Abstract
Empathy is a core prerequisite for human social behavior. Relatively, little is known about how empathy is influenced by social stress and its associated neuroendocrine alterations. The current study was designed to test the impact of acute stress on emotional and cognitive empathy. Healthy male participants were exposed to a psychosocial laboratory stressor (trier social stress test, (TSST)) or a well-matched control condition (Placebo-TSST). Afterwards they participated in an empathy test measuring emotional and cognitive empathy (multifaceted empathy test, (MET)). Stress exposure caused an increase in negative affect, a rise in salivary alpha amylase and a rise in cortisol. Participants exposed to stress reported more emotional empathy in response to pictures displaying both positive and negative emotional social scenes. Cognitive empathy (emotion recognition) in contrast did not differ between the stress and the control group. The current findings provide initial evidence for enhanced emotional empathy after acute psychosocial stress.
Introduction
For humans, a threat to our social status or reputation is highly stressful (Dickerson & Kemeny, Citation2004). Laboratory paradigms containing elements of social evaluative threat are thus potent stressors. They stimulate the two major stress systems, the sympathetic nervous system (SNS) and the hypothalamic–pituitary–adrenal (HPA) axis (Joels & Baram, Citation2009). In contrast, experiencing prosocial behavior in the form of social support is a potent stress buffer (Ditzen & Heinrichs, Citation2014). Surprisingly, little is known on how the experience of a stressful episode influences the cognitive and emotional abilities to understand and feel for another person, that is, empathy.
Early concepts of the stress response focusing on the rapid response of the sympathetic nervous system have characterized this first response as the fight or flight response (Cannon, Citation1932). However, later in the course of the stress response, a different response pattern characterized by tending and befriending might occur, especially in females (Taylor et al., Citation2000). The latter concept predicts enhanced prosocial behavior and enhanced empathy in the aftermath of stress. Importantly, it has been argued that this effect should occur in men as well (Geary & Flinn, Citation2002). Indeed, recent studies provided initial evidence in support of this notion (Buchanan & Preston, Citation2014). For example, von Dawans et al. (Citation2012) observed enhanced prosocial behavior in economic games in young healthy men after exposure to a group version of the TSST (von Dawans et al., Citation2011). No effects on antisocial behavior were found. However, it has to be acknowledged that the findings are heterogeneous and that reductions in empathy after stress have been reported (Buruck et al., Citation2014; Tomova et al., Citation2014).
There is converging agreement that empathy should not be considered a single process but in contrast a construct consisting of at least two separable aspects, namely emotional and cognitive empathy (Blair, Citation2005). Cognitive empathy, often referred to as mindreading or mentalizing, refers to the capacity to consciously understand and interpret the affective state of another person (Frith & Frith, Citation2007). In contrast, emotional empathy describes an observer's emotional response to another person's emotional state (Singer & Lamm, Citation2009). Patient studies (Adolphs, Citation2009; Blair, Citation2005) and human neuroimaging experiments (Fan et al., Citation2011; Lamm et al., Citation2011) have contributed substantially during the past decades in characterizing the neural correlates involved in these processes (Gonzalez-Liencres et al., Citation2013).
The idea of enhanced prosocial behavior after stress exposure appears to be in contrast to findings obtained in rodents, primates and humans linking stress to antisocial behavior such as aggression or violence (Craig, Citation2007; Honess & Marin, Citation2006; Sandi & Haller, Citation2015). These contradictory results emphasize the need for additional research trying to characterize the circumstances leading to prosocial versus antisocial behavior after stress.
In this study, we tested the impact of an acute psychosocial stressor on emotional and cognitive aspects of empathy. We therefore employed a potent laboratory stressor, the TSST (Kirschbaum et al., Citation1993) and an empathy test allowing the differentiation of cognitive and emotional empathy (multifaceted empathy test, (MET)) (Dziobek et al., Citation2008). We hypothesized that enhanced emotional empathy might be one mechanism underlying the initial evidence for prosocial behavior after stress (von Dawans et al., Citation2012). Moreover, there is recent pharmacological evidence for enhanced emotional but not cognitive empathy after stimulation of the mineralocorticoid receptor (Wingenfeld et al., Citation2014). Based on these studies, we expected enhanced emotional empathy after stress exposure, even though it has to be acknowledged that alternative predictions (less empathy after stress) are conceivable (Decety & Lamm, Citation2006; Tomova et al., Citation2014).
Methods
Participants
Participants had to be between 18 and 40 years old. Regular smoking, a body mass index (BMI) out of the normal range (below 19 or above 26 kg/m2) and acute or chronic diseases led to exclusion. In addition, we excluded students who had previously participated in the TSST. Participants were randomly assigned to the stress or control condition using a randomization list. Initially, 102 male students were recruited. Three participants had to be excluded. Two were excluded because of a technical failure during the administration of the MET. Another one admitted at the end of the study that he had already participated in a TSST study in the past. Therefore, data from 99 participants could be used (control group = 50; stress group = 49).
Participants had a mean age of 24.4 ± 4.43 (SD) years and a mean BMI of 23.6 ± 2.32 kg/m2. Only men were included in this initial study in order to reduce the impact of variations in gonadal hormone concentrations on the HPA stress response (Kirschbaum et al., Citation1999) and on social cognitive capacities (Ball et al., Citation2013; Taylor et al., Citation2000). All participants refrained from smoking, caffeine, meals, and all kinds of beverages except for water at least 1 h prior to testing. The study was approved by the local ethics committee, and all students provided written informed consent before participation. Participants received a small financial reimbursement for study participation.
Procedure and stress induction
Experimental sessions started between 1 p.m. and 5 p.m. First, participants signed the consent form and filled out a demographic questionnaire. Afterward, they rested for 30 min (while filling out questionnaires) before they collected the first saliva sample (baseline). Subsequently, they were brought to another room where the stress or control task was performed. Approximately, 15 min after the stress or control treatment, participants completed the empathy test.
Stress and control treatment
The TSST was used to induce stress (Kirschbaum et al., Citation1993). After a five-min preparation period, participants should perform an oral presentation (a faked job interview) and an arithmetic task (counting backwards in steps of 17) for a total of 10 min. They are evaluated by a panel (one woman and one man dressed in white coats) that deliberately refrains from any sort of feedback. Additionally, participants are videotaped. The TSST is known to reliably activate the SNS and the HPA axis (Dickerson & Kemeny, Citation2004). The nonstressful control condition called the placebo-TSST (P-TSST; Het et al., Citation2009) also consists of an oral presentation and an arithmetic task, but participants do not perform in front of an audience and are not videotaped. It thus lacks the stressful components of the TSST (social evaluative threat and uncontrollability) and does not elicit an HPA response (Het et al., Citation2009).
Neuroendocrine stress measurement
Saliva samples were taken at four different times; at baseline as well as 1 min, 10 min and 30 min after completion of the TSST or placebo TSST. Saliva was collected using Salivette collection devices (Sarstedt, Nuernbrecht, Germany). Cortisol concentrations served as a measure of HPA activity and were determined by a commercially available immunoassay (IBL, Hamburg, Germany). Salivary alpha amylase (sAA; an indirect marker of adrenergic activity) was measured using a quantitative enzyme kinetic method as described in detail elsewhere (Rohleder & Nater, Citation2009). Inter- and intracoefficients of variations were below 10%.
Psychometric stress measurement
Furthermore, the German version of the positive and negative affect schedule (PANAS (Watson et al., Citation1988)) was applied to assess positive and negative affect. Participants fill the intensity of 20 feelings and emotions on a five-point scale. Items can be subdivided into negative affect (NA consisting of 10 items) and positive affect (PA consisting of 10 items). We used the mean score as outcome measure. This score can thus range between 1 and 5. The questionnaire was applied before and after the respective experimental treatment.
Multifaceted empathy test – condensed and revised
To assess cognitive and emotional empathy, the multifaceted empathy test (MET) (Dziobek et al., Citation2008) was administered in its condensed and revised version (MET-core) which has been shown to be a reliable and sensitive measure of empathy in previous studies involving healthy participants and those with psychiatric disorders (Dziobek et al., Citation2011; Hurlemann et al., Citation2010; Wingenfeld et al., Citation2014). The version used contains a total of 30 pictures showing people in emotionally charged situations serving as ecologically valid stimuli, that is, they depict everyday life scenes conveying information on emotional mental states via facial expressions, body language, and context. Pictures contain either a single person displaying one emotion (a crying man) or one person interacting with another person (a child hugging its father). Stimuli can be subdivided according to their valence (positive: n = 13, negative: n = 17). In the positive category, the pictures display seven women/girls and six men/boys. In the negative category, the pictures display 10 women/girls and seven men/boys.
To assess cognitive empathy (CE), participants are required to infer the mental state of the individual in each scene and indicate the correct one from a list of four alternatives. For emotional empathy, participants are asked to rate for the same pictures in different blocks how much they feel for the person (Emotional Empathy; EE) on a Likert scale ranging from 0 = not at all to 9 = very much. Correct responses in the CE condition are scored as one point and a sum score is computed. For EE, an average rating score is calculated.
Statistical analysis
Psychometric, neuroendocrine and behavioral data were analyzed using mixed-model analysis of variances (ANOVAs) as described in detail in the respective result sections. Greenhouse-Geisser corrections were applied when indicated. Post hoc tests were performed using Bonferroni-adjusted t-tests. In addition, regression analyses were conducted.
Results
Affective response to stress
A repeated measures ANOVA of the PANAS scores with the factor time (pre- and post-treatment) and the between-subjects factor group (control group vs. stress group) was computed separately for negative and positive affect. The analysis revealed a significant time x group interaction for negative affect (F(1,97) = 35.92, p < 0.01). Negative affect increased in the stress group (pre 1.43 ± 0.57; post 1.86 ± 0.73) while it decreased in the control group (pre 1.25 ± 0.36; post 1.17 ± 0.22). The ANOVA with positive affect also revealed a significant time × group interaction (F(1,97) = 4.03, p < 0.05). Positive affect decreased in the stress group (pre 3.03 ± 0.57; post 2.89 ± 0.69), while it remained stable in the control group (pre 2.99 ± 0.73; post 3.05 ± 0.84).
sAA response to stress
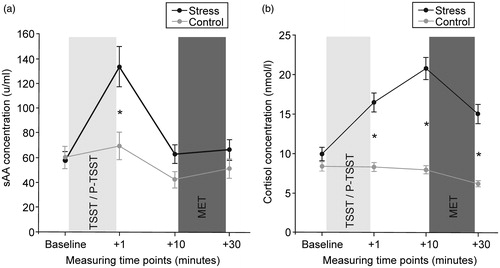
Due to an insufficient amount of saliva, sAA concentrations were missing from two participants. A repeated measures ANOVA with the factors time (baseline, + 1 min, + 10 min, + 30 min) and group (control group vs. stress group) was performed. As expected, the analysis revealed higher sAA concentrations in the stress (TSST) group (). The ANOVA indicated a main effect of group (F (1,94) = 4.67, p < 0.05), and a significant time × group interaction (F (3,282) = 12.19, p < 0.001). Post hoc tests using Bonferroni-adjusted t-tests revealed that the stress group (compared to the control group) only displayed significantly larger sAA concentrations immediately after stress exposure (p < 0.01).
Figure 1. (a) sAA and (b) cortisol response to the TSST and the control condition (P-TSST). Data are presented as mean (±SEM). A significant interaction between stress and time occurred in the ANOVAs for both stress markers. sAA levels were significantly elevated compared to the control condition immediately after the TSST (* < 0.05 in post hoc t-tests). Cortisol concentrations were significantly elevated at all three time points after stress cessation (* < 0.05 in post hoc t-tests).
Cortisol response to stress
Analysis of saliva samples revealed higher cortisol concentrations in the stress (TSST) group (). Again, a repeated measures ANOVA with the factors time (baseline, + 1 min, + 10 min, + 30 min) and group (control group vs. stress group) was performed. The ANOVA indicated a main effect of group (F (1,97) = 45.62, p < 0.001) and a significant time × group interaction (F (3,291) = 54.07, p < 0.001). Post hoc tests using Bonferroni-adjusted t-tests demonstrated that the stress group (compared to the control group) had significantly larger cortisol concentrations at all three post-treatment time points (p < 0.001).
Emotional empathy
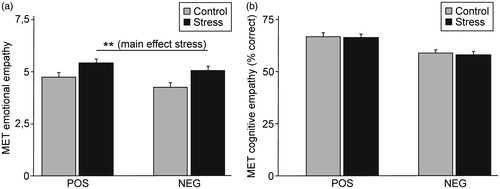
Stressed participants reported more emotional empathy in response to both positive and negative stimuli (). A repeated measures ANOVA with the factors group (control group vs. stress group) and valence (positive vs. negative stimuli) revealed a main effect of group (F (1,97) = 8.19, p < 0.01). Moreover, a main effect of valence (F (1,97) = 6.68, p < 0.05) was detected, with participants reporting more emotional empathy in response to pictures displaying positive emotions. The interaction (valence × group) was not significant (F < 1). The effect sizes obtained for the comparison between the stress and the control group were small to medium (Cohen’s d = 0.44 for positive stimuli and 0.53 for negative stimuli).
Figure 2. (a) Effect of stress on affective empathy (mean ± SEM). Stressed participants reported more affective empathy as indicated by a main effect of stress (** p < 0.01). This was independent of valence (no significant interaction between stress and valence). (b) Effect of stress on cognitive empathy (mean ± SEM). Stress had no influence on the capability of recognizing the displayed emotions.
Cognitive empathy
The two groups did not differ in their cognitive empathy (). The ANOVA neither revealed a main effect of group nor a group × valence interaction (both F values < 1). A main effect of valence was apparent (F (1,97) = 22.36, p < 0.01). Participants were better in recognizing positive compared to negative emotions.
Associations between the stress markers and the empathy measures
A linear regression analysis was conducted to test whether changes in the biological stress markers (sAA and cortisol) or negative affect significantly predicted empathy within the stress group. For cognitive and emotional empathy, separate regression models were calculated using the entry method. The initial model included the predictors increase in cortisol and alpha amylase (both were operationalized as area under the curve with respect to increase (AUCi) according to (Pruessner et al., Citation2003)), and the change in negative affect (post minus pre). In a second, extended model, the interactions between increase in cortisol, increase in alpha amylase and increase in negative affect were included as additional predictors.
For cognitive empathy, the three initial predictors explained a significant amount of variance (F (3,44) = 3.84, p < 0.05, R2 = 0.21, = 0.15). The analysis showed that the increase in negative affect significantly predicted cognitive empathy (b = −2.56, standardized β = −0.44, t(44) = −2.82, p < 0.01) while neither the increase in cortisol nor that in alpha amylase did so (both ps > 0.10). Extending the model by including the interactions between the predictors did not significantly improve the overall fit of the model (F (6,41) = 2.03, p = 0.08, R2 = 0.23,
= 0.12).
Regarding emotional empathy, the predictors did not explain a significant amount of variance in the mean emotional rating of all stimuli, neither in the initial nor in the extended model (both ps > 0.10).
Discussion
The current study tested the effects of acute stress exposure in a well-controlled laboratory setting on emotional and cognitive empathy as assessed with the MET. Stressed participants reported more emotional empathy. Interestingly, this was the case for pictures displaying both positive and negative emotions. In contrast, stress had no apparent impact on cognitive empathy.
Our study adds to the growing evidence for increased prosocial behavior in the aftermath of acute stress (Buchanan & Preston, Citation2014; von Dawans et al., Citation2012). The findings are thus in contrast to the literature linking stress to antisocial behavior such as aggression (Craig, Citation2007; Honess & Marin, Citation2006; Sandi & Haller, Citation2015) and illustrate an alternative, so far underappreciated social response to stress in humans.
In fact, enhanced emotional empathy could be one underlying mechanism of the prosocial effects observed. However, it needs to be emphasized that many steps lie between an empathic response and prosocial behavior (Decety & Lamm, Citation2006; Gonzalez-Liencres et al., Citation2013). At a broader conceptual level, our data appear to fit the tend-and-befriend hypothesis originally proposed by Taylor and colleagues for the biobehavioral stress response in women (Taylor et al., Citation2000). The current findings support the emerging notions that the stress response in men (under the circumstances studied in our experiment) can be characterized by tending and befriending as well. This conclusion is in line with some of the theoretical arguments provided by Geary and Finn. They argued that male befriending in times of stress has evolved to allow for the formation of kin-based coalitions (Geary & Flinn, Citation2002).
The observed stress-induced enhancement of emotional empathy further is in line with a recent pharmacological study using the mineralocorticoid receptor (MR) agonist fludrocortisone (Wingenfeld et al., Citation2014). The study also used the MET and observed that, similar to our current study, MR stimulation was associated with enhanced emotional but not cognitive empathy. The enhanced emotional empathy observed in the current study might thus reflect rapid nongenomic effects of the membrane-bound MR. Studies in rodents support the notion of an important role of the MR for social-cognitive functioning (Ter Horst et al., Citation2014). However, findings derived from stress studies do not allow conclusions about the underlying neuroendocrine mechanisms, and a possible role of the glucocorticoid receptor (GR) in mediating the observed stress effects cannot be ruled out. Therefore, additional pharmacological studies are needed in order to disentangle the underlying mechanisms (Martin et al., Citation2015).
Another recent study employing the MET observed enhanced emotional empathy after treatment with the neuropeptide oxytocin (Hurlemann et al., Citation2010). Results supported the emerging evidence for prosocial effects of this hormone (Meyer-Lindenberg et al., Citation2011). Since stress is thought to increase oxytocin concentrations (Taylor et al., Citation2000), this could constitute an additional or alternative mechanism for the effects observed in the current study.
Importantly, the enhancement in affective empathy was observed for pictures displaying both positive and negative emotions. This argues against a state-dependent interpretation of our findings (e.g., I feel bad and thus can better empathize with someone else who feels similar). The missing association between the increase in negative affect and the affective empathy ratings is in further support of this conclusion. Previous studies reported an enhanced negativity bias (attentional bias toward threatening or aversive stimuli) in stressed participants (Oei et al., Citation2012; Mogg et al., Citation1990). As mentioned previously, our current empathy study failed to find valence-specific effects in the aftermath of acute psychosocial stress. The timing or the specifics of the empathy task might underlie these discrepancies.
At the group level, stress had no impact on cognitive empathy. The observed effects on affective empathy can thus not be explained by a stress-induced increase in cognitive empathy. A previous study from our laboratory (Smeets et al., Citation2009) using the movie for the assessment of social cognition (MASC) (Dziobek et al., Citation2006)) similarly failed to find an overall effect of stress on social cognition, even though in this study, sex-specific associations with the individual cortisol response were detected.
The present study indicates that the emotional aspect of empathy is more heavily influenced by stress. Recent neuroimaging research has helped characterize the neural correlates of emotional empathy. Subcortical regions, such as the insula and the amygdala, appear to be especially important for emotional empathy (Blair, Citation2005; Dziobek et al., Citation2011; Singer & Lamm, Citation2009). Neuroimaging studies by our group have repeatedly observed that those regions are influenced by stress or cortisol administration (Merz et al., Citation2010, Citation2012a, Citation2013). Cognitive empathy in contrast appears to be more closely linked to cortical regions such as the temporo-parietal junction and the superior temporal sulcus (Bahnemann et al., Citation2010; Blair, Citation2005; Wolf et al., Citation2010). It is conceivable that those regions might be less sensitive to stress. Future functional neuroimaging studies are needed in order to characterize the neural correlates of the stress-induced increase in emotional empathy observed in the current study.
It has to be acknowledged that the stress-induced enhancement of emotional empathy observed in our study appears to be in contrast to some recent findings. Tomova and colleagues observed enhanced egocentricity in men exposed to a group version of the TSST (Tomova et al., Citation2014). In women, the opposite pattern was observed. Their findings thus resembled closely the prediction derived from the Taylor model (Taylor et al., Citation2000). The increased egocentricity in situations of strong personal distress is less resource demanding and might thus be initially adaptive (Decety & Lamm, Citation2006; Tomova et al., Citation2014). We suggest as a possible compromise that the relationship between stress and other-relatedness/prosocial feelings is likely not linear but might reflect an inverted U-shaped function. While at both low and high levels of stress individuals show reduced levels of prosocial orientation, midrange stress levels might lead an individual to orient toward other individuals to seek and provide affiliation, respectively. Thus, we do not challenge the idea that under high levels of personal distress other-oriented feelings and prosocial behaviors might occur less likely as predicted by Decety’s empathy model (Decety & Lamm, Citation2006) as well as resource allocation models. As discussed earlier, we believe that the stress levels induced in our study by means of the TSST were in the midrange and thus resulted in an increased levels of other-relatedness. In addition, subjects’ empathic responses were assessed with some temporal distance to the TSST (see below for an extension on this issue) in an external task, that is, the MET, and were thus not related to the people who caused the stress response. This might have further prevented excess self-orientedness.
In another study, Buruck and colleagues reported reduced empathic reactions to other’s pain after exposure to the TSST (Buruck et al., Citation2014). Of note, however, participants were asked to rate the intensity of pain that they thought persons in different pictures would experience. Although the authors assumed that this would trigger empathic feelings, this measure in fact approximates an understanding of other’s emotions, that is, cognitive empathy, much more closely than emotional empathy. As such, the results are not in conflict with the findings of the current study. The discussion of these studies illustrates the complexity of the topic and the need for additional research.
In recent years, the impact of the complex temporal dynamics of the acute stress response has become a focus of interest. Rapid SNS-mediated arousal increases, followed by fast nongenomic glucocorticoid (GC) signals and finally slower genomic GC signals might have in part opposing effects on cognition (Joels et al., Citation2011). For example, we could recently demonstrate that decision making was impaired in the aftermath of stress, when cortisol concentrations were high and SNS markers had returned to baseline. In contrast, immediately before or after the stressor, at times when the SNS signal dominates enhanced decision making was observed (Pabst et al., Citation2013). It thus would be interesting to characterize the impact of stress on empathy at different time-points after stress exposure. It is conceivable that the initial SNS activation in men is associated with fight or flight behavior (Taylor et al., Citation2000) while the somewhat delayed HPA activation might be associated with prosocial behavior (enhanced empathy and befriending). It is of note that in the study conducted by Tomova et al. (Citation2014), the empathy measures were in contrast to our study taken in close proximity to the stressor.
The conducted regression analyses did not detect a strong association between changes in cortisol, sAA or affect and the observed enhancement in emotional empathy. Interindividual differences in glucocorticoid sensitivity (Rohleder et al., Citation2010) could for example underlie the missing associations. Moreover, the two stress systems (SNS and HPA) might interact at multiple levels in a complex nonlinear manner (Roozendaal et al., Citation2009). Last but not least effects mediated via other stress-sensitive neurotransmitter systems (e.g., serotonin, oxytocin or CRH) have to be considered as well (Sandi & Haller, Citation2015; Taylor et al., Citation2000).
The current study tested only male participants so conclusions about the impact of stress on empathy in women cannot be drawn. For the first study, on this topic, we wanted to ascertain sufficient power since we expected the effects to be of medium size only. We thus focused on one sex (males) only. Given the conceptual (Taylor et al., Citation2000) and empirical (Kirschbaum et al., Citation1999; Smeets et al., Citation2009; Tomova et al., Citation2014; Wolf, Citation2013) evidence for sex differences in the neuroendocrine and behavioral stress response the inclusion of both sexes in future studies on this topic appears warranted (Tomova et al., Citation2014). Last but not least researchers interested in sex or gender differences should be aware of the fact that the impact of stress on the human brain may change during the menstrual cycle and appears to be further modulated by the usage of hormonal contraceptives (Merz et al., Citation2012b).
There are two further methodological issues that need to be discussed. We used Salivette sampling devices for saliva collection. The usage of these devices has been criticized for not taking into account saliva flow rate. Moreover, the lack of standardized usage of the devices by the participants might additionally increase the variance of the results obtained (Bosch et al., Citation2011). A study by Rohleder explicitly addressing the issue of flow rate suggested that it was not a major determined of the obtained sAA concentrations (Rohleder et al., Citation2006). These authors concluded that valid sAA measurements can be obtained with Salivettes. The response pattern observed in our current study (and multiple previous studies) with a rapid rise and rapid decline of sAA in response to acute stress fits very well to previous studies using different sampling approaches (Rohleder et al., Citation2006). Nevertheless, we cannot exclude the possibility that a different sampling approach might have been associated with a different outcome, especially with less variance.
In line with most of our previous experimental stress studies, we have used a between group design with random group allocations (Kinner et al., Citation2014; Quesada et al., Citation2012; Smeets et al., Citation2009). The advantage of this design is the exclusion of transfer, habituation or other forms of carry over effects. However, repeated measurement studies have the advantage to reduce the impact of interindividual differences. It would be reassuring if similar results as reported in the current manuscript would be obtained in a within subject design study as well.
Taken together the present study demonstrates enhanced emotional empathy in young healthy men after exposure to a psychosocial laboratory stressor. The results support the notion that men’s biobehavioral stress response might under specific circumstances be characterized in part by empathetic prosocial responses.
Declaration of interest
This study was funded by the German Research Foundation (DFG) project B4 of the Collaborative Research Center (SFB) 874 “Integration and Representation of Sensory Processes” and awarded to Oliver T. Wolf. The authors declare that they do not have a conflict of interest.
References
- Adolphs R. (2009). The social brain: neural basis of social knowledge. Annu Rev Psychol 60:693–716
- Bahnemann M, Dziobek I, Prehn K, Wolf I, Heekeren HR. (2010). Sociotopy in the temporoparietal cortex: common versus distinct processes. Soc Cogn Affect Neurosci 5:48–58
- Ball A, Wolf CC, Ocklenburg S, Herrmann BL, Pinnow M, Brune M, Wolf OT, et al. (2013). Variability in ratings of trustworthiness across the menstrual cycle. Biol Psychol 93:52–7
- Blair RJ. (2005). Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious Cogn 14:698–718
- Bosch JA, Veerman EC, de Geus EJ, Proctor GB. (2011). alpha-Amylase as a reliable and convenient measure of sympathetic activity: don't start salivating just yet! Psychoneuroendocrinology 36:449–53
- Buchanan TW, Preston SD. (2014). Stress leads to prosocial action in immediate need situations. Front Behav Neurosci 8:5
- Buruck G, Wendsche J, Melzer M, Strobel A, Dorfel D. (2014). Acute psychosocial stress and emotion regulation skills modulate empathic reactions to pain in others. Front Psychol 5:517
- Cannon W. (1932). Wisdom of the body. New York: W.W. Norton & Company
- Craig IW. (2007). The importance of stress and genetic variation in human aggression. Bioessays 29:227–36
- Decety J, Lamm C. (2006). Human empathy through the lens of social neuroscience. ScientificWorldJournal 6:1146–63
- Dickerson SS, Kemeny ME. (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 130:355–91
- Ditzen B, Heinrichs M. (2014). Psychobiology of social support: the social dimension of stress buffering. Restor Neurol Neurosci 32:149–62
- Dziobek I, Fleck S, Kalbe E, Rogers K, Hassenstab J, Brand M, Kessler J, et al. (2006). Introducing MASC: A movie for the assessment of social cognition. J Autism Dev Disord 36:623–36
- Dziobek I, Preissler S, Grozdanovic Z, Heuser I, Heekeren HR, Roepke S. (2011). Neuronal correlates of altered empathy and social cognition in borderline personality disorder. Neuroimage 57:539–48
- Dziobek I, Rogers K, Fleck S, Bahnemann M, Heekeren HR, Wolf OT, Convit A. (2008). Dissociation of cognitive and emotional empathy in adults with asperger syndrome using the multifaceted empathy test (MET). J Autism Dev Disord 38: 464–73
- Fan Y, Duncan NW, de Greck M, Northoff G. (2011). Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev 35:903–11
- Frith CD, Frith U. (2007). Social cognition in humans. Curr Biol 17:R724–32
- Geary DC, Flinn MV. (2002). Sex differences in behavioral and hormonal response to social threat: commentary on Taylor et al. (2000). Psychol Rev 109:745–50
- Gonzalez-Liencres C, Shamay-Tsoory SG, Brune M. (2013). Towards a neuroscience of empathy: ontogeny, phylogeny, brain mechanisms, context and psychopathology. Neurosci Biobehav Rev 37:1537–48
- Het S, Rohleder N, Schoofs D, Kirschbaum C, Wolf OT. (2009). Neuroendocrine and psychometric evaluation of a placebo version of the ‘Trier Social Stress Test'. Psychoneuroendocrinology 34:1075–86
- Honess PE, Marin CM. (2006). Behavioural and physiological aspects of stress and aggression in nonhuman primates. Neurosci Biobehav Rev 30:390–412
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Dziobek I, et al. (2010). Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci 30:4999–5007
- Joels M, Baram TZ. (2009). The neuro-symphony of stress. Nat Rev Neurosci 10:459–66
- Joels M, Fernandez G, Roozendaal B. (2011). Stress and emotional memory: a matter of timing. Trends Cogn Sci 15:280–288
- Kinner VL, Het S, Wolf OT. (2014). Emotion regulation: exploring the impact of stress and sex. Front Behav Neurosci 8:397
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine 61:154–62
- Kirschbaum C, Pirke KM, Hellhammer DH. (1993). The ‘trier social stress test' – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81
- Lamm C, Decety J, Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54:2492–502
- Martin LJ, Hathaway G, Isbester K, Mirali S, Acland EL, Niederstrasser N, Slepian PM, et al. (2015). Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Curr Biol 25:326–32
- Merz CJ, Stark R, Vaitl D, Tabbert K, Wolf OT. (2012a). Stress hormones are associated with the neuronal correlates of instructed fear conditioning. Biol Psychol 92:82–9
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. (2010). Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology 35:33–46
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. (2012b). Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Horm Behav 62:531–38
- Merz CJ, Wolf OT, Schweckendiek J, Klucken T, Vaitl D, Stark R. (2013). Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology 38:2529–41
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. (2011). Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 12:524–38
- Mogg K, Mathews A, Bird C, Macgregor-Morris R. (1990). Effects of stress and anxiety on the processing of threat stimuli. J Pers Soc Psychol 59:1230–37
- Oei NY, Veer IM, Wolf OT, Spinhoven P, Rombouts SA, Elzinga BM. (2012). Stress shifts brain activation towards ventral ‘affective' areas during emotional distraction. Soc Cogn Affect Neurosci 7:403–412
- Pabst S, Brand M, Wolf OT. (2013). Stress and decision making: a few minutes make all the difference. Behav Brain Res 50:39–45
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–31
- Quesada AA, Wiemers US, Schoofs D, Wolf OT. (2012). Psychosocial stress exposure impairs memory retrieval in children. Psychoneuroendocrinology 37:125–136
- Rohleder N, Nater UM. (2009). Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology 34:469–85
- Rohleder N, Wolf JM, Maldonado EF, Kirschbaum C. (2006). The psychosocial stress-induced increase in salivary alpha-amylase is independent of saliva flow rate. Psychophysiology 43:645–52
- Rohleder N, Wolf JM, Wolf OT. (2010). Glucocorticoid sensitivity of cognitive and inflammatory processes in depression and posttraumatic stress disorder. Neurosci Biobehav Rev 35:104–114
- Roozendaal B, McEwen BS, Chattarji S. (2009). Stress, memory and the amygdala. Nat Rev Neurosci 10:423–33
- Sandi C, Haller J. (2015). Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci 16:290–04
- Singer T, Lamm C. (2009). The social neuroscience of empathy. Ann N Y Acad Sci 1156:81–96
- Smeets T, Dziobek I, Wolf OT. (2009). Social cognition under stress: differential effects of stress-induced cortisol elevations in healthy young men and women. Horm Behav 55:507–13
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. (2000). Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev 107:411–29
- Ter Horst JP, van der MM, Kentrop J, Arp M, van der Veen R, De Kloet ER, Oitzl MS. (2014). Deletion of the forebrain mineralocorticoid receptor impairs social discrimination and decision-making in male, but not in female mice. Front Behav Neurosci 8:26
- Tomova L, von DB, Heinrichs M, Silani G, Lamm C. (2014). Is stress affecting our ability to tune into others? Evidence for gender differences in the effects of stress on self-other distinction. Psychoneuroendocrinology 43:95–104
- von Dawans B, Fischbacher U, Kirschbaum C, Fehr E, Heinrichs M. (2012). The social dimension of stress reactivity: acute stress increases prosocial behavior in humans. Psychol Sci 23:651–60
- von Dawans B, Kirschbaum C, Heinrichs M. (2011). The trier social stress test for groups (TSST-G): a new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology 36:514–22
- Watson D, Clark LA, Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54:1063–70
- Wingenfeld K, Kuehl LK, Janke K, Hinkelmann K, Dziobek I, Fleischer J, Otte C, Roepke S. (2014). Enhanced emotional empathy after mineralocorticoid receptor stimulation in women with Borderline Personality Disorder and healthy women. Neuropsychopharmacology 38:1799–04
- Wolf I, Dziobek I, Heekeren HR. (2010). Neural correlates of social cognition in naturalistic settings: a model-free analysis approach. Neuroimage 49:894–04
- Wolf OT. (2013). Effects of stress on learning and memory: evidence for sex differences in humans. In: Conrad CD, editor. The handbook of stress: neuropsychological effects on the brain. Chichester, West Sussex, UK: Wiley-Blackwell. p 545–59
