may be more tuned to the manner in which body weight changes, rather than the extent of weight change per se. This likely occurs, at least in part, because bariatric surgery reduces the defended body weight (i.e. the rats could eat more but they choose not to) (Stefater et al., Citation2010), whereas food restriction does not (i.e. the rats would choose to eat more if given the opportunity) (Campfield & Smith, Citation1986; Heiderstadt et al., Citation2000). In this context, the present results indicate that counter-regulatory systems like the HPA axis may be engaged during food restriction in an attempt to restore body weight to the defended level, while this may not occur after bariatric surgery. To date, minimal work has been done to assess HPA axis function following weight loss after bariatric surgery in people, but there are reports that cortisol secretion is reduced following the types of bariatric surgery used here (Bevilacqua et al., Citation2010; Ruiz-Tovar et al., Citation2013). Additional studies will be needed to determine whether HPA axis activity is differentially regulated according to the weight loss method in humans, and the extent to which this contributes to the long-term maintenance of weight loss.
It should be noted that VSG and RYGB did not have identical effects on the HPA axis. For example, the increased basal HPA tone (i.e. elevated morning plasma corticosterone) that occurred with food restriction-induced weight loss was completely diminished by VSG but only partially by RYGB. Additionally, RYGB decreased the plasma corticosterone response to acute stress, increased adrenal weight normalized to body weight, and decreased thymus weight, whereas VSG did not. The effects of RYGB on adrenal and thymus weights resemble those that occur following chronic stress (Ulrich-Lai et al., Citation2006a). However, chronic stress typically also increases hypothalamic expression of CRH and AVP mRNA and facilitates the HPA response to a novel acute stressor (Ulrich-Lai & Herman, Citation2009), effects that are not observed in RYGB-treated rats. Interestingly, RYGB primarily reduced the post-stress plasma corticosterone concentration at 60 min after stress onset, implicating a faster rate of recovery to pre-stress levels. This suggests a possibility for increased corticosterone clearance, and/or increased corticosterone negative feedback following RYGB. Collectively, these data suggest that RYGB has complex, divergent effects on the HPA axis.
The reasons for the differing effects of RYGB and VSG on HPA function are not clear. The present data indicate that this does not result from variable effects on hypothalamic expression of metabolic (agrp, pomc)- and stress-regulatory (crh, avp, nr3c1, nr3c2) genes. It may be possible that differential actions of VSG and RYGB may result from effects on other stress-regulatory brain regions. For instance, VSG and RYGB differentially affect neuroinflammation in the hippocampus (Grayson et al., Citation2014). Moreover, VSG and RYGB may have dissimilar effects on metabolic hormones that in turn regulate HPA function, as VSG interferes with the ghrelin-producing portion of the stomach resulting in larger reductions in circulating ghrelin than RYGB (Grayson et al., Citation2014) and circulating ghrelin might then act to modify HPA axis activity. In addition, recent evidence suggests that VSG and RYGB may differentially affect glucose metabolism or insulin secretion under some circumstances (Roslin et al., Citation2014), which could then impact HPA function (Ulrich-Lai & Ryan, Citation2014).
Perspectives
Behavioral modifications such as reducing caloric intake are the first line of treatment for obesity. These approaches have a relatively low risk, but long-term compliance and efficacy are also low. In contrast, bariatric surgical procedures are invasive and relatively risky but result in greater enduring weight loss. It is possible that the differences in the success of these approaches may be due in part to their differing effects on stress response systems. Thus, increased HPA activation during “dieting” could undermine compliance and limit success, whereas this hurdle may not occur with bariatric surgery-induced weight loss.
Declaration of interest
This research was supported in part by NIH Awards DK56863, DK57900, U01CA141464, DK082480, MH069860, DK091425 and also work with Ethicon Endo-Surgery Inc., F. Hoffman-La Roche Ltd., Pfizer Inc. and Novo Nordisk A/S. BEG is also supported by NIH Award 1F32HD68103.BEG, APF, MK, HPL, AEE, IBR, SCW, JPH and YMU have no conflicts of interest to disclose. RJS and SCB have received research support from Ethicon Endo-Surgery. RJS also receives support from Mannkind, Novo Nordisk, Ablaris, Pfizer and Roche. RJS has served on scientific advisory boards for Ethicon Endo-Surgery, Angiochem, Novartis and Novo Nordisk, RJS is a paid speaker for Merck, Ethicon Endo-Surgery, Pfizer and Novo Nordisk.
Acknowledgements
The authors thank Jose Berger, Alfor Lewis, Ken Parks, Kathi Smith and Mouhamadoul Toure for their surgical expertise. We thank Dr William C. Engeland (University of Minnesota) for providing the ACTH antiserum. We also thank Benjamin Packard and Kristin Ludwick for technical assistance with the radioimmunoassays.
References
- Bevilacqua M, Dominguez LJ, Righini V, Vago T, Foschi D, Corsi F, Trabucchi E, et al. (2010). Acute parathyroid hormone increase by oral peptones administration after roux-en-Y gastric bypass surgery in obese subjects: role of phosphate in the rapid control of parathyroid hormone release. Surgery 147:655–61
- Bhatnagar S, Dallman M. (1998). Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84:1025–39
- Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. (2008). Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab 19:175–80
- Campfield LA, Smith FJ. (1986). Functional coupling between transient declines in blood glucose and feeding behavior: temporal relationships. Brain Res Bull 17:427–33
- Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N. (2005). Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci 25:7792–800
- Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Perez HE, Stefater MA, Gaitonde SG, et al. (2011). Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141:950–8
- Dallman MF. (2010). Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab 21:159–65
- Dimitrijevic M, Stanojevic S, Kustrimovic N, Leposavic G. (2012). End-point effector stress mediators in neuroimmune interactions: their role in immune system homeostasis and autoimmune pathology. Immunol Res 52:64–80
- Elenkov IJ, Chrousos GP. (2002). Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci 966:290–303
- Engeland WC, Miller P, Gann DS. (1989). Dissociation between changes in plasma bioactive and immunoreactive adrenocorticotropin after hemorrhage in awake dogs. Endocrinology 124:2978–85
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. (2003). The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci 18:2357–64
- Flak JN, Jankord R, Solomon MB, Krause EG, Herman JP. (2011). Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol Behav 104:228–34
- Francis LA, Granger DA, Susman EJ. (2013). Adrenocortical regulation, eating in the absence of hunger and BMI in young children. Appetite 64:32–8
- Goodwin GM, Fairburn CG, Keenan JC, Cowen PJ. (1988). The effects of dieting and weight loss upon the stimulation of thyrotropin (TSH) by thyrotropin-releasing hormone (TRH) and suppression of cortisol secretion by dexamethasone in men and women. J Affect Disord 14:137–44
- Grayson BE, Fitzgerald MF, Hakala-Finch AP, Ferris VM, Begg DP, Tong J, Woods SC, et al (2014). Improvements in hippocampal-dependent memory and microglial infiltration with calorie restriction and gastric bypass surgery, but not with vertical sleeve gastrectomy. Int J Obes (Lond) 38:349–56
- Green PK, Wilkinson CW, Woods SC. (1992). Intraventricular corticosterone increases the rate of body weight gain in underweight adrenalectomized rats. Endocrinology 130:269–75
- Heiderstadt KM, McLaughlin RM, Wright DC, Walker SE, Gomez-Sanchez CE. (2000). The effect of chronic food and water restriction on open-field behaviour and serum corticosterone levels in rats. Lab Anim 34:20–8
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai YM, Ostrander MM, Choi DC, Cullinan WE. (2003). Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24:151–80
- Herman JP, Watson SJ. (1995). Stress regulation of mineralocorticoid receptor heteronuclear RNA in rat hippocampus. Brain Res 677:243–9
- Honma K, Honma S, Hirai T, Katsuno Y, Hiroshige T. (1986). Food ingestion is more important to plasma corticosterone dynamics than water intake in rats under restricted daily feeding. Physiol Behav 37:791–5
- Kamara K, Eskay R, Castonguay T. (1998). High-fat diets and stress responsivity. Physiol Behav 64:1–6
- Lu Q, Tao F, Hou F, Zhang Z, Sun Y, Xu Y, Xu S, et al. (2014). Cortisol reactivity, delay discounting and percent body fat in Chinese urban young adolescents. Appetite 72:13–20
- McEwen BS. (2001). Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci 933:265–77
- Mitchell DR, Lyles KW. (1990). Glucocorticoid-induced osteoporosis: mechanisms for bone loss; evaluation of strategies for prevention. J Gerontol 45:M153–8
- Pankevich DE, Teegarden SL, Hedin AD, Jensen CL, Bale TL. (2010). Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. J Neurosci 30:16399–407
- Patschan D, Loddenkemper K, Buttgereit F. (2001). Molecular mechanisms of glucocorticoid-induced osteoporosis. Bone 29:498–505
- Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, et al. (1995). Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 222:339–50
- Roslin MS, Dudiy Y, Brownlee A, Weiskopf J, Shah P. (2014). Response to glucose tolerance testing and solid high carbohydrate challenge: comparison between Roux-en-Y gastric bypass, vertical sleeve gastrectomy, and duodenal switch. Surg Endosc 28:91–9
- Ruiz-Tovar J, Oller I, Galindo I, Llavero C, Arroyo A, Calero A, Diez M, et al. (2013). Change in levels of C-reactive protein (CRP) and serum cortisol in morbidly obese patients after laparoscopic sleeve gastrectomy. Obes Surg 23:764–9
- Sapolsky RM, Krey LC, McEwen BS. (1985). Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci 5:1222–7
- Schwabe L, Wolf OT. (2009). Stress prompts habit behavior in humans. J Neurosci 29:7191–8
- Sharma S, Fernandes MF, Fulton S. (2013). Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int J Obes (Lond) 37:1183–91
- Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques F, Palha JA, Cerqueira JJ, et al. (2012). Stress-induced changes in human decision-making are reversible. Transl Psychiatry 2:131–8
- Stefater MA, Perez-Tilve D, Chambers AP, Wilson-Perez HE, Sandoval DA, Berger J, Toure M, et al. (2010). Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 138:2426–36
- Stefater MA, Wilson-Perez HE, Chambers AP, Sandoval DA, Seeley RJ. (2012). All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev 33:595–622
- Tafet GE, Bernardini R. (2003). Psychoneuroendocrinological links between chronic stress and depression. Prog Neuropsychopharmacol Biol Psychiatry 27:893–903
- Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ. (1997). High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol 273:E1168–77
- Tomiyama AJ, Mann T, Vinas D, Hunger JM, Dejager J, Taylor SE. (2010). Low calorie dieting increases cortisol. Psychosom Med 72:357–64
- Ulrich-Lai YM, Engeland WC. (2002). Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology 76:79–92
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. (2006a). Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab 291:E965–73
- Ulrich-Lai YM, Herman JP. (2009). Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409
- Ulrich-Lai YM, Ryan KK. (2014). Neuroendocrine circuits governing energy balance and stress regulation: functional overlap and therapeutic implications. Cell Metab 19:910–25
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, et al. (2007). Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology 148:1823–34
- Ulrich-Lai YM, Xie W, Meij JT, Dolgas CM, Yu L, Herman JP. (2006b). Limbic and HPA axis function in an animal model of chronic neuropathic pain. Physiol Behav 88:67–76
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio DA, et al. (2005). Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 289:E823–8
- Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, et al. (2011). Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 34:1481–6
- Witbracht MG, Laugero KD, Van Loan MD, Adams SH, Keim NL. (2012). Performance on the Iowa Gambling Task is related to magnitude of weight loss and salivary cortisol in a diet-induced weight loss intervention in overweight women. Physiol Behav 106:291–7
