Abstract
Inconsistencies exist in the current literature regarding hypothalamic–pituitary–adrenal (HPA) regulation following exposure to repeated stressful events. These inconsistencies stem, in part, from the limitations imposed by measuring cortisol in saliva or plasma (i.e. “point measures” of HPA activity). The present study used a cross-sectional, correlational design to examine the relationship between childhood stress (assessed using the adverse childhood experiences [ACEs] questionnaire) and hair cortisol (a biomarker of chronic HPA activity) in 55 healthy 18–24-year-old college students. Dichotomous ACE score for two models using different cut-points was significantly, inversely related to hair cortisol level (B = 1.03, p = 0.046 and B = 1.09, p = 0.031). These results are consistent with theoretical models where exposure to repeated stressful events results in chronic HPA dysregulation, which may include down-regulation under certain conditions.
Introduction
Adverse childhood experiences (ACEs) are harmful and distressing events within a child’s family or social environment that vary in severity and often persist over time (Kalmakis & Chandler, Citation2014). Examples of ACEs are physical abuse, sexual abuse, emotional neglect, witnessing violence in the home or neighborhood and living with an alcohol-addicted parent. ACEs can be a source of physiologic stress and are associated with a number of health problems that manifest later in life (Chartier et al., Citation2010; Greenfield & Marks, Citation2009). Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis is associated with disease outcomes (Black & Garbutt, Citation2002; Taylor et al., Citation2004), suggesting that such dysregulation may be an important mediator of the relationship between early adversity and adult disease. This possibility is supported by evidence that cortisol, the primary hormone of the human HPA axis, is important in regulating the central nervous, metabolic and immune systems (McEwen & Gianaros, Citation2010). This research adds to current knowledge by demonstrating chronic HPA activity following repeated stressful childhood events. This knowledge can serve to further clarify the long-term effects of early adversity on health.
Inconsistencies exist in the current literature regarding HPA regulation following exposure to repeated stressful events (Struber et al., Citation2014). One potential source of this variation is the time interval between the stressor and HPA assessment (Miller et al., Citation2007). For example, children from low-income households were found to have significantly higher salivary cortisol levels than their counterparts from high-income households from ages 6 to 10 (Lupien et al., Citation2001); however, this difference in acute cortisol levels did not persist into the teen years, suggesting that the HPA axis adapts in the presence of chronic stressful environments. Similarly, a study of children living in poverty found elevated salivary cortisol levels in infants, but these levels declined by four years of age (Blair et al., Citation2011).
Another source of variation in the literature is the direction of the cortisol change. When exposed to an acute stressor, salivary cortisol levels were elevated in children who experienced multiple childhood adversities (Carrion et al., Citation2002), yet blunted in adults with a history of childhood adversity (Elzinga et al., Citation2008). The challenge to researchers in this area is to determine the long-term effects of chronic early adversity on the body’s stress systems (Taylor et al., Citation2011).
Cortisol may be measured using various bodily fluids including saliva and blood. However, these biomarkers provide only momentary cortisol levels and are affected by circadian rhythms and mechanisms of homeostasis (McEwen, Citation2007). Thus, the use of momentary cortisol measures may not be suitable to examine chronic HPA activity following repeated stressful events. In contrast, hair cortisol analysis has provided retrospective cortisol measurement in studies examining both physical and psychosocial stressors in humans (Dettenborn et al., Citation2010; Karlen et al., Citation2011; Luo et al., Citation2012; Pereg et al., Citation2011). The ability to extract cortisol from hair represents a promising measure of HPA activity following exposure to chronic conditions. Evidence from numerous studies in humans as well as various animal species supports the general validity and reliability of hair cortisol as an index of chronic HPA activity that can provide insight into longer-term stress-related perturbations of this endocrine system (Stalder & Kirschaum, 2012). Importantly, Stalder et al. (Citation2012) recently demonstrated a high degree of intraindividual stability of hair cortisol levels using a test–retest paradigm (“r”s from 0.68 to 0.79), thereby showing that this measure can serve as a trait-like marker of HPA system activity.
The purpose of this study was to assess chronic HPA activity following a history of repeated stressful experiences in childhood. To accomplish this aim, we determined the relationship between hair cortisol levels and scores on the ACEs questionnaire in a cohort of young adults.
Methods
Setting and sample
Following approval from appropriate institutional review boards, a convenience sample of young adults attending general undergraduate psychology courses on an urban college campus was recruited. To be included in the study, individuals had to be 18–24 years old (providing a sample of individuals at similar periods of development), have hair at least 3-cm long, and be able to read English.
Procedures
Students were recruited in-person during class over a 6-week period. Those who consented to participate in the study were given study identifiers, which they used to complete an on-line questionnaire for childhood adversity. Participants next attended one of five prescheduled sessions on campus to provide hair samples for analysis of cortisol levels (n = 55). Hair questionnaires were collected just prior to hair sampling. Hair was obtained from a 1-cm2 area in the posterior vertex region of the head using scissors to cut close to the scalp. Hair samples were weighed at the time of collection and placed in a clean envelope that was clearly labeled with the participant’s study identifier and taken within 48 h to the laboratory for storage and later analysis.
Measures
Chronic HPA activity
Cortisol levels were determined in 3-cm hair samples collected from the posterior vertex of participants’ scalps, a collection method found to minimize the possible loss of cortisol due to hair washing, hair treatments and exposure to sun (Meyer & Novak, Citation2012). Hair grows at approximately rate of 1 cm per month, thus the hair samples provided approximately 3 months of cortisol deposition. The posterior vertex has been shown to have the lowest coefficient of variation (15.6%) compared to other areas (30.5%) (Sauve et al., Citation2007). Cortisol was analyzed in hair samples as previously described (Meyer et al., Citation2014). Assays were conducted in duplicate with standards and quality controls, and data were converted to pg cortisol per mg hair weight. The intra- and inter-assay coefficients of variation were 1.6% and 4.0% respectively. The laboratory personnel were blinded to participants’ histories.
Variables influencing hair cortisol
To account for possible variation in cortisol levels resulting from metabolic abnormalities (e.g. diabetes) or environmental influences (e.g. hair washing and chemical treatments) (Meyer & Novak, Citation2012), participants completed a brief six-item questionnaire at the time of hair collection. The questions pertained to their health history, medications, hair care and exposure to hair chemicals. Participants were excluded from the study if they suffered from any endocrine, psychiatric or other illness known to affect HPA activity.
Childhood stress
Data on early childhood adversity were collected using a 19-item adapted self-report ACE study questionnaire (Dube et al., Citation2004). Participants indicated their exposure to physical, emotional or sexual abuse (six items), neglect (four items) or household dysfunction (nine items). This widely used questionnaire has been validated (Dong et al., Citation2004), and the test–retest reliability over a 1-year interval has been reported as 0.64 (Dube et al., Citation2004).
Analysis
All data from on-line questionnaires were checked for missing data and valid values. Missing data were noted for three participants, each of whom failed to respond to one ACE question. This was handled by substituting the participant’s mean ACE score for the missing response.
Review of hair sample questionnaries did not result in the elimination of any samples due to metabolic or environmental influences on hair cortisol. Hair cortisol data were log transformed due to right skewedness.
Total ACE score was obtained by summing the total number of ACEs reported. Curve estimation was used to identify the nature of the relationship. Based on the results of this analysis, multiple logistic regression analyses were performed to further evaluate the ACE total score threshold and the relationship between ACE score and hair cortisol. Each dichotomous ACE score variable (cut at total score thresholds of 1–6) was evaluated in a separate logistic regression model controlling for age group, gender and race (white vs. non-white). A dichotomous race score (white and non-white) was used, as cell sizes of other racial categories were too small. All covariates were simulatenously entered into the model.
Results
Fifty-seven participants completed questionnaires and provided hair samples. Fifty-five samples fell within the typical range of hair cortisol values recorded by our laboratory for healthy human participants (see Meyer et al., Citation2014) and were used for statistical analysis. Two samples provided abnormally high cortisol values (>2500 pg/mg) that were well outside this range and were more than 70 SD above the mean of the other samples. Consequently, these extreme outliers were dropped prior to analysis.
Student characteristics are shown in . Of the fifty-five participants whose hair cortisol levels were used, 23.6% were male and 58.2% were white. Forty-five students (81.8%) reported at least one ACE (out of a possible 19) and 9.1% reported 10 or more; the highest ACE score was 16 reported by one participant. The average number of ACEs reported was just over 3 (mean = 3.2, SD = 3.6). Mean cortisol level of hair samples was 16.2 pg/mg (SD = 34.9, range = 2.2–260.8 pg/mg).
Table 1. Participants’ demographic characteristics (N = 55).
Non-parametric regression was performed to provide an initial evaluation of model fit (). In non-parametric regression, regression lines are fitted locally to each region of the data. Then, a smoothing spline is used to produce a smooth curve from these short lines to identify the shape of the distribution, which allows for visual inspection for the presence of a threshold. Results identified that the cubic model was the best fit (p = 0.03; incremental R2 = 0.08). includes observed cases along with linear, quadratic and cubic models. All model results are presented in .
Figure 1. Curve estimation analysis of model fit. The figure illustrates results of a non-parametric regression analysis of log hair cortisol concentration as a function of total ACE score. A smoothing spline was used to visually inspect for the presence of a threshold ACE value. The analysis identified the cubic model as the best fit (incremental R2 = 0.08, F = 3.16, p = 0.033). Other model results are presented in .
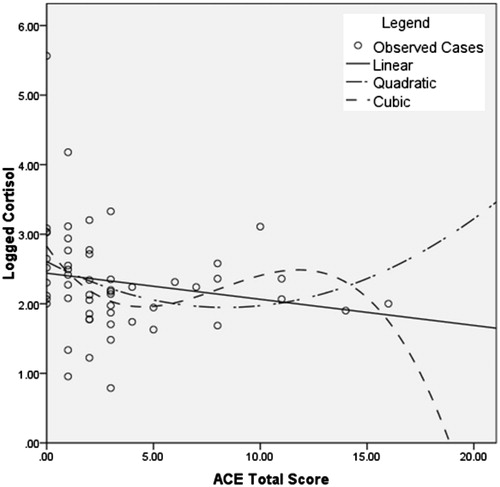
Table 2. Curve fitting model summary.
To explore potential threshold, four dichotomous ACE scores were computed. For each ACE grouped variable a different cut-point for low versus high trauma was used (e.g. ACE score of 0 vs. ACE score 1 or more, ACE score of 0–1 vs. ACE score of 2 or more). Each dichotomous variable was included in separate logistic regression models controlling for age group, gender and race (white vs. non-white). The logistic regression analyses are presented in . The dichotomous ACE score for the first two models (cut-points 1 and 2) was significantly, inversely related to hair cortisol level (B = 1.03, p = 0.046 and B = 1.09, p = 0.031, respectively). Participants with low cortisol values were 2.79 times more likely to be in the high trauma group using a cut-point of 1; using a cut-point of 2, subjects were 2.97 times more likely to be in the high trauma group using a cut-point of 2. Using a cut-point of 3 (0–2 vs. 3 or more) yielded a non-significant trend in the same direction (B = 0.93, p = 0.065, odds ratio = 2.54). None of the remaining models identified significant results.
Table 3. Logistic regression analyses by dichotomous ACE variable.
Discussion
To our knowledge, this is the first study to use hair cortisol (a biomarker of chronic adrenocortical activity) to assess the influence of stress earlier in life on HPA axis function in healthy young adults. We found that a history of childhood stress was significantly related to chronic low levels of cortisol in adjusted models. Other recent studies have found that prior trauma, either during adulthood or childhood, is similarly associated with reduced hair cortisol concentrations (Hinkelmann et al., Citation2013; Steudte et al., Citation2013). Taken together, these findings are important because they help to clarify the earlier inconsistent results (obtained using point measures such as salivary or plasma cortisol) that found either elevated, reduced or unchanged cortisol levels in individuals who were severely stressed earlier in life (Struber et al., Citation2014). These more recent results using a biomarker of chronic HPA activity collectively support the hypothesis that repeated stressful experiences lead, over time, to HPA axis dysregulation in the form of reduced cortisol levels (Miller et al., Citation2007). Consequently, conditions characterized by prolonged activation of the stress response, such as childhood adversity, might produce illness as a result of insufficient glucocorticoid signaling later in life (Raison & Miller, Citation2003) rather than over stimulation, which has been found in children (Blair et al., Citation2011; Carrion et al., Citation2002). Chronically deficient cortisol is suspected in the development of chronic fatigue syndrome (Demitrack et al., Citation1991), chronic pelvic pain (Heim et al., 2000) and treatment-resistant post-traumatic stress disorder (Yehuda et al., Citation2009). Low cortisol is believed to negatively affect health because the body is not prepared to cope effectively with stressors (Sapolsky et al., Citation2000).
Some limitations of the present study were noted. First, the sample size was insufficient to test a graded relationship between ACEs and cortisol for participants with high ACE scores. Second, African American and Hispanic males were disproportionally excluded from the study due to short hair length. Third, we did not account for acute stress within the 3 months before hair collection, which may have confounded the results. However, it is important to note that current stressful life events such as being unemployed or being a caregiver for an elderly demented patient have been shown to increase, rather than decrease, hair cortisol concentrations (Dettenborn et al., Citation2010; Stalder et al., Citation2014). Future studies using both acute and chronic measures of cortisol to gauge HPA activity over time would add to knowledge in this area. Fourth, because the ACE questionnaire includes a range of experiences that vary in intensity, we do not know whether low cortisol levels were particularly associated with severely stressful events. In this regard, a previous study found that severe abuse during childhood was related to reduced morning salivary cortisol in adulthood, whereas participants who had experienced more moderate abuse showed elevated cortisol levels compared to non-abused controls (van der Vegt et al., Citation2008). Fifth, although chronic hypo-activation of the HPA system was found, this was a small sample and included a narrow age range. Future studies should include a larger sample size and a wider range of participant ages to test more strongly the hypothesis that HPA activity declines in relation to the time since occurrence of prior repeated stressful experiences. Lastly, it is possible that recall bias may have influenced the findings. However, retrospective responses to ACEs have been found to be generally stable over time (Dube et al., Citation2004).
In conclusion, results provide evidence that cortisol measured in hair samples, a biomarker for chronic HPA activity, is significantly reduced in young adults with a history of repeated stressful events in childhood. Our findings add to the mounting evidence that repeated or prolonged activation of the stress response, such as occurs with ACEs, can result in insufficient glucocorticoid signaling (Miller et al., Citation2007; Raison & Miller, Citation2003). Further research is needed to determine whether reduced hair cortisol levels reflect overall lower adrenocortical activity (leading to decreased baseline circulating cortisol concentrations) or normal baseline activity but reduced responsiveness to recurring daily life stressors.
Acknowledgements
The authors would like to acknowledge the contribution of Kendra Rosenberg, Lyna Mekhilef and Megan Sutton for their assistance in processing and analyzing the hair samples.
Declaration of interest
A University of Massachusetts Amherst Faculty Research Grant/Healey Endowment Grant supported this research. The authors report no conflict of interest. The authors alone are responsible for the content and writing of this manuscript.
References
- Black PH, Garbutt, LD. (2002). Stress, inflammation and cardiovascular disease. J Psychosom Res 52(1):1–23
- Blair C, Raver CC, Granger D, Mills-Koonce R, Hibel L. (2011). Allostasis and allostatic load in the context of poverty in early childhood. Dev Psychopathol 23:845–57
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. (2002). Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biol Psychiatry 51(7):575–82
- Chartier MJ, Walker JR, Naimark B. (2010). Separate and cumulative effects of adverse childhood experiences in predicting adult health and health care utilization. Child Abuse Negl 34:454–64
- Demitrack MA, Dale JK, Straus SE, Laue L, Listwak SJ, Kruesi MJ, Chrousos GP, Gold PW. (1991). Evidence for impaired activation of the hypothalamic–pituitary–adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab 73(6):1224–34
- Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. (2010). Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology 35(9):1404–9
- Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, Loo CM, Giles WH. (2004). The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abus Negl 28(7):771–84
- Dube SR, Williamson DF, Thompson T, Felitti VJ, Anda RF. (2004). Assessing the reliability of retrospective reports of adverse childhood experiences among adult HMO members attending a primary care clinic. Child Abuse Negl 28(7):729–37
- Elzinga B, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. (2008). Diminished cortisol responses to psychosocial stress associated with lifetime adverse events. A study among healthy young adults. Psychoneuroendocrinology 33:227–37
- Greenfield EA, Marks NF. (2009). Profiles of physical and psychological violence in childhood as a risk factor for poorer adult health: evidence from the 1995–2005 National Survey of Midlife in the United States. J Aging Health 21(7):943–66
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 2000;25(1):1–35
- Hinkelmann K, Muhtzb C, Dettenbornc L, Agorastosd A, Wingenfelda K, Spitzere C, Gao W, et al. (2013). Association between childhood trauma and low hair cortisol in depressed patients and healthy control subjects. Biol Psychiatry 74:e15–17
- Kalmakis KA, Chandler GE. (2014). Adverse childhood experiences: towards a clear conceptual meaning. J Adv Nurs 70(7):1489–501
- Karlen J, Ludvigsson J, Frostell A, Theodorsson E, Faresjo T. (2011). Cortisol in hair measured in young adults – a biomarker of major life stressors? BMC Clin Pathol 11(12):1–6
- Luo H, Hu X, Liu X, Ma X, Guo W, Qiu C, Wang Y, et al. (2012). Hair cortisol level as a biomarker for altered hypothalamic–pituitary–adrenal activity in female adolescents with posttraumatic stress disorder after the 2008 Wenchuan earthquake. Biol Psychiatry 72(1):65–9
- Lupien SJ, King S, Meaney MJ, McEwen BS. (2001). Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol 13(3):653–76
- McEwen BS. (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904
- McEwen BS, Gianaros PJ. (2010). Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann NY Acad Sci 1186(1):190–222
- Meyer JS, Novak MA. (2012). Minreview: hair cortisol: a novel biomarker of hypothalamic–pituitary–adrenocortical activity. Endocrinology 153(9):4120–7
- Meyer JS, Novak MA, Hamel A, Rosenberg K. (2014). Extraction and analysis of cortisol from human and monkey hair. J Vis Exp 83: e50882(1–6)
- Miller GE, Chen E, Zhou ES. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychol Bull 133(1):25–45
- Pereg D, Gow R, Mosseri M, Lishner M, Rieder M, Van Uum S, Koren G. (2011). Hair cortisol and the risk for acute myocardial infarction in adult men. Stress 14(1):73–81
- Raison CL, Miller AH. (2003). When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160(9):1554–65
- Sapolsky RM, Romero LM, Munck AU. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21(1):55–89
- Sauve B, Koren G, Tokmakejian S, Van Uum SHM. (2007). Measurement of cortisol in human hair as a biomarker of systematic exposure. Clin Invest Med 30(5):E183–91
- Stalder T, Kirschaum C. Analysis of cortisol in hair – state of the art and future directions. Brain Behav Immun 2012;26:1019–29
- Stalder T, Steudte S, Miller R, Skoluda N, Dettenborn L, Kirschbaum C. Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology 2012;37(5):602–10
- Stalder T, Tietze A, Steudte S, Alexander N, Dettenborn L, Kirschbaum C. (2014). Elevated hair cortisol levels in chronically stressed dementia caregivers. Psychoneuroendocrinology 47:26–30
- Steudte S, Kirschbaum C, Gao W, Alexander N, Schonfeld S, Hoyer J, Stalder T. (2013). Hair cortisol as a biomarker for traumatization in healthy individuals and posttraumatic stress disorder patients. Biol Psychiatry 74:639–46
- Struber N, Struber D, Roth G. (2014). Impact of early adversity on glucocorticoid regulation and later mental disorders. Neurosci Biobehav Rev 38:17–37
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. (2004). Early environment, emotions, responses to stress, and health. J Pers 72(6):1365–94
- Taylor SE, Way BM, Seeman TE. (2011). Early adversity and adult health outcomes. Dev Psychopathol 23(3):939–54
- van der Vegt E, van der Ende J, Kirschbaum C, Verhulst FC, Tiemeir H. (2008). Early neglect and abuse predict diurnal cortisol patterns in adults: a study of international adoptees. Psychoneuroendocrinology 34:660–9
- Yehuda R, Bierer LM, Sarapas C, Makotkine I, Andrew R, Seckl JR. (2009). Cortisol metabolic predictors of response to psychotherapy for symptoms of PTSD in survivors of the World Trade Center attacks on September 11, 2001. Psychoneuroendocrinology 34(9):1304–13
