Abstract
Life events induce stress, which is considered to negatively impact the course of disease in patients with bipolar disorder (BD), its effects being predominantly mediated by cortisol. Cortisol in scalp hair has been identified as a biomarker for assessing long-term cortisol levels, and allows clarifying the relation between life events, hair cortisol concentrations (HCC), and clinical course over time. In 71 BD patients, we analyzed the proximal 3 cm of hair, reflecting 3 months of cortisol production, and investigated the association between HCC, the number of life events, the amount of social support, and mood in the 3 months prior to the hair assessment and between HCC and mood in the subsequent 3 months. Although the total number of life events was not associated with HCC (p > 0.05), the number of negative life events was associated with increased HCC (r2 = 0.04, p = 0.02). Social support showed an inverse association with HCC in patients reporting negative life events (r2 = 0.07, p = 0.03). HCC and mood were not associated in the 3 months prior to hair sampling or in the subsequent 3 months. This study indicates that patients who experienced recent negative life events have increased hair cortisol levels, which seem to be attenuated by social support.
Introduction
Evidence for stress as cause or amplifier of a wide range of somatic and mental disorders has been firmly established (Sapolsky, Citation2004). Individuals with a mental illness such as bipolar disorder (BD) are more likely to report the experience of a stressful life event and may also be more affected by the consequences of stress than healthy persons (Hosang et al., Citation2010). BD is a chronic mental illness that causes people to have one or more episodes of high (manic) and low (depressed) mood. The occurrence of life events as well as the hypothalamic-pituitary-adrenal (HPA) axis has been reported to play a potential role in the induction of a new affective episode (Daban et al., Citation2005, Johnson & Roberts, Citation1995). A life event is defined as a “dateable occurrence representing discrete changes in the subject’s social or personal environment that is external and verifiable rather than internal or psychological” (Paykel, Citation1994; Rafanelli et al., Citation2005). The availability or lack of social support is one of those environmental factors that have been widely recognized as a factor that may promote psychological well-being and physical health (Boutin-Foster, Citation2005; Taylor, Citation2011). Social support has been defined as the actuality and the perception that one is cared for and valued, has assistance available from other people, and that one is part of a supportive social network. It can involve emotional support as well as instrumental support, information and companionship (Bridges et al., Citation2002; Cobb, Citation1976; Van Sonderen, Citation1991). Social support can buffer the impact of stressful experiences and it can function as a coping mechanism (Hostinar & Gunnar, Citation2013).
BD has been associated with a dysregulation of the endocrine stress system, the HPA axis (Daban et al., Citation2005). Conflicting data have been published on cortisol levels, the end product of the HPA axis. Normal as well as elevated basal salivary and serum cortisol levels have been reported (Cervantes et al., Citation2001; Deshauer et al., Citation2006; Hardoy et al., Citation2006; Havermans et al., Citation2011). These inconsistent findings could partly be caused by methodological differences between the studies but also by differential exposure to stressful life events.
The possibility to measure cortisol in scalp hair has the advantage of being non-invasive and, unlike measurements in blood, saliva or urine, insensitive to daily variation, short-term transient stress, and oral contraceptives (Dettenborn et al., Citation2012), and to reflect the cortisol concentration of several weeks or months (Manenschijn et al., Citation2011).
In our previous study on long-term cortisol in bipolar patients, no difference in hair cortisol concentrations (HCC) between mood episodes was found, however, increased HCC were observed in patients with a late age of onset (>30 years) (Manenschijn et al., 2012). Recently, evidence was provided that the occurrence of stressful life events increases HCC in healthy young adults as well as in crack cocaine users (Grassi-Oliveira et al., Citation2012; Karlen et al., Citation2011).
Until now, it is not known whether patients with BD show increased HCC after a major life event, and whether increased HCC after a life event are associated with mood symptoms. Initial results in a longitudinal study on our cohort showed an effect of life events on mood symptoms and functional impairment, especially in patients with BD I as compared to BD II (Koenders et al., Citation2014).
We aimed to explore the effects of life events and social support on hair cortisol levels in bipolar patients and to investigate whether HCC are associated with mood in the subsequent three months.
Materials and methods
Study design
This is a cross-sectional and 3-month prospective study among outpatients with a diagnosis of BD. The current project is part of “The Bipolar Stress Study”, which is a cross-sectional and 24 month longitudinal study that aims on identifying risk factors that have an impact on the clinical course of BD and treatment of patients with BD. In the present study, we focused on the association between life events and HCC in patients with BD, and explore whether HCC in patients with life events are predictive of mood in the subsequent three months.
The study was approved by the local medical ethics committee and carried out in accordance with the declaration of Helsinki. After complete description of the study, all patients gave their informed consent.
Participants
Patients with BD from the same cohort whose hair cortisol characteristics have previously been published in our article concerning cross-sectional data (Manenschijn et al., 2012) were included in this study. All patients were participants of The Bipolar Stress Study and were treated in the local outpatient Department of Mood Disorders in The Hague, the Netherlands. During their regular visits every three months at the outpatient clinic, information on mood and life events was assessed. The hair sample was obtained at the seventh measurement (after 21 months of participation); the second last assessment in this study. Detailed description of the assessment methods of the patients has been described elsewhere (Manenschijn et al., 2012, Spijker et al., Citation2009). Patients were eligible for inclusion if they had not been using glucocorticoids in the 6 months prior to hair sample collection and if they had sufficient hair growth at the posterior vertex. The first 100 patients that fulfilled these requirements were asked to participate and to provide a hair sample.
Patients were interviewed by trained psychologists to collect socio-demographic data and disease characteristics. Diagnoses of BD and psychiatric co-morbidities were based on DSM-IV criteria and were assessed with a standardized diagnostic interview (Sheehan et al., Citation1998) using the Dutch version of the MINI International Neuropsychiatric Interview Plus (version 5.00-R; MINI-PLUS; van Vliet & de Beurs, Citation2007). Patients were classified in subtypes according to the DSM-IV-TR: bipolar I disorder, bipolar II disorder, cyclothymia, and bipolar disorder NOS, which vary in severity and frequency of mood episodes. BD I is characterized by one or more manic episodes, whereas BD II is defined by no manic episodes, but one or more hypomanic episodes and one or more major depressive episode. The Questionnaire for Bipolar Illness, Dutch translation (Leverich et al., Citation2001; Suppes et al., Citation2001) was used to identify subtypes of BD, its course over time and detailed information about age of onset of first symptoms regarding hypomanic, manic and depressive episodes.
Hair sample collection, preparation, and cortisol measurement
From all patients, approximately 100–150 hairs were cut from the posterior vertex as close to the scalp as possible. Hair sample preparation has been described in detail elsewhere (Manenschijn et al., Citation2011). In short, the hair was taped to a paper and stored until preparation. The 3 cm hair segments most proximal to the scalp were weighted in separate glass vials and then minced with small surgical scissors. One ml of methanol was added to extract cortisol from the hair samples and incubated for 16 h at 52 °C while gently shaking. Afterwards, the methanol was transferred to a clean glass vial and was evaporated under a constant nitrogen stream until completely dry. The samples were then dissolved in 250 μl phosphate buffered saline (pH 8.0) and vortexed until thoroughly mixed. Cortisol levels in the hair extracts were measured using a commercial ELISA kit for salivary cortisol (DRG Instruments GmbH, Marburg, Germany). Cross reactivity of other steroids with the kit’s antibodies was reported as follows: Corticosterone (29.00%), Cortisone (3.00%), 11-Deoxycortisol (<1.00%), 17-OH Progesterone (<0.50%), other hormones (<0.10%). Intra-assay variation was below 5% and the inter-assay variation below 8% as reported by the supplier. The low-end detection limit for this assay is 1.5 nmol/l. HCC are reported as median and interquartile range (IQR).
Life events
The occurrence of life events in the previous three months was assessed with Paykel’s self-report questionnaire consisting of 61 life events (Paykel et al., Citation1971). Patients indicated which events on the list occurred within the preceding three months. The 61 single life event items were a priori grouped into three main categories: negative life events, positive life events, and ambiguous life events. Events were categorized by independent raters, since patients’ rating of an event may be influenced by their current mood state. A total of 39 different negative life events consisted of life events such as increasing arguments with the spouse, relationship break-up, business failure, serious illness of a family member, failure of an important exam, demotion at work, and unemployment for one month. A total of eleven positive life events consisted of life events such as promotion at work, engagement, marriage, and a desired pregnancy. Eleven life events were rated as neutral or ambiguous events (change of work field, change of work hours, moving).
Social support
The presence and degree of social support was assessed with the Social Support List (Sociale Steun Lijst (SSL; Van Sonderen, Citation1991). This list quantifies two aspects of social support: “frequency of social support” measures the frequency of social support that the patient receives; and “perceived lack of social support” is the perceived difference in social support between that which is desired and what is received by the patient. Patients rated items from the three subscales using a 4-point scale to indicate the frequency in which they received social support (“frequency”). For the “perceived” score, patients rated the perceived discrepancy between the desired and received level of social support on a 4-point scale, ranging from too little to too much. The scores were summed for each variable, with a higher score indicating a higher amount of social support.
Mood
Illness severity was assessed in two ways. Symptom severity as well as the functional impact of the mood disturbances was measured. The observer based Young Mania Rating Scale (YMRS, (Young et al., Citation1978)) was used to assess the number and severity of mania symptoms. The Quick Inventory of Depressive Symptoms (QIDS, (Rush et al., Citation2003)) was administered to assess the number and severity of depression symptoms. Both the YMRS and the QIDS were administered during the visit at the outpatient clinic. The functional impact of mood disturbances was measured with the monthly retrospective life chart method (LCM-r) by the National Institute of Mental Health (NIMH) (Roy-Byrne et al., Citation1985). This instrument was used to assess medication use and monthly functional impairment resulting from mania or depression symptoms in the subsequent three months after collecting the hair sample and was administered by the research psychologist during every visit at the outpatient clinic. Based on the life chart data, the mean severity of functional impairment was calculated by averaging the monthly severity scores for depression and mania of every three-month period between the visits. A similar method has been used previously (Post et al., Citation2003).
Statistical analysis
SPSS 20.0 for Windows was used for statistical analysis. Hair cortisol levels, the number of life events, social support, age, and BMI were continuous variables, and gender, dyeing of hair, bleaching of hair, age of onset before/after the age of 30, comorbidities, and medication use were used as dichotomous variables. Pearson Chi-Square tests were used to compare frequency distributions. After log transformation, hair cortisol levels were normally distributed. Linear regression analyses were performed to analyze the association between the number of life events and HCC, between social support measures and HCC, and between HCC and mood. Backward stepwise regression was used to identify the best predictors of the following variables: gender, age, BMI, natural hair color, frequency of hair wash, use of hair products, use of lithium, use of antiepileptics, use of antidepressants, use of antipsychotics, use of benzodiazepines, mood phase, bipolar disorder subtype, psychiatric comorbid disorders (except for panic disorder), comorbid panic disorder, age of onset, the presence of metabolic syndrome, and the presence of an endocrine disease. The final model consisted of the age of onset, presence of comorbid disorders, hair treatments, and use of benzodiazepines to account for their influences, and the variable in question, such as number of life events. The results of the linear regression are reported as standardized coefficients (beta), t-value of the life event variable, and r2 change (increase in explained variation that the life event variable adds to the model). The association between life events and mood was analyzed using non-parametric (Spearman) correlation. The temporal relations are illustrated in .
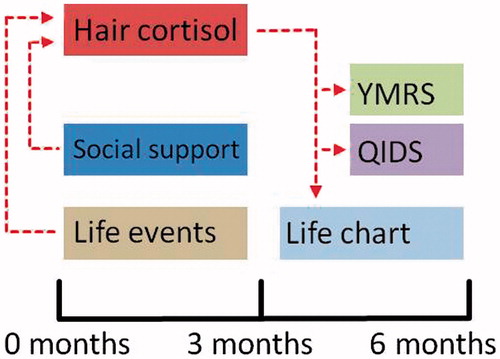
Figure 1. Schematic overview of the assessment moments and the analyzed relationships between the different measurements. We investigated the influence of life events, social support, stability (life chart) and mood (QIDS, YMRS) on hair cortisol levels that were assessed at the 3-month assessment, and whether hair cortisol levels from the 3-month assessment had an influence on mood and stability as reported at the 6-month assessment.
Results
Hair cortisol measurements were available in 100 patients. For 96 of these patients, life event data were available. Of these 96 patients, 71 patients had complete information on the final set of covariates and constituted our final study sample. Ten patients reported a treated endocrine disease (n = 2 diabetes, n = 7 hypothyroidism, n = 1 diabetes and hypothyroidism), and a total of 17 patients presented with metabolic syndrome. Group characteristics are shown in . The median HCC was 30.28 pg/mg with an interquartile range from 22.73 to 48.96. Hair cortisol levels of patients with either metabolic syndrome or an endocrine disease were not different from the hair cortisol concentrations of the other patients (F(1,66) = 0.87, p = 0.35 and F(1,69) = 0.03, p = 0.86) respectively, and controlling for these variables did not alter the results.
Table 1. Group characteristics.
Life events and hair cortisol
Descriptive information of the life events is provided in . The total number of life events was not associated with HCC (). The number of negative life events was associated with increased HCC (β = 0.22, t(64) = 2.07, r2 = 0.04, p = 0.04). Selecting the patients that only experienced negative life events (excluding all patients that reported any positive or ambiguous life events in addition to negative events), the results became even more significant (β = 0.31, t(64) = 2.54, r2 = 0.08, p = 0.02), as illustrated in . For positive life events and ambiguous life events, the analyses could not be performed separately due to small sample sizes.
Figure 2. First row: The number of negative life events is associated with an increase in hair cortisol concentrations in patients with only negative life events, whereas in presence of positive or neutral life events, this association was not significant. This graph shows the inverse log-transformed cortisol values. Second row: The score of the frequency measurement of the Social Support List is associated with a decrease in hair cortisol concentrations in the presence of negative life events, whereas no association was found between social support and hair cortisol concentrations in patients with also positive or neutral life events.
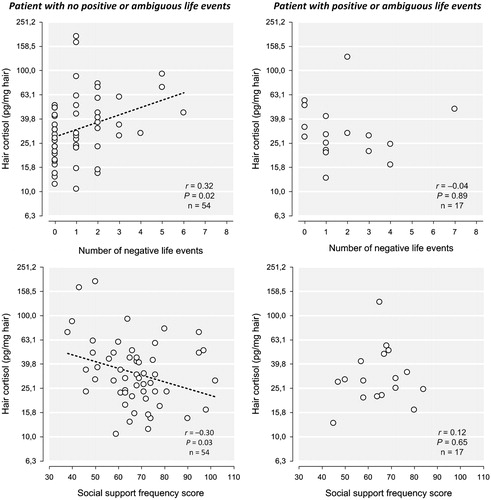
Table 2. Descriptive information on the occurrence of 61 life events.
Table 3. The effect of life events on HCC for the whole group and stratified for subgroups.
Based on the recent finding that life events relate to mood in BD I but not in BD II patients (Koenders et al., Citation2014), analyses were stratified for subtype of BD. We found a trend for an effect of the total number of life events on hair cortisol for patients with BD I (β = 0.25, t(47) = 1.91, r2 = 0.05, p = 0.06) whereas this was not significant for patients with BD II/NOS. More specifically, an increase in HCC was only seen in patients with BD I for the number of only negative events (β = 0.31, t(47) = 2.18, r2 = 0.07, p = 0.04) but not in patients with BD II.
As our group showed before that age of onset before or after 30 years of age is associated with different HCC (Manenschijn et al., 2012), analyses were also stratified for age of onset before or after 30. The total number of life events was not associated with HCC in either the early age of onset or the late age of onset patients. The number of negative life events was associated with increased HCC in patients with an early age of onset (β = 0.23, t(39) = 2.04, r2 = 0.05, p = 0.05; only negative events: β = 0.28, t(39) = 2.28, r2 = 0.07, p = 0.03), but not in patients with a late age of onset. Age of onset was not associated with BD subtype, and the frequency distribution was not different for the groups (χ (1, n = 71) = 0.02, p = 0.90).
Hair cortisol and mood
Patients reported a mean score of 0.54 (SD = 1.94) on the YMRS (range of potential scores: 0–60) and a mean score of 6.90 (SD = 5.18) on the QIDS (range of potential scores: 0–27). The mean functional impairment as assessed with the LCM-r was 0.52 (SD = 0.67) for the depressive symptoms and 0.17 (SD = 0.39) for the manic symptoms. For both LCM-r measurements, the range of potential scores is 0 to 4. HCC were not associated with mood symptoms in the three months prior to the hair collection (corresponding to the time frame captured by the hair sample). HCC were also not predictive of mood in the three months after taking the hair sample (all p > 0.05). The disorder state in the corresponding three months has earlier been shown to not be associated with HCC (Manenschijn et al., 2012).
Life events and mood
The number of all life events correlated significantly with the QIDS score in the corresponding three months, ρ = 0.26, p = 0.03. The number of negative life events in presence of other life events also showed this association; ρ = 0.29, p = 0.02. Reducing the sample to the patients with only negative life events made the association even stronger, ρ = 0.33, p = 0.02. The relation between life events (all, negative, only negative), the YRMS score and the functional impairment for depressive symptoms and manic symptoms did not reach significance (all p > 0.05).
Hair cortisol, life events, and social support
The frequency of social support and the perceived social support showed a mean score of 66.58 (SD = 13.81) and −34.68 (SD = 12.14), respectively. The frequency of social support was inversely associated with HCC (β = −0.21, t(64) = −2.04, r2 = 0.04, p = 0.05), as was the perceived social support (β = −0.22, t(64) = −2.06, r2 = 0.04, p = 0.04). Further analyses revealed that the decreasing effect of the frequency of social support on hair cortisol concentrations was only present in patients who experienced negative life events (β = −0.28, t(38) = −2.21, r2 = 0.07, p = 0.03), as illustrated in , and not in patients who did not experience life events (). Perceived social support did not reach significance when stratifying for the occurrence of negative life events.
Table 4. The effect of social support on HCC in presence and absence of life events.
As with life events, stratification for type of bipolar disorder showed that the frequency of social support in presence of negative life events was trend-associated with decreased HCC in patients with BD I (β = −0.30, t(29) = −1.97, r2 = 0.08, p = 0.06, only negative events: β = −0.40, t(20) = −2.29, r2 = 0.13, p = 0.03) but not in patients with BD II/NOS or in patients with no negative life events. Stratification for age of onset showed that the frequency of social support in presence of negative life events showed no effect on HCC in patients with an early age of onset, but a trend in patients with only negative events: β = −0.28, t(16) = −1.85, r2 = 0.07, p = 0.08) and decreased HCC in patients with a late age of onset and with negative life events (β = −0.62, t(9) = −2.85, r2 = 0.33, p = 0.02, only negative events: β = −1.18, t(4) = −3.02, r2 = 0.45, p = 0.04).
Discussion
The focus of our research project “The Bipolar Stress Study” is to identify the influence of biological and psychological stress on characteristics and course of bipolar disorder. In the current study, we focused on the association between life events and HCC in patients with BD, and to explore whether HCC in patients with life events are predictive of mood in the subsequent three months. For this purpose, we benefited from earlier findings regarding the same cohort (Koenders et al., Citation2014; Manenschijn et al., 2012,) as we could account for the previously reported relations such as age of onset and subtype of bipolar disorder, and extended these findings.
In this study, we found an association between the number of negative life events and increased HCC, in particular in patients with the BD I subtype or in those who had an age of onset before 30 years. The observed increased HCC after life events are consistent with current literature on the influence of life events on hair cortisol levels (Grassi-Oliveira et al., Citation2012; Karlen et al., Citation2011). However, we showed that it is not the total number of life events but in particular the number of negative life events, which significantly increases HCC in the corresponding months. Negative life events might aggravate rumination that may exacerbate the already adverse experience (Michaud et al., Citation2008), thereby potentiating the effect of life events. Interestingly, it has previously been reported that bipolar patients show normal initial cortisol responses to stressful life events, but that they have more difficulty in terminating the stress response and returning to a stable baseline mood. Whereas healthy participants reported that it took them on average 2.3 days to return to pre-event mood and behavioral levels, cyclothymic and dysthymic patients stated that their average recovery time was 3.9 days and 7.7 days, respectively (Goplerud & Depue, Citation1985). It is essential that an individual is able to terminate the cortisol response appropriately to prevent hypercortisolemic effects (Sapolsky et al., Citation2000). A failure to do so would reflect in higher mean cortisol levels over a prolonged period of time, which would contribute to higher HCC levels.
Regarding social support, our first analyses indicated that the frequency of social support and perceived social support were associated with decreases in HCC. Further analyses revealed that the attenuating effect of the frequency of social support on HCC was only present in patients with BD I that experienced (negative) life events and not in patients without any life events, suggesting that social support could partially attenuate the increasing HCC after negative life events. One might speculate that mitigating against increased long-term cortisol levels might protect from new mood episodes, as the “social zeitgeber theory” has proposed that life events might induce a new mood episode through disruptions in social and biological rhythms (Grandin et al., Citation2006). Through this mechanism, increased social support may stabilize the social and thereby also the biological rhythm.
Stratifying the analyses led to interesting findings, i.e. that the number of negative life events leads to HCC increases in BD I patients and in patients with an early age of onset. Stronger effects of life events on HCC in patients with BD I and an early age of onset could be due to various reasons. One reason might be a power problem that reflects the smaller number of participants in BD II and with a late age of onset rather than an underlying difference between the subtypes. This seems probable, as the standardized coefficients of BD II and a late age of onset are greater than the standardized coefficients of BD I and early age of onset, but fail to reach significance. However, other possibilities need to be acknowledged. A stronger effect of life events on mood symptoms and functional impairment on patients with BD I patients as compared to BD II patients has already been found in a longitudinal study on this cohort (Koenders et al., Citation2014). One possibility might be the different course trajectory of the subtypes. BD II is associated with a more chronic course and fluctuations in mood (Mantere et al., Citation2008). This is also consistent with the kindling theory, suggesting that stress triggers the initial episodes and recurrences, but that successive episodes are progressively induced independently of stressors (Post, Citation1992). With more episodes and a more chronic course, the association between stress and a new mood episode may already be too weak to be detected. For age of onset, an early onset BD has been suggested to be associated with a genetic vulnerability (Leboyer et al., Citation2005), whereas late onset BD is thought to be initially triggered by a major life event preceding the first episode (Johnson et al., Citation2000). A late onset is therefore more likely associated with a chronic HPA axis disturbance, which is in line with the earlier reported higher HCC in patients with a late age of onset compared to patients with a younger age of onset in this cohort (Manenschijn et al., 2012). Therefore, another major life event might not influence HCC as much as it would in patients with early age of onset and hypothetically more normal HPA axis functioning.
The question arises, how these results have to be interpreted, i.e. whether this increase reflects a healthy reaction of the HPA axis to a stressor, or whether it is a risk factor for a new mood episode. The same holds true for the potential absent HCC increase in BD II patients and patients with a late age of onset, and whether this reflects the failure to start a stress reaction to a life event, or whether it is a protective property that these patients have. An answer to this question would need a more frequent monitoring of the patients to assess exactly when a life event has happened, and a smaller time frame covered by the hair sample, for example 1 cm instead of 3 cm. This would yield insight in whether an increase in HCC happens in all patients and that some patient groups are faster in ending the prolonged stress response than others, or whether the increase is a feature associated with different patient characteristics. In our study, this frequent monitoring was not possible due to logistic reasons.
In the current study, we found no association between hair cortisol concentrations and mood. However, for investigating the influence of HCC on mood and vice versa, the presence of distinct mood symptoms is required. Our well-treated sample only showed minor mood fluctuations and reported few clinical symptoms, thereby questioning the validity of our negative findings.
Several strengths and limitations of the present study need to be mentioned. The patients that participated in this research projects were seen regularly and frequently, i.e. every 3 months, and the occurrence of life events and presence of mood disorder symptoms were inquired upon every visit. For research on life events this is of high value, as in long periods between visits, patients may either forget to report life events, or may aggravate the meaning of events in order to explain for example a new mood episode, a process called “effort after meaning” (Johnson & Roberts, Citation1995). However, we have to acknowledge the already mentioned small sample size of patients with either only positive or only ambiguous events, and the lack of a control group, i.e. HCC of healthy controls who also provided information on life events and mood. Furthermore, our sample presented with sparse mood symptoms which could be related to the high number of patients that used psychotropic medication. This treatment might be another limitation for this study as the effects of psychotropic medication on HCC have not been extensively examined until now; however, it seems an almost inevitable factor when dealing with this patient group. A related point is that patients with serious mood problems at the moment of assessment might not come to the outpatient department (Kessing et al., Citation2000). Our timeframe of observation amounted to six months, one period of three months that comprised the hair sample and the information on mood and life events of the corresponding three months, and the period of the subsequent three months, that comprised information on mood. Seeing that manic patients report on average 0.4–0.7 mood episodes per year (Angst & Sellaro, Citation2000), our design was likely too short to appropriately capture major mood changes.
To summarize, in the present study we showed that the number of negative life events is associated with increased hair cortisol concentrations in bipolar patients. These results indicate that even in the presence of a disorder that is associated with a dysregulation of the HPA-axis, exposure to negative life events might induce further HPA-axis disturbances. This relationship seems to be modified by social support, pointing towards the flexibility of the HPA-axis in both directions. Furthermore, these are first indications that there might be subtypes of bipolar patients that react differently to life events in terms of cortisol secretion, which might on the long term warrant different approaches to coping with stress or treatment of these patients.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This project is supported by the Netherlands Brain Foundation (grant number F2011(1)-12).
References
- Angst J, Sellaro R. (2000). Historical perspectives and natural history of bipolar disorder. Biol Psychiatry 48(6):445–57
- Boutin-Foster C. (2005). In spite of good intentions: patients’ perspectives on problematic social support interactions. Health Qual Life Outcomes 3:52–8
- Bridges KR, Sanderman R, van Sonderen E. (2002). An english language version of the social support list: preliminary reliability. Psychol Rep 90(3 Pt 1):1055–8
- Cervantes P, Gelber S, Kin FN, Nair VN, Schwartz G. (2001). Circadian secretion of cortisol in bipolar disorder. J Psychiatry Neurosci 26(5):411–16
- Cobb S. (1976). Presidential address-1976. Social support as a moderator of life stress. Psychosom Med 38(5):300–14
- Daban C, Vieta E, Mackin P, Young AH. (2005). Hypothalamic-pituitary-adrenal axis and bipolar disorder. Psychiatr Clin North Am 28(2):469–80
- Deshauer D, Duffy A, Meaney M, Sharma S, Grof P. (2006). Salivary cortisol secretion in remitted bipolar patients and offspring of bipolar parents. Bipolar Disord 8(4):345–9
- Dettenborn L, Tietze A, Kirschbaum C, Stalder T. (2012). The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress 15(6):578–88
- Goplerud E, Depue RA. (1985). Behavioral response to naturally occurring stress in cyclothymia and dysthymia. J Abnorm Psychol 94(2):128–39
- Grandin LD, Alloy LB, Abramson LY. (2006). The social zeitgeber theory, circadian rhythms, and mood disorders: review and evaluation. Clin Psychol Rev 26(6):679–94
- Grassi-Oliveira R, Pezzi JC, Daruy-Filho L, Viola TW, Francke ID, Leite CE, Brietzke E. (2012). Hair cortisol and stressful life events retrospective assessment in crack cocaine users. Am J Drug Alcohol Abuse 38(6):535–8
- Hardoy MC, Serra M, Carta MG, Contu P, Pisu MG, Biggio G. (2006). Increased neuroactive steroid concentrations in women with bipolar disorder or major depressive disorder. J Clin Psychopharmacol 26(4):379–84
- Havermans R, Nicolson NA, Berkhof J, deVries MW. (2011). Patterns of salivary cortisol secretion and responses to daily events in patients with remitted bipolar disorder. Psychoneuroendocrinology 36(2):258–65
- Hosang GM, Korszun A, Jones L, Jones I, Gray JM, Gunasinghe CM, McGuffin P, Farmer AE. (2010). nAdverse life event reporting and worst illness episodes in unipolar and bipolar affective disorders: measuring environmental risk for genetic research. Psychol Med 40(11):1829–37
- Hostinar CE, Gunnar MR. (2013). future directions in the study of social relationships as regulators of the hpa axis across development. J Clin Child Adolesc Psychol 42(4):564–75
- Johnson L, Andersson-Lundman G, Aberg-Wistedt A, Mathe AA. (2000). Age of onset in affective disorder: its correlation with hereditary and psychosocial factors. J Affect Disord 59(2):139–48
- Johnson SL, Roberts JE. (1995). Life events and bipolar disorder: implications from biological theories. Psychol Bull 117(3):434–49
- Karlen J, Ludvigsson J, Frostell A, Theodorsson E, Faresjo T. (2011). Cortisol in hair measured in young adults – a biomarker of major life stressors? BMC Clin Pathol 11:12
- Kessing LV, Andersen EW, Andersen PK. (2000). Predictors of recurrence in affective disorder–analyses accounting for individual heterogeneity. J Affect Disord 57(1–3):139–45
- Koenders MA, Giltay EJ, Spijker AT, Hoencamp E, Spinhoven P, Elzinga BM. (2014). Stressful life events in bipolar I and II disorder: cause or consequence of mood symptoms? J Affect Disorders 161:55–64
- Leboyer M, Henry C, Paillere-Martinot ML, Bellivier F. (2005). Age at onset in bipolar affective disorders: a review. Bipolar Disord 7(2):111–18
- Leverich GS, Nolen WA, Rush AJ, McElroy SL, Keck PE, Denicoff KD, Suppes T, et al. (2001). The stanley foundation bipolar treatment outcome network. I. Longitudinal methodology. J Affect Disord 67(1–3):33–44
- Manenschijn L, Koper JW, Lamberts SWJ, van Rossum EFC. (2011). Evaluation of a method to measure long term cortisol levels. Steroids 76(10–11):1032–6
- Manenschijn L, Spijker AT, Koper JW, Jetten AM, Giltay EJ, Haffmans J, Hoencamp E, van Rossum EF. (2012). Long-term cortisol in bipolar disorder: associations with age of onset and psychiatric co-morbidity. Psychoneuroendocrinology 37(12):1960–8
- Mantere O, Suominen K, Valtonen HM, Arvilommi P, Leppamaki S, Melartin T, Isometsa E. (2008). Differences in outcome of DSM-IV bipolar I and II disorders. Bipolar Disord 10(3):413–25
- Michaud K, Matheson K, Kelly O, Anisman H. (2008). Impact of stressors in a natural context on release of cortisol in healthy adult humans: a meta-analysis. Stress 11(3):177–97
- Paykel ES. (1994). Life events, social support and depression. Acta Psychiatr Scand Suppl 377:50–8
- Paykel ES, Prusoff BA, Uhlenhuth EH. (1971). Scaling of life events. Arch Gen Psychiatry 25(4):340–7
- Post RM. (1992). Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry 149(8):999–1010
- Post RM, Denicoff KD, Leverich GS, Altshuler LL, Frye MA, Suppes TM, Rush AJ, et al. (2003). Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J Clin Psychiatry 64(6):680–90 (quiz 738–9)
- Rafanelli C, Roncuzzi R, Milaneschi Y, Tomba E, Colistro MC, Pancaldi LG, Di Pasquale G. (2005). Stressful life events, depression and demoralization as risk factors for acute coronary heart disease. Psychother Psychosom 74(3):179–84
- Roy-Byrne P, Post RM, Uhde TW, Porcu T, Davis D. (1985). The longitudinal course of recurrent affective illness: life chart data from research patients at the NIMH. Acta Psychiatr Scand Suppl 317:1–34
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, et al. (2003). The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 54(5):573–83
- Sapolsky RM. (2004). Why zebras don’t get ulcers – the acclaimed guide to stress, stress-related diseases, and coping. New York: Owl Books
- Sapolsky RM, Romero LM, Munck AU. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21(1):55–89
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, et al. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33 (quiz 4–57)
- Spijker AT, van Rossum EF, Hoencamp E, DeRijk RH, Haffmans J, Blom M, Manenschijn L, et al. (2009). Functional polymorphism of the glucocorticoid receptor gene associates with mania and hypomania in bipolar disorder. Bipolar Disord 11(1):95–101
- Suppes T, Leverich GS, Keck PE, Nolen WA, Denicoff KD, Altshuler LL, McElroy SL, et al. (2001). The stanley foundation bipolar treatment outcome network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disord 67(1–3):45–59
- Taylor SE. (2011). Social support: a review. In: Friedman HS, editor. Oxford handbook of health psychology. New York: Oxford University Press; 2011. p. 189–214
- Van Sonderen FLP. (1993). Het meten van sociale steun met de Sociale Steun Lijst-Interacties (SSL-I) en Sociale Steun Lijst Discrepanties (SSL-D): een handleiding. Groningen: Noordelijk Centrum voor Gezondheidsvraagstukken. Rijksuniversiteit Groningen
- van Vliet IM, de Beurs E. (2007). The MINI-International Neuropsychiatric Interview. A brief structured diagnostic psychiatric interview for DSM-IV en ICD-10 psychiatric disorders. Tijdschr Psychiatr 49(6):393–7
- Young RC, Biggs JT, Ziegler VE, Meyer DA. (1978). A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133:429–35
