Abstract
Chronic stress causes hypothalamo–pituitary–adrenal (HPA) axis hyperactivity and cardiovascular dyshomeostasis. Noradrenergic (NA) neurons in the nucleus of the solitary tract (NTS) are considered to play a role in these changes. In this study, we tested the hypothesis that NTS NA A2 neurons are required for cardiovascular and HPA axis responses to both acute and chronic stress. Adult male rats received bilateral microinjection into the NTS of 6-hydroxydopamine (6-OHDA) to lesion A2 neurons [cardiovascular study, n = 5; HPA study, n = 5] or vehicle [cardiovascular study, n = 6; HPA study, n = 4]. Rats were exposed to acute restraint stress followed by 14 d of chronic variable stress (CVS). On the last day of testing, rats were placed in a novel elevated plus maze (EPM) to test post-CVS stress responses. Lesions of NTS A2 neurons reduced the tachycardic response to acute restraint, confirming that A2 neurons promote sympathetic activation following acute stress. In addition, CVS increased the ratio of low-frequency to high-frequency power for heart rate variability, indicative of sympathovagal imbalance, and this effect was significantly attenuated by 6-OHDA lesion. Lesions of NTS A2 neurons reduced acute restraint-induced corticosterone secretion, but did not affect the corticosterone response to the EPM, indicating that A2 neurons promote acute HPA axis responses, but are not involved in CVS-mediated HPA axis sensitization. Collectively, these data indicate that A2 neurons promote both cardiovascular and HPA axis responses to acute stress. Moreover, A2 catecholaminergic neurons may contribute to the potentially deleterious enhancement of sympathetic drive following chronic stress.
Introduction
Stress generates autonomic and endocrine responses that promote physiological coping (Chrousos & Gold, Citation1992; Myers et al., Citation2014). Stress responses are initiated, in part, by brainstem noradrenergic (NA) neurons in the nucleus of the solitary tract (NTS; A2 region), as depletion of A2 NA neurons reduces pressor responses to psychosocial stressors (Daubert et al., Citation2012). Cardiovascular stress responses are likely mediated by rich A2 projections to central cardioregulatory circuits, including the ventrolateral medulla (Ross et al., Citation1985), preautonomic subdivisions of hypothalamic paraventricular nucleus (PVN) and/or the anteroventral subdivisions of the bed nucleus of the stria terminalis (BST) (Ricardo & Koh, Citation1978; Riche et al., Citation1990; Sawchenko & Swanson, Citation1982). The A2 region also sends direct projections to medial parvocellular corticotropin-releasing hormone (CRH) neurons (Sawchenko & Swanson, Citation1982), which are responsible for activation of hypothalamo–pituitary–adrenal (HPA) axis responses (Plotsky, Citation1987). Pharmacological and lesion studies confirm that ascending medullary NA neurons are required for generation of HPA axis responses to stressful stimuli (Gaillet et al., Citation1993; Li et al., Citation1996), mediated by adrenergic receptors in the parvocellular PVN (Day et al., Citation1999).
Long-term (chronic) stress exposure produces marked changes in endocrine and cardiovascular function. In rats, chronic stress elevates basal corticosterone (Herman et al., Citation1995), facilitates endocrine responses to novel stressors (Dallman et al., Citation1992) and increases PVN expression of CRH and arginine vasopressin mRNAs (Herman et al., Citation1995; Imaki et al., Citation1991). Chronic variable stress (CVS) also enhances NA innervation of CRH neurons (Flak et al., Citation2009), indicating a role for A2 neurons in chronic HPA axis activation. Functional studies suggest that chronic stress-induced HPA axis sensitization is mediated at least, in part, by NA innervation of A2 targets, including the PVN and BST (Rinaman, Citation2011). Chronic stress also results in resting tachycardia with reduced heart rate (HR) variability in the time domain, reflecting increased sympathetic drive and/or decreased vagal tone in rats (Grippo et al., Citation2002). In contrast to the HPA axis, little is known about the role of NA cell groups in the process of chronic stress-induced cardiovascular changes.
Although A2 neurons are in position to coordinate autonomic and endocrine reactivity to chronic stress, the functional contribution of this cell group remains to be determined. This study tests the hypothesis that NTS NA neurons are required for control of cardiovascular as well as HPA axis responses to acute stress and CVS exposure. Our data are consistent with a role for A2 NA neurons in cardiovascular and HPA axis excitation and indicate that they may play an important role in pathophysiological cardiovascular responses to chronic stress.
Methods
Animals
Adult male Sprague–Dawley rats (n = 33; 250–275 g; Harlan, Indianapolis, IN) were single-housed and maintained in a temperature- and humidity-controlled room on a 12/12-h light/dark cycle (lights on at 06:00 h; lights off at 18:00 h). Rats were given standard chow (Tekland, Madison, WI) and water ad libitum. Rats were acclimated to the housing facility for at least one week prior to experimental manipulations. All procedures for animal use were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
There were four groups of rats: two groups for cardiovascular studies, 6-hydroxydopamine (6-OHDA) lesion of NTS neurons and vehicle controls; and two similarly treated groups for corticosterone response studies.
Surgical procedures
6-OHDA lesion
6-OHDA was used to preferentially target NTS NA neurons in area A2 (Kostrzewa & Jacobwitz, Citation1974). Rats were anesthetized by intraperitoneal injection of a mixture of ketamine/xylazine (50/5 mg/kg) followed by a pre-emptive analgesic, butorphanol (0.5 mg/kg, s.c.) and antibiotic, gentamicin (6 mg/kg, i.m.) and placed in a stereotaxic surgical apparatus (Kopf, Tujunga, CA). Bilateral injections of vehicle (1 µl/side of 0.2% ascorbic acid/0.9% saline) or 6-OHDA (Sigma Aldrich, St. Louis, MO) (1 µl/side, at a concentration of 10 µg/µl dissolved in 1 µl of 0.2% ascorbic acid in saline) were made using a 26-gauge stainless steel needle and a 5 -µl Hamilton syringe (Reno, NV) at AP: −14.3 mm, ML: ±0.6 mm from bregma and DV– 8.3 mm from the skull surface according to the Paxinos & Watson (Citation1998) rat brain atlas. The 6-OHDA or vehicle was manually infused into the targeted NTS region over a 10-min period, followed by another 5 min waiting period to allow diffusion and minimize dorsal spread of injection up the needle track. The syringes were withdrawn over a 1-min period. Rats were allowed to recover for at least seven days before further treatment. The rats were handled daily and body weight (BW) was monitored.
Radio transmitter implantation for cardiovascular studies
One week after NTS 6-OHDA or vehicle microinjection, rats were implanted with radio telemetry transmitters (TA11PA-C40, Data Sciences International (DSI), St. Paul, MN) as described previously (Flak et al., Citation2011). Briefly, under inhalational isoflurane anesthesia, the descending aorta was exposed via an abdominal incision. A catheter (polyethylene), filled with biocompatible gel at its outer tip, extending from the transmitter capsule was placed into the descending aorta so that the catheter tip was below the renal arteries. The device was secured with tissue adhesive (Vetbond, St. Paul, MN) and a cellulose patch. The transmitter capsule was sutured to abdominal musculature. The abdominal musculature was sutured and wound clips were used to close the skin incision. Rats were allowed to recover for two weeks with BW and food intake monitored to ensure that they were not in post-surgical discomfort prior to experimentation.
Cardiovascular data and stress protocols
Following recovery from the telemetry surgery (two weeks), 6-OHDA- (n = 5) and vehicle- (n = 6) injected rats were left undisturbed in their home cage for three days to record baseline cardiovascular data [HR, mean arterial pressure (MAP) and heart rate variability (HRV)] (see below for detailed method of collection and analysis), and locomotor activity. Following baseline recording, we first assessed cardiovascular and autonomic parameter responses to acute restraint. Briefly, each rat was placed into a restrainer, a well-ventilated transparent Plexiglas cylinder (approximately 6 cm in diameter and 19 cm in length) for 30 min. At the end of the 30 min, rats were released from the restrainers into their home cages. We noted the exact time at which rats were placed into the restrainer and considered this as time = 0. Cardiovascular and HRV data were analyzed for (i) baseline (60-min prior to the test), (ii) during the restraint test (30 min) and (iii) throughout the recovery period (90 min after the restraint). For each 10-min bin, a separate estimation of HR, MAP, locomotor activity and HRV indices were measured in the frequency domain (see below).
To assess cardiovascular adaptations to chronic stress, rats were subsequently subjected to a CVS protocol (the above noted restraint exposure constituted the initial stressor in the CVS regimen). The CVS protocol consisted of twice-daily (morning and afternoon) exposure to one of the several randomly assigned stressors for 14 d, including shaker stress (1 h at 100 rpm on a platform orbital shaker), 20 min warm (31–33 °C) swim, 10-min cold (16–18 °C) swim, 1 h in a cold (4 °C) room and 30-min hypoxia (8% oxygen and 92%nitrogen) (see Supplementary Table I for schedule). Morning stressors were applied between 09:00 h and 11:00 h and afternoon stressors between 14:00 h and 17:00 h. CVS is a well-characterized chronic stress paradigm that attenuates BW gain, causes thymic atrophy and leads to adrenal hypertrophy (Herman et al., Citation1995). To determine the influence of A2 neuronal lesion on these somatic markers, we monitored BW throughout the study and assessed thymus and adrenal weight at the conclusion of this study.
Following exposure to CVS, we assessed cardiovascular responses to a novel elevated plus maze (EPM) facing one of the open arms (5 min) on day 15. Cardiovascular parameters and HRV were assessed as described below.
Cardiovascular data acquisition and analysis
For measurement of daily cardiovascular rhythm (baseline conditions), HR, MAP, indices of HRV (see below for quantification of HRV) and locomotor activity were recorded continuously for three days. These data were averaged as the mean values of 12-h periods (06:00 h–18:00 h and 18:00 h–06:00 h), omitting 11:00 h–15:00 h, which is when daily animal husbandry was performed. MAP, HR and activity were determined using A.R.T. Platinum software (DSI) as previously described (Flak et al., Citation2011).
HR, MAP, HRV and activity data from the acute restraint experiment were calculated as follows: data were averaged into 10-min bins starting 60 min prior to the restraint challenge and continuing through 90 min after the termination of the restraint. For each bin, separate estimates of HR, MAP, HRV indices (see below for quantification of HRV) and activities were generated.
For the measurement of cardiac and autonomic parameters during CVS exposure, HR, MAP, indices of HRV (see below for quantification of HRV) and activity were recorded continuously and averaged as the mean values of 24 h, excluding recordings from 11:00 h to 15:00 h.
Cardiovascular and activity data from the novel EPM exposure were calculated as follows: data were averaged into 10-min bins starting at 60 min prior to exposure and continuing through 120 min after the termination of EPM exposure, except for during the 5-min period of actual testing.
Quantification of HRV
Spectral (frequency-domain) analysis of HRV was performed using a fast Fourier transform method. The total power of the spectrum (ms2) reflects all the components responsible for variability, and the power of the low frequency (LF; 0.25–1 Hz) and high frequency (HF; 1–3 Hz) in absolute values (ms2). The power of LF is considered to reflect both sympathetic and parasympathetic influences (Reyes Del Paso et al., Citation2013), whereas the HF component is linked to parasympathetic activity (Berntson et al., Citation1997). The ratio of LF to HF (LF/HF) estimates the fractional distribution of power and represents sympathovagal balance, with higher LF/HF ratio associated with a relative increase in sympathetic drive (Carnevali & Sgoifo, Citation2014; Task Force, Citation1996). The LF and HF were represented in normalized units that represent the relative proportion of each of the components as a ratio of the total power (Carnevali & Sgoifo, Citation2014; Task Force, Citation1996).
Corticosterone data and stress protocols
Following chronic stress exposure, the HPA axis typically exhibits sensitized responses to subsequent novel stress (Bhatnagar & Dallman, Citation1998). To determine whether A2 neurons contribute to this HPA response, the response to a mild novel environment stress (EPM) was assessed in 6-OHDA (vs. vehicle) rats at the conclusion of the CVS paradigm. For the assessment of plasma corticosterone responses, a separate cohort of 6-OHDA- (n = 5) and vehicle- (n = 4) treated rats were subjected to an acute 30-min restraint, during the circadian nadir of corticosterone secretion. For blood sampling, rats were restrained and blood samples were collected before (0 min) and 15, 30, 60 and 120 min after initiation of the restraint. Sampling was performed in less than 3 min from first touching the home cage to ensure assessment of basal, unstressed plasma hormone (Vahl et al., Citation2005). This cohort was run in parallel with the cardiovascular cohort and thus received exposure to the same stressors in the same order.
Assessment of corticosterone responses to the novel EPM was performed on day 15, as noted above. For blood sampling, rats were brought individually from the housing room (between 09:00 h and 11:00 h) into the procedure room and a pre-stress tail-blood sample was quickly collected. Rats were then placed onto a Plexiglas EPM apparatus facing one of the open arms. After 5 min, rats were returned to their home cages and additional tail-blood samples were collected from freely moving rats at 15, 30, 60 and 120 min after the onset of the EPM exposure. As the EPM was used as a probe for physiological sensitization, behavior was not recorded.
Blood collection and corticosterone assay
Tail-blood samples (approximately 250 µl) were collected in chilled tubes containing 10 µl of 100 mM EDTA by a tail clip procedure as described previously (Vahl et al., Citation2005). Briefly for tail clips, the distal millimeter of the tail was removed using a sterile scalpel blade. Each blood sample was collected into EDTA tubes within three minutes to avoid any increase in corticosterone levels due to sampling alone and was immediately placed on ice. Serial samples were obtained by gentle removal of the clot from the tip of the tail using sterile gauze. Blood samples were centrifuged at 3000 g for 15 min at 4 °C and plasma samples were stored at −20 °C for subsequent hormone analysis. Plasma corticosterone concentration was measured with a 125I radioimmunoassay kit from ICN Biochemicals (Cleveland, OH) as described previously (Ulrich-Lai et al., Citation2006). All samples were run in duplicate in the same assay. The assay has an intra-assay coefficient of variation of 8.6% and an inter-assay coefficient of variation of 13.6% and a minimum sensitivity of 12.5 ng/ml.
Brain and organ collection
Two hours after the termination of the novel stressor (EPM), all rats were overdosed with sodium pentobarbital (150 mg/kg, i.p.) and intracardially perfused with 100 ml of 0.9% saline followed by 250–300 ml of 4% paraformaldehyde, pH 7.6. Brains were collected and post fixed in 4% paraformaldehyde overnight and then transferred to 30% sucrose (4 °C). In order to determine the effects of 6-OHDA lesion on somatic markers of stress, adrenal and thymus glands were removed, cleaned and weighed.
Immunohistochemistry
Coronal brain sections (25 µm) from all 6-OHDA and vehicle rats were sectioned in a series of 1–6. Sections were stored in cryoprotectant (30% sucrose, 1% polyvinylpyrrolidone and 30% ethylene glycol in 0.1 M phosphate buffer) at −20 °C until used for immunohistochemistry. Brain sections at the level of −13.8 to −14.4 mm caudal to the bregma were identified using the Paxinos & Watson (Citation1998) rat brain atlas, and immunolabeled for dopamine-β-hydroxylase (DBH) and neuronal nuclei (NeuN). For DBH and NeuN immunohistochemistry, sections were transferred from cryoprotectant to 50 mM potassium phosphate-buffered saline (KPBS) and rinsed (5 × 5 min) at room temperature (RT) on a platform shaker. Afterwards, sections were incubated in blocking solution (0.1% bovine serum albumin and 0.2 % Triton X-100 in KPBS) for 1 h at RT on the shaker. Sections were incubated overnight at 4 °C with a monoclonal antibody against DBH (Flak et al., Citation2009) (Chemicon, Temecula, CA) diluted 1:2500 in blocking solution. The following morning, sections were rinsed in KPBS (5 × 5 min) and incubated in Cy-3 conjugated donkey anti-mouse secondary antibody (1:500, Jackson Immuno Research Laboratories, West Grove, PA) for 1 h at RT on the shaker. Sections were rinsed in KPBS (5 × 5 min) and then incubated with a monoclonal antibody against NeuN (1:200; Millipore, Billerica, MA) overnight in the dark. Sections were then rinsed in KPBS (5 × 5 min) followed by incubation in Alexa 488-labeled goat anti-mouse secondary antibody (1:500; Invitrogen, Eugene, OR) for 1 h at RT in the dark. Following the final antibody incubation, sections were rinsed (5 × 5 min) in KPBS at RT, mounted and cover slipped using anti-fading DABCO medium (Fluka, Sigma).
Quantification of DBH neurons
For verification of the 6-OHDA lesion, the number of DBH immunopositive neurons within the NTS was counted. Digital images of the NTS region were captured at 20 × magnification with a Carl Zeiss Imager Z.1 (Carl Zeiss Microimaging, Thornwood, NY). The number of DBH-immunopositive neurons was counted manually within the three levels of the NTS (i.e. bregma levels: −14.6, −14.3 and −14.08) (Paxinos & Watson, Citation1998), using 1−3 images (at each level) per rat. 6-OHDA lesions were considered “hits” if the injection site was within the targeted NTS region. Lesions that were centered outside of the targeted NTS region were considered “misses”, and these rats were removed from this study. Thus the final group size for the cardiovascular study was n = 5 for the 6-OHDA group and n = 6 for the vehicle group; and for the HPA study was n = 5 for the 6-OHDA group and n = 4 for the vehicle group.
DBH fiber density in A2-targeted regions
DBH immunofluorescence in known targets of the A2 cell group was quantified as described previously (Zhang et al., Citation2010) with minor modification. These regions include (i) anteroventral subdivision of the BST (avBST) (approximately −0.26 mm from bregma), (ii) medial parvocellular PVN (mpPVN) (approximately −1.8 mm from bregma) and (iii) dorsomedial hypothalamic nucleus (DMH) (approximately −3.12 mm from bregma), and were identified using the Paxinos & Watson (1998) rat brain atlas. For analysis of DBH fiber density, digital images of each brain region (2–3 sections/rat for each region) were captured using a Carl Zeiss Imager Z.1 at a magnification to distinguish immunoreactivity from the background (40 × for each region). For each region, z-stacks were collected containing dense DBH immunoreactivity, and after thresholding to ensure that there was no bias for intensity of staining, the occupied field area was calculated using the measurement function of Axiovision 4.6 software (Zeiss MicroImaging, Jena, Deutschland). The threshold was calculated by analysis of several random images and was held constant for all images analyzed. For each rat, the percentage of field area occupied by DBH staining was determined by averaging across the z-stacks. Finally, the percent field area was averaged across rats by treatment group (vehicle vs. lesion).
Statistical analysis
Data are reported as mean ± SEM. All statistical analyses were done using Sigma Stat (SYSTAT, San Jose, CA) software. Two-way repeated measures ANOVA [with group (lesions vs. vehicle) as between subject factor] × time (time being the repeated factor) were performed where appropriate. Follow-up analyses were done by protected Fisher’s post hoc analysis. Student’s t tests were used for comparisons between 6-OHDA- and vehicle-treated rats. Statistical significance was set at p < 0.05 for all analyses.
Results
6-OHDA decreased DBH immunoreactivity
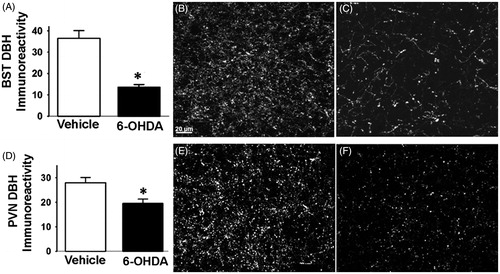
To assess the extent of 6-OHDA lesion in the targeted NTS region, we quantified the number of DBH immunoreactive cells present in the NTS A2 region (). In both experiments [cardiovascular () and (ii) corticosterone ()], we observed a significant reduction in the number of NTS NA (i.e. DBH-positive) neurons in 6-OHDA rats (p < 0.05) compared to vehicle (). We found that 6-OHDA caused marked loss of DBH-positive cells in the NTS [cardiovascular (t (9) = 2.39, p < 0.05), −14.6 mm; (t (9) = 4.13, p < 0.05, −14.30 mm; (t (9) = 2.46, p < 0.05), −14.03 mm and HPA (t (8) = 5.52, p < 0.05), −14.6 mm; (t (8) = 3.39, p < 0.05, −14.30 mm; (t (8) = 7.58, p < 0.05), −14.03 mm] (representative images of vehicle and 6-OHDA rats are depicted in () and (), respectively. This observation was supported by DBH fiber density quantification in regions implicated in cardiovascular and endocrine control of stress responses (Herman et al., Citation2003; Rinaman, Citation2011) and also known to be targeted by the NTS A2 neurons, including the DMH, avBST and mpPVN. DBH-positive fiber densities did not vary between the corticosterone and cardiovascular cohorts, so the groups were pooled for analysis. There was decreased DBH immunoreactivity in avBST (t (18) = 4.38, p < 0.0003; ) and mpPVN (t (18) = 2.74, p < 0.01; ). There was no significant difference in the DBH immunoreactivity in the DMH between vehicle and 6-OHDA rats (data not shown). The data demonstrate that 6-OHDA effectively reduced the number of DBH neurons in the targeted NTS region and resulted in removal of DBH fibers from major stress regulatory forebrain regions.
Figure 1. 6-Hydroxydopamine decreased dopamine beta-hydroxylase (DBH) immunoreactivity within the targeted nucleus tractus solitarii (NTS) region. Representative images of DBH (red)/neuronal nuclei (NeuN; green) immunoreactivity from different rostral-caudal levels of NTS of vehicle- (A, C and E) and 6-hyoxydopamine (6-OHDA)-injected rats (B, D and F). Quantification of DBH neurons at the conclusion of cardiovascular and hypothalamo-pituitary adrenal (HPA) studies revealed significant reduction in DBH neurons in the 6-OHDA rats [cardiovascular (G, I and K) and HPA (H, J and L) studies]. Figure A, B, G and H = −14.6 mm from Bregma; Figure C, D, J and L = −14.3 mm from Bregma; Figure E, F, K and L = −14.08 mm from Bregma. Scale bar in (A) (scale represents100 μm) also applies to (B–F). #Indicates significant difference, p < 0.05, from vehicle: t-test. Data are represented as means ± SEM with n = 4–6 per group.
![Figure 1. 6-Hydroxydopamine decreased dopamine beta-hydroxylase (DBH) immunoreactivity within the targeted nucleus tractus solitarii (NTS) region. Representative images of DBH (red)/neuronal nuclei (NeuN; green) immunoreactivity from different rostral-caudal levels of NTS of vehicle- (A, C and E) and 6-hyoxydopamine (6-OHDA)-injected rats (B, D and F). Quantification of DBH neurons at the conclusion of cardiovascular and hypothalamo-pituitary adrenal (HPA) studies revealed significant reduction in DBH neurons in the 6-OHDA rats [cardiovascular (G, I and K) and HPA (H, J and L) studies]. Figure A, B, G and H = −14.6 mm from Bregma; Figure C, D, J and L = −14.3 mm from Bregma; Figure E, F, K and L = −14.08 mm from Bregma. Scale bar in (A) (scale represents100 μm) also applies to (B–F). #Indicates significant difference, p < 0.05, from vehicle: t-test. Data are represented as means ± SEM with n = 4–6 per group.](/cms/asset/8b0dab22-8bcf-4b68-8df2-a984f7eabcde/ists_a_1013531_f0001_c.jpg)
Figure 2. Dopamine beta-hydroxylase (DBH) immunoreactivity in anterior bed nucleus of stria terminalis (avBST) and medial parvocellular paraventricular nucleus of the hypothalamus (mpPVN). 6-Hydroxydopamine (6-OHDA)-injected rats exhibited lower levels of DBH immunoreactive fibers and terminals in the avBNST (A–C) and in the mpPVN (D–F). Representative projection images of DBH immunoreactivity in the avBST of vehicle (B) and 6-OHDA (C) rats. Representative projection images of DBH immunoreactivity in the mpPVN of vehicle (E) and 6-OHDA (F) rats. Scale bar (scale bar = 20 μm) in (B) also applies to (C, E and F). *Indicates significant difference, p < 0.05, from vehicle: t test. Data are represented as means ± SEM with n = 4–6 per group.
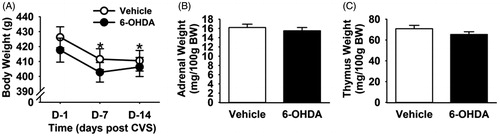
BW and organ weights were not differentially affected by 6-OHDA lesion in chronically stressed rats
Body and organ weight data for the cardiovascular and HPA studies were pooled as there were no statistically significant differences between the two cohorts. Repeated measures two-way ANOVA (lesion × time, time being a repeated measure) revealed significant effects of time on BW, (F (1,59) = 84.52, p < 0.001). A significant reduction in BW was noted on fourth day of the CVS and BW remained lower than pre-stress levels until the end of CVS (). There was no interactive effect within the BW measurement, suggesting that CVS exposure decreased BW regardless of the lesion. Following two weeks of CVS exposure, adrenal () and thymus () weights did not differ between lesion and control rats, indicating that loss of A2 neurons did not differentially affect these endpoints; effects of CVS per se could not be assessed as control rats not exposed to CVS were not included in this study.
Figure 3. Body weight, adrenal weight and thymus weight following chronic variable stress (CVS) in 6-hydroxydopamine (OHDA) and vehicle rats. CVS decreased body weight in both vehicle and 6-OHDA groups (A). Adrenal (B) and thymus weight (C) showed no significant differences with A2 lesion following CVS. *Indicates significant difference, p < 0.05, from CVS day 1: two-way ANOVA with repeated measures followed by protected Fisher’s post hoc analysis. Data are shown as mean ± SEM with n = 10–12 per group.
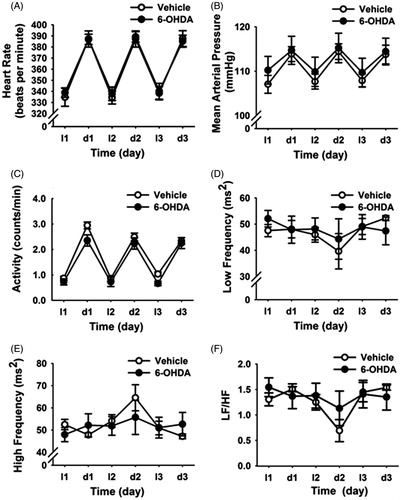
Baseline cardiovascular parameters were not altered by 6-OHDA lesion
Vehicle and 6-OHDA rats had similar mean HR, MAP and activity values across the entire light (l)–dark (d) cycle (). Frequency-domain analysis of HRV in both light and dark phases indicated that LF and HF power spectra were not altered by A2 neuronal lesion (). In addition, there was no difference between groups for the LF/HF values (). Overall, the data indicate that selective loss of A2 neurons did not affect daily rhythms of HR, MAP and HRV parameters in basal (non-stress) conditions.
Figure 4. Time course of changes in cardiovascular and heart rate variability (HRV) parameters during the light and dark phase of baseline daily rhythms (three days). A2 6-hydroxydopamine (6-OHDA) lesions did not alter daily rhythms of heart rate (A), mean arterial pressure (B), locomotor activity (C), low frequency (LF) HRV (D), high frequency (HF) HRV (E) and LF/HF ratio (F) under resting conditions. Data are expressed as mean ± SEM (n = 5 for 6-OHDA and n = 6 for vehicle).
Cardiovascular and locomotor activity responses to acute restraint stress
Acute restraint stress led to rapid increases in HR and MAP. Two-way repeated measures ANOVA revealed effects of time (F (21,210) = 54.64, p < 0.05) and a lesion by time interaction (F (21,210) = 1.82, p < 0.05). As expected, post-hoc analysis showed that both vehicle and 6-OHDA rats had increased HR response to restraint relative to their baseline HR (p < 0.05; ). However, the 6-OHDA lesion blunted the increased HR response to restraint (p < 0.05), indicating that A2 neuronal lesion attenuated the restraint-induced peak tachycardia. In addition, the HR response at the 90-min post-stress time point was also lower in the 6-OHDA rats than the controls (p < 0.05). These data indicate that A2 neurons contribute to stress-induced tachycardia. The acute restraint also increased MAP regardless of treatment (F (21,210) = 44.21, p < 0.05; ). Two-way repeated measures ANOVA also revealed a main effect of restraint on locomotor activity (F (21,210) = 29.70, p < 0.05; ). Post-hoc analysis revealed that in both groups, post-restraint activity was increased and remained elevated for up to 50 min after restraint onset, with no effect of lesion. Together, these findings indicate that differences in the stress-induced HR response between the lesion and the vehicle groups were not confounded by effects on locomotion.
Figure 5. Time course of changes in cardiovascular and heart rate variability (HRV) parameters during and after exposure to a 30-min acute restraint. 6-hydroxydopamine (6-OHDA)-injected rats exhibited lower heart rate, beats per minute (BPM) during the first 30 min of restraint stress and at 60 min after stress cessation (A). A2 lesion did not alter mean arterial pressure (MAP), mmHg (B), locomotor activity, counts per minute, CPM (C), normalized low frequency (LFnorm) HRV (D), normalized high frequency (HFnorm) HRV (E) or ratio of LF/HF (F) responses to restraint. #Indicates significant difference, p < 0.05, from vehicle group: two-way ANOVA with repeated measures followed by protected Fisher’s post hoc analysis. Data are expressed as mean ± SEM with n = 5–6 per group.
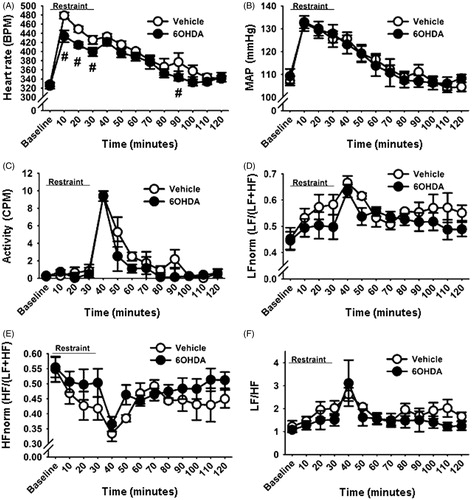
Restraint also increased normalized LF (F (21,210) = 20.10, p < 0.05; ) and reduced normalized HF (F (21,210) = 16.22, p < 0.05; ) components of HRV regardless of A2 lesion status. The restraint-induced changes in the LF and HF components also increased the ratio of LF/HF relative to the pre-stress ratio in both 6-OHDA and vehicle groups (p < 0.05; ), indicating that the A2 lesion in the targeted NTS regions did not differentially modify restraint-induced spectral parameters of HRV.
Cardiovascular responses during CVS
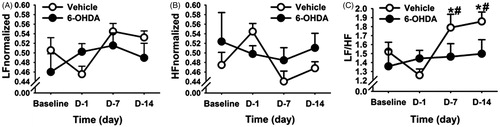
In order to determine the effects of CVS on basal cardiovascular tone, home cage HRV was assessed between 06:00 h and 08:00 h (i.e., prior to the onset of each day’s morning stressor exposure) on days 1, 7 and 14 of the CVS paradigm. Respiratory activity may affect HRV in the rodent, particularly during acute stress (Bondarenko et al., Citation2014). To avoid this possible confound, we performed all analyses of baseline HRV during the inactive phase of the diurnal cycle, temporally isolated from stress exposures by at least 12 h. Two-way repeated measures ANOVA revealed main effects of time on both the LF (F (3,27) = 3.38, p < 0.05; ) and HF (F (3,27) = 3.80, p < 0.05; ) components of HRV. Moreover, the LF/HF ratio (an estimate of autonomic balance (Malliani et al., Citation1991)) showed a main effect of time (F (3,27) = 12.92, p < 0.05) and a lesion × time interaction (F (3,27) = 3.10, p < 0.05). Before exposure to CVS, baseline LF/HF ratio was similar between the two treatment groups (). However, post-hoc analysis revealed that CVS caused a significant increase in the LF/HF ratio on days 7 and 14 relative to their pre-stress time points (i.e. baseline and day 1; p < 0.05) in the vehicle rats (). In contrast, 6-OHDA rats showed no changes in their LF/HF ratio on days 7 and 14, indicating that NTS A2 neurons may alter the balance of sympathetic and parasympathetic tone during chronic stress. In addition, vehicle-treated rats had increased normalized LF activity on the seventh day (compared to baseline and CVS day 1, p < 0.05) and 14th day (compared to CVS day 1, p < 0.05) of CVS. They also had decreased normalized HF activity on the seventh day (compared to CVS day 1, p < 0.05). Resting HR and MAP were not different between 6-OHDA and vehicle rats, indicating that the discrete A2 lesion did not differentially alter resting HR and blood pressure during CVS.
Figure 6. Time course of changes in heart rate variability (HRV) parameters during chronic variable stress (CVS) in vehicle- and 6-hydroxydopamine (6-OHDA)-treated rats. Normalized low frequency (LF) (A) and normalized high frequency (HF) (B) parameters of HRV showed no significant differences with stress or 6-OHDA lesion. (C) CVS increased LF:HF ratio component of HRV in the vehicle group, and this effect was prevented by 6-OHDA lesion. #Indicates significant difference, p < 0.05, from pre stress values. *Indicates significant difference, p < 0.05, from CVS day 1:two-way ANOVA with repeated measures followed by protected Fisher’s post hoc analysis. Data are expressed as mean ± SEM with n = 5–6 per group.
Post-CVS cardiovascular and activity responses to a novel environment stress
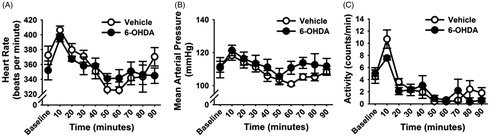
In order to test post-CVS cardiovascular responses to a novel stress, rats were placed in a novel EPM. Two-way repeated measures ANOVA revealed an effect of EPM exposure on MAP (F (18,162) = 4.23, p < 0.05), HR (F (18,162) = 3.3, p < 0.05) and locomotor activity (F (18,162) = 5.71, p < 0.05). More specifically, post-hoc analysis showed that in both experimental groups, all three parameters were increased 10 min after exposure to the EPM, and in vehicle-treated rats MAP and HR remained elevated until 20 min after stress cessation (). However, there was no effect of 6-OHDA lesion. No changes in HRV parameters were observed after EPM exposure in either experimental group (data not shown).
Figure 7. Cardiovascular responses to novel stress in chronically stressed vehicle- and 6-hydroxydopamine (6-OHDA)-treated rats. Heart rate (A), mean arterial pressure (MAP) (B) and locomotor activity (C) of vehicle and 6-OHDA rats in response to novel exposure to the elevated plus maze (EPM) following chronic variable stress (CVS). Heart rate and MAP were increased slightly after the EPM exposure, with increased locomotor activity. There were no significant differences between the vehicle- and 6-OHDA treated rats. Data are expressed as mean ± SEM with n = 5–6 per group.
Plasma corticosterone responses to acute restraint stress
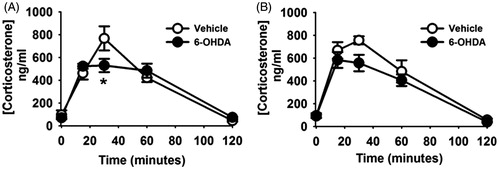
Two-way repeated measures ANOVA revealed an effect of time (F (2,28) = 69.83, p < 0.05) and a time by lesion interaction (F (4,28) = 4.01, p < 0.05) on plasma corticosterone concentrations after acute restraint. Post-hoc comparisons indicated that acute restraint caused significant elevations of corticosterone in vehicle and 6-OHDA rats at 15, 30 and 60 min, compared to both pre-stress and 120-min time points () as expected. Importantly, 6-OHDA-treatment reduced plasma corticosterone at 30 min after restraint onset (), indicating that A2 neurons normally act to promote HPA activation by acute restraint stress.
Figure 8. A2 lesion attenuated neuroendocrine responses to acute stress. (A). Compared with vehicle-treated rats, 6-hydroxydopamine (6-OHDA)-treated rats exhibited a reduced plasma corticosterone concentration at 30 min after onset of restraint. (B). Following chronic variable stress (CVS) exposure, the plasma corticosterone response to novel exposure to the elevated plus maze was not significantly different between 6-OHDA lesioned and vehicle treated rats. Data are mean ± SEM with n = 5–6 per group. *Indicates significant difference, p < 0.05, from vehicle group. Two-way ANOVA with repeated measures followed by protected Fisher’s post hoc analysis.
Plasma corticosterone response to a novel exposure to the EPM in rats with prior CVS exposure
Two-way repeated measures ANOVA revealed an effect of time (F (4,28) = 65.38, p < 0.05) on plasma corticosterone after exposure to the EPM. Post-hoc tests revealed significantly elevated corticosterone concentrations at 15, 30 and 60 min after EPM exposure compared to both pre-EPM exposure and the 120-min time point. There was no effect of lesion and no lesion × time interaction (). The apparent decrease in corticosterone concentration in the 6-OHDA group at 30-min after onset of the EPM was not statistically significant (p > 0.05).
Discussion
Our study supports a central role for A2 neurons in driving autonomic responses to chronic stress. Importantly, the integrity of A2 neurons is required for appropriate cardiovascular responses to acute stress and is required for chronic stress-related sympathetic overdrive. In contrast, while A2 neurons are required for stimulation of corticosterone responses to acute stress, they are evidently not necessary for activation of the HPA axis to a novel stress in chronically stressed rats. Thus, A2 neurons appear to be particularly important in controlling autonomic balance (rather than HPA axis activation) over the course of long-term challenge by stressors.
We assessed the involvement of A2 NA cells in autonomic and neuroendocrine stress responses using discrete 6-OHDA lesions. 6-OHDA produces a profound and long-lasting depletion of brain catecholamine after being administered into the brain (Uretsky & Iversen, Citation1970). We specifically targeted the caudal NTS, which is considered to be preferentially involved in cardiovascular and HPA axis regulation (Rinaman, Citation2011). Importantly, the A2 cell group is not intermixed with phenylethanolamine N-methyltransferase-positive adrenergic neurons of the C2 cell group in this region (Rinaman, Citation2011). Our lesions caused cell loss, accompanied by marked depletion of DBH terminals in the avBST, indicating that our A2 lesions damaged ascending fibers that preferentially project to this brain region (Shin et al., Citation2008). We also observed a significant (though more limited) loss of DBH fibers in the mpPVN, likely due to overlapping innervation of this region by other non-lesioned catecholaminergic cell groups (e.g. A1 and A6) (Cunningham & Sawchenko, Citation1988). There was no significant loss of DBH fiber density in the DMH, probably due to preferential innervation by non-A2 catecholamine neurons in the A1 and C1 regions that are spared by the 6-OHDA lesion (Card et al., Citation2006; Thompson & Swanson, Citation1998). Indeed, the partial denervation of the PVN as well as lack of DBH fiber loss in DMH supports the notion that the lesions selectively compromised ascending A2 projections.
Previous studies indicate that systemic and emotional stressors activate different pathways to drive HPA axis responses (Herman & Cullinan, Citation1997). Systemic stimuli are considered to traverse reflexive pathways (spinal cord, brainstem and lamina terminalis continuum), whereas forebrain processing and integration is necessary for responses to psychological (emotional) stimuli (Herman et al., Citation2003). Our results indicate that A2 cells are required for HPA axis responses to psychological stress (i.e. blunted corticosterone response at 30 min after initiation of restraint), probably through (partial) denervation of mpPVN CRH neurons. Our findings are in agreement with previous evidence reporting attenuated restraint-induced Fos immunoreactivity in PVN neurons following total NTS lesion (Dayas et al., Citation2001) and decreased plasma corticosterone level during restraint following anti-dopamine-b-hydroxylase saporin lesions of the NTS (Daubert et al., Citation2012).
Although damage to A2 NA neurons affects acute HPA axis responsiveness, our data do not support a major role for this pathway in HPA adaptations to chronic stress. Following CVS, there is no effect of 6-OHDA lesion on adrenal gland weight relative to vehicle-treated rats, suggesting that there are no major differences in long-term exposure of the adrenals to ACTH (Ulrich-Lai et al., Citation2006). Post-CVS thymus weight was not affected by 6-OHDA, indicating that cumulative corticosteroid exposure was not dramatically different over the course of CVS. These findings are in agreement with our previous study (Flak et al., Citation2014) demonstrating that norepinephrine/epinephrine neurons do not play a critical role in CVS-induced regulation of body and organ weight. In addition in the CVS group, neither post-CVS baseline nor novel stress-induced corticosterone release was affected by 6-OHDA lesions. The decrease in corticosterone observed at the 30-min time point was not statistically significant, likely due to the limited sample size. Together, our data indicate that while A2 neurons are necessary for cardiovascular reactivity to chronic stress, they are not required for prolonged HPA axis adaptations seen during chronic stress (at least for the CVS paradigm).
Acute restraint stress also elicits autonomic responses, characterized by increased HR and MAP. Previous studies demonstrated that increased cardiac sympathetic nerve activity as well as vagal withdrawal contribute to HR responses to acute stress (Barron & Van Loon, Citation1989; Ngampramuan et al., Citation2008). In agreement with these findings, we observed increased HR responses following acute restraint in both groups of rats (vehicle- and 6-OHDA-treated), but further demonstrated that lesions of A2 neurons attenuate the acute stress-induced HR increase, consistent with a role in generating cardiovascular stress responses.
In contrast to the robust response seen following acute restraint (first stress exposure), cardiovascular responses to the final novel stressor were small in both 6-OHDA and vehicle groups. This indicates that EPM exposure, considered to represent a mild stressor, may not be sufficient to recruit a robust cardiovascular response. Assessment of autonomic function using a more severe stress paradigm will be required to test the necessity for the A2 neurons in mediating such chronic stress responses. Many studies provide evidence that links prolonged exposure and responsiveness to stressors with depressive syndromes and depression with heart disease (Bidzińska, Citation1984; Freedland et al., Citation2003; Johnson & Grippo, Citation2006). Rats exposed to chronic mild stress have resting tachycardia and reduced HRV, indicative of increased sympathetic drive (Grippo et al., Citation2002). Analysis of HRV in the frequency domain revealed noticeable effects of chronic stress on the LF and HF components. CVS provoked an increase in the ratio of LF to HF, which is considered to be indicative of a shift in sympathovagal balance. Importantly, A2 lesions evidently blocked changes in this ratio, indicating that this region is critical for the shift in sympathovagal tone seen following chronic stress. While our results suggest lesion effects on HRV, there are some limitations for this interpretation. The DSI software does not permit analysis of HRV in the time domain, hence we could not verify HRV changes using an alternative method. Thus, while our data support previous studies showing enhanced sympathetic drive following stress (Grippo et al., Citation2002), our analysis methods cannot provide definitive differentiation of sympathetic vs. parasympathetic contributions (Sgoifo et al., Citation1997).
These findings are largely consistent with previous studies documenting cardiovascular consequences of chronic stress. For example, Carnevali et al. (Citation2011) demonstrated that subchronic (i.e. five days) footshock exposure causes a substantial and enduring decrease in HR that is mediated via an increase in cardiac vagal tone. They speculated that the sustained vagal activation observed after subchronic stress exposure may be a transient compensatory phenomenon that initially overcomes the commonly observed stress-induced sympathetic hyperactivity, but after prolonged exposure to stress, this adaptation might fail so that the sympathetic effect becomes dominant. Costoli et al. (Citation2004) also showed a shift of sympathovagal balance toward sympathetic dominance following repeated exposure of mice to social stressors. Unlike in our study, increased sympathetic drive waned with repeated exposures (Costoli et al., Citation2004), likely due to habituation to homotypic stress exposure that does not occur in the CVS model.
Lesions of A2 neurons did not result in any significant changes in pre-stress HR or MAP, either in the morning or evening. These findings are in agreement with reports showing no MAP change after NTS lesions (Itoh et al., Citation1992; Lin et al., Citation2013; Snyder et al., Citation1978; Talman et al., 1980). However, we should note that other studies indicate increased MAP (Daubert et al., Citation2012; Duale et al., Citation2007) after NTS cell loss. This apparent contradiction may be due to unequal extent of A2 lesion or to damage to the NTS astrocytes, which play a critical role in mediating cardiovascular reflexes (Lin et al., Citation2013). Though causing clear pathology in the region of the NTS and loss of fiber staining in its targets, our 6-OHDA lesions did not result in complete cell loss. Thus, our failure to observe alterations in resting HR and blood pressure may be related to sparing of sufficient neurons to maintain basal function, or to sparing of key NA cardioregulatory neurons distant from our injection site.
The loss of DBH fibers and terminals in the anteroventral BST (avBST) by A2 lesions indicates this region is critical for A2 neuronal regulation of HPA axis and autonomic stress responses, both acutely and over time. It should be noted that the role of the BST in stress regulation is complex, and the valence of HPA axis regulation varies across different subnuclei. For example, lesions of the posterior divisions of the BST increase the magnitude of HPA axis stress responses to restraint, whereas lesions of the anteroventral component decrease stress reactivity (Choi et al., Citation2008a,Citationb). Given the marked diminution of DBH fibers in the anteroventral region, our data suggest that loss of noradrenaline may serve to decrease drive of the HPA axis by stress, resulting in the observed decrease in corticosterone response after A2 lesions.
Overall, the present data indicate that A2 NA neurons are critical for control of stress reactivity, both acutely and over time. These neurons participate in the generation of acute HPA axis and autonomic responses to psychogenic stress, and control long-term changes in sympathetic tone that may be of importance to cardiac pathologies associated with stress-related diseases such as depression. Moreover, our data highlight the importance of the hindbrain as a critical integrator of psychogenic stress processing, likely mediated via descending projections from the forebrain.
Supplementary material available online
Supplementary Table I.
Supplemental Material.pdf
Download PDF (7.5 KB)Acknowledgements
The authors thank Jessica McKlveen and Dr. Brent Myers for comments on the manuscript preparation as well as Mark Dolgas and Dayna Wick for their help with blood and tissue collection.
Declaration of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
This research work is supported by US National Institute of Health Grant MH069860 to J. P. H.
References
- Barron BA, Van Loon GR. (1989). Role of sympathoadrenomedullary system in cardiovascular response to stress in rats. J Auton Nerv Syst 28:179–87
- Bhatnagar S, Dallman M. (1998). Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84:1025–39
- Bidzińska EJ. (1984). Stress factors in affective diseases. Br J Psychiatry 144:161–6
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, et al. (1997). Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 34:623–48
- Bondarenko E, Hodgson DM, Nalivaiko E. (2014). Amygdala mediates respiratory responses to sudden arousing stimuli and to restraint stress in rats. Am J Physiol Regul Integr Comp Physiol 306:R951–9
- Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sveb AF. (2006). Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: implications for the central control of cardiovascular regulation. J Comp Neurol 499:840–59
- Carnevali L, Bondarenko E, Sgoifo A, Walker FR, Head GA, Lukoshkova EV, Day TA, Nalivaiko E. (2011). Metyrapone and fluoxetine suppress enduring behavioral but not cardiac effects of subchronic stress in rats. Am J Physiol Regul Integr Comp Physiol 301:R1123–31
- Carnevali L, Sgoifo A. (2014). Vagal modulation of resting heart rate in rats: the role of stress, psychosocial factors, and physical exercise. Front Physiol 5:118
- Choi DC, Evanson NK, Furay AR, Ulrich-Lai YM, Ostrander MM, Herman JP. (2008a). The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology 149:818–26
- Choi DC, Furay AR, Evanson NK, Ulrich-Lai YM, Nguyen MM, Ostrander MM, Herman JP. (2008b). The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology 33:659–69
- Chrousos G, Gold P. (1992). The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267:1244–52
- Costoli T, Bartolomucci A, Graiani G, Stilli D, Laviola G, Sgoifo A. (2004). Effects of chronic psychosocial stress on cardiac autonomic responsiveness and myocardial structure in mice. Am J Physiol Heart Circ Physiol 286:H2133–40
- Cunningham ET, Sawchenko PE. (1988). Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol 274:60–76
- Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker CD, Strack AM, Casio CS. (1992). Stress, feedback, and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol 4:517–26
- Daubert DL, McCowan M, Erdos B, Scheuer DA. (2012). Nucleus of the solitary tract catecholaminergic neurons modulate the cardiovascular response to psychological stress in rats. J Physiol 590:4881–95
- Day HE, Campeau S, Watson SJ, Akil H. (1999). Expression of alpha(1b) adrenoceptor mRNA in corticotropin-releasing hormone-containing cells of the rat hypothalamus and its regulation by corticosterone. J Neurosci 19:10098–106
- Dayas CV, Buller KM, Day TA. (2001). Medullary neurons regulate hypothalamic corticotropin-releasing factor cell responses to an emotional stressor. Neuroscience 105:707–19
- Duale H, Waki H, Howorth P, Kasparov S, Teschemacher AG, Paton JFR. (2007). Restraining influence of A2 neurons in chronic control of arterial pressure in spontaneously hypertensive rats. Cardiovasc Res 76:184–93
- Gaillet S, Alonso G, Le Borgne R, Barbanel G, Malaval F, Assenmacher I, Szafarczyk A. (1993). Effects of discrete lesions in the ventral noradrenergic ascending bundle on the corticotropic stress response depend on the site of the lesion and on the plasma levels of adrenal steroids. Neuroendocrinology 58:408–19
- Flak JN, Ostrander MM, Tasker JG, Herman JP. (2009). Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol 517:156–65
- Flak JN, Jankord R, Solomon MB, Krause EG, Herman JP. (2011). Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol Behav 104:228–34
- Flak JN, Myers B, Solomon MB, McKlveen JM, Krause EG, Herman JP. (2014). Role of paraventricular nucleus-projecting norepinephrine/epinephrine neurons in acute and chronic stress. Eur J Neurosci 39:1903–11
- Freedland KE, Rich MW, Skala JA, Carney RM, Dávila-Román VG, Jaffe AS. (2003). Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med 65:119–28
- Grippo AJ, Moffitt JA, Johnson AK. (2002). Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol 282:R1333–41
- Herman JP, Adams D, Prewitt C. (1995). Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology 61:180–90
- Herman JP, Cullinan WE. (1997). Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20:78–84
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. (2003). Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24:151–80
- Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. (1991). Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci 11:585–99
- Itoh H, Alper RH, Buñag RD. (1992). Baroreflex changes produced by serotonergic or catecholaminergic lesions in the rat nucleus tractus solitarius. J Pharmacol Exp Ther 261:225–33
- Johnson AK, Grippo AJ. (2006). Sadness and broken hearts: neurohumoral mechanisms and co-morbidity of ischemic heart disease and psychological depression. J Physiol Pharmacol 11:5–29
- Kostrzewa RM, Jacobowitz DM. (1974). Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev 26:199–288
- Li HY, Ericsson A, Sawchenko PE. (1996). Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc Natl Acad Sci U S A 93:2359–64
- Lin LH, Moore SA, Jones SY, McGlashon J, Talman WT. (2013). Astrocytes in the rat nucleus tractus solitarii are critical for cardiovascular reflex control. J Neurosci 33:18608–17
- Malliani A, Pagani M, Lombardi F, Cerutti S. (1991). Cardiovascular neural regulation explored in the frequency domain. Circulation 84:482–92
- Myers B, McKlveen JM, Herman JP. (2014). Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Front Neuroendocrinol 35:180–96
- Ngampramuan S, Baumert M, Beig MI, Kotchabhakdi N, Nalivaiko E. (2008). Activation of the 5-HT(1A) receptors attenuates tachycardia induced by restraint stress in rats. Am J Physiol Regul Integr Comp Physiol 294:R132–41
- Paxinos G, Watson C. (1998). The rat brain in stereotaxic coordinates. 4th ed. San Diego, CA: Academic Press
- Plotsky PM. (1987). Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic pathways or central norepinephrine injection. Endocrinology 121:924–30
- Reyes Del Paso GA, Langewitz W, Mulder LJ, Van Roon A, Duschek S. (2013). The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology 50:477–87
- Ricardo JA, Koh ET. (1978). Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res 153:1–26
- Riche D, De Pommery J, Menetrey D. (1990). Neuropeptides and catecholamines in efferent projections of the nuclei of the solitary tract in the rat. J Comp Neurol 293:399–424
- Rinaman L. (2011). Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol 300:R222–35
- Ross CA, Ruggiero DA, Reis DJ. (1985). Projections from the nucleus tractus solitarii to the rostral ventrolateral medulla. J Comp Neurol 242:511–34
- Sawchenko PE, Swanson LW. (1982). The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res 257:275–325
- Sgoifo A, de Boer SF, Westenbroek C, Maes FW, Beldhuis H, Suzuki T, Koolhaas JM. (1997). Incidence of arrhythmias and heart rate variability in wild-type rats exposed to social stress. Am J Physiol 273:H1754–60
- Shin JW, Geerling JC, Loewy AD. (2008). Inputs to the ventrolateral bed nucleus of the stria terminalis. J Comp Neurol 511:628–57
- Snyder DW, Nathan MA, Reis DJ. (1978). Chronic lability of arterial pressure produced by selective destruction of the catecholamine innervation of the nucleus tractus solitarii in the rat. Circ Res 3:662–71
- Talman WT, Perrone MH, Reis DJ. (1980). Evidence for L-glutamate as the neurotransmitter of baroreceptor afferent nerve fibers. Science 209:813–15
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. (1996). Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circ Res 93:1043–65
- Thompson RH, Swanson LW. (1998). Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res Rev 27:89–118
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. (2006). Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab 291:E965–73
- Uretsky NJ, Iversen LL. (1970). Effects of 6-hydroxydopamine on catecholamine containing neurons in the rat brain. J Neurochem 17:269–78
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA, Herman JP. (2005). Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 289:E823–8
- Zhang R, Jankord R, Flak JN, Solomon MB, D’Alessio DA, Herman JP. (2010). Role of glucocorticoids in tuning hindbrain stress integration. J Neurosci 30:14907–14
