Abstract
Several studies have shown that acute stress affects working memory (WM) in young adults, but the effect in older people is understudied. As observed in other types of memory, older people may be less sensitive to acute effects of stress on WM. We performed two independent studies with healthy older men and women (from 55 to 77 years old) to investigate the effects of acute stress (Trier Social Stress Test; TSST) and cortisol on WM. In study 1 (n = 63), after the TSST women (but not men) improved their performance on Digit Span Forward (a measure of the memory span component of WM) but not on Digit Span Backward (a measure of both memory span and the executive component of WM). Furthermore, in women, cortisol levels at the moment of memory testing showed a positive association with the memory span component of WM before and after the TSST, and with the executive component of WM only before the stress task. In study 2 (n = 76), although participants showed a cortisol and salivary alpha-amylase (sAA) response to the TSST, stress did not affect performance on Letter-Number Sequencing (LNS; a task that places a high demand on the executive component of WM). Cortisol and sAA were not associated with WM. The results indicate that circulating cortisol levels at the moment of memory testing, and not the stress response, affect memory span in older women, and that stress and the increase in cortisol levels after stress do not affect the executive component of WM in older men and women. This study provides further evidence that older people may be less sensitive to stress and stress-induced cortisol response effects on memory processes.
Introduction
Acute stress can modulate memory performance through the action of cortisol on glucocorticoid and mineralocorticoid receptors (GR and MR, respectively), especially those located in the prefrontal cortex (PFC), hippocampus and amygdala (Roozendaal et al., Citation2009). Most studies in young adults have shown that acute stress impairs working memory (WM; e.g. Duncko et al., Citation2009; Luethi et al., Citation2009; Oei et al., Citation2006; Schoofs et al., Citation2008, Citation2009), but there is also evidence to the contrary (e.g. Cornelisse et al., Citation2011; Smeets et al., Citation2006; Stauble et al., Citation2013). Memory-enhancing effects have only been reported in men (see Schoofs et al., Citation2013). WM is a PFC-dependent ability that includes both a memory span component (maintenance of a limited amount of information) and an executive component (manipulation of this information) (D'Esposito, Citation2007). Along these lines, the executive component seems more prone to being affected by acute stress than the memory span component (Schoofs et al., Citation2009). In addition, a study with young men has shown that stress may enhance the initial encoding of information in the WM, a cognitive function common to all WM tasks (Stauble et al., Citation2013).
However, few studies have investigated the effects of cortisol on WM in olderFootnote1 people. Using pharmacological approaches, previous findings showed no cortisol effects on WM in older men (Porter et al., Citation2002; Wolf et al., Citation2001; Yehuda et al., Citation2007). These results coincide with the idea that older people may be less sensitive to acute effects of cortisol on memory due to a loss and/or dysfunction of corticoid receptors in the aging brain (Giordano et al., 2005; Heffelfinger & Newcomer, Citation2001; Perlman et al., Citation2007; Porter et al., Citation2002). However, the effects of acute psychosocial stress on WM tasks are still unknown. Using a task designed to assess declarative memory, we found that stress improved memory span and impaired retroactive interference (a PFC-dependent executive ability) in women from 54 to 72 years old (Almela et al., Citation2011a), but not in young adults (Hidalgo et al., Citation2014), suggesting that stress may have a sex-dependent effect on WM in older people.
We present the results of two studies designed to investigate whether acute stress affects memory span (study 1) and the executive component of WM (studies 1 and 2) in older people. Based on pharmacological studies, we did not expect stress and cortisol to affect memory span (study 1) or the executive component of WM (studies 1 and 2), at least in men. However, based on our previous results using a psychosocial stressor, we expected that, in women, stress would enhance memory span (study 1) but impair the executive component of WM (Studies 1 and 2). Thus, sex differences in acute stress effects on specific components of WM are expected.
Materials and methods
Participants
Participants of both studies belonged to a study program at the University of Valencia for people older than 55 years. There was not overlap between participants in study 1 and those in study 2. Exclusion criteria were as follows: smoking more than 10 cigarettes a day, alcohol or other drug abuse, visual or hearing problems, diabetes, presence of an HPA-axis, neurological or psychiatric disease and using any medication directly related to emotional or cognitive functioning or able to influence hormonal levels, such as glucocorticoids, psychotropic substances or sleep medications. Use of anti-hypertensive medications was allowed (study 1: men = 9 and women = 5; study 2: men-stress = 7, women-stress = 5, men-control = 4 and women-control = 8), but including these participants did not change the statistical conclusions of this study. None of the participants had been under general anesthesia in the past year. Only postmenopausal women who had had their last menstrual period more than one year prior to the study were allowed to participate.
Study 1
The sample in the first study was composed of 63 participants (30 men and 33 women) ranging from 55 to 77 years old (M = 63.40, SD = 4.42). Their subjective socioeconomic status (SES scale; Adler et al., Citation2000) was medium-high, and over half of the participants (60.30%) had an educational level beyond high school. There were no significant differences in age between men and women (t(61) = −0.561; p = 0.577). Men had a higher body mass index (BMI; men, M = 27.80, SD = 3.96; Women: M = 25.57, SD = 3.55; t(61) = 2.352; p = 0.022), a higher SES (t(61) = 2.013; p = 0.049) and a slightly higher educational level than women (U = 363.5; p = 0.059).
Study 2
The sample in the second study was composed of 76 participants (38 men and 38 women) ranging from 56 to 76 years old (M = 64.26, SD = 4.10). Their SES was medium-high, and most of them (84.20%) had an educational level beyond high school. In study 2, participants were randomly assigned to a stress (19 men and 18 women) or control condition (19 men and 20 women). There were no significant differences between men and women in age, SES or educational level (p > 0.168), but men had higher BMI (Men, M = 27.83, SD = 3.34; Women, M = 25.99, SD = 3.67; F(1,72) = 3.727, p = 0.026). There were no significant differences between the stress and control groups in age, BMI, SES or educational level (all p > 0.163).
Procedure
When they arrived at the laboratory, participants' weight and height were measured, and the experimenter checked to see whether they had followed the instructions given to them previously: the day before the session, they had to maintain their general habits, sleep as long as usual and refrain from heavy physical activity; they could not consume alcohol the night before, and two hours prior to the session, they could not drink (except water), eat, smoke or take any stimulants, such as coffee, cola, caffeine, tea or chocolate. The sessions were carried out individually and started between 16:00 and 18:00 h in a laboratory at the Faculty of Psychology. This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Research Ethics Committee of the University of Valencia. All participants provided written informed consent to participate in this study.
Study 1
In this study, we measure WM of the participants before and after they performed a stress task. The procedure began with a habituation phase of 15 min to allow participants to adapt to the laboratory setting. After the habituation phase, participants completed the State Anxiety Inventory (STAI-S; Spielberger et al., Citation1970) to measure baseline anxiety scores (STAI-pre), and they performed the first version of the Digit Span. Following the Digit Span, participants were exposed to the stress task. After this, they completed the STAI-S for the second time (STAI-post), and after a recovery phase of 10 min, participants performed the second version of the Digit Span (the timeline for the session is represented in ).
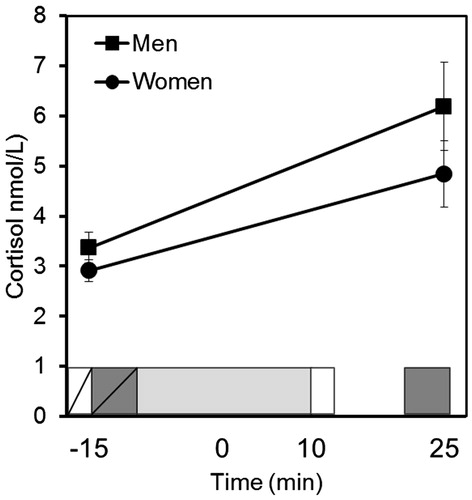
Figure 1. Study 1: salivary cortisol concentrations for men and women. After a 15-min habituation phase (not represented in the figure), participants completed the STAI-pre (white rectangle with diagonal line). Afterwards, they performed the pre-stress Digit Span (dark gray rectangle with diagonal line). Next, they performed the TSST (light gray rectangle): (i) they were introduced to the task, (ii) they prepared the free speech and (iii) they performed the free speech and arithmetic tasks. Immediately after that, they completed the STAI-post (white rectangle), and after 10 min recovery, they performed the post-stress Digit Span (dark grey rectangle). The 0-min time point was fixed at the beginning of the free speech task.
Study 2
This procedure was similar to the one described for the first study, but in study 2, we included a control condition, and we measured the performance on the WM task after the stress or control tasks. Upon arrival at the laboratory, participants began a habituation phase of 15 min. After the habituation phase, the participants completed the STAI-pre and then they remained seated until they were introduced to the stress or control task. Immediately after the stress or control task, participants completed the STAI-post, and after a recovery phase of 10 min, they performed the LNS (the timeline for the session is represented in ). After the LNS, and as part of a larger study performed to investigate the effect of acute stress on different memory processes in older people, participants in study 2 performed three more memory tasks to measure long-term memory retrieval and they provided three more salivary samples (results not included in this study, but shown in Pulopulos et al., Citation2013).
Stress task
Study 1
The Trier Social Stress Test (TSST; Kirschbaum et al., Citation1993) was used to provoke acute stress. After an introduction phase (5 min) in which participants were informed about the procedure for the stress task in front of a committee, participants had 5 min to prepare for the task. After this phase, participants carried out a 5-min free speech task and a 5-min arithmetic task, standing at a distance of 1.5 m from the committee. The participants were informed that the speech and arithmetic tasks would be filmed with a video camera and a microphone, which were clearly visible. The committee was composed of a man and a woman, and interactions with participants were always performed by the committee member of the opposite sex.
Study 2
The stress task was the same as the one described for study 1, but with a preparation phase of 3 min instead of 5 min (Kudielka et al., Citation2007). The control task consisted of 5 min of talking aloud about a recent non-emotional experience, and 5 min counting by five aloud. The control task was performed in the same room as the stress task, but none of the stressful elements (video camera, microphone and committee) were present.
WM task
Study 1
Both the Digit Span Forward (DS-Forward) and Digit Span Backward (DS-Backward) subtests of the Wechsler Memory Scale III (Wechsler, Citation1997) were applied before and after the stress task. These tests require participants to listen to a series of numbers of increasing lengths (ranging from 0 to 9). Participants have to repeat the numbers in the same order (DS-Forward) or the reverse order (DS-Backward) in which they were presented. Each set length was tested twice, and for each correctly repeated digit set, the number of digits was added up. The maximum score possible in each test condition is 16. Two parallel versions of the test were administered. The order of presentation was counterbalanced, and performance on the two versions of each subtest was similar (p > 0.112). The DS-Forward is a task used to measure the memory span component of WM and attentional processes, and the DS-Backward is used to measure the executive component of WM (Conklin et al., Citation2000).
Study 2
The LNS from the Wechsler Memory Scale III (Wechsler, Citation1997) was used to assess WM performance. This test requires participants to listen to a sequence of alternating digits (ranging from 0 to 9) and letters (from A to Z) of increasing length. Then, they have to repeat the digits and letters from the sequence, beginning with the digits in numerical order, followed by the letters in alphabetical order. The LNS test requires participants to categorize alternating letters and numbers into separate classes and re-order the stimuli within each class. The maximum score possible is 16. Activation of the orbital frontal lobe, dorsolateral PFC and posterior parietal cortex has been observed during this test (Haut et al., Citation2000).
Anxiety
The Spanish version of the STAI-S was used to assess state anxiety in both studies (Spielberger et al., Citation1970). This test contains 20 sentences responded to on a four-point Likert scale ranging from 0 (not at all) to 3 (extremely). The Cronbach's alpha for the Spanish version of the scale ranges from 0.90 to 0.93 (Seisdedos, Citation1988).
Saliva samples and biochemical analyses
Study 1
Participants provided two saliva samples by depositing 3 ml of saliva in plastic vials in order to measure cortisol levels immediately before the first Digit Span assessment (−15 min) and immediately after the second Digit Span assessment ( + 25 min).
Study 2
In study 2, we measured both salivary cortisol and salivary alpha-amylase (sAA) levels (using Salivettes; Sarstedt, Nümbrecht, Germany). Given that the increase in sAA after the onset of exposure to the stressor is faster than the increase in cortisol, as is recovery to baseline (Almela et al., Citation2011b; Nater et al., Citation2005), in study 2, we included one salivary sample immediately after the speech task and another one immediately after the arithmetic task, in order to have a more complete picture of the sAA response. The samples were provided 15 min before the TSST (−15 min), between the speech and arithmetic tasks of the TSST (+5 min), immediately after the TSST (+10 min) and immediately after the LNS (+25 min).
Salivary samples in both studies were analyzed to measure cortisol levels in duplicate through a competitive solid phase radioimmunoassay (tube coated) using the commercial kit Spectria Cortisol RIA (cat. Nu 06119) from Orion Diagnostica (Espoo, Finland). Assay sensitivity was 0.8 nmol/L, and the intra- and inter-assay variation coefficients were all below 8%. The sAA concentration in study 2 was measured through an enzyme kinetic method using the commercial sAA assay kit (cat. no 1-1902, 1-1902-5) from Salimetrics (State College, PA). Assay sensitivity was 0.4 U/mL. Inter- and intra-assay variation coefficients were all below 10%.
Statistical analysis and data management
Because cortisol (studies 1 and 2) and sAA (study 2) did not show normal distributions, they were log transformed. Stress response (study 1: cortisol and STAI-S; study 2: cortisol, sAA and STAI-S) and stress effects on the DS-Forward and DS-Backward (study 1) were assessed using repeated-measures ANOVAs with sex as a between-subject factor and time (study 1: for cortisol = −15 min and +25 min; for STAI-S and Digit Span = pre and post; study 2: for cortisol and sAA = −15 min, + 5 min, + 10 min, and +25 min; for STAI-S = pre and post) as a within-subject factor. We used Greenhouse–Geisser when the requirement of sphericity in the repeated-measures ANOVA was violated. Two-way ANOVAs were performed to test the effect of stress on LNS performance (study 2), with group (stress vs. control) and sex as between-subject factors. Post-hoc planned comparisons were performed using Bonferroni adjustments for the p values. Partial eta squared (partial η2) is reported as a measure of effect sizes for ANOVAs (Cohen, Citation1973). Partial correlations with SESFootnote2 as covariate were used to investigate the relationship between cortisol (studies 1 and 2), sAA (study 2) and WM (study 1: DS-Forward and DS-Backward; study 2: LNS) in men and women.
Observed power was calculated using G*Power (Faul et al., Citation2007). Previous studies in young adults have shown medium to large effect sizes of stress on WM (Oei et al., Citation2006; Porcelli et al., Citation2008; Schoofs et al., Citation2009, Citation2013). Thus, with a medium-large effect size (Cohen's f = 0.33), the power of our studies were 0.84 and 0.081 for studies 1 and 2, respectively. The power of both studies was sufficient to detect an effect like the one observed in young adults, if present in the data. Two outliers in study 1 (cortisol: one woman and one man) and three outliers in study 2 (cortisol: one woman and one man in the control group; sAA: one man in the stress group) were removed from the cortisol and sAA analyses because their concentrations differed by more than three SD from the total sample mean. When not otherwise specified, the results shown are means ± SEM.
Results of study 1
Stress response
Anxiety
The repeated-measures ANOVA revealed significant effects of Time (F(1,61) = 18.550, p < 0.001, partial η2 = 0.233) and sex (F(1,61) = 11.753, p = 0.001, partial η2 = 0.150) and the interaction between time and sex (F(1,61) = 10.768, p = 0.002, partial η2 = 0.162). Men and women had similar anxiety scores before the stress task (p = 0.182, partial η2 = 0.029). Women increased their anxiety scores after the TSST (p < 0.001, partial η2 = 0.331), but men did not (p = 0.481, partial η2 = 0.008). Therefore, after the stress task, anxiety was higher in women than in men (p < 0.001, partial η2 = 0.225).
Cortisol
shows the mean cortisol values for men and women. The repeated-measures ANOVA showed the main effects of time (F(1,59) = 19.990, p < 0.001, partial η2 = 0.253). After the stress task, cortisol levels were higher than baseline levels in both men and women. The factor sex (F(1,59) = 2.358, p = 0.130, partial η2 = 0.038) and the interaction between time and sex were not significant (F(1,59) = 0.588, p = 0.446, partial η2 = 0.010).
Stress effects on DS-Forward and DS-Backward
shows the performance on the DS-Forward and DS-Backward before and after the stress task. For the DS-Forward, there was a significant effect of Time (F(1,61) = 5.929, p = 0.018, partial η2 = 0.089). Neither the effect of sex (F(1,61) = 3.611, p = 0.062, partial η2 = 0.056) nor the interaction between time and sex (F(1,62) = 3.685, p = 0.085, partial η2 = 0.048) reached statistical significance. Post hoc exploration of the interaction between time and sex showed that men had a similar performance before and after the stress task (p = 0.639, partial η2 = 0.004), while women improved their performance after the stress task (p = 0.004, partial η2 = 0.131).
Figure 2. Study 1: performance on Digit Span Forward (left) and Digit Span Backward (right) before (gray) and after (black) the stress task. *Women improved their performance on Digit Span Forward after the stress task (p = 0.004).
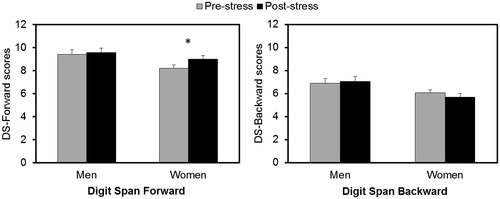
For the DS-Backward, results showed a main effect of sex (F(1,61) = 6.728, p = 0.012, partial η2 = 0.099). Overall, men performed better on this test than women. Neither the factor time (F(1,61) = 0.137, p = 0.713, partial η2 = 0.002) nor the interactions between time and sex were significant (F(1,61) = 0.992, p = 0.323, partial η2 = 0.016).
Relationship between cortisol and DS-Forward and DS-Backward
Using partial correlation analyses, we analyzed (i) the relationship between pre-stress cortisol and pre-stress Digit Span; (ii) the relationship between post-stress cortisol and post-stress Digit Span; and (iii) the relationship between cortisol response (change in cortisol levels) and the change in Digit Span performance. Cortisol response was calculated by saving the unstandardized residual scores from the regression analyses, using pre-stress cortisol as a predictor and post-stress cortisol as the dependent variable for men and women separately (Mehta et al., Citation2008; van der Meij et al., Citation2012). The same method was used to calculate the change in DS-Forward and DS-Backward performance. We used residual scores to control for the influence of baseline values on the magnitude of possible change (e.g. smaller increases in cortisol levels and Digit Span in participants with higher baseline scores; Cohen et al., Citation2000).
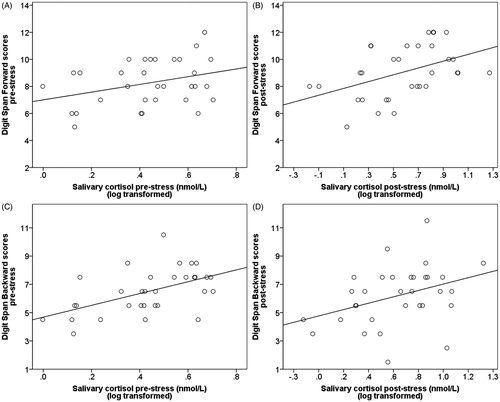
In men, none of the partial correlation analyses showed significant results (all p > 0.210). In women, there was a positive relationship (approaching significance) between pre-stress cortisol levels and pre-stress DS-Forward (r(29) = 0.349, p = 0.054; ), and a positive significant association between post-stress cortisol levels and post-stress DS-Forward (r(29) = 0.443, p = 0.013; ). The relationship between cortisol response and change in DS-Forward performance was not significant (r(29) = 0.301, p = 0.099). Results for DS-Backward showed a positive significant relationship between pre-stress cortisol levels and pre-stress DS-Backward (r(29) = 0.526, p = 0.002; ) and between post-stress cortisol levels and post-stress DS-Backward (r(29) = 0.369, p = 0.041; ). No significant association was observed between cortisol response and change in DS-Backward performance (r(29) = 0.289, p = 0.144).
Figure 3. Study 1: scatter plots for the association between acute cortisol levels and Digit Span performance in women. (A) Relationship between acute cortisol levels and Digit Span Forward pre-stress (r = 0.349, p = 0.054). (B) Relationship between acute cortisol levels and Digit Span Forward post-stress (r = 0.443, p = 0.013). (C) Relationship between acute cortisol levels and Digit Span Backward pre-stress (r = 0.526, p = 0.002); this latter relationship remained significant after controlling for Digit Span Forward (r = 0.462, p = 0.010). (D) Relationship between acute cortisol levels and Digit Span Backward post-stress (r = 0.369, p = 0.041); this latter relationship did not remain significant after controlling for Digit Span Forward (r = 0.216, p = 0.252).
In addition, given that DS-Backward is a task that contains both a memory span component (i.e. participants have to keep the numbers in mind for a short period of time) and an executive component (i.e. participants have to mentally change the order of the numbers presented), we explored the association between cortisol and DS-Backward more thoroughly. To find out whether the relationship between cortisol and DS-Backward might be due to the effect of cortisol on memory span (as observed for DS-Forward), we included pre-stress DS-Forward, post-stress DS-Forward and change in DS-Forward outcomes as covariates, respectively, in the partial correlations. With these analyses, we controlled for the effect of cortisol on memory span, thus focusing only on the executive function of manipulating numbers in memory. In men, all the associations remained non-significant (p > 0.408). In women, the results showed that the association between pre-stress cortisol levels and pre-stress DS-Backward remained significant (r(28) = 0.462, p = 0.010). However, neither the association between post-stress cortisol and post-stress DS-Backward (r(28) = 0.216, p = 0.252) nor the association between cortisol response and change in DS-Backward (r(28) = 0.073, p = 0.700) was significant when controlling for DS-Forward outcomes.
Results of study 2
Study 1 showed that higher levels of cortisol after the stress task improved memory span only in older women; however, they did not affect the executive component of WM in older men or women. This lack of association between post-stress cortisol levels and/or cortisol response and WM executive processes may be due to the low sensitivity of the DS-Backward test to these effects in older people. Therefore, in a second study, we investigated whether stress affects executive processes in older people by using a task that places more demands on the executive component of WM. In this second study, we used the LNS, a test that is more cognitively demanding than the DS-Backward test, and it is considered a measure of the executive component of both verbal and visual WM (Crowe, Citation2000; Haut et al., Citation2000). Furthermore, previous studies in young adults found effects of stress on the executive component of WM when comparing the performance of participants exposed to the TSST to the performance of participants exposed to a control condition (e.g. Duncko et al., Citation2009; Oei et al., Citation2006; Schoofs et al., Citation2009, Citation2013). Therefore, in study 2, the procedure was similar to the one described for the first study, but in this case, the participants were exposed to either the TSST or a control task, and we compared the WM performance of the stress and control groups. Based on the results of study 1, we do not expect to find an effect of stress on LNS performance.
Furthermore, in addition to the activation of the HPA-axis, previous studies have shown that stress-induced activation of the sympathetic nervous system (SNS) is necessary in order to observe stress effects on memory performance (Elzinga & Roelofs, Citation2005; Roozendaal et al., Citation2004; Schwabe et al., Citation2009). Thus, to explore whether the stress task provoked an activation of the SNS in our participants, during the session, we measured the levels of sAA, an oral cavity enzyme that is considered a sensitive biomarker of sympathetic-adrenal-medullary system activity (i.e. higher sAA levels indicate higher SNS activity; for reviews see Nater & Rohleder, Citation2009). Higher basal levels of sAA have been observed in older people, and sex differences are not expected for the TSST-induced sAA response (Almela et al., Citation2011b).
Stress response
Anxiety
The repeated-measures ANOVA showed the main effects of Time (F(1,71) = 22.689, p < 0.001, partial η2 = 0.242), sex (F(1,71) = 11.315, p = 0.001, partial η2 = 0.137) and group (F(1,71) = 6.852, p = 0.011, partial η2 = 0.088) and the interaction between time and group (F(1,71) = 14.904, p < 0.001, partial η2 = 0.174). Overall, women showed higher anxiety scores than men (p = 0.001). The stress and control groups had similar anxiety scores before the stress task (p = 0.659, partial η2 = 0.003). The stress group increased their anxiety after the TSST (p < 0.001, partial η2 = 0.341), whereas the control group did not (p = 0.522, partial η2 = 0.006).
Cortisol
shows the mean cortisol values for men and women in the stress and control groups. The results showed the main effects of time (F(1.678,117.433) = 17.074, p < 0.001, partial η2 = 0.196), sex (F(1,70) = 11.132, p = 0.001, partial η2 = 0.137), group (F(1,70) = 14.516, p < 0.001, partial η2 = 0.172) and the interaction between time and group (F(1.678,117.433) = 21.305, p < 0.001, partial η2 = 0.233) and between time and sex (F(1.678,117.433) = 6.971, p = 0.003, partial η2 = 0.091). The interaction between time, group and sex was not significant (F(1.678,117.433) = 0.228, p = 0.877, partial η2 = 0.003).
Figure 4. Study 2: salivary cortisol concentrations for men and women in the stress and control groups. After a habituation of 15 min (not represented in the figure), they completed the STAI-pre (white rectangle with diagonal line). Next, they were exposed to the TSST or control task (light grey rectangle): (i) they were introduced to the task, (ii) they prepared the free speech and (iii) they performed the free speech and arithmetic/counting tasks. Immediately after that, they completed the STAI-post (white rectangle) and after a recovery of 10 min, they performed the LNS test (dark gray rectangle). The 0-min time point was fixed at the beginning of the free speech. *Cortisol levels were higher in the stress group than in the control group in samples +5 min, +10 min and +25 min (all p < 0.011).
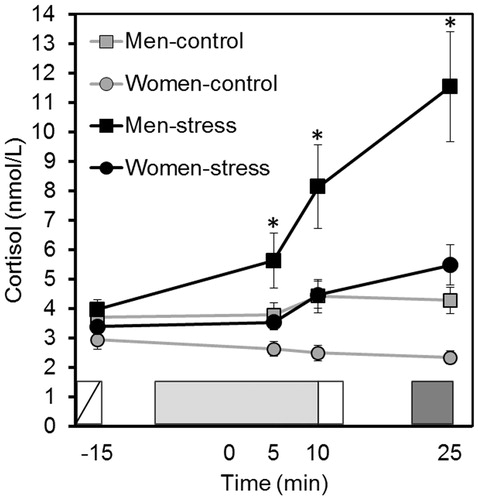
The stress and control groups had similar cortisol levels in the first saliva sample (p = 0.138). In the stress group, cortisol levels were higher than baseline in the two samples provided after the stress task (all p < 0.001). In the control group, cortisol levels did not change in any sample provided (all p > 0.999). Cortisol levels were higher in the stress group than in the control group in samples +5 min, + 10 min and +25 min (all p < 0.011). Finally, considering the stress and control groups together, men had higher cortisol levels than women (all p < 0.045).
sAA
Repeated measures ANOVA indicated a main effect of time (F(2.167,153.860) = 16.945, p < 0.001, partial η2 = 0.193). The factors sex (F(1,71) = 1.524, p = 0.221, partial η2 = 0.021) and group (F(1,71) = 0.099, p = 0.754, partial η2 = 0.001) and the interactions between these factors (all p > 0.176, partial η2 < 0.024) were not significant. In both groups, sAA levels were above baseline 5 min after the onset of the task (−15 vs. + 5: p < 0.001), and then participants recovered baseline levels 10 min after the onset of the task (−15 vs. + 10: p = 0.904). Although the interaction between group and time was non-significant, a one-way ANOVA with group (stress vs. control) and sex as between-subject factors showed that the increase in sAA levels, computed as the total response curve with respect to the increase (AUCi, Pruessner et al., Citation2003), was higher in the stress group than in the control group (F(1,72) = 4.327, p = 0.041, partial η2 = 0.057). Neither the factor sex nor the interaction between group and sex was significant (F(1,72) > 0.027, p > 0.716, partial η2 < 0.002). These results confirmed that the TSST was able to provoke a stronger sympathetic-adrenal-medullary system response than the control task, and that there were no differences between men and women.
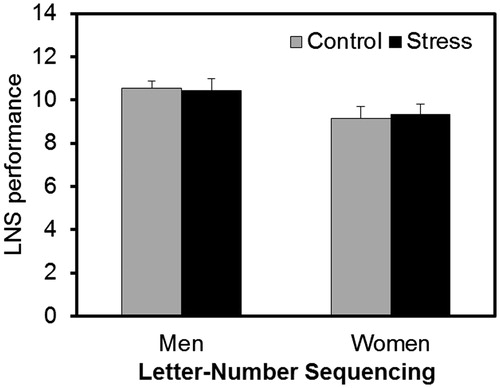
Stress effects on LNS
shows performance on LNS after the stress or control task. There was a significant effect of sex (F(1,72) = 6.323, p = 0.014, partial η2 = 0.081), as men showed better performance than women. Neither the factor group (F(1,72) = 0.006, p > 0.937, partial η2 < 0.001) nor the interaction between group and sex was significant (F(1,72) = 0.0087, p > 0.769, partial η2 = 0.001).
Relationship between cortisol, sAA and LNS
For participants in the stress group, partial correlations were used to explore (i) the relationship between post-stress cortisol (+25 min) and post-stress LNS performance and (ii) the relationship between cortisol response (unstandardized residual scores using cortisol levels at −15 min as pre-stress outcome and cortisol levels at +25 min as post-stress outcome) and post-stress LNS performance. In addition, to explore whether sAA is related to LNS, the same partial correlation analyses were performed for sAA. Because the peak of the stress-induced sAA increase in our participants occurred immediately after the speech task (+5 min), we used the sAA levels at the +5 min sampling point for the partial correlation analyses (instead of the + 25 min sampling point used for the cortisol data)Footnote3 . Thus, the partial correlation analyses for sAA were (i) sAA levels immediately after the free speech task (+5 min) and LNS and (ii) between the sAA response (unstandardized residual scores using sAA levels at −15 min as pre-stress outcome and sAA levels at +5 min as post-stress outcome) and LNS.
These analyses showed that there were no significant relationships between cortisol levels after the stress task (+25 min) and LNS performance in men (r(16) = −0.156, p = 0.536) or women (r(15) = 0.159, p = 0.542). Cortisol response to stress was not related to LNS performance in men (r(16) = −0.153, p = 0.543) or women (r(15) = 0.074, p = 0.778). Partial correlation analyses with sAA showed that LNS was not related to sAA post-stress (men: r(15) = −0.49, p = 0.851; women: r(15) = 0.249, p = 0.336) or the sAA response (men: r(15) = 0.019, p = 0.943; women: r(15) = 0.229, p = 0.376).
Discussion
We investigated whether acute stress affects WM performance in men and women between 55 and 77 years of age. To this end, we performed two studies, using two different memory tests to assess WM.
In study 1, we observed that older women, but not men, improved their performance on the DS-Forward (a measure of the memory span component of WM) after the stress task. The correlation analyses showed a positive association between DS-Forward and cortisol levels at the moment of testing, but not between cortisol response and change in DS-Forward. Thus, although cortisol seems to be a main contributor to this finding, stress itself would not affect memory span and might not be necessary to achieve the effects observed. These results coincide with a previous study by our group that showed an enhanced memory span in older women after a stress task. As in this study, this effect was not related to the stress-induced cortisol increase (Almela et al., Citation2011a). Given that cortisol may increase dopamine's actions in the PFC (a catecholamine that influences WM) (Arnsten, Citation2009), it is possible that, regardless of stress, changes in cortisol levels interact with dopamine levels, affecting WM. However, it is also possible that the memory span enhancement observed is related to other changes that occur with stress (e.g. increase in general arousal) but are not measured in our study. Thus, further research is needed to examine whether changes in memory span would be observed in older women after a pharmacologically induced cortisol increase, without other effects of stress. In addition, we performed these studies in the late afternoon, when endogenous cortisol levels are low. Thus, given that the relationship between cortisol and memory may follow an inverted U-shaped pattern (e.g. de Veld et al., Citation2014; Lupien et al., Citation2002; Schilling et al., Citation2013), further studies could also examine whether stress and cortisol can impair memory span in the morning, when endogenous cortisol levels are higher, and a greater GR and MR occupation is observed.
Our results show that cortisol affected memory span in women, but not in men. Sex-differences in cortisol receptor expression might explain these results. Although no differences in MR and GR between young males and females have been reported in the PFC (Elaković et al., Citation2011; Peijie et al., Citation2004), estrogen has been shown to down-regulate corticosteroid receptor expression in the brain (Bangasser, Citation2013). Thus, after a drastic reduction in estrogen levels due to menopause, older women might show slightly higher cortisol receptor expression than older men, which might affect the relationship between circulating cortisol levels and WM. Another possible explanation would be the higher number of dopamine receptors in the PFC in women (Shansky & Lipps, Citation2013), which would make them more sensitive to the effects of cortisol levels on WM.
Results of studies 1 and 2 showed, in men and women, that stress and the cortisol response to stress did not affect the executive component of the WM (DS-Backward and LNS) and that sAA was not related to LNS. These results agree with prior research in healthy older men showing that pharmacologically induced cortisol increases did not affect performance on the DS-Backward (Porter et al., Citation2002; Wolf et al., Citation2001) or the LNS (Yehuda et al., Citation2007). Therefore, contrary to what has been observed in most studies with young adults (e.g. Duncko et al., Citation2009; Luethi et al., Citation2009; Oei et al., Citation2006; Schoofs et al., Citation2009), neither a stress-induced cortisol and SNS response nor high circulating cortisol levels after stress affect the executive component of WM in healthy older people.
Our findings support the idea that older people are less sensitive than young adults to the acute effects of stress and cortisol on memory processes. In contrast to the clear effects observed in young adults (for a review see Wolf, Citation2009), previous studies in older animals and humans did not show any acute effects on spatial memory (Buechel et al., Citation2014), learning, short-term declarative or non-declarative memory (Porter et al., Citation2002), or long-term memory retrieval of pictures, words and stories (Pulopulos et al., Citation2013). However, we have observed a specific effect of stress-induced cortisol response on retroactive interference (i.e. impairment in memory due to the interference of previously learned material) in middle-aged people (Almela et al., Citation2011a), but not in young adults (Hidalgo et al., Citation2014). Similarly, Lupien et al. (Citation1997) and Wolf et al. (Citation2001) found an impairing effect of cortisol increase on short-term word list recall. In these studies, word-list recall was measured after other memory tasks were performed (e.g. a different word-list recall); therefore, the weakening of word-list recall could also be due to the enhancement of retroactive interference. Therefore, most of the memory processes might be unaffected by stress in older people.
One possible explanation for these age-related differences would be a loss and/or dysfunction of MR and GR in the aging brain (Giordano et al., 2005; Mizoguchi et al., Citation2009; Lupien et al., Citation2002; Perlman et al., Citation2007), which could affect HPA axis regulation (Garrido et al., Citation2012) and the acute effects of cortisol response on memory performance. Along these lines, our results showed that, in older women, the executive component of the DS-Backward was related to acute cortisol levels at baseline, when there is only a moderate MR occupation; however, this association was not observed after the stress task, when cortisol levels are high and a greater occupation of MR and GR is needed to observe stress effects on memory (Oitz et al., Citation1997). Supporting the idea of low sensitivity, patients with major depression disorder show both a reduction in GR sensitivity, and no effects of acute cortisol increases on declarative memory and WM (Terfehr et al., Citation2011a,Citationb).
Interestingly, these age-related changes may have negative consequences for older individuals' adaptation. Roozendaal (Citation2002) proposed that the effect of stress on memory observed in young individuals is an adaptive mechanism that blocks some memory processes (e.g. long-term memory retrieval) to facilitate others (e.g. consolidation). It has been suggested that this mechanism would diminish retroactive interference, allowing the brain to learn new important information to be used in the future (e.g. dangerous places in animals) (Joëls et al., Citation2006). Following this line of thinking, if older individuals are less sensitive to the effects of stress on several memory processes, and at the same time stress might increase retroactive interference, this condition would make them more vulnerable to the environment, since they would not benefit from learning necessary information to avoid potential problems.
Stress response in men in study 2, and in women in both studies, is in accordance with previous studies in young and older people (Almela et al., Citation2011b; Kudielka et al., Citation2009). By contrast, men in study 1 showed a moderated cortisol response compared to men in study 2. One possible explanation for these difference across-studies would be that men in study 1 reported higher SES than men in study 2. Thus, factors related to low SES (e.g. fewer psychological resources in stressful social interactions) might affect the stress response (Derry et al., Citation2013). This effect would especially be observed in men because they reported higher SES than women. Most importantly, our results indicate that the lack of stress and cortisol response effects on WM is independent from the magnitude of the cortisol response.
One limitation of study 1 is that we did not include a stress-free control condition. Thus, other factors not controlled for may have affected the increase in DS-Forward performance in women. However, the habituation phase was long enough to consider the first cortisol and WM assessments as a baseline measure to be compared to scores after stress, and the results observed coincide with previous studies (Almela et al., Citation2011a; Porter et al., Citation2002; Wolf et al., Citation2001). In addition, the focus of these studies was to investigate the effects of stress-induced cortisol increases on WM. Therefore, WM was measured 10 min after the TSST, when cortisol levels were high; however, SNS activity had returned to basal levels at that moment. Previous research in young adults has shown cortisol effects on WM, even when the control and stress groups did not differ on SNS activity (e.g. Cornelisse et al., Citation2011; Schoofs et al., Citation2013). However, there is also evidence that the effects of cortisol response on WM require the concurrent activation of the SNS (Elzinga & Roelofs, Citation2005). Future studies should investigate whether stress can affect WM in older people when high cortisol levels and SNS activity coincide.
In conclusion, our results showed that older women, but not men, increased their memory span after stress. This effect was related to acute circulating cortisol levels, but not to the magnitude of the stress-induced cortisol increase. In addition, in both older men and women, cortisol and stress response did not affect the executive component of the WM. Together, our findings provide empirical support for the idea that healthy older people may be less sensitive than younger people to the acute effects of stress-induced cortisol increases on several types of memory processes.
Acknowledgements
We are grateful to Ms. María Salvador, Ms. Marta Garcia Lluch, Ms. Teresa Montoliu, Dr. Eva Lira, Dr. Leander van der Meij and Dr. Lucas Monzani for their support in the research process and Ms. Cindy DePoy for the revision of the English text.
Declaration of interest
The authors state that there are no conflicts of interest associated with this research.
This research was supported by the Spanish Education and Science Ministry (PSI2010/21343, FPU AP2010-1830, FPU AP2009-4713, FPU12/04597 and FPI/BES-2008-004224) and Generalitat Valenciana (ACOMP/2013/0200, PROMETEO 2011/048 and ISIC/2013/01).
Notes
1Previous studies that investigated the effect of acute stress and/or cortisol increases on memory in older people have included samples with a mean age of older than 60 years and an age range from 52 to 83 years old (e.g. an age range from 54 to 72 in Almela et al. (2011a); from 52 to 81 in Yehuda et al. (2007); and from 59 to 76 in Wolf et al. (2001)). To be consistent with the terms used in most of these studies, in this article, we refer to the participants as older people; however, it should be noted that in the present study and previous studies some participants are in the second half of the age range called middle-aged people (from 50 to 60 years old); therefore, the results and conclusions should also be applicable to them.
2 We performed two-ways ANOVAs with study (study 1 vs. study 2) and sex as between-subject factors for Age, BMI, SES and educational level to explore differences in the demographic characteristics between participants in the two studies. Participants in study 1 reported higher SES than participants in study 2 (F(1,94) = 9.098; p = 0.003). No significant differences were observed for Age, BMI and educational level (p > 0.189). Overall, men showed higher SES, BMI and educational level (p < 0.051) than women. None of the interactions between the factors study and sex were significant (p > 0.282). In addition, we performed correlation analyses for men and women (participants in both studies together) to explore whether these variables were related to the cortisol response to stress. Only in men, there was a significant negative association between cortisol response and SES (r(48) = −0.321; p = 0.026). None of the other associations for men and women were significant (p > 0.247). Thus, to compare the relationship between cortisol and WM across the two studies, we included SES as a covariate in the correlation analyses of both studies to control for its effect on the cortisol response to stress.
3 The statistical conclusions of the partial correlations for sAA are the same if we perform these analyses using sAA levels at +10 min or +25 min.
1 Previous studies that investigated the effect of acute stress and/or cortisol increases on memory in older people have included samples with a mean age of older than 60 years and an age range from 52 to 83 years old (e.g. an age range from 54 to 72 in Almela et al. (Citation2011a); from 52 to 81 in Yehuda et al. (Citation2007); and from 59 to 76 in Wolf et al. (Citation2001)). To be consistent with the terms used in most of these studies, in this article, we refer to the participants as older people; however, it should be noted that in the present study and previous studies some participants are in the second half of the age range called middle-aged people (from 50 to 60 years old); therefore, the results and conclusions should also be applicable to them.
2 We performed two-ways ANOVAs with study (study 1 vs. study 2) and sex as between-subject factors for Age, BMI, SES and educational level to explore differences in the demographic characteristics between participants in the two studies. Participants in study 1 reported higher SES than participants in study 2 (F(1,94) = 9.098; p = 0.003). No significant differences were observed for Age, BMI and educational level (p > 0.189). Overall, men showed higher SES, BMI and educational level (p < 0.051) than women. None of the interactions between the factors study and sex were significant (p > 0.282). In addition, we performed correlation analyses for men and women (participants in both studies together) to explore whether these variables were related to the cortisol response to stress. Only in men, there was a significant negative association between cortisol response and SES (r(48) = –0.321; p = 0.026). None of the other associations for men and women were significant (p > 0.247). Thus, to compare the relationship between cortisol and WM across the two studies, we included SES as a covariate in the correlation analyses of both studies to control for its effect on the cortisol response to stress.
3 The statistical conclusions of the partial correlations for sAA are the same if we perform these analyses using sAA levels at +10 min or + 25 min.
References
- Adler NE, Epel ES, Castellazzo G, Ickovics JR. (2000). Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy, white women. Health Psychol 19(6):586
- Almela M, Hidalgo V, Villada C, Espín L, Gómez-Amor J, Salvador A. (2011a). The impact of cortisol reactivity to acute stress on memory: sex differences in middle-aged people. Stress 14(2):117–27
- Almela M, Hidalgo V, Villada C, van der Meij L, Espín L, Gómez-Amor J, Salvador A. (2011b). Salivary alpha-amylase response to acute psychosocial stress: the impact of age. Biol Psychol 87(3):421–9
- Arnsten AF. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10(6):410–22
- Bangasser DA. (2013). Sex differences in stress-related receptors: “micro” differences with “macro” implications for mood and anxiety disorders. Biol Sex Differ 4:2
- Buechel HM, Popovic J, Staggs KH, Anderson KL, Thibault O, Blalock E. (2014). Aged rats are hypo-responsive to acute restraint: implications for psychosocial stress in aging. Front Aging Neurosci 6:13
- Cohen J. (1973). Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educ Psychol Meas 33:107–12
- Cohen S, Hamrick NM, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. (2000). The stability of and intercorrelations among cardiovascular, immune, endocrine, and psychological reactivity. Ann Behav Med 22(3):171–9
- Conklin HM, Curtis CE, Katsanis J, Iacono WG. (2000). Verbal working memory impairment in schizophrenia patients and their first-degree relatives: evidence from the digit span task. Am J Psychiatry 157(2):275–7
- Cornelisse S, van Stegeren AH, Joëls M. (2011). Implications of psychosocial stress on memory formation in a typical male versus female student sample. Psychoneuroendocrinology 36(4):569–78
- Crowe SF. (2000). Does the letter number sequencing task measure anything more than digit span? Assessment 7(2):113–17
- de Veld DM, Riksen-Walraven JM, de Weerth C. (2014). Acute psychosocial stress and children's memory. Stress 17(4):305–13
- Derry HM, Fagundes CP, Andridge R, Glaser R, Malarkey WB, Kiecolt-Glaser JK. (2013). Lower subjective social status exaggerates interleukin-6 responses to a laboratory stressor. Psychoneuroendocrinology 38:2676–85
- D'Esposito M. (2007). From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci 362(1481):761–72
- Duncko R, Johnson L, Merikangas K, Grillon C. (2009). Working memory performance after acute exposure to the cold pressor stress in healthy volunteers. Neurobiol Learn Mem 91(4):377–81
- Elaković I, Djordjevic A, Adzic M, Djordjevic J, Radojčić M, Matić G. (2011). Gender-specific response of brain corticosteroid receptors to stress and fluoxetine. Brain Res 1384:61–8
- Elzinga BM, Roelofs K. (2005). Cortisol-induced impairments of working memory require acute sympathetic activation. Behav Neurosci 119(1):98–103
- Faul F, Erdfelder E, Lang AG, Buchner A. (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–91
- Garrido P, De Blas M, Giné E, Santos Á, Mora F. (2012). Aging impairs the control of prefrontal cortex on the release of corticosterone in response to stress and on memory consolidation. Neurobiol Aging 33(4):827.e1–9
- Giordano R, Bo M, Pellegrino M, Vezzari M, Baldi M, Picu A, Balbo M, et al. (2005). Hypothalamus-pituitary-adrenal hyperactivity in human aging is partially refractory to stimulation by mineralocorticoid receptor blockade. J Clin Endocrinol Metab 90(10):5656–62
- Haut MW, Kuwabara H, Leach S, Arias RG. (2000). Neural activation during performance of number-letter sequencing. Appl Neuropsychol 7(4):237–42
- Heffelfinger AK, Newcomer JW. (2001). Glucocorticoid effects on memory function over the human life span. Dev Psychopathol 13(3):491–513
- Hidalgo V, Almela M, Villada C, Salvador A. (2014). Acute stress impairs recall after interference in older people, but not in young people. Horm Behav 65(3):264–72
- Joëls M, Pu Z, Wiegert O, Oitzl, MS, Krugers HJ. (2006). Learning under stress: how does it work? Trends Cog Sci 10(4):152–8
- Kirschbaum C, Pirke KM, Hellhammer DH. (1993). The ‘Trier social stress Test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28(1–2):76–81
- Kudielka BM, Hellhammer DH, Kirschbaum C. (2007). Ten years of research with the trier social stress test revisited. In: Harmon-Jones E, Winkielman P, editors. Social neuroscience: integrating biological and psychological explanations of social behavior. New York: Guilford Press (Chapter 4). pp. 56–83
- Kudielka BM, Hellhammer DH, Wüst S. (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology 34(1):2–18
- Luethi M, Meier B, Sandi C. (2009). Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Front Behav Neurosci 2:5
- Lupien S, Gaudreau S, Tchiteya B, Maheu F, Sharma S, Nair N, Hauger R, et al. (1997). Stress-induced declarative memory impairment in healthy elderly subjects: relationship to cortisol reactivity. J Clin Endocrinol Metabol 82(7):2070–5
- Lupien SJ, Wilkinson CW, Briere S, Ng Ying Kin NMK, Meaney MJ, Nair NPV. (2002). Acute modulation of aged human memory by pharmacological manipulation of glucocorticoids. J Clin Endocrinol Metabol 87(8):3798–807
- Mehta PH, Jones AC, Josephs RA. The social endocrinology of dominance: basal testosterone predicts cortisol changes and behavior following victory and defeat. J Pers Soc Psychol 2008;94:1078–93
- Mizoguchi K, Ikeda R, Shoji H, Tanaka Y, Maruyama W, Tabira T. (2009). Aging attenuates glucocorticoid negative feedback in rat brain. Neuroscience 159(1):259–70
- Nater UM, Rohleder N, Gaab J, Berger S, Jud A, Kirschbaum C, Ehlert U. (2005). Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int J Psychophysiol 55(3):333–42
- Nater UM, Rohleder N. (2009). Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology 34:486–96
- Oei N, Everaerd W, Elzinga B, Van Well S, Bermond B. (2006). Psychosocial stress impairs working memory at high loads: an association with cortisol levels and memory retrieval. Stress 9(3):133–41
- Oitz MS, van Haarst AD, de Kloet ER. (1997). Behavioral and neuroendocrine responses controlled by the concerted action of central mineralocorticoid (MRS) and glucocorticoid receptors (GRS). Psychoneuroendocrinology 22:S87–93
- Peijie C, Zicai D, Haowen X, Renbao X. (2004). Effects of chronic and acute training on glucocorticoid receptors concentrations in rats. Life Sci 75:1303–11
- Perlman WR, Webster MJ, Herman MM, Kleinman JE, Weickert CS. (2007). Age-related differences in glucocorticoid receptor mRNA levels in the human brain. Neurobiol Aging 28(3):447–58
- Porcelli AJ, Cruz D, Wenberg K, Patterson MD, Biswal BB, Rypma B. (2008). The effects of acute stress on human prefrontal working memory systems. Physiol Behav 95(3):282–9
- Porter RJ, Barnett NA, Idey A, McGuckin EA, O'Brien JT. (2002). Effects of hydrocortisone administration on cognitive function in the elderly. J Psychopharmacol 16(1):65–71
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–31
- Pulopulos MM, Almela M, Hidalgo V, Villada C, Puig-Perez S, Salvador A. (2013). Acute stress does not impair long-term memory retrieval in older people. Neurobiol Learn Mem 104:16–24
- Roozendaal B, McEwen BS, Chattarji S. (2009). Stress, memory and the amygdala. Nat Rev Neurosci 10(6):423–33
- Roozendaal B, McReynolds JR, McGaugh JL. (2004). The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci 24(6):1385–92
- Roozendaal B. (2002). Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem 78:578–95
- Schilling TM, Kölsch M, Larra MF, Zech CM, Blumenthal TD, Frings C, Schächinger H. (2013). For whom the bell (curve) tolls: cortisol rapidly affects memory retrieval by an inverted U-shaped dose–response relationship. Psychoneuroendocrinology 38(9):1565–72
- Schoofs D, Pabst S, Brand M, Wolf OT. (2013). Working memory is differentially affected by stress in men and women. Behav Brain Res 241:144–53
- Schoofs D, Preuß D, Wolf OT. (2008). Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology 33(5):643–53
- Schoofs D, Wolf OT, Smeets T. (2009). Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behav Neurosci 123(5):1066–75
- Schwabe L, Römer S, Richter S, Dockendorf S, Bilak B, Schächinger H. (2009). Stress effects on declarative memory retrieval are blocked by a β-adrenoceptor antagonist in humans. Psychoneuroendocrinology 34(3):446–54
- Seisdedos N. (1988). Adaptación española del STAI, cuestionario de ansiedad estado-rasgo [spanish adaptation of the STAI, state-trait anxiety inventory]. Madrid: Tea Ediciones
- Shansky RM, Lipps J. (2013). Stress-induced cognitive dysfunction: hormone-neurotransmitter interactions in the prefrontal cortex. Front Hum Neurosci 7:123
- Smeets T, Jelicic M, Merckelbach H. (2006). The effect of acute stress on memory depends on word valence. Int J Psychophysiol 62(1):30–7
- Spielberger CD, Gorsuch RL, Lushene RE. (1970). Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologist Press
- Stauble MR, Thompson LA, Morgan G. (2013). Increases in cortisol are positively associated with gains in encoding and maintenance working memory performance in young men. Stress 16(4):402–10
- Terfehr K, Wolf OT, Schlosser N, Fernando SC, Otte C, Muhtz C, Beblo T, et al. (2011b). Hydrocortisone impairs working memory in healthy humans, but not in patients with major depressive disorder. Psychopharmacology 215(1):71–9
- Terfehr K, Wolf OT, Schlosser N, Fernando SC, Otte C, Muhtz C, Beblo T, et al. (2011a). Effect of acute hydrocortisone administration on declarative memory in patients with major depression disorder: a placebo-controlled, double-blind crossover study. J Clin Psychiatry 72(12):1644–50
- van der Meij L, Almela M, Buunk AP, Fawcett TW, Salvador A. (2012). Men with elevated testosterone levels show more affiliative behaviours during interactions with women. Proc R Soc B 279(1726):202–8
- Wechsler DA. (1997). WMS–III technical manual. San Antonio, TX: Psychological Corporation
- Wolf OT. (2009). Stress and memory in humans: twelve years of progress? Brain Res 1293:142–54
- Wolf O, Convit A, McHugh P, Kandil E, Thorn E, De Santi S, McEwen B, De Leon M. (2001). Cortisol differentially affects memory in young and elderly men. Behav Neurosci 115(5):1002–11
- Yehuda R, Harvey PD, Buchsbaum M, Tischler L, Schmeidler J. (2007). Enhanced effects of cortisol administration on episodic and working memory in aging veterans with PTSD. Neuropsychopharmacology 32(12):2581–91