Abstract
Clinical and pre-clinical studies have shown that early-life adversities, such as abuse or neglect, can increase the vulnerability to develop psychopathologies and cognitive decline later in life. Remarkably, the lasting consequences of stress during this sensitive period on the hypothalamic–pituitary–adrenal axis and emotional function closely resemble the long-term effects of early malnutrition and suggest a possible common pathway mediating these effects. During early-life, brain development is affected by both exogenous factors, like nutrition and maternal care as well as by endogenous modulators including stress hormones. These elements, while mostly considered for their independent actions, clearly do not act alone but rather in a synergistic manner. In order to better understand how the programming by early-life stress takes place, it is important to gain further insight into the exact interplay of these key elements, the possible common pathways as well as the underlying molecular mechanisms that mediate their effects. We here review evidence that exposure to both early-life stress and early-life under-/malnutrition similarly lead to life-long alterations on the neuroendocrine stress system and modify emotional functions. We further discuss how the different key elements of the early-life environment interact and affect one another and next suggest a possible role for the early-life adversity induced alterations in metabolic hormones and nutrient availability in shaping later stress responses and emotional function throughout life, possibly via epigenetic mechanisms. Such knowledge will help to develop intervention strategies, which gives the advantage of viewing the synergistic action of a more complete set of changes induced by early-life adversity.
Environmental factors present during early-life and their role in the development of psychopathology later in life
Early-life (EL) is a critical period for brain development. Exposure to adverse experiences during this sensitive period is associated with lasting changes in later brain structure and function (Lupien et al., Citation2009; Teicher et al., Citation2012) as well as with an increased vulnerability to develop psychopathologies, cognitive deficits and anxiety disorders later in life (Abe et al., Citation2007; Bale et al., Citation2010; Gershon et al., Citation2013 ; Glover, Citation2011; Kroupina et al., Citation2012; Loman et al., Citation2010). Several epidemiologic studies strongly support a direct association between early-life stress (ELS) exposure and the development of psychopathology in adulthood, as shown for example by childhood sexual abuse (Danese & Tan, Citation2014; Heim et al., Citation2010; Maselko et al., Citation2011; McGowan et al., Citation2009; Neigh et al., Citation2009), poverty (Chugani et al., Citation2001) and maternal depression (Stevens et al., Citation2008), which are all accompanied by cognitive decline and an increased vulnerability to develop psychopathology later in life. However, the exact mechanisms underlying these programming effects are not fully understood. Important questions in this respect are: (1) which key elements in the early environment play a role? And: (2) what are the cellular and molecular mechanisms responsible for the lifelong programming of the brain?
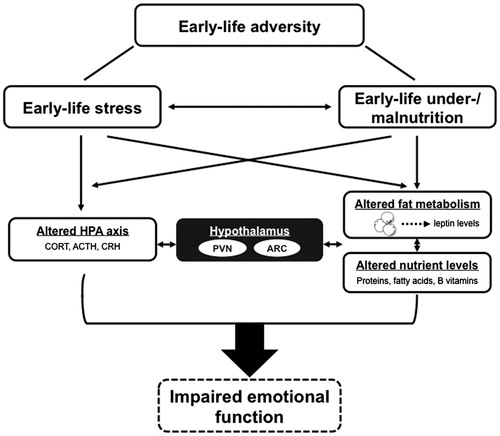
The EL environment encompasses several elements, including sensory stimuli from the mother, neuroendocrine signals and nutrition (Baram et al., Citation2012; Joëls & Baram, Citation2009; Korosi & Baram, Citation2009; Lucassen et al., Citation2013). Alterations in either one or multiple of these elements will impact brain development during specific critical periods and thereby affect brain structure and function later in life. For example, (pre)clinical studies show that disruption in maternal care influence the development of emotional and cognitive functioning throughout life (Baram et al., Citation2012; Heim et al., Citation2008; Maselko et al., Citation2011; Tyrka et al., Citation2008). It is remarkable that also adverse nutrition-related changes early in life affect the stress system and pre-dispose an individual to emotional disturbances in adulthood as well (Brown et al., Citation2000; de Rooij et al., Citation2006; Heijmans et al., Citation2008; Painter et al., Citation2005). For example, exposure to famine in the Dutch Hunger Winter during the first trimester resulted in a 2-fold increase in the risk to develop schizophrenia, while exposure to famine in the second and third trimester is associated with an increased risk to develop affective psychosis (Brown & Susser, Citation2008; Brown et al., Citation1995; Hulshoff Pol et al., Citation2000). What is not yet clear is whether and how these seemingly uncoordinated sensory stimuli, the stress-induced hormonal changes and the nutritional environment interact, affect one another, and whether there might be a common pathway via which these inputs affect and program our brain ().
Figure 1. Schematic representation of the interaction of early-life stress and early-life nutrition in the programming of the HPA axis and emotional function by early-life adversity. This figure shows the complexity of the closely interrelated factors that are able to influence each other and contribute to the outcome of developing lasting effects on emotional behavior. However, to what extent alterations in the fat metabolism and nutrient levels play a role in the development of impaired emotional functions is not yet clear. ACTH, adrenocorticotropic hormone; ARC, arcuate nucleus; CORT, corticosterone; CRH, corticotropin-releasin hormone; HPA axis, hypothalamic–pituitary–adrenal axis; PVN, paraventricular nucleus.
Clearly, the early stressful environment is able to affect the nutritional status of the offspring and adverse nutritional environments can be considered as a stress condition. Stress and nutrition are indeed closely interrelated factors (Hoeijmakers et al., Citation2015; Lucassen et al., Citation2013; Spencer, Citation2013; Walker, Citation2010). An extreme clinical example that illustrates the close interplay between stress and nutrition is a pediatric condition named non-organic failure to thrive (NOFTT), the most frequent (up to 90%) form of failure to thrive (Cole & Lanham, Citation2011). NOFTT consists of a decelerated or arrested physical growth that involves a state of undernutrition due to inadequate caloric intake and absorption, which however develops without implications of major metabolic diseases and is directly caused by behavioral or psychosocial problems. In fact, postnatal factors, such as economic status and parental child abuse/neglect, are associated with NOFFT (Black et al., Citation1995; Cole & Lanham, Citation2011; Wright, Citation2006). Moreover, there is some evidence pointing towards a positive correlation between the severity of growth deficiency in children who experienced NOFTT and the development of cognitive, mental or emotional disturbances later in life (Corbett & Drewett, Citation2004; Glaser et al., Citation1968; Muñoz-Hoyos et al., Citation2011), but see (Rudolf, Citation2005).
In this review, we will discuss the effects of ELS and under-/malnutrition on the developing brain with a focus on the programming of the neuroendocrine stress system and emotional function. We will examine in detail the evidence from clinical and pre-clinical studies on the effects of ELS and early-life nutrition on these systems and cover the specific role of stress, metabolic hormones, maternal sensory stimuli as well as nutrition and highlight their interaction in the lasting effects (). Finally, we will address some of the epigenetic mechanisms that might mediate these alterations.
Long-term consequences of early-life stress on emotional behavior and the neuroendocrine stress system
Development of the neuroendocrine stress system
The neuroendocrine stress system is largely under control of the hypothalamus. Both physical and psychological stressors activate the hypothalamic–pituitary–adrenal (HPA) axis by rapidly triggering the release of the corticotropin-releasing hormone (CRH) from the paraventricular nucleus (PVN), which in turn causes the secretion of adrenocorticotropic hormone (ACTH) from the pituitary into the blood (de Kloet et al., Citation2005). ACTH stimulates the synthesis and secretion of glucocorticoids (GCs) from the adrenal cortex, i.e. corticosterone (CORT) and cortisol in rodents and humans, respectively. The HPA axis is under negative feedback control of GCs binding to glucocorticoid receptors (GRs) in the hypothalamus and pituitary gland, leading to the inhibition of CRH and ACTH release and causing tight regulation of the stress response system.
The development of this important hypothalamic circuit occurs until the first 2–3 postnatal weeks of a rodent life (Morgane et al., Citation2002), while in humans it takes place throughout the entire gestation and is not completed until the last trimester (Bouret & Simerly, Citation2006; Koutcherov et al., Citation2003). The HPA axis of rodents in early postnatal life undergoes a period of reduced responsiveness (Pryce et al., Citation2002), termed the stress hyporesponsive period (SHRP), which is largely controlled by maternal care (Levine, Citation2001). The human equivalent to the rodent SHRP appears to take place at the end of the first year of life (Gunnar & Quevedo, Citation2007). The SHRP is characterized by an active suppression to secrete ACTH and CORT in response to various stressors. However, this suppression is dependent on the nature of the stressor, for example, specific age-appropriate stressors such as the absence of the mother, inadequate maternal care or cold exposure can still elicit an HPA axis activation during the SHRP (Avishai-Eliner et al., Citation1995; Dent et al., Citation2000). Consequently, manipulations that disturb the dam–pup relationship are very effective in inducing ELS conditions, particularly during the first 2 weeks of a rodent life when the pups depend on the dam for nutrition, warmth and sensory stimulation (see next section for more details).
In the next sections, we will consider how adverse experiences during the perinatal period can affect long-term HPA axis activity and emotional functions and present the evidence for the modulatory role of key elements (sensory stimuli, stress as well as metabolic hormones and nutrition) and their interaction during adverse EL experiences on the development and function of the neuroendocrine stress system and emotional behavior.
Lasting effects of early-life stress on the hypothalamic–pituitary–adrenal axis and emotional behavior
Early-life stress is associated with an altered responsiveness of the HPA axis in adulthood. This has been established both in clinical as well as pre-clinical studies. Indeed, humans exposed to ELS have increased HPA axis reactivity in response to physiological and pharmacological stressors (Lupien et al., Citation2009; Pesonen et al., Citation2010; Teicher et al., Citation2012; Tyrka et al., Citation2008) as well as a higher incidence of developing cognitive and emotional impairments in adulthood (Abe et al., Citation2007; Bale et al., Citation2010; Glover, Citation2011; Gershon et al., Citation2013; Heim et al., Citation2008; Loman et al., Citation2010; Pesonen et al., Citation2007). Similarly, experimentally induced modulations of the EL environment in rodents affect several modulators of the neuroendocrine stress system on the long-term, e.g. through lasting changes in the expression of CRH and GR genes, which will be discussed in detail in this section (Avishai-Eliner et al., Citation2001; Brunson et al., Citation2001; Chen et al., Citation2010; Korosi & Baram, Citation2010; Rincón-Cortés & Sullivan, Citation2014). The effects of ELS on HPA axis activity and emotional function of the offspring have also been extensively studied in primate (Pryce et al., Citation2002), however, we will here review the studies based on rodent postnatal stress models (Naninck et al., Citation2015; Oomen et al., Citation2011; Workel et al., Citation2001) ().
Table 1. Examples of early-life interventions affecting emotional behavior and the neuroendocrine system.
Most postnatal stress models consist of disrupting the dam–pup interaction by a single prolonged separation for 24 h (maternal deprivation; MD) or by repeated daily separations of the dam and pups for 3–8 h (maternal separation; MS). For example, following MD in rats at postnatal (P) day 5 or 8, basal expression of CRH mRNA in the PVN was reported to be either unchanged (Avishai-Eliner et al., Citation1995; Korosi et al., Citation2010) or reduced (Schmidt et al., Citation2004), accompanied by reduced CRH receptor 2 (CRFR2) mRNA expression in the ventromedial hypothalamus (Eghbal-Ahmadi et al., Citation1997). In addition, ACTH and CORT responses to a stressor, such as cold exposure or restraint, were markedly increased in pre-pubertal rats (Avishai-Eliner et al., Citation1995; Dent et al., Citation2000; Vázquez et al., Citation1996). Moreover, repeated periodic MD of 180 min from P3 to P10 in rats leads to later anxiety-related behavior that was accompanied with elevated ACTH response after elevated plus maze exposure (Wigger & Neumann, Citation1999). Changes in HPA axis activity in response to stress are still present in adulthood, but the direction of the effect largely depends on the age of the animal and on the testing conditions (Workel et al., Citation2001).
Similarly, repeated daily MS in rats for several hours during the first 2 postnatal weeks augments the hormonal stress response in rodents throughout life and is accompanied by decreased GR binding in both hippocampus and hypothalamus (Chen et al., Citation2012; Ladd et al., Citation2000) but see (Lehmann et al., Citation2002; Pryce et al., Citation2001), associated with increased fearfulness in tests of novelty and anxiety (Caldji et al., Citation1998; de Kloet et al., Citation2005; Huot et al., Citation2001; Ladd et al., Citation2004; Romeo et al., Citation2003; Wigger & Neumann, Citation1999) and depressive-like behavior at an adult age (Huot et al., Citation2002; Ladd et al., Citation2000).
Interestingly, also the expression level of several genes that are essential regulators of the stress response are persistently altered in repeatedly separated rats. This includes for example increased CRH expression in the amygdala, locus coeruleus (LC) and parabrachial nucleus, increased stress-induced CRH hnRNA expression in the PVN (Chen et al., Citation2012) and increased CRH receptor 1 (CRFR1) mRNA expression levels in the LC and raphe nucleus (Ladd et al., Citation1996; Pryce et al., Citation2002). Furthermore, levels of arginine vasopressin (AVP) in the PVN and bed nucleus of the stria terminalis (BnST) have been reported to be either increased (Levine, Citation2001; Veenema & Neumann, Citation2009) or decreased by MS (Desbonnet et al., Citation2008; Gunnar & Quevedo, Citation2007). This discrepancy in AVP level changes are possibly caused by sex specific effects (Desbonnet et al., Citation2008).
Next to artificial modulation of dam–pup interaction, natural variations in maternal care combined with selected breeding programs have been widely used to study the effects of differential maternal care on the HPA axis. Low amounts of Licking and Grooming (LG) in rats lead to increased ACTH and CORT release in response to stress in adulthood as well as lasting increased hypothalamic CRH expression and reduced hippocampal GR expression (Liu, Citation1997; Weaver et al., Citation2004). On the emotional level, low LG increases anxiety-like behavior in rats (Caldji et al., Citation1998).
So far, we have described how MS/MD and natural variation in maternal care affect the HPA axis and emotional function later in life. However, these models all involve acute or recurrent stress, and as such lack some important aspects of ELS present in humans, in which stress is typically chronic and the mother/caregiver is present but unable to give her child the appropriate care (Gaudin et al., Citation1996; Kendall-Tackett, Citation2007; Koenen et al., Citation2003). Therefore, an animal model of actual chronic ELS was developed to recapitulate important elements of the human EL adverse condition (Avishai-Eliner et al., Citation2001; Baram et al., Citation2012; Ivy et al., Citation2008; Liao et al., Citation2014; Rice et al., Citation2008; Rincón-Cortés & Sullivan, Citation2014; Wang et al., Citation2013). In this model, the amount of nesting and bedding material is limited during the first postnatal week, inducing chronic stress in the dam which results in erratic, fragmented maternal care (i.e. shortened bouts of nurturing behavior and frequent shifts between behaviors). The fragmentation of maternal care subsequently causes chronic ELS in the pups, as is evident from their elevated basal plasma CORT levels, a transient reduction in weight gain and increased adrenal weight by the end of the 1-week stress period at P9. These physiological changes at P9 are accompanied by reduced CRH receptor binding in the pituitary, reduced in CRH and GR mRNA expression in the PVN and reduced CRFR1 expression in the hippocampus as well as GR expression in the frontal cortex, while hypothalamic AVP levels are unaffected (Avishai-Eliner et al., Citation2001; Gilles et al., Citation1996). While the ELS-induced reduction in hypothalamic CRH was found to be transient in rats (Avishai-Eliner et al., Citation2001), it was still present in adult mice (Rice et al., Citation2008).
These above-summarized studies clearly highlight the importance of maternal presence and the quality and quantity of her care that appear to define the development and later life function of the neuroendocrine stress system and emotional function. Mother-offspring interaction clearly encompasses multiple elements including sensory stimuli, but also nutrition. The importance of maternal tactile stimulation is clearly highlighted in the above-reviewed studies. However, interestingly, there is some evidence supporting rather a synergistic effect of tactile stimulation and nutrition. For example, stroking the rat pups to mimic maternal tactile stimulation prevented several effects of early stress, including on metabolic derangements (Haley et al., Citation2013; Moyer-Mileur et al., Citation2011; van Oers et al., Citation1999). Remarkably, the adverse effects of MD on HPA axis function in rats were reversible only when combining stroking with food administration (van Oers et al., Citation1999). This highlights the possible critical involvement of the nutritional element as well, next to the well-investigated roles of sensory stimuli, stress related hormones and neuropeptides in mediating the above-described effects. The role of the nutritional element in this context, however, has been largely ignored. In the next section, we review the available information on the role of EL nutrition in regulating the stress system and emotional functions.
Long-term consequences of perinatal nutrition on emotional behavior and the neuroendocrine stress system
Nutritional deficiency, i.e. under- and malnutrition during critical developmental periods leads to permanent effects on the susceptibility of an individual to develop psychopathology later in life (Langley-Evans, Citation2009; Prado & Dewey, Citation2014). Undernutrition is defined as a state of chronic inadequate (amount of) nutrition, with all the required nutrients present in the diet. In contrast, malnutrition is the lack of one or more essential nutrients from the diet (Benton, Citation2010). Both perinatal under- and malnutrition increase the vulnerability to develop psychiatric disorders, e.g. schizophrenia and depression (Brown et al., Citation2000; Lesage et al., Citation2006). Considering the extraordinary need for nutrients during early development, it is not surprising that any sort of deficiency in this realm during these sensitive developmental periods might lastingly affect emotional functions later in life or for example on the hypothalamic circuits development ().
One way to model under- or over-nutrition is by modulating litter size. For instance, reducing the litter sizes of rats to three or four pups leads to higher body fat mass and metabolic deregulations in adulthood compared to normal litter sizes (Morris et al., Citation2005). These changes in small litter-raised rats are accompanied by long-term alterations in emotional functions as demonstrated by reduced anxious behavior when compared to rats raised in large litter sizes (Spencer & Tilbrook, Citation2009). This behavioral phenotype is accompanied by faster HPA axis maturation early in life, which was evidenced by increased basal levels of circulating ACTH and CORT at P14 and P21. Also, the decrease of CRH and increase of GR mRNA expressions levels in hypothalamic PVN was accelerated at P8 and P14 in rats (Boullu-Ciocca et al., Citation2005). Furthermore, rats that underwent the small litter size model exhibited increased amounts of neuronal activation (c-FOS expression) of the CRH expressing PVN neurons in response to restraint stress (Dayas et al., Citation1999; Spencer & Tilbrook, Citation2009). On the metabolic level, small litter-raised rats showed higher GR and 11β-HSD1 expression in fat tissue, accompanied by increased circulating leptin levels (Boullu-Ciocca et al., Citation2005). These changes are possibly explained by the fact that the reduction in litter size led to an increase of 20–30% in milk consumption. To what extent this manipulation affects maternal care was uninvestigated. In contrast, rats raised in large litters consumed less maternal milk, exhibited slower growth, were associated with reduced stress-induced HPA axis responses and returned faster to baseline levels (Spencer, Citation2013).
Several other animal models have been used to study how alterations in the early nutritional environment affect the stress system and emotional functions. The most widely studied ones are 50% food restriction (FR50), high fat diet (HF) and maternal protein restriction (PR) during gestation, lactation or both. Rat offspring of mothers that underwent FR50 are characterized by increased depressive-like and anxious behavior at P28 (Jahng et al., Citation2007), increased hippocampal MR and GR gene expression, accompanied by decreased ACTH plasma levels at weaning period and without alterations in basal CORT or CRH (Leonhardt et al., Citation2002; Vieau et al., Citation2007). In adulthood, the increase in hippocampal GR mRNA expression, but not MR, returns to control levels (Sebaai et al., Citation2004). In addition to the effects on emotion and neuroendocrine stress system, rat offspring raised by mothers exposed to FR50 during the perinatal period were also affected metabolically. This was evidenced by growth retardation in offspring, reduced amounts of different fat depots and reduced circulating leptin levels at weaning (Leonhardt et al., Citation2003). In addition, exposure to prenatal maternal undernutrition followed by postnatal HF diet in rats resulted in increased expression of orexigenic genes, causing a net result of increased appetite (Ikenasio-Thorpe et al., Citation2007).
These examples clearly show the close relationship between alterations in nutrition, metabolism and the development of long-term regulation of the HPA axis and emotional function (). However, how do these changes modulate one another? In the following sections, we will discuss in detail the role of metabolic hormones as well as the effects of the lack or excess of specific nutrients on the neuroendocrine stress system.
Role of metabolic hormones in regulating the hypothalamic–pituitary–adrenal axis and brain function
A key hormone in regulating processes associated with the hypothalamic feeding circuitry and the neuroendocrine system is the anorexigenic hormone leptin (Morrison, Citation2009). Leptin release from fat cells causes a loss of appetite and enhances energy expenditure via its actions in the arcuate nucleus of the hypothalamus (ARH) (Mainardi et al., Citation2013; Morton et al., Citation2006). Importantly, levels of leptin during the sensitive period of brain development are of utmost importance. In fact, there is a leptin surge occurring between P4 and P16 in rodents that is necessary to form proper connectivity within the hypothalamic circuitry. The absence of the leptin surge in mice during this specific developmental period leads to lasting alterations in feeding behavior and stress responses (Ahima et al., Citation1998; Granado et al., Citation2012), and leptin injections within this period in mice affect the development of neural ARH projections (Bouret et al., Citation2004). Indeed, advancing the time of onset of this leptin surge at P2 in rats results in a decrease of food intake and ablating the surge at P9 leads to reduced body weight and fat mass in adulthood (Granado et al., Citation2011). There is evidence that this leptin surge is not only crucial for the ARH-feeding circuit development, but also for correct development and function of the neuroendocrine system. Indeed, chronic leptin treatment early in life in rats leads to lifelong altered stress-induced HPA axis activity, accompanied by reduced PVN-CRF expression and increased GR expression in the hippocampus and PVN (Oates et al., Citation2000; Proulx et al., Citation2001). The fact that the nutrition-related metabolic hormone leptin can act on the stress system is not surprising considering the high expression of leptin receptors in brain areas related to stress, such as the hypothalamus and hippocampus (Li et al., Citation2002; Scott et al., Citation2009).
Based on the evidence presented above, it appears that ELS and EL malnutrition lastingly program the neuroendocrine stress system. In fact, the two main hypothalamic nuclei, PVN and ARH, involved in regulating the neuroendocrine stress system and the metabolism as well as feeding behavior, respectively, are neuroanatomical strikingly close (Bouret et al., Citation2004). In addition, there is increasing evidence (presented above) that the metabolic hormone leptin is able to affect and program these systems as well. These evidence altogether strongly points towards an intense cross-talk between these two systems. Thus, an important question that arises is whether ELS-induced changes in metabolic hormones might possibly play a role in the programming of the stress system. This could be viewed in two ways: (1) ELS induced alterations in HPA axis activity might trigger metabolic changes, resulting in changes in peripheral metabolic hormones, which could then be involved in reshaping the hypothalamic feeding and/or stress circuitries and/or (2) ELS might result in undernutrition or decreased caloric intake, which in turn results in reducing circulating leptin levels that could then reshape HPA axis responses to stress. Based on the current knowledge, we are unable to clarify the order of causality and clearly more research in this area is needed. However, there is some evidence that metabolic hormones might play a role in programming the neuroendocrine system and/or hypothalamic circuits.
Indeed, stress-related hormones are able to affect the functioning of metabolic hormones (Joung et al., Citation2014) and MS on P8 from 4 to 12 h in mice leads to reductions of circulating glucose as well as leptin levels and increases in ghrelin levels early in life (Schmidt et al., Citation2006). Further pointing to a key modulating role of these metabolic hormones in the programming of the HPA axis; blocking the MS-induced ghrelin increase could reverse the MS effects on the HPA axis as well. Long-term effects were not presented in this study, however, MD in rats leads to long-term reductions in leptin levels at the age of P75 (Viveros et al., Citation2010). How exactly glucocorticoid and metabolic hormones interact is not resolved, but importantly glucocorticoids play a pivotal role in regulating lipid homeostasis and are one of the most potent stimulators of leptin expression in white adipose tissues (Bradley & Cheatham, Citation1999; Havel, Citation2004).
How exactly are these changes in metabolic mediators involved in the programming of the stress system? One possibility is that the ELS-induced reduction in leptin interferes with the leptin surge (see above) and may thereby possibly influence the proper developmental trajectory of the hypothalamus (Bouret & Simerly, Citation2006; Bouret et al., Citation2004), ultimately affecting neuroendocrine and eating behavior regulating centers. However, further testing of this possibility is required.
So far, we have discussed the effects of stress and metabolic factors on the neuroendocrine stress system and emotional function. However, next to stress and metabolic hormones, ELS and malnutrition clearly affect specific nutrient intake/availability. Considering the enormous demand for nutrients in the EL period, nutrition might play a crucial role in the lasting effects induced by EL adversities. In the next section, we will discuss the role of (the lack of) specific macro- and micronutrients on the development and function of the neuroendocrine stress system and emotional function.
Nutrients and the stress system
There are several key nutrients that are extremely important during development including among others, not only proteins, fatty acids, choline but also essential micronutrients such as B vitamins and essential amino acids, e.g. methionine (Benton, Citation2011; van de Rest et al., Citation2012; Walker, Citation2005). The information on how the availability of these nutrients affects brain development, in particular how it programs the hypothalamic system, has been addressed only to some extent and is fragmented, indicating the need for more detailed research in this context. We will here discuss the described effects of imbalanced perinatal protein and fat on HPA axis programming.
Lifelong effects of perinatal low protein diet on the hypothalamic–pituitary–adrenal axis and emotional function
The importance of protein intake early in life has been largely studied using maternal protein restriction (PR) during gestation and/or lactation. Maternal PR in rats induces a blunted diurnal pattern of ACTH plasma concentrations, elevated GR expression in the hippocampus and no changes in basal CORT (Langley-Evans et al., Citation1996). In addition, perinatal PR in rat dams has been associated with alterations of adhesion molecules in the vascular endothelium of the placenta as well as reduced expression levels of placental 11β-hydroxysteroid dehydrogenase 2 (11β-HSD2) (Langley-Evans, Citation2009; Langley-Evans et al., Citation1996), resulting in higher placental permeability. Since 11β-HSD2 enzymes convert active GCs into inactive forms (Edwards et al., Citation1996; Seckl et al., Citation2000), this reduction could lead to high GC exposure from the maternal compartment to fetal tissues. As a result, these high GC levels might lead to, among others, disrupted HPA axis development and a higher CORT response upon encountering stress in adulthood (Lesage et al., Citation2006). Interestingly, on the level of emotional functions, offspring mice of dams that were protein-malnourished show increased anxious and depressive-like behavior (Belluscio et al., Citation2014). Altogether, these examples indicate that alterations in protein availability early in life can program the HPA axis and might therefore play a role in increasing the risk to develop stress-related disorders later in life.
Effects of fat on the hypothalamic–pituitary–adrenal axis and emotional behavior
The potential of fatty acids to affect the HPA axis depends on the type of fat. Fatty acids can be divided into four classes: saturated, trans, polyunsaturated and monounsaturated (Morris, Citation2011). The polyunsaturated type of fatty acids are essential for, among others, molecular signaling, synaptogenesis, energy storage as well as for membrane functions and are entirely obtained from the diet (Rapoport et al., Citation2007). Particularly unsaturated, and not saturated fatty acids, stimulate steroidogenesis in adrenocortical cells in vitro (Sarel & Widmaier, Citation1995). In addition, the synthesis of corticosteroids requires cholesterol from fat, which needs to be obtained via the diet, indicating that fatty acids potentially could have a strong influence on the HPA axis. Polyunsaturated fatty acids (PUFAs) are further divided into omega-3 fatty acids termed α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). DHA is the most abundant type of PUFAs in the central nervous system and, importantly, is indispensable for cell membrane building as well as proper functioning of the brain (Innis, Citation2008). PUFAs are also pivotal components in maternal milk, further supporting their importance for critical developmental processes during EL (Innis, Citation2014; Nishimura et al., Citation2014).
Depletion of omega-3 fatty acids and HF diets perinatally has been the two main experimental manipulations to explore the effects of fats on brain development and (partly) to study its effects on HPA axis functioning and emotional function. Reducing omega-3 fatty acid in the diet for two generations in rats has been shown to reduce DHA levels in several brain regions, including the cerebellum, medulla, hypothalamus, striatum, hippocampus, cortex and midbrain (Xaio et al., Citation2006), suggesting that levels of fatty acid content of the brain can be manipulated through diet.
Docosahexaenoic acid (DHA) deficiency in rats during the pre-weaning, but not post-weaning period, resulted in lasting increased responsiveness of the HPA axis to stress, as indicated by increased and prolonged stress-induced CORT levels and heart rate changes in adulthood. This was accompanied by enhanced anxiety- and depressive-like behavior after forced swim test and elevated plus-maze, respectively (Chen & Su, Citation2013). Moreover, omega-3 PUFA deficiency in mice after weaning disrupts the GR mediated signaling pathway and causes HPA axis hyperactivity as shown by elevated plasma CORT levels at 3 months of age (Larrieu et al., Citation2014). Interestingly, depressive-like behavior induced by prenatal stress in rats could be reduced by omega-3 treatment during pregnancy and lactation (Borsonelo et al., Citation2011). The tight interaction between nutrition and stress is further highlighted by studies showing that rats exposed to omega-3 PUFA-deficient diet early in life and MS, were more anxious and fearful in inescapable situations compared to the ones that were exposed to MS alone (Mathieu et al., Citation2008, Citation2011). In addition, rats that underwent MS and omega-3 deficient diet exhibited metabolic alterations, such as increased abdominal fat, circulating leptin and insulin levels and increased sucrose consumption when compared to unhandled control animals that obtained sufficient omega-3 in their diet (Bernardi et al., Citation2013; Mathieu et al., Citation2008).
Interestingly, next to depletion of omega-3 fatty acid, increasing the amount of fats in general by HF diets early in life has lasting effects on the HPA axis, metabolism and emotional functions of the offspring as well. Indeed, HF feeding of rat dams during lactation led to increased fatty acids levels in maternal milk (Trottier et al., Citation1998). This in turn led to reductions in HPA axis responsiveness in the rat offspring up to the age of P21, as evidenced by diminished ACTH and faster recovery to baseline CORT levels in response to stress, and showed increased levels of leptin. After weaning, rat pups from the HF mothers had lower plasma leptin levels and increased stress-induced ACTH secretion. The inverse relationship between circulating leptin levels and HPA responses in pups creates the possibility that the effects of the HF diet on stress responsiveness are mediated by changes in leptin exposure during development (Trottier et al., Citation1998). Concerning the emotional behavior, mice offspring of dams fed a HF diet for 9 weeks (prior to gestation, gestation and lactation) showed higher anxiety-like behavior in adulthood (Peleg-Raibstein et al., Citation2012). In line with these findings on emotional behavior, early maternal exposure to HF diet in rats leads to an increased susceptibility to depressive behavior (Giriko et al., Citation2013).
Taken together, both stress and nutrition play profound roles during the early perinatal period, causing alterations in activity and sensitivity of the HPA axis, and thus modifying the vulnerability to develop psychopathologies later in life. Importantly, as highlighted above, not only do these adversities often occur simultaneously, they are often interrelated, thereby indicating the need to consider these elements as acting synergistically rather than independently. Most likely, common mechanistic pathways underlie the effects of stress and nutritional stimuli, possibly involving epigenetic mechanisms. The next section will be devoted to discuss the role of epigenetic mechanisms in the lasting effects of ELS and EL nutrition and how specific nutrients might modulate the epigenome.
Epigenetic mechanisms underlying the lasting effects of early-life experiences
Effects of ELS on the epigenome
Over the past few years, a growing body of epidemiological as well as pre-clinical studies has demonstrated the role of epigenetic mechanisms in determining the lasting effects of EL experiences (Vaiserman, Citation2015). Epigenetics is a phenomenon that alters gene expression without eliciting changes in DNA sequence. Epigenetic modifications encompass among others DNA methylations, post-translational histone modifications and transcriptional non-coding RNA regulations. These modifications occur through alterations in the structure of the chromatin or the DNA, leading to the expression or repression of gene transcription, which is determined by the state of chromatin compaction: an open chromatin structure is transcriptionally active (euchromatin), whereas a condensed chromatin with tightly coiled DNA is associated with a transcriptional inactive state (heterochromatin). In general, DNA methylation predominantly leads to gene silencing, whereas histone acetylation usually promotes transcription. In mammals, DNA methylation occurs at cytosines through the effects of methyl-binding proteins (MeCP1, MeCP2, MBD1, MBD2, MBD3 and MBD4) and methylated CpG islands in the promoter region of genes repress gene expression by preventing transcription factors to bind at the promoter region. In addition, silencing via MeCP2 occurs partly by recruiting histone deacetylase (HDAC) activity, resulting in chromatin remodeling (Fuks et al., Citation2003).
Lasting effects of ELS on gene expression in the offspring are associated with epigenetic alterations. Indeed, ELS in mice induces DNA hypomethylation at the AVP gene, resulting in increased levels of AVP levels (Murgatroyd et al., Citation2009). Also, EL adversity from P1 to P7 by stress-abusive rat dams results in reduced brain-derived neurotrophic factor (BDNF) expression due to increased methylation at the BDNF exon IV promoter in the prefrontal cortex later in life (Roth et al., Citation2009). Differences in the amount of maternal care are also able to cause lasting modifications in DNA methylation and chromatin structure. For example, low-LG rat offspring show reduced GR gene expression in the hippocampus that is related to increased DNA methylation in exon 17 GR promoter at the region around the nerve growth factor inducible factor A (NGFIA) consensus sequence, resulting in increased histone acetylation of the GR (Weaver et al., Citation2004). Intriguingly, this programming is reversible by cross-fostering offspring of low-LG to high-LG mothers, which leads to hypomethylation of cytosines at the 5′CpG dinucleotide of the NGFIA consensus sequence (Weaver et al., Citation2004).
Accordingly, in the case of handling, where pups are separated daily from the mother for 15 min on P2 for at least 1 week or up to 3 weeks (Fenoglio et al., Citation2006; Francis & Meaney, Citation1999; Meaney et al., Citation1985), the changes induced by reductions in CRH expression levels are maintained by epigenetic mechanisms (Chen et al., Citation2012). Reduction in CRH levels in rats are accompanied with permanent increased levels of the neuron restrictive silencing factor [NRSF; also designated as repressor element 1-silencing transcription factor (REST)] (Korosi et al., Citation2010), which is a transcriptional repressor that has a binding site within the CRH gene (Seth & Majzoub, Citation2001) and concomitantly recruits the epigenetic machinery by driving histone deacetylation (Roopra et al., Citation2000). The short lack of maternal care during handling is not associated with deleterious effects, leading to a significantly higher amount of maternal sensory stimulation, such as LG towards the offspring after reunion.
In addition to these epigenetic changes on specific genes induced by these various EL experiences, there is evidence that these effects are affecting the epigenome more globally (Levine et al., Citation2012; McGowan et al., Citation2011).
Effects of early-life nutrition on the epigenome
As in the case of stress, the effects of early nutrition might also be mediated by modulation of the epigenome (He et al., Citation2014; McNeil et al., Citation2008). This has mostly been addressed in the context of metabolic programming, which is in contrast to the scarce evidence of epigenetic effects of early nutritional manipulations in the brain. For example, changes in the methylation of the hypothalamic proopiomelanocortin (POMC) promoter have been found in rat pups that were allocated to small litter sizes (Plagemann et al., Citation2009). Furthermore, in both rats and mice, administering a FR50 diet to pregnant rodents resulted in elevated homocysteine concentrations in the earliest stages of gestation (Petrie et al., Citation2007). In rats, HF resulted in high methylation of the leptin promoter in adipocytes (Milagro et al., Citation2009). On the other hand, maternal PR diet leads to reduced expression of DNA (cytosine-5)-methyltransferase 1 and 2) (Dnmt1) and increased hypomethylation of the GR110 promoter, which in turn caused increases in GR expression (Lillycrop et al., Citation2007). Interestingly, maternal supplementation with folic acid counteracted this reduced expression of Dnmt1 enzymes (James et al., Citation2002).
Another possible epigenetic process that could be important in mediating the lasting effects of ELS and EL malnutrition is histone acetylation. ELS and EL malnutrition has been shown to affect acetylation of histones (Burdge et al., Citation2007; Levine et al., Citation2012; Portha et al., Citation2014; Xie et al., Citation2013). In fact, fatty acids are potential donors of acetyl groups (Burdge & Lillycrop, Citation2014). As discussed in the previous sections, ELS might lead to alterations in fat metabolism (Danese et al., Citation2014; Schmidt et al., Citation2006; Viveros et al., Citation2010) and thereby altering circulating and available fatty acids. Moreover, manipulations of fat contents early in life have been shown to affect HPA axis functioning as well (Chen & Su, Citation2013; Larrieu et al., Citation2014; Trottier et al., Citation1998). Therefore, in future research, it will be important to consider acetylation as a possible mechanism in mediating these effects.
The following sub-section will highlight the possible importance of a selection of B-vitamins, such as vitamin B6, B9 (folate), B12 (cobalamin) as well as methionine and choline in mediating the lasting effects of ELS and EL malnutrition. These micronutrients play a crucial role in neuronal cell growth and development during the perinatal period and are also key co-factors and metabolites of the one-carbon (1C) metabolism. These 1C metabolites (referred further in the text as methyl donors) are required for methylation of DNA and histones and therefore associated to the epigenetic machinery.
Even though it is not known yet how the (lack of) these 1C metabolites early in life might specifically program HPA axis and emotional function, we will discuss the possibilities that it might involve the modulation of the epigenetic modifications and/or DNA synthesis as well as DNA repair processes.
Effects of dietary methyl donor nutrients on the brain and the epigenetic machinery
Vitamin B6, B9 [folate and its active form 5-methyl-tetrahydrofolate (5-MTHF)] and B12 (cobalamin) belong to the family of B vitamins and in addition to their role as suppliers of methyl donors, deficits of these micronutrients can lead to cognitive impairments and anxiety. Indeed, male weanling mice receiving diets deficient in B vitamins for 10 weeks displayed impaired spatial learning and memory (Troen et al., Citation2008). Furthermore, rats exposed to prenatal vitamin B6 deficient diets displayed functional and structural impairments in the hippocampus (Krishna & Ramakrishna, Citation2004). On the emotional level, dietary methyl donor deficiency in rats during pregnancy leads to lasting increased anxiety levels, without changes in GR or 11β-HSD2 methylation in the hippocampus (Konycheva et al., Citation2011). The brain can additionally be affected by pyridoxal phosphate (PLP), the active coenzyme of vitamin B6. It influences brain development due to its role in the synthesis of nucleic acids as well as playing a role in the 1C metabolism by producing 5,10-methylene tetrahydrofolate (5,10-CH2-THF) from THF. Interestingly, vitamin B6 also modulates the HPA axis by regulating multiple members of the steroid hormone receptor family, among others, the GR (Allgood et al., Citation1990; Oka, Citation2001). These studies clearly indicate the need to gain further knowledge on how these nutrients might regulate hypothalamic development and function as well.
Folate (vitamin B9) plays a major role throughout life and is involved in brain development. Folate is a well-known nutrient due to its crucial role in the first trimester of pregnancy where its inadequacy leads to congenital neural tube defects (Morris, Citation2011). In addition, folate plays important roles in the metabolism of DNA and erythrocytes as well as the synthesis of methionine and S-adenosylmethionine (SAM). The activation of methionine to SAM ensues using the folate-derived methyl groups in numerous DNA methylation reactions. The rate-limiting enzyme in the methylation cycle named 5,10-methylene tetrahydrofolate reductase (MTHFR), converts 5,10-methylene THF to 5-MTHF. Interestingly, in humans, the specific C677T single-nucleotide polymorphism in the MTHFR gene is accompanied with decreased DNA methylation capacity and increased oxidative stress, which aggravates the outcome on developing major depressive disorder in adulthood when traumatic childhood was encountered early in life (Lok et al., Citation2013). The importance of folate is further demonstrated in humans by the association of low maternal folate status during early pregnancy and emotional problems in the offspring later in life (Steenweg-de Graaff et al., Citation2012). In addition, folate also contributes to the synthesis of purine and pyrimidine (Duthie & Hawdon, Citation1998; Nimmo-Smith, Citation1954). Thus, altered folate levels could also affect DNA synthesis. Next to the epigenetic effects, impaired DNA synthesis could be involved in the effects of (possibly early-stress-induced) lack of these nutrients on brain function. In line with this, there is some evidence that maternal folate depletion affects DNA repair in adult mice offspring (Langie et al., Citation2013) and that early-adversity is associated with altered mitochondrial DNA copy number and telomere length (Picard et al., Citation2014; Tarry-Adkins & Ozanne, Citation2014; Tyrka et al., Citation2015).
Choline also belongs to the group of 1C metabolites (Zeisel et al., Citation2003). Choline, the precursor of the neurotransmitter acetylcholine, is of particular importance during perinatal life due to its pivotal role in cholinergic neurotransmission and phospholipid synthesis, having the ability to affect stem cell proliferation as well as apoptosis in the brain (Blusztajn & Mellott, Citation2012; Zeisel, Citation2004; Zeisel & Niculescu, Citation2006). Different from the other 1C metabolites that we discussed, choline can be synthesized to a limited extent by mammalian organisms, however, this is mostly insufficient and dietary intake of it is required (Zeisel & da Costa, Citation2009). Alterations in dietary choline levels affect the neuroendocrine system as well as emotional functions. For example, dietary choline supplementation during pregnancy and lactation mitigates the anxiety-related behaviors in rats (Schulz et al., Citation2014) and high choline in humans reduces CRH and cortisol levels in placenta and cord plasma, respectively (Jiang et al., Citation2012). Choline has also been reported to modulate DNA methylation (Mehedint et al., Citation2010). In fact, these effects of choline are at least partly due to epigenetic modifications, as DNA methylation of CRH and NR3C1 (nuclear receptor subfamily 3, group C, member 1; alias GR) promoters are increased in placental tissue.
The 1C metabolites mentioned in this section, i.e. choline, methionine and B vitamins, are metabolically interrelated in such a way that deficiency in one of these nutrients subsequently affects levels of other nutrients involved in the cycle and methylation. For example, vitamin B12 deficiency leads to hypomethylation due to accumulations of homocysteine and 5-MTHF, leading to the arrest of the methylation cycle and resulting in manifestations of folate deficiency due to a build-up of 5-MTHF. Interestingly, it appears that dietary choline is able to partially reverse the effects of folate deficiency during late gestation on neurogenesis and apoptosis in the fetal mouse brain (Craciunescu et al., Citation2010).
Finally, to add further to the complexity of these interactions, there is also evidence for a tight interaction between these methyl donors and fatty acids. Indeed, changes in folate status have been associated with alterations in lipid metabolism (Teng et al., Citation2011) and imbalances in maternal folic acid and vitamin B12 during gestation reduces offspring brain DHA levels (Rao et al., Citation2006; Roy et al., Citation2012).
To conclude, a lack of dietary methyl donors occurring in the window of critical development during EL may cause permanent effects through alterations in neurogenesis and the epigenetic machinery. Realizing the importance of these micronutrients, in particular during early development and considering the above-described effects of methyl donor and their deficiencies, raises the interesting possibility for nutritional interventions to modulate some of these effects. In fact, there is some evidence that supplementation with these metabolites can have lasting effects. For example, methyl donor supplementation diminishes adverse effects by decreasing global hypomethylation in the mice offspring of dams fed with a HF diet (Carlin et al., Citation2013). In addition, folic acid supplementation prevented hypomethylation on hepatic GR promoters in rat offspring of dams fed a PR diet during pregnancy (Lillycrop et al., Citation2005). On the cognitive level, methionine supplementation in rats is able to prevent the cognitive impairments caused by folate deficiency (Troen et al., Citation2008). Also, consequences of maternal behavior on DNA methylation in rats are reversible by infusing l-methionine (the precursor of SAM) in later life (Weaver, Citation2005). This preliminary evidence supports the possibility that supplementation with methyl donors may be effective to modulate lasting effects of EL adversities.
Conclusions
In summary, EL adversity during critical developmental periods can program the developing brain and increase the vulnerability to develop psychopathologies in adulthood. We review intriguing evidence that ELS and EL nutrition lead to similar outcomes with respect to the resulting stress reactivity and emotional behavior. While it is out of the scope of our review, it is important to realize that many of the described effects of the various early environments might be part of a predictive adaptive response and may in fact prepare the organism to respond optimally under comparable situations encountered later in life, a concept known as the match-mismatch theory (Nederhof & Schmidt, Citation2012; Oomen et al., Citation2010, Citation2011). As ELS and nutrition are elements that interact and affect one another, it is difficult to distinguish the effects caused by each factor independently. One possibility is that nutrition, metabolic and stress hormones act synergistically in programming the stress system. To further explore this, we need to gain further knowledge as to how these factors affect one another, possibly involving epigenetic mechanisms. Elucidating the underlying mechanisms will not only advance our knowledge about the critical mediators, but also create the opportunity to further develop nutrition as a potential non-invasive intervention.
Declaration of interest
The authors declare that the work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. For financial support, we would like to acknowledge NWO Meervoud, Food & Cognition and ISAO grants to AK and ISAO, Alzheimer Nederland and HersenStichting grants to PJL.
Notes
* This article is based on work presented at the 5th Brain and Behavior: "Good Stress, Bad Stress and Very Bad Stress", February 2014.
References
- Abe H, Hidaka N, Kawagoe C, Odagiri K, Watanabe Y, Ikeda T, Ishizuka Y, et al. (2007). Prenatal psychological stress causes higher emotionality, depression-like behavior, and elevated activity in the hypothalamo-pituitary-adrenal axis. Neurosci Res 59(2):145–51
- Ahima RS, Prabakaran D, Flier JS. (1998). Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest 101(5):1020–7
- Allgood VE, Powell-Oliver FE, Cidlowski JA. (1990). The influence of vitamin B6 on the structure and function of the glucocorticoid receptor. Ann N Y Acad Sci 585:452–65
- Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. (2001). Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J Neuroendocrinol 13:799–807
- Avishai-Eliner S, Yi SJ, Newth CJ, Baram TZ. (1995). Effects of maternal and sibling deprivation on basal and stress induced hypothalamic-pituitary-adrenal components in the infant rat. Neurosci Lett 192:49–52
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, et al. (2010). Early life programming and neurodevelopmental disorders. Biol Psychiatry 68(4):314–19
- Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A, Stem H. (2012). Fragmentation and unpredictability of early-life experience in mental disorders. Am J Psychiatry 169(9):907–15
- Belluscio LM, Berardino BG, Ferroni NM, Ceruti JM, Cánepa ET. (2014). Early protein malnutrition negatively impacts physical growth and neurological reflexes and evokes anxiety and depressive-like behaviors. Physiol Behav 129:237–54
- Benton D. (2010). The influence of dietary status on the cognitive performance of children. Mol Nutr Food Res 54(4):457–70
- Benton D. (2011). Vitamins and neural and cognitive developmental outcomes in children. Proc Nutr Soc 71(01):14–26
- Bernardi JR, Ferreira CF, Senter G, Krolow R, de Aguiar BW, Portella AK, Kauer-Sant'anna M, et al. (2013). Early life stress interacts with the diet deficiency of omega-3 fatty acids during the life course increasing the metabolic vulnerability in adult rats. PLoS One 8(4):e62031
- Black MM, Dubowitz H, Hutcheson J, Berenson-Howard J, Starr RH. (1995). A randomized clinical trial of home intervention for children with failure to thrive. Pediatrics 95(6):807–14
- Blusztajn JK, Mellott TJ. (2012). Choline nutrition programs brain development via DNA and histone methylation. Cent Nerv Syst Agents Med Chem 12(2):82–94
- Borsonelo EC, Suchecki D, Galduróz JCF. (2011). Effect of fish oil and coconut fat supplementation on depressive-type behavior and corticosterone levels of prenatally stressed male rats. Brain Res Elsevier B.V 1385(C):144–50
- Boullu-Ciocca S, Dutour A, Guillaume V, Achard V, Oliver C, Grino M. (2005). Postnatal diet-induced obesity in rats upregulates systemic and adipose tissue glucocorticoid metabolism during development and in adulthood its relationship with the metabolic syndrome. Diabetes 54(1):197–203
- Bouret SG, Draper SJ, Simerly RB. (2004). Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci 24(11):2797–805
- Bouret SG, Simerly RB. (2006). Developmental programming of hypothalamic feeding circuits. Clin Genet 70(4):295–301
- Bradley RL, Cheatham B. (1999). Regulation of ob gene expression and leptin secretion by insulin and dexamethasone in rat adipocytes. Diabetes 48(2):272–8
- Brown AS, Susser ES. (2008). Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull 34(6):1054–63
- Brown AS, Susser ES, Lin SP, Neugebauer R, Gorman JM. (1995). Increased risk of affective disorders in males after second trimester prenatal exposure to the Dutch hunger winter of 1944–45. Br J Psychiatry 166(5):601–6
- Brown AS, van Os J, Driessens C, Hoek HW, Susser ES. (2000). Further evidence of relation between prenatal famine and major affective disorder. Am J Psychiatry 157(2):190–5
- Brunson KL, Avishai-Eliner S, Hatalski CG, Baram TZ. (2001). Neurobiology of the stress response early in life: evolution of a concept and the role of corticotropin releasing hormone. Mol Psychiatry 6(6):647–56
- Burdge GC, Hanson MA, Slater-Jefferies JL, Lillycrop KA. (2007). Epigenetic regulation of transcription: a mechanism for inducing variations in phenotype (fetal programming) by differences in nutrition during early life? Br J Nutr 97(6):1036–46
- Burdge GC, Lillycrop KA. (2014). Fatty acids and epigenetics. Curr Opin Clin Nutr Metab Care 17(2):156–61
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. (1998). Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA 95(9):5335–40
- Carlin J, George R, Reyes TM. (2013). Methyl donor supplementation blocks the adverse effects of maternal high fat diet on offspring physiology. PLoS One 8(5):e63549
- Chen H-F, Su H-M. (2013). Exposure to a maternal n-3 fatty acid-deficient diet during brain development provokes excessive hypothalamic–pituitary–adrenal axis responses to stress and behavioral indices of depression and anxiety in male rat offspring later in life. J Nutr Biochem Elsevier Inc 24(1):70–80
- Chen J, Evans AN, Liu Y, Honda M, Saavedra JM, Aguilera G. (2012). Maternal deprivation in rats is associated with corticotrophin-releasing hormone (CRH) promoter hypomethylation and enhances CRH transcriptional responses to stress in adulthood. J Neuroendocrinol 24(7):1055–64
- Chen Y, Rex CS, Rice CJ, Dubé CM, Gall CM, Lynch G, Baram TZ. (2010). Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci USA 107(29):13123–8
- Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, Chugani DC. (2001). Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage 14(6):1290–301
- Cole SZ, Lanham JS. (2011). Failure to thrive: an update. Am Fam Physician 83(7):829–34
- Corbett SS, Drewett RF. (2004). To what extent is failure to thrive in infancy associated with poorer cognitive development? A review and meta-analysis. J Child Psychol Psychiatry 45(3):641–54
- Craciunescu CN, Johnson AR, Zeisel SH. (2010). Dietary choline reverses some, but not all, effects of folate deficiency on neurogenesis and apoptosis in fetal mouse brain. J Nutr 140(6):1162–6
- Danese A, Dove R, Belsky DW, Henchy J, Williams B, Ambler A, Arseneault L. (2014). Leptin deficiency in maltreated children. Transl Psychiatry 4:e446
- Danese A, Tan M. (2014). Childhood maltreatment and obesity: systematic review and meta-analysis. Mol Psychiatry 19(5):544–54
- Dayas CV, Buller KM, Day TA. (1999). Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci 11(7):2312–22
- de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt M. (2005). Stress, genes and the mechanism of programming the brain for later life. Neurosci Biobehav Rev 29(2):271–81
- de Rooij SR, Painter RC, Phillips DIW, Osmond C, Tanck MWT, Bossuyt PMM, Roseboom TJ. (2006). Cortisol responses to psychological stress in adults after prenatal exposure to the Dutch famine. Psychoneuroendocrinology 31(10):1257–65
- Dent GW, Smith MA, Levine S. (2000). Rapid induction of corticotropin-releasing hormone gene transcription in the paraventricular nucleus of the developing rat. Endocrinology 141(5):1593–8
- Desbonnet L, Garrett L, Daly E, McDermott KW, Dinan TG. (2008). Sexually dimorphic effects of maternal separation stress on corticotrophin-releasing factor and vasopressin systems in the adult rat brain. Int J Dev Neurosci 26(3–4):259–68
- Duthie SJ, Hawdon A. (1998). DNA instability (strand breakage, uracil misincorporation, and defective repair) is increased by folic acid depletion in human lymphocytes in vitro. FASEB J 12(14):1491–7
- Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. (1996). 11 beta-Hydroxysteroid dehydrogenases: key enzymes in determining tissue-specific glucocorticoid effects. Steroids 61(4):263–9
- Eghbal-Ahmadi M, Hatalski CG, Avishai-Eliner S, Baram TZ. (1997). Corticotropin releasing factor receptor type II (CRF2) messenger ribonucleic acid levels in the hypothalamic ventromedial nucleus of the infant rat are reduced by maternal deprivation. Endocrinology 138(11):5048–51
- Fenoglio KA, Chen Y, Baram TZ. (2006). Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. J Neurosci 26(9):2434–42
- Francis DD, Meaney MJ. (1999). Maternal care and the development of stress responses. Curr Opin Neurobiol 9(1):128–34
- Fuks F, Hurd PJ, Wolf D, Nan X, Bird AP, Kouzarides T. (2003). The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J Biol Chem 278(6):4035–40
- Gaudin JM, Polansky NA, Kilpatrick AC, Shilton P. (1996). Family functioning in neglectful families. Child Abuse Negl 20(4):363–77
- Gershon A, Sudheimer K, Tirouvanziam R, Williams LM, O'Hara R. (2013). The long-term impact of early adversity on late-life psychiatric disorders. Curr Psychiatry Rep 15(4):352. doi: 10.1007/s11920-013-0352-9
- Gilles EE, Schultz L, Baram TZ. (1996). Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol 15(2):114–19
- Giriko CÁ, Andreoli CA, Mennitti LV, Hosoume LF, Souto TDS, Silva AVD, Mendes-da-Silva C. (2013). Delayed physical and neurobehavioral development and increased aggressive and depression-like behaviors in the rat offspring of dams fed a high-fat diet. Int J Dev Neurosci 31(8):731–9
- Glaser HH, Heagarty MC, Bullard DM Jr., Pivchik EC. (1968). Physical and psychological development ofchildren with early failure to thrive. J Pediatr 73(5):690–8
- Glover V. (2011). Annual research review: prenatal stress and the origins of psychopathology: an evolutionary perspective. J Child Psychol Psychiatry 52(4):356–67
- Granado M, Fuente-Martín E, García-Cáceres C, Argente J, Chowen JA. (2012). Leptin in early life: a key factor for the development of the adult metabolic profile. Obes Facts 5(1):138–50
- Granado M, García-Cáceres C, Fuente-Martín E, Díaz F, Mela V, Viveros M-P, Argente J, Chowen JA. (2011). Effects of acute changes in neonatal leptin levels on food intake and long-term metabolic profiles in rats. Endocrinology 152(11):4116–26
- Gunnar M, Quevedo K. (2007). The neurobiology of stress and development. Annu Rev Psychol 58(1):145–73
- Haley S, Neff K, Gulliver K, Gough G, Slater H, Lane RH, Moyer-Mileur LJ. (2013). Mechanical-tactile stimulation (MTS) intervention in a neonatal stress model alters adult adipose tissue deposition and prevents hyperinsulinemia in male rats. Early Hum Dev 89(6):387–92
- Havel PJ. (2004). Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes 53(Suppl 1):S143–51
- He F, Lupu DS, Niculescu MD. (2014). Perinatal α-linolenic acid availability alters the expression of genes related to memory and to epigenetic machinery, and the Mecp2 DNA methylation in the whole brain of mouse offspring. Int J Dev Neurosci 36:38–44
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. (2008). Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA 105(44):17046–9
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. (2008). The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33(6):693–710
- Heim C, Shugart M, Craighead WE, Nemeroff CB. (2010). Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol 52(7):671–90
- Hoeijmakers L, Lucassen PJ, Korosi A. (2015). The interplay of early-life stress, nutrition, and immune activation programs adult hippocampal structure and function. Front Mol Neurosci 7:103. doi: 10.3389/fnmol.2014.00103
- Hulshoff Pol HE, Hoek HW, Susser E, Brown AS, Dingemans A, Schnack HG, van Haren NE, et al. (2000). Prenatal exposure to famine and brain morphology in schizophrenia. Am J Psychiatry 157(7):1170–2
- Huot R, K T, Meaney M, Plotsky P. (2001). Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology [Springer-Verlag] 158(4):366–73
- Huot RL, Plotsky PM, Lenox RH, McNamara RK. (2002). Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res 950(1–2):52–63
- Ikenasio-Thorpe BA, Breier BH, Vickers MH, Fraser M. (2007). Prenatal influences on susceptibility to diet-induced obesity are mediated by altered neuroendocrine gene expression. J Endocrinol 193(1):31–7
- Innis SM. (2008). Dietary omega 3 fatty acids and the developing brain. Brain Res 1237:35–43
- Innis SM. (2014). Impact of maternal diet on human milk composition and neurological development of infants. Am J Clin Nutr 99(3):734S–41S
- Ivy AS, Brunson KL, Sandman C, Baram TZ. (2008). Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience 154(3):1132–42
- Jahng JW, Kim JG, Kim HJ, Kim B-T, Kang D-W, Lee J-H. (2007). Chronic food restriction in young rats results in depression- and anxiety-like behaviors with decreased expression of serotonin reuptake transporter. Brain Res 1150:100–7
- James SJ, Melnyk S, Pogribna M, Pogribny IP, Caudill MA. (2002). Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. J Nutr 132(8 Suppl):2361S–6S
- Jen KLC, Church MW, Wang C, Moghaddam M, Dowhan L, Laja F, Sherman J. (2009). Perinatal n-3 fatty acid imbalance affects fatty acid composition in rat offspring. Physiol Behav 98:17--24
- Jiang X, Yan J, West AA, Perry CA, Malysheva OV, Devapatla S, Pressman E, et al. (2012). Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB J 26(8):3563–74
- Joëls M, Baram TZ. (2009). The neuro-symphony of stress. Nat Rev Neurosci 10(6):459–66
- Joung KE, Park K-H, Zaichenko L, Sahin-Efe A, Thakkar B, Brinkoetter M, Usher N, et al. (2014). Early life adversity is associated with elevated levels of circulating leptin, irisin, and decreased levels of adiponectin in midlife adults. J Clin Endocrinol Metab 99(6):E1055–60
- Kendall-Tackett KA. (2007). Violence against women and the perinatal period: the impact of lifetime violence and abuse on pregnancy, postpartum, and breastfeeding. Trauma Violence Abuse 8(3):344–53
- Koenen KC, Moffitt TE, Caspi A, Taylor A, Purcell S. (2003). Domestic violence is associated with environmental suppression of IQ in young children. Dev Psychopathol 15(2):297–311
- Konycheva G, Dziadek MA, Ferguson LR, Kraegeloh CU, Coolen MW, Davison M, Breier BH. (2011). Dietary methyl donor deficiency during pregnancy in rats shapes learning and anxiety in offspring. Nutr Res 31(10):790–804
- Korosi A, Baram TZ. (2009). The pathways from mother's love to baby's future. Front Behav Neurosci 3:27. doi: 10.3389/neuro.08.027.2009
- Korosi A, Baram TZ. (2010). Plasticity of the stress response early in life: mechanisms and significance. Dev Psychobiol 52(7):661–70
- Korosi A, Shanabrough M, McClelland S, Liu ZW, Borok E, Gao XB, Horvath TL, Baram TZ. (2010). Early-Life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J Neurosci 30(2):703–13
- Koutcherov Y, Mai JK, Paxinos G. (2003). Hypothalamus of the human fetus. J Chem Neuroanat 26(4):253–70
- Krishna AP, Ramakrishna T. (2004). Effect of pyridoxine deficiency on the structural and functional development of hippocampus. Indian J Physiol Pharmacol 48:304–10
- Kroupina MG, Fuglestad AJ, Iverson SL, Himes JH, Mason PW, Gunnar MR, Miller BS, et al. (2012). Adoption as an intervention for institutionally reared children: HPA functioning and developmental status. Infant Behav Dev 35(4):829–37
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. (2000). Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res 122:81–103
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. (2004). Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biol Psychiatry 55(4):367–75
- Ladd CO, Owens MJ, Nemeroff CB. (1996). Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology 137(4):1212–18
- Langie SAS, Achterfeldt S, Gorniak JP, Halley-Hogg KJA, Oxley D, van Schooten FJ, Godschalk RW, et al. (2013). Maternal folate depletion and high-fat feeding from weaning affects DNA methylation and DNA repair in brain of adult offspring. FASEB J 27(8):3323–34
- Langley-Evans SC. (2009). Nutritional programming of disease: unravelling the mechanism. J Anat 215(1):36–51
- Langley-Evans SC, Gardner DS, Jackson AA. (1996). Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis. J Nutr 126(6):1578–85
- Larrieu T, Hilal LM, Fourrier C, De Smedt-Peyrusse V, Sans N, Capuron L, Laye S (2014). Nutritional omega-3 modulates neuronal morphology in the prefrontal cortex along with depression-related behaviour through corticosterone secretion. Transl Psychiatry 4:e437
- Lehmann J, Pryce CR, Jongen-Rêlo AL, Stöhr T, Pothuizen HHJ, Feldon J. (2002). Comparison of maternal separation and early handling in terms of their neurobehavioral effects in aged rats. Neurobiol Aging 23(3):457–66
- Leonhardt M, Lesage J, Croix D, Dutriez-casteloot I, Beauvillain JC, Dupouy JP. (2003). Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty. Biol Reprod 68(2):390–400
- Leonhardt M, Lesage J, Dufourny L, Dickès-Coopman A, Montel V, Dupouy J-P. (2002). Perinatal maternal food restriction induces alterations in hypothalamo-pituitary-adrenal axis activity and in plasma corticosterone-binding globulin capacity of weaning rat pups. Neuroendocrinology 75(1):45–54
- Lesage J, Sebaai N, Leonhardt M, Dutriez-casteloot I, Breton C, Deloof S, Vieau D. (2006). Perinatal maternal undernutrition programs the offspring hypothalamo–pituitary–adrenal (HPA) axis. Stress 9(4):183–98
- Levine A, Worrell TR, Zimnisky R, Schmauss C. (2012). Early life stress triggers sustained changes in histone deacetylase expression and histone H4 modifications that alter responsiveness to adolescent antidepressant treatment. Neurobiol Dis 45(1):488–98
- Levine S. (2001). Primary social relationships influence the development of the hypothalamic--pituitary--adrenal axis in the rat. Physiol Behav 73(3):255–60
- Li X-L, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. (2002). Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience 113(3):607–15
- Liao X-M, Yang X-D, Jia J, Li J-T, Xie X-M, Su Y-A, Wang XD. (2014). Blockade of corticotropin-releasing hormone receptor 1 attenuates early-life stress-induced synaptic abnormalities in the neonatal hippocampus. Hippocampus 24(5):528–40
- Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. (2005). Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr 135(6):1382–6
- Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. (2007). Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr 97(06):1064
- Liu D. (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277(5332):1659–62
- Lok A, Bockting CLH, Koeter MWJ, Snieder H, Assies J, Mocking RJT, Vinkers CH, et al. (2013). Interaction between the MTHFR C677T polymorphism and traumatic childhood events predicts depression. Nature Publishing Group. Transl Psychiatry 3(7):e288–8
- Loman MM, Gunnar MR, Early Experience, Stress, and Neurobehavioral Development Center. (2010). Early experience and the development of stress reactivity and regulation in children. Neurosci Biobehav Rev 34(6):867–76
- Lucassen PJ, Naninck EFG, van Goudoever JB, Fitzsimons C, Joëls M, Korosi A. (2013). Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends Neurosci 36:621–31
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10(6):434–45
- Mainardi M, Pizzorusso T, Maffei M. (2013). Environment, leptin sensitivity, and hypothalamic plasticity. Neural Plast 2013:438072
- Maselko J, Kubzansky L, Lipsitt L, Buka SL. (2011). Mother's affection at 8 months predicts emotional distress in adulthood. J Epidemiol Commun Health 65(7):621–5
- Mathieu G, Denis S, Lavialle M, Vancassel S. (2008). Synergistic effects of stress and omega-3 fatty acid deprivation on emotional response and brain lipid composition in adult rats. Prostaglandins Leukot Essent Fatty Acids 78(6):391–401
- Mathieu G, Oualian C, Denis I, Lavialle M, Gisquet-Verrier P, Vancassel S. (2011). Dietary n-3 polyunsaturated fatty acid deprivation together with early maternal separation increases anxiety and vulnerability to stress in adult rats. Prostaglandins Leukot Essent Fatty Acids 85(3–4):129–36
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Publishing Group. Nat Neurosci 12(3):342–8
- McGowan PO, Suderman M, Sasaki A, Huang TCT, Hallett M, Meaney MJ, Szyf M. (2011). Broad epigenetic signature of maternal care in the brain of adult rats. PLoS One 6(2):e14739
- McNeil CJ, Hay SM, Rucklidge GJ, Reid M, Duncan G, Maloney CA, Rees WD. (2008). Disruption of lipid metabolism in the liver of the pregnant rat fed folate-deficient and methyl donor-deficient diets. Br J Nutr 99(2):262–71
- Meaney MJ, Aitken DH, Bodnoff SR, Iny LJ, Tatarewicz JE, Sapolsky RM. (1985). Early postnatal handling alters glucocorticoid receptor concentrations in selected brain regions. Behav Neurosci 99(4):765–70
- Mehedint MG, Craciunescu CN, Zeisel SH. (2010). Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proc Natl Acad Sci USA 107(29):12834–9
- Milagro FI, Campión J, García-Díaz DF, Goyenechea E, Paternain L, Martínez JA. (2009). High fat diet-induced obesity modifies the methylation pattern of leptin promoter in rats. J Physiol Biochem 65(1):1–9
- Morgane PJ, Mokler DJ, Galler JR. (2002). Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci Biobehav Rev 26(4):471–83
- Morris MC. (2011). Nutritional determinants of cognitive aging and dementia. Proc Nutr Soc 71(01):1–13
- Morris MJ, Velkoska E, Cole TJ. (2005). Central and peripheral contributions to obesity-associated hypertension: impact of early overnourishment. Exp Physiol 90(5):697–702
- Morrison CD. (2009). Leptin signaling in brain: a link between nutrition and cognition? Biochim Biophys Acta 1792(5):401–8
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. (2006). Central nervous system control of food intake and body weight. Nature 443(7109):289–95
- Moyer-Mileur LJ, Haley S, Gulliver K, Thomson A, Slater H, Barrett B, Joss-Moore LA, et al. (2011). Mechanical-tactile stimulation (MTS) during neonatal stress prevents hyperinsulinemia despite stress-induced adiposity in weanling rat pups. Early Hum Dev 87(3):159–63
- Muñoz-Hoyos A, Molina-Carballo A, Augustin-Morales M, Contreras-Chova F, Naranjo-Gómez A, Justicia-Martínez F, Uberos J. (2011). Psychosocial dwarfism: psychopathological aspects and putative neuroendocrine markers. Psychiatry Res 188(1):96–101
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmühl Y, Fischer D, Holsboer F, et al. (2009). Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nature Publishing Group. Nat Neurosci 12(12):1559–66
- Naninck EFG, Hoeijmakers L, Kakava-Georgiadou N, Meesters A, Lazic SE, Lucassen PJ, Korosi A. (2015). Chronic early-life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus 25:309–28
- Nederhof E, Schmidt MV. (2012). Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol Behav 106:691–700
- Neigh GN, Gillespie CF, Nemeroff CB. (2009). The neurobiological toll of child abuse and neglect. Trauma Violence Abuse 10(4):389–410
- Nimmo-Smith RH. (1954). The function of folic acid in the biosynthesis of purine and pyrimidine derivatives. In: Wolstenholme GEW, editor. Ciba Foundation Symposium – Chemistry and biology of pteridines. Chichester, UK: Wiley
- Nishimura RY, Barbieiri P, Castro GSF de, Jordão AA, Perdoná GDSC, Sartorelli DS. (2014). Dietary polyunsaturated fatty acid intake during late pregnancy affects fatty acid composition of mature breast milk. Nutrition 30(6):685–9
- Oates M, Woodside B, Walker CD. (2000). Chronic leptin administration in developing rats reduces stress responsiveness partly through changes in maternal behavior. Horm Behav 37(4):366–76
- Oka T. (2001). Modulation of gene expression by vitamin B6. Nutr Res Rev 14(2):257–66
- Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EMM, Joëls M, et al. (2010). Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J Neurosci 30(19):6635–45
- Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EMM, Joëls M, et al. (2011). Early maternal deprivation affects dentate gyrus structure and emotional learning in adult female rats. Psychopharmacology 214(1):249–60
- Painter RC, Roseboom TJ, Bleker OP. (2005). Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol 20:345–52
- Peleg-Raibstein D, Luca E, Wolfrum C. (2012). Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav Brain Res 233(2):398–404
- Pesonen A-K, Räikkönen K, Feldt K, Heinonen K, Osmond C, Phillips DIW, Barker DJ, et al. (2010). Childhood separation experience predicts HPA axis hormonal responses in late adulthood: a natural experiment of World War II. Psychoneuroendocrinology 35(5):758–67
- Pesonen A-K, Räikkönen K, Heinonen K, Kajantie E, Forsén T, Eriksson JG. (2007). Depressive symptoms in adults separated from their parents as children: a natural experiment during World War II. Am J Epidemiol 166(10):1126–33
- Petrie L, Duthie SJ, Rees WD, McConnell JML. (2007). Serum concentrations of homocysteine are elevated during early pregnancy in rodent models of fetal programming. Br J Nutr 88(05):471--7
- Picard M, Juster R-P, McEwen BS. (2014). Mitochondrial allostatic load puts the “gluc” back in glucocorticoids. Nat Rev Endocrinol 10(5):303–10
- Plagemann A, Harder T, Brunn M, Harder A, Roepke K, Wittrock-Staar M, Ziska T, et al. (2009). Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J Physiol 587(20):4963–76
- Portha B, Fournier A, Kioon MDA, Mezger V, Movassat J. (2014). Early environmental factors, alteration of epigenetic marks and metabolic disease susceptibility. Biochimie 97:1–15
- Prado EL, Dewey KG. (2014). Nutrition and brain development in early life. Nutr Rev 72(4):267–84
- Proulx K, Clavel S, Nault G, Richard D, Walker CD. (2001). High neonatal leptin exposure enhances brain GR expression and feedback efficacy on the adrenocortical axis of developing rats. Endocrinology 142(11):4607–16
- Pryce CR, Bettschen D, Feldon J. (2001). Comparison of the effects of early handling and early deprivation on maternal care in the rat. Dev Psychobiol 38(4):239–51
- Pryce CR, Rüedi-Bettschen D, Dettling AC, Feldon J. (2002). Early life stress: long-term physiological impact in rodents and primates. News Physiol Sci 17:150–5
- Rao S, Joshi S, Kale A, Hegde M, Mahadik S. (2006). Maternal folic acid supplementation to dams on marginal protein level alters brain fatty acid levels of their adult offspring. Metabolism 55(5):628–34
- Rapoport SI, Rao JS, Igarashi M. (2007). Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fatty Acids 77(5–6):251–61
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. (2008). A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 149(10):4892–900
- Rincón-Cortés M, Sullivan RM. (2014). Early life trauma and attachment: immediate and enduring effects on neurobehavioral and stress axis development. Front Endocrinol (Lausanne)5:33. doi: 10.3389/fendo.2014.00033
- Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. (2003). Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav 43(5):561–7
- Roopra A, Sharling L, Wood IC, Briggs T, Bachfischer U, Paquette AJ, Buckley NJ. (2000). Transcriptional repression by neuron-restrictive silencer factor is mediated via the Sin3-histone deacetylase complex. Mol Cell Biol 20(6):2147–57
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. (2009). Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry 65(9):760–9
- Roy S, Kale A, Dangat K, Sable P, Kulkarni A, Joshi S. (2012). Maternal micronutrients (folic acid and vitamin B(12)) and omega 3 fatty acids: implications for neurodevelopmental risk in the rat offspring. Brain Dev 34(1):64–71
- Rudolf MCJ. (2005). What is the long term outcome for children who fail to thrive? A systematic review. Arch Dis Childh 90(9):925–31
- Sarel I, Widmaier EP. (1995). Stimulation of steroidogenesis in cultured rat adrenocortical cells by unsaturated fatty acids. Am J Physiol 268(6 Pt 2):R1484–90
- Schmidt M, Enthoven L, van Woezik JHG, Levine S, de Kloet ER, Oitzl MS. (2004). The dynamics of the hypothalamic-pituitary-adrenal axis during maternal deprivation. J Neuroendocrinol 16(1):52–7
- Schmidt MV, Levine S, Alam S, Harbich D, Sterlemann V, Ganea K, de Kloet ER, et al. (2006). Metabolic signals modulate hypothalamic-pituitary-adrenal axis activation during maternal separation of the neonatal mouse. J Neuroendocrinol 18(11):865–74
- Schulz KM, Pearson JN, Gasparrini ME, Brooks KF, Drake-Frazier C, Zajkowski ME, Kreisler AD, et al. (2014). Dietary choline supplementation to dams during pregnancy and lactation mitigates the effects of in utero stress exposure on adult anxiety-related behaviors. Behav Brain Res 268:104–10
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. (2009). Leptin targets in the mouse brain. J Comp Neurol 514(5):518–32
- Sebaai N, Lesage J, Breton C, Vieau D, Deloof S. (2004). Perinatal food deprivation induces marked alterations of the hypothalamo-pituitary-adrenal axis in 8-month-old male rats both under basal conditions and after a dehydration period. Neuroendocrinology 79(4):163–73
- Seckl JR, Cleasby M, Nyirenda MJ. (2000). Glucocorticoids, 11beta-hydroxysteroid dehydrogenase, and fetal programming. Kidney Int 57(4):1412–17
- Seth KA, Majzoub JA. (2001). Repressor element silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) can act as an enhancer as well as a repressor of corticotropin-releasing hormone gene transcription. J Biol Chem 276(17):13917–23
- Spencer SJ. (2013). Perinatal programming of neuroendocrine mechanisms connecting feeding behavior and stress. Front Neurosci 7:109. doi: 10.3389/fnins.2013.00109
- Spencer SJ, Tilbrook A. (2009). Neonatal overfeeding alters adult anxiety and stress responsiveness. Psychoneuroendocrinology 34(8):1133–43
- Steenweg-de Graaff J, Roza SJ, Steegers EA, Hofman A, Verhulst FC, Jaddoe VW, Tiemeier H. (2012). Maternal folate status in early pregnancy and child emotional and behavioral problems: the Generation R Study. Am J Clin Nutr 95(6):1413–21
- Stevens SE, Sonuga-Barke EJS, Kreppner JM, Beckett C, Castle J, Colvert E, Groothues C, et al. (2008). Inattention/overactivity following early severe institutional deprivation: presentation and associations in early adolescence. J Abnorm Child Psychol 36(3):385–98
- Tarry-Adkins JL, Ozanne SE. (2014). The impact of early nutrition on the ageing trajectory. Proc Nutr Soc 73(2):289–301
- Teicher MH, Anderson CM, Polcari A. (2012). Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA 109(9):E563–72
- Teng Y-W, Mehedint MG, Garrow TA, Zeisel SH. (2011). Deletion of betaine-homocysteine S-methyltransferase in mice perturbs choline and 1-carbon metabolism, resulting in fatty liver and hepatocellular carcinomas. J Biol Chem 286(42):36258–67
- Troen AM, Chao WH, Crivello NA, D'Anci KE, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. (2008). Cognitive impairment in folate-deficient rats corresponds to depleted brain phosphatidylcholine and is prevented by dietary methionine without lowering plasma homocysteine. J Nutr 138(12):2502–9
- Trottier G, Koski KG, Brun T, Toufexis DJ, Richard D, Walker C-D. (1998). Increased fat intake during lactation modifies hypothalamic-pituitary-adrenal responsiveness in developing rat pups: a possible role for leptin 1. Endocrinology 139(9):3704–11
- Tyrka AR, Parade SH, Price LH, Kao H-T, Porton B, Philip NS, Welch ES, Carpenter LL. (2015). Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol Psychiatry. [Epub ahead of print]. doi: http://dx.doi.org/10.1016/j.biopsych.2014.12.025
- Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. (2008). Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry 63(12):1147–54
- Vaiserman AM. (2015). Epigenetic programming by early-life stress: evidence from human populations. Dev Dyn 244(3):254–65
- van de Rest O, van Hooijdonk LWA, Doets E, Schiepers OJG, Eilander A, de Groot LCPGM. (2012). B vitamins and n-3 fatty acids for brain development and function: review of human studies. Ann Nutr Metab 60(4):272–92
- van Oers HJJ, de Kloet ER, Levine S. (1999). Persistent effects of maternal deprivation on HPA regulation can be reversed by feeding and stroking, but not by dexamethasone. J Neuroendocrinol 11(8):581–8
- Vázquez DM, Van Oers H, Levine S, Akil H. (1996). Regulation of glucocorticoid and mineralocorticoid receptor mRNAs in the hippocampus of the maternally deprived infant rat. Brain Res 731(1–2):79–90
- Veenema AH, Neumann ID. (2009). Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psychoneuroendocrinology 34(3):463–7
- Vieau D, Sebaai N, Leonhardt M, Dutriez-casteloot I, Molendi-Coste O, Laborie C, Breton C, et al. (2007). HPA axis programming by maternal undernutrition in the male rat offspring. Psychoneuroendocrinology 32(Suppl 1):S16–20
- Viveros M-P, Llorente R, Díaz F, Romero-Zerbo SY, Bermudez-Silva FJ, Rodríguez de Fonseca F, Argente J, Chowen JA. (2010). Maternal deprivation has sexually dimorphic long-term effects on hypothalamic cell-turnover, body weight and circulating hormone levels. Horm Behav 58(5):808–19
- Walker C-D. (2005). Nutritional aspects modulating brain development and the responses to stress in early neonatal life. Prog Neuro-Psychopharmacol Biol Psychiatry 29(8):1249–63
- Walker C-D. (2010). Maternal touch and feed as critical regulators of behavioral and stress responses in the offspring. Dev Psychobiol 52(7):638–50
- Wang X-D, Su Y-A, Wagner KV, Avrabos C, Scharf SH, Hartmann J, Wolf M, et al. (2013). Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nature Publishing Group. Nat Neurosci 16(6):706–13
- Weaver ICG. (2005). Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci 25(47):11045–54
- Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, et al. (2004). Epigenetic programming by maternal behavior. Nat Neurosci 7(8):847–54
- Wigger A, Neumann ID. (1999). Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav 66(2):293–302
- Workel JO, Oitzl MS, Fluttert M, Lesscher H, Karssen A, de Kloet ER. (2001). Differential and age-dependent effects of maternal deprivation on the hypothalamic-pituitary-adrenal axis of brown norway rats from youth to senescence. J Neuroendocrinol 13(7):569–80
- Wright CM. (2006). The influence of maternal socioeconomic and emotional factors on infant weight gain and weight faltering (failure to thrive): data from a prospective birth cohort. Arch Dis Childh 91(4):312–17
- Xaio Y, Wang L, Xu R-J, Chen Z-Y. (2006). DHA depletion in rat brain is associated with impairment on spatial learning and memory. Biomed Environ Sci 19:474–80
- Xie L, Korkmaz KS, Braun K, Bock J. (2013). Early life stress-induced histone acetylations correlate with activation of the synaptic plasticity genes Arc and Egr1 in the mouse hippocampus. J Neurochem 125(3):457–64
- Zeisel SH. (2004). Nutritional importance of choline for brain development. J Am Coll Nutr 23(6 Suppl):621S–6S
- Zeisel SH, da Costa K-A. (2009). Choline: an essential nutrient for public health. Nutr Rev 67(11):615–23
- Zeisel SH, Mar M-H, Howe JC, Holden JM. (2003). Concentrations of choline-containing compounds and betaine in common foods. J Nutr 133(5):1302–7
- Zeisel SH, Niculescu MD. (2006). Perinatal choline influences brain structure and function. Nutr Rev 64(4):197–203
