Abstract
Postnatal treatment with bacterial endotoxin lipopolysaccharide (LPS) changes the activity of the hypothalamic-pituitary-gonadal (HPG) axis and the gonadotropin-releasing hormone (GnRH) surge in rats. Exposure to an immune challenge in the critical periods of development has profound and long-lasting effects on the stress response, immune, metabolic, and reproductive functions. Prenatal LPS treatment delays the migration of GnRH neurons associated with increased cytokine release in maternal and fetal compartments. We investigated the effects of a single maternal exposure to LPS (18 μg/kg, i.p.) on day 12 (embryonic day (E)12) of pregnancy on reproductive parameters in rat offspring. Hypothalamic GnRH content, plasma luteinizing hormone (LH), testosterone, and estradiol concentrations were measured in both male and female offsprings at different stages of postnatal development by RIA and ELISA (n = 10 each per group). Body weight and in females day of vaginal opening (VO) were recorded. In offspring exposed to LPS prenatally, compared with controls, body weight was decreased in both sexes at P5 and P30; in females, VO was delayed; hypothalamic GnRH content was decreased at postnatal days 30–60 (P30–P60) in both sexes; plasma LH concentration was decreased at P14–P60 in females; plasma concentrations of testosterone/estradiol were increased at P14 in females, and plasma estradiol was increased at P14 in males. Hence activation of the maternal immune system by LPS treatment at a prenatal critical period leads to decreased GnRH and LH levels in pre- and postpubertal life and sex steroid imbalance in the prepubertal period, and delayed sexual maturation of female offspring.
Introduction
Reciprocal relationships between the hypothalamic–pituitary–gonadal (HPG) axis and the immune system have an extensive impact on development and functioning of both systems (Sominsky et al., Citation2012; Zakharova & Izvolskaya, Citation2012). Administration of the bacterial endotoxin lipopolysaccharide (LPS), as an experimental model of immune/inflammatory stress, leads to disruptions in physiological and behavioral outcomes (Spencer et al., Citation2006; Walker et al., Citation2009), metabolism (Iwasa et al., Citation2009), and brain morphology (Amath et al., Citation2012). Inflammation induced by LPS may affect the reproductive system at the hypothalamic level, by modulating the activity of gonadotropin-releasing hormone (GnRH) neurons, and at the pituitary level, by inhibiting luteinising hormone (LH) secretion in adults (Herman & Tomaszewska-Zaremba, Citation2010; Li et al., Citation2015). LPS induces synthesis of proinflammatory cytokines (interleukin [IL] 1β, IL-6, and tumor necrosis factor-alpha [TNFα] in the hypothalamic medial preoptic area, where cell bodies of GnRH neurons are located; McCann et al., Citation2000). Proinflammatory cytokines act either directly or via modulating secretion of opioid peptides, prostaglandins, catecholamines, gamma-aminobutyric acid (GABA), or nitric oxide (Kalra et al., Citation1998; Kimura et al., Citation1997; McCann et al., Citation2000).
Maternal immunological stress at day 12 of embryonic development (E12), which is the day of the beginning of GnRH neuron migration from the olfactory epithelium to the forebrain, delays this migration in mouse and rat fetuses (Sharova et al., Citation2013,Citation2015). The influence of maternal inflammatory stress is mediated by the regulation of IL-6, monocyte chemotactic protein (MCP)-1, and leukemia-inhibiting factor (LIF) secretion in the maternal-fetal system (Chattopadhyay et al., Citation2007; Dozio et al., Citation2009; Magni et al., Citation2007). LPS injection in pregnant females increases all proinflammatory cytokine levels in fetal cerebrospinal fluid and blood and disorganizes the initial steps of GnRH neuron migration (Sharova et al., Citation2015). Delay in GnRH neuron migration affects the establishment of axonal connections, with consequent disturbances in the crucial points of reproductive system development with consequent impaired fertility in adult animals. GnRH neurons start neurite extension immediately after reaching the forebrain (Low et al., Citation2012). In the adult brain, they have long distance projections and may be regulated by axonal outputs at the level of dendrite projections within and near the median eminence (Herde et al., Citation2013).
The question about the possibility of transplacental LPS transfer from the mother to the fetus remains open, since the available data are contradictory (Ashdown et al., Citation2006; Cai et al., Citation2000). The placenta can produce proinflammatory cytokines that act on fetal tissues and inhibit fetal neurite growth (Straley et al., Citation2014). LPS treatment of pregnant mice also increases corticosterone level in maternal blood and damages the placenta resulting in abnormalities of fetal development (Kirsten et al., Citation2013).
The aim of the present study was to test the hypothesis that the retarded GnRH neuron migration could adversely impact the HPG axis of postnatal offspring at the level of hypothalamic GnRH content, plasma LH and sex steroid hormone levels, and delay sexual maturation in females (time of vaginal opening [VO]), and reduce body weight gain.
Methods
Animals and experimental design
The study was carried out on female Wistar rats initially weighing 200–260 g (Stolbovaya Breeding Center, Moscow, Russia). Females were housed overnight with adult males from the same strain and supplier. The day sperm detected in a vaginal smear was considered to be E1. Timed pregnant rats were housed at 3–4 females per cage under specific pathogen-free standard conditions with a 12 h light/dark cycle (lights-on at 07:00 h) and supplied with ad libitum food and water. All the rat manipulation protocols were approved by the Animal Care Committee of the Institute of Developmental Biology of the Russian Academy of Sciences.
Twenty-eight pregnant rats were divided into two groups (14 rats per group). One group was injected intraperitoneally (i.p.) between 08:30 h and 10:00 h with LPS (E. coli, Sigma-Aldrich, Steinheim, Germany, 18 μg/kg body in 500 μl of pyrogen-free 0.9% saline) at E12 (LPS-treated group). LPS concentration was chosen according to previously published data (Sharova et al., Citation2011), and overall the lethality was less than 25–30% of fetuses. The second group was injected i.p. with the same volume of pyrogen-free 0.9% saline at the same time and day of pregnancy (E12, control group).
Six pregnant rats (three from each group) were killed by conscious decapitation without anesthetic at E21; fetuses were removed and divided into female and male groups according to the visual assessment of anogenital distance (26 females and 29 males), which were killed by decapitation for blood collection. All manipulations were carried out from 10:00 h to 12:00 h. Fetal blood was collected from hearts into the sample tubes with heparin (5000 U/ml); the samples were pooled from two to three fetuses of the same sex and litter and centrifuged (2000 × g, 20 min, 20 °C) and plasma was snap-frozen and stored (−80 °C) until LH and sex steroid determinations by ELISA. Fetal brain samples of anterior preoptic area (APOA) and mediobasal hypothalamus (MBH) were dissected immediately on ice pads at 4 °C under a stereomicroscope. The dissection of the forebrain was carried out according to Altman and Bayer’s atlas (Altman & Bayer, Citation1987). Brains were gently removed from the skulls and immediately placed individually in a matrix with the ventral surface facing up. Coronal sections were made, with the anterior border of the optic chiasm as the anterior boundary and the pituitary stalk as a posterior landmark. Then the ventro-medial rectangular parts of the blocks were rapidly microdissected under a microscope, using the anterior commissure as the dorsal boundary and lateral preoptic area as lateral boundary landmarks. Then the samples were processed as described previously (Caraty et al., Citation1994). Briefly, the samples were weighed and homogenized in 0.2-M acetic acid with an ultrasonic homogenizer; tissue weight/acid volume ratio was 1:20. Homogenates were placed in a water bath (5 min, 100 °C) and then centrifuged (3000 × g, 15 min, 20 °C). The supernatants were stored at −80 °C until the time of GnRH determination by RIA.
After birth, the litters from the remaining 22 pregnant rats (11 per group) were weighed on day 5 of postnatal development (P5), and on P14 and P30; day of birth was designated as P0. In total, 30 male and 50 female offsprings of mothers given i.p. saline prenatally, and 30 males and 51 females of mothers given i.p. LPS prenatally were used in this study. The rats (10 females and 10 males per group from at least three different litters) were killed by decapitation at P5, P14, and P30, and plasma/brain samples were obtained according to the above-described procedure. The remaining female rats (20 control and 21 prenatally LPS-treated females) were monitored daily for VO from P29. Ten males from each group, 20 control, and 21 prenatally LPS-treated females were killed by decapitation on P60, and plasma/brain samples were obtained as above.
GnRH determination
Hypothalamic GnRH concentrations were estimated in duplicate using RIA (Caraty et al., Citation1994). GnRH for iodination and standards were purchased from UCB-Bioproducts (Brussels, Belgium). All samples were measured in the same assay. Intra- and inter-assay coefficients of variations were lower than 10% and 12%, respectively. The minimum detectable concentration was 2 pg/ml. As shown previously in our laboratory, the total protein concentration in different organs varied significantly during prenatal and postnatal development, for this reason we normalized the GnRH content to tissue weight (mg) (Melnikova et al., Citation2010).
LH and sex steroid determinations
Plasma LH concentrations were determined in duplicate using a rat LH ELISA kit (CSB-E12654r, Cusabio, China), according to the manufacturer’s instructions. Intra- and inter-assay coefficients of variations were less than 15%. The minimum detectable concentration was 0.15 mlU/ml. LH concentration (mlU/ml) was calculated using standards provided with the kit and Sigma Stat software.
Plasma testosterone and estradiol concentrations were determined in duplicate using a Direct Elisa kit (Diagnostics Biochem Canada Inc., Ontario, Canada), according to the manufacturer’s instructions (Check, Citation1995). Intra- and inter-assay coefficients of variations were less than 17% for testosterone and 10% for estradiol; the minimum detectable concentrations were 0.17 and 10 pg/ml, respectively. Standard curves were plotted using standards provided with the kits. Optical density was measured at 450 nm using a universal microplate reader (STAT FAX® 2100 Awareness Technology, Inc., Palm City, FL).
Statistical analysis
Results are expressed as mean (M) ± SEM for each group. All data sets met the assumption of a normal distribution and homogeneity of variance. To analyze the effects due to prenatal LPS treatment on hormone (GnRH, LH, estradiol, and testosterone) concentrations at different time points, a two-way analysis of variance (ANOVA) was used with prenatal treatment (LPS/saline) as one variable and developmental age as the other. We used also one-way analysis of variance to analyze differences between control and LPS-treated groups. Tukey’s test was performed to seek any interaction, and significance level was set at p < 0.05. The mean of day of VO is presented using Kaplan–Meier curves; a log-rank test was used to compare the day of VO between groups. A p value of < 0.05 was considered statistically significant. All analyses were performed using Statistica version 10 (Statsoft Inc., Tulsa, OK).
Results
Effects of prenatal LPS exposure on hypothalamic GnRH content in offspring
Hypothalamic GnRH content increased continually from E21 and reached a maximum at P60 in control males and females. Two-way ANOVA showed a significant effect of developmental age (males: n = 10, df = 4, F2.47 = 73.6 p < 0.001; females: n = 10, df = 4, F2.47 = 78.7, p < 0.001), LPS treatment (males: n = 10, df = 1, F3.94 = 44.39, p < 0.001; females: n = 10, df = 1, F3.95 = 37.57, p < 0.001) on GnRH concentration in the brains and a LPS-treatment with age interaction (males: n = 10, df = 4, F2.47 = 21.56, p < 0.001; females: n = 10, df = 4, F2.47 = 17.96, p < 0.001). Therefore, we compared the differences between control and LPS prenatally treated groups by the analysis of differences at each time point of development by Tukey’s post hoc test. We observed significant decreases in GnRH content at P14 (F4.41 = 37.36, p < 0.001) and P60 (F4.41 = 63.34, p < 0.001) of female rats treated with LPS prenatally in comparison to saline controls (). Moreover, prenatal LPS treatment significantly decreased GnRH content in male rats at P30 (F4.41 = 15.69, p < 0.001) and P60 (F4.41 = 43.02, p < 0.001; ). In both sexes at P60, GnRH content was reduced after prenatal LPS treatment. However, there was no significant difference in GnRH content in both sexes at early developmental stages (E21 and P5; ).
Figure 1. Plasma concentrations of hypothalamic (a, b) gonadotropin-releasing hormone (GnRH) (pg/ml) and (c, d) luteinising hormone (LH) (mlU/ml) in male (a, c) and female (b, d) rat offspring at E22 and P5, 14, 30, 60 after saline (control) or lipopolysaccharide (LPS; 18 μg/kg, i.p. on E12) administration to the mother on day 12 of pregnancy; data are M ± SD. Statistical analysis: two-way analysis of variance (details in Results text), followed analysis of differences at each time point of development by Tukey’s post hoc test; *p < 0.05 between groups at same age (n = 10 per group).
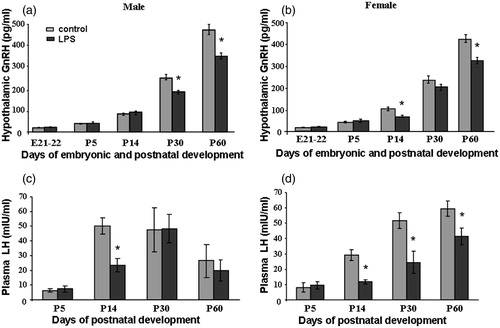
Effects of prenatal LPS exposure on plasma LH concentrations in male and female offsprings
Plasma LH concentration increased from P5 to P14, remained constant at P30, and then was decreased at P60 in control males (). LH concentration in blood plasma increased progressively from P5 to P60 in control females (). The effect of age on LH concentration was significant in both sexes in control groups (; males: n = 10, df = 1, F3.97 = 58.42, p < 0.001; ; females: n = 10, df = 3, F3.97 = 44.56, p < 0.001), and in LPS-treated males and females (males: n = 10, df = 3, F2.73 = 15.26, p < 0.001; females: n = 10, df = 3, F2.73 = 49.19, p < 0.001). There was a significant LPS treatment with age interaction (males: n = 10, df = 3, F2.73 = 7.23, p < 0.001; females: n = 10, df = 3, F2.73 = 8.7, p < 0.001). Plasma LH concentrations were significantly lower at P14 (F4.41 = 43.19, p < 0.001), P30 (F4.41 = 81.75, p < 0.001), P60 (F4.41 = 44.21, p < 0.001) in females, and at P14 (F4.41 = 53.38, p < 0.001) in males after prenatal exposure to LPS compared to controls, as observed by Tukey’s test ().
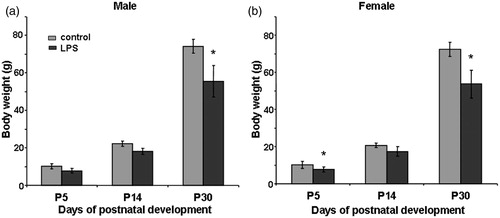
Effects of prenatal LPS exposure on body weights of offspring
Body weights did not differ between control males and females of the same age, but increased progressively from P5 to P60 (). The body weight range of adult males and females of control and treated groups was 250–300 g in males and 200–270 g in females (data not shown). The body weight was significantly lower in LPS-treated females at P5 (F4.11 = 36.5, p < 0.001) () and in LPS-treated males and females at P30 (males: F4.05 = 62.44, p < 0.001, females F4.07 = 36.17, p < 0.001; ) as detected by one-way ANOVA. Body weight did not differ significantly between control and LPS-treated females and males at P14.
Figure 2. Effect of prenatal exposure to lipopolysaccharide (LPS; 18 μg/kg, i.p. on E12) on body weight in male (a) and female (B) offspring at P5 (n = 30 per group), P14 (n = 20 per group), and P30 (n = 10 per group). Data are M ± SEM. Statistical analysis: one-way analysis of variance: *р < 0.01 between groups at same age.
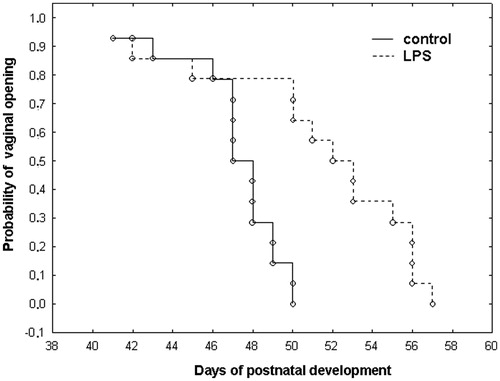
Effect of prenatal LPS exposure on day of vaginal opening in female offspring
Prenatal exposure to LPS resulted in delayed VO (LPS: 50.0 ± 5.6 days; saline 45.0 ± 3.9 d). The Kaplan–Meier plots show the significant differences appearing at P50–P57 when 100% of saline-treated rats showed VO, as compared to 47% in the LPS-treated group (); a log-rank test revealed a statistically significant difference between control and LPS-treated groups (p = 0.004).
Effects of prenatal LPS exposure on plasma testosterone and estradiol concentrations in male and female offspring
Plasma testosterone concentration in control males remained unchanged between P5 and P30, then increased 4-fold by P60. Two-way ANOVA showed a significant effect on plasma testosterone concentration of development age (males: n = 10, df = 3, F2.73 = 58.84 p < 0.001; females: n = 10, df = 3, F2.73 = 63.56, p < 0.001), LPS treatment (males: n = 10, df = 1, F3.97 = 45.51, p < 0.001; females: n = 10, df = 1, F3.97 = 63.56, p < 0.001), and a LPS treatment with age interaction (males: n = 10, df = 4, F2.73 = 18.59, p < 0.001; females: n = 10, df = 4, F2.47 = 30.97, p < 0.001). Plasma testosterone concentration was greater in control males as compared to females at P60.
Prenatal LPS exposure resulted in significant decreases in plasma testosterone concentrations in both sexes at P60 (males: F4.41 = 11.82, p < 0.001; females F4.41 = 86.17, p < 0.001; ). Prenatal LPS treatment increased concentrations of testosterone in prepubertal (P14, F4.41 = 30.31, p < 0.001) and peripubertal (P30, F4.41 = 39.17, p < 0.001) females (), whereas plasma testosterone concentration was unchanged in male rats at the same ages ().
Figure 4. Concentrations of (a, b) free testosterone (pg/ml) and (c, d) estradiol (pg/ml) in male (a, c) and female (b, d) rat offspring at P5, P14, P30, and P60 after saline (control) or lipopolysaccharide (LPS) (18 μg/kg, i.p. at E12) administration to the mother on day 12 of pregnancy, data are M ± SD, (n = 10 per group). Statistical analysis: two-way ANOVA (details in “Results” text), followed by one-way analysis of differences at each time point of development by Tukey’s post hoc test. *p < 0.01 between groups at same age.
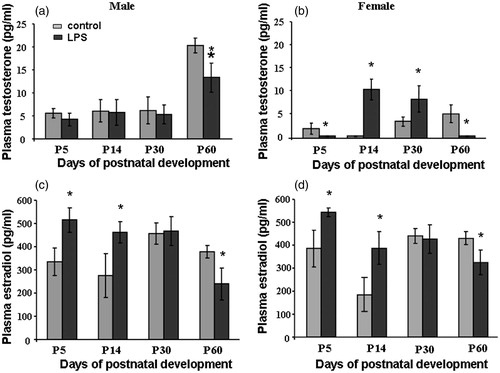
Plasma estradiol concentration was not significantly changed in control groups during postnatal development (). Prenatal LPS exposure significantly decreased plasma testosterone concentrations in both sexes at P60 (males: F4.41 = 51.74, p < 0.001; females F4.41 = 18.19, p < 0.001)) (). Prenatal LPS treatment significantly increased plasma estradiol concentrations in both females and males at P5 (males: F4.41 = 29.15, p < 0.001; females F4.41 = 26.14, p < 0.001) and P14 (males: F4.41 = 30.17, p < 0.001; females F4.41 = 39.64, p < 0.001; ).
Discussion
Several experimental approaches were used in this study to determine whether maternal exposure to a single low dose (Sharova et al., Citation2015) of LPS at an early stage of pregnancy has a severe impact on the reproductive system of rat offspring. We found decreased GnRH content in the hypothalamus of pre- and postpubertal males and females after prenatal immune challenge with LPS. The decrease in GnRH level was associated with decreased plasma LH concentration in female rats from P14 to P60. Plasma LH concentration in males was significantly reduced by prenatal LPS at P14, but later this effect of prenatal LPS treatment was absent. Sex steroid hormone concentrations in plasma were decreased in adult females and males by prenatal LPS exposure. Increased plasma testosterone concentration was detected after prenatal LPS in prepubertal females (P14–P30). By contrast, we observed increased plasma estradiol concentration in early postnatal life in males and females exposed to prenatal LPS. Female offspring showed delayed VO after prenatal LPS as compared to the control group. Prenatal LPS treatment decreased gain of body weight of females at P5 and females and males at P30.
Maternal LPS stress and hypothalamic GnRH content in postnatal offspring
First, we focused our study on GnRH content in the hypothalamus of prenatal and postnatal offsprings of LPS-treated mothers. We did not find a decrease of GnRH content in the hypothalamus of prenatal fetuses at E21–22, but we demonstrated a long-lasting negative effect on the GnRH system in the peripubertal period.
Previous studies provide evidence that exposure to LPS in early postnatal development affects the program of reproductive health and sexual behavior in adult offspring. In the critical period of GnRH system development in rats (P3–P7), LPS treatment inhibits Kiss-1 gene expression in the hypothalamic medial preoptic area, which leads to a significant delay in puberty (Knox et al., Citation2009).
According to our recent results, LPS administration to pregnant mice at the initial stage of intensive neuronal migration leads to increased number of GnRH neurons in the nasal and olfactory bulb compartments in E14.5 fetuses, but GnRH neuron distribution was restored by E18.5 (Sharova et al., Citation2015). LPS predominantly acts in the maternal compartment and together with auxiliary receptor molecules CD14 and MD2 initiates the mechanism of a “hazardous signal” transferred through Toll-like receptors (TLR4; Nagai et al., Citation2002). LPS treatment increases the maternal serum proinflammatory cytokine (IL-6, MCP-1, LIF, and TNF-α) levels. The levels of IL-6, MCP-1, LIF, and TNF-α are elevated in the fetal blood serum and in amniotic fluid (Sharova et al., Citation2015). Cytokines cross the placenta and are involved in the processes of fetal brain injury (Ashdown et al., Citation2006; Cai et al., Citation2000; Mouihate & Mehdawi, Citation2015). A recent substantial study provided evidence that LPS does not cross the placenta barrier as radiolabelled LPS injected to pregnant dams was detected in only low concentration in fetal tissue (Ashdown et al., Citation2006). TLR4 is expressed in fetal mouse brain from E15, but TLR4 is not functional in fetal neurons but is evidently functionally active on fetal astrocytes and microglia (Breen et al., Citation2012). TLR4 signaling in glial cells plays a significant role after birth in rats and mediates the production of proinflammatory cytokines, which in turn, contributes to damage in the developing brain (Yao et al., Citation2013). After birth, a suppressive action of LPS on GnRH and LH secretion becomes evident around P25 in rats (Munkhzaya et al., Citation2015).
In the present work, the total GnRH content in the hypothalamus of perinatal rats (E21–22 and P5) was unchanged as compared to the control groups. Although GnRH mRNA level was not studied in this work, we can suppose that GnRH content in the hypothalamus could be maintained by more intensive GnRH synthesis or decreased GnRH secretion.
GnRH neurons may have a limited time window to migrate in mice, as they are committed to stop at particular positions along the migratory route; early-generated neurons preferentially populate rostral structures in the hypothalamus, whereas later-generated GnRH neurons come to reside more caudally (Jasoni et al., Citation2009). Location of GnRH neurons in the rostro-caudal continuum is correlated with the development of neuronal inputs (mainly using catecholamines and GABA; Zakharova & Izvolskaya, Citation2012). Maternal LPS injections could decrease dopamine and serotonin levels (Wang et al., Citation2009) and alter glutamate decarboxylase synthesis (Basta-Kaim et al., Citation2015) in the brains of postnatal offspring and in this indirect manner decrease GnRH content in the forebrain of postnatal offspring.
The development of the neuronal network controlling GnRH neuron activity takes place after birth in rodents and continues to P21 (Ben-Ari, Citation2001; Ben-Ari et al., Citation1997). In this study, we demonstrated that LPS-treated female offspring had decreased GnRH level in the early prepubertal period at P14 whereas, males by contrast, showed a decrease in GnRH level just before puberty at P30. This decrease could be due to the altered distribution of GnRH neurons within the hypothalamus and malformations of neuronal inputs to GnRH neurons. As a result, GnRH content in the hypothalamus was decreased by prenatal LPS in adults of both sexes.
LPS maternal stress and LH secretion in postnatal offspring
The postnatal decrease in plasma LH concentration in females observed after prenatal LPS exposure in the present work is clearly associated with the decrease in GnRH content in the hypothalamus in females, whereas in males the decrease in LH level was detected just at P14. Gonadotrope cells are developed and able to release LH in response to GnRH stimulation from E17 in the rat pituitary (Nemeskeri et al., Citation1984), and GnRH receptors are expressed in the pituitary beginning from this stage of development (Alarid et al., Citation1996). Pulsatile GnRH release from the median eminence begins in the perinatal period in rats (Yamanaka et al., Citation1999). Further activation of the HPG system occurs in the pubertal period. LH level rapidly increases from P7 to P11 in male rats and normal feedback control of LH secretion in males requires aromatization of testosterone and activation of estrogen receptors in the gonadotropes (Lindzey et al., Citation1998). The second week of postnatal life is a critical point of male gonadal development (Dygalo et al., Citation2014).
Unchanged serum LH content in pre- and postpubertal males after prenatal LPS treatment has been published previously by Wang et al. (Citation2014), who showed that prolonged LPS treatment from E13 to E18 resulted in unchanged LH concentration. However, in another model of prenatal stress (maternal restraint stress) a decrease in serum LH concentration was shown at P28, whereas this was unchanged at P75 (Pallarés et al., Citation2013).
Pituitary development also is regulated by LIF, (Yano et al., Citation1998) which has been shown to inhibit gonadotrope, thyrotrope, lactotrope, and somatotrope lineages. Transgenic mice overexpressing LIF exhibited hypogonadism and low follicle-stimulating hormone (FSH) level (Yano et al., Citation1998). According to our previous study, LIF concentration is strongly elevated in fetal tissue after LPS treatment of pregnant mothers (Sharova et al., Citation2015), and in this environmental condition, the development of gonadotropes as well as further LH synthesis could be suppressed in offspring of LPS-treated rats.
LPS maternal stress and sex hormones in postnatal offspring
Testosterone level was low in prepubertal males and females and increased at P60 in males as sexual differentiation is completed. Estradiol level in control female and male groups remained unchanged throughout the time-course of postnatal development. The relatively high-estradiol concentration in blood plasma of males could be provided by the adrenal glands (Weisz & Gunsalus, Citation1973) that produce dehydroepiandrosterone (DHEA), a metabolic precursor of androgens and estrogens (Luu-The & Labrie, Citation2010). Estradiol is synthesized locally in different tissues from androgens by aromatase (Carreau et al., Citation2012; Gonzales et al., Citation2007), and average plasma level is relatively high in both sexes.
In our study, we observed decreased testosterone level in prepubertal females (P5), but this was elevated after this until puberty (P14, P30), and we found elevated estradiol levels in prepubertal (P5, P14) males and females after LPS treatment. In general, early maternal immune stress had an unbalancing effect on the sex steroid milieu in the prepubertal period in males and females. As shown previously, prenatal restraint or under-nutrition stress leads to increased testosterone level in serum or testis of prepubertal male offspring, and testosterone level was decreased in adult animals (Pallarés et al., Citation2013; Teixeira et al., Citation2007). Prepubertal sex steroid synthesis by the gonads regulates the development of gametogenesis, sexual behavior, and secondary sexual characteristics, and seems to be regulated by an independent adaptive mechanism after prenatal maternal stress. For example, the expression of CYP19 (aromatase, a key enzyme for estrogen biosynthesis) is not only regulated normally by steroidogenic factor (SF)-1 expression in the developing testis from E11.5 in mice but also stimulated by cytokines (IL-11, oncostatin-M, IL-6, and LIF) in human adipose tissue (Simpson et al., Citation1997). So during embryonic development with the excess of cytokines in fetal tissue after maternal LPS treatment the ontogeny of CYP19 expression in gonads may be dysregulated. This can lead to aromatase over-expression and increased estrogen production in prepubertal animals. Puberty initiates regular HPG-system control of testicular and ovarian steroid production as shown by our present work or other studies (Pallarés et al., Citation2013; Teixeira et al., Citation2007; Wang et al., Citation2014), and circulating estradiol and testosterone levels are decreased in adult offspring after prenatal immune stress.
The influence of maternal LPS stress on the body weight and vaginal opening in postnatal offspring
The second step of our investigation was directed to the evaluation of prenatal maternal LPS treatment on reproductive phenotype of postnatal offspring. We demonstrated a slight reduction in total body weight at P5 and P14 in males and females after a single maternal exposure to LPS and a significantly lower increase in body weight than in controls was observed in both sexes at P30. However, adult prenatally LPS-treated offspring did not differ in body weight from the control group. As shown previously, LPS administration to pregnant mothers on the 21st day of pregnancy results in reduced testis weight and disturbances in sexual behavior in adult male offspring (Knox et al., Citation2009). Reduced increase in body weight of juvenile and prepubertal males after prolonged prenatal LPS treatment was described previously (Wang et al., Citation2014).
According to our data, maternal LPS-induced inflammation resulted in delayed VO. Previously, it was shown that neonatal LPS treatment resulted in delayed VO in Sprague–Dawley rats (Knox et al., Citation2009; Wu et al., Citation2011), but in Wistar rats, VO was advanced (Sominsky et al., Citation2012). Sominsky et al. (Citation2012) observed VO at P34–38, but in our laboratory using Wistar strain rats the peak of VO was closer to the day published for the Sprague–Dawley rats. In this way, our results for delayed VO are in agreement with Knox et al (Citation2009) and Wu et al. (Citation2011).
An increase in serum estradiol level in prepubertal females delays puberty and leads to anovulatory syndrome in postpubertal rats (Pinilla et al., Citation1993). Here we demonstrated that in rats exposed prenatally to LPS, the sex steroid level was increased prepubertally, and this was associated with delayed puberty and sexual maturation.
Conclusions
This study demonstrated that prenatal exposure to LPS leads to reduced hypothalamic content of GnRH, and reduced circulating LH in adult females, and reduced sex steroid hormones in blood plasma in adult male and female offsprings. Delay in GnRH neurons arriving in the forebrain after a single LPS injection in pregnant mothers on day 12 of pregnancy described previously, evidently leads to disorganization of control of the GnRH system and the HPG axis in postnatal life. The decreased hypothalamic GnRH evidently reduces LH production by the pituitary of postnatal offspring of LPS-treated females; VO was also delayed in these female offspring, and sex steroid levels were reduced in these females as adults. In adult male offspring of LPS-treated mothers, sex steroid levels were also reduced, but LH levels were not reduced. The future direction of our studies is to investigate GnRH synthesis and synaptic input formation during the final steps of GnRH neurone migration in fetuses after LPS treatment of pregnant mothers, and actions of proinflammatory cytokines on these processes. Further studies of postnatal mechanisms based on sex steroid perturbations will be necessary for an understanding of the long-lasting effect of prenatal immune challenge on the HPG axis.
Acknowledgements
The authors are thankful to Didier Lomet (Physiologie de la Reproduction et des Comportements, UMR 7247 INRA CNRS Universite de Tours PRC INRA, Nouzilly, France) for help in carrying out the GnRH assay.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
This work was supported by the Russian Foundation for Basic Research 13-04-00191.
References
- Alarid ET, Windle JJ, Whyte DB, Mellon PL. (1996). Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122(10):3319–29
- Altman J, Bayer SA. (1987). Atlas of prenatal rat brain development. Boca Raton, FL: CRC Press
- Amath A, Foster JA, Sidor MM. (2012). Developmental alterations in CNS stress-related gene expression following postnatal immune activation. Neuroscience 220:90–9
- Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. (2006). The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry 11:47–55
- Basta-Kaim A, Fijał K, Ślusarczyk J, Trojan E, Głombik K, Budziszewska B, Leśkiewicz M, et al (2015). Prenatal administration of lipopolysaccharide induces sex-dependent changes in glutamic acid decarboxylase and parvalbumin in the adult rat brain. Neuroscience 287:78–92
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. (1997). GABAA, NMDA and AMPA receptors: a developmentally regulated ‘ménage à trois’. Trends Neurosci 20:523–9
- Ben-Ari Y. (2001). Developing networks play a similar melody. Trends Neurosci 24:353–60
- Breen K, Brown A, Burd I, Chai J, Friedman A, Elovitz MA. (2012). TLR-4-dependent and -independent mechanisms of fetal brain injury in the setting of preterm birth. Reprod Sci 19:839–50
- Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. (2000). Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res 47:64–72
- Caraty A, Locatelli A, Moenter SM, Karsch FJ. (1994). Sampling of hypophyseal portal blood of conscious sheep for direct monitoring of hypothalamic neurosecretory substances. In: Levine JE, editor. Methods in neuroscience. San Diego, CA: Academic Press. p. 163al–bl
- Carreau S, Bouraima-Lelong H, Delalande C. (2012). Estrogen, a female hormone involved in spermatogenesis. Adv Med Sci 57(1):31–6
- Chattopadhyay N, Jeong KH, Yano S, Huang S, Pang JL, Ren X, Terwilliger E, et al (2007). Calcium receptor stimulates chemotaxis and secretion of MCP-1 in GnRH neurons in vitro: potential impact on reduced GnRH neuron population in CaR-null mice. Am J Physiol Endocrinol Metab 292(2):E523–32
- Check JN. (1995). Falsely elevated steroidal assay levels repeated to heterophile antibodies against various animal species. Gynecol Obstet Invest 40:139–40
- Dozio E, Ruscica M, Galliera E, Corsi MM, Magni P. (2009). Leptin, ciliary neurotrophic factor, leukemia inhibitory factor and interleukin-6: class-I cytokines involved in the neuroendocrine regulation of the reproductive function. Curr Protein Pept Sci 10:577–84
- Dygalo NN, Shemenkova TV2, Kalinina TS, Shishkina GT. (2014). A critical point of male gonad development: neuroendocrine correlates of accelerated testicular growth in rats during early life. PLoS One 9(4):e93007
- Gonzales RJ, Ansar S, Duckles SP, Krause DN. (2007). Androgenic/estrogenic balance in the male rat cerebral circulation: metabolic enzymes and sex steroid receptors. J Cereb Blood Flow Metab Off J Int Soc 27:1841–52
- Herde MK, Iremonger KJ, Constantin S, Herbison AE. (2013). GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J Neurosci 33(31):12689–97
- Herman AP, Tomaszewska-Zaremba D. (2010). Effect of endotoxin on the expression of GnRH and GnRHR genes in the hypothalamus and anterior pituitary gland of anestrous ewes. Anim Reprod Sci 120:105–11
- Iwasa T, Matsuzaki T, Kinouchi R, Fujisawa S, Murakami M, Kiyokawa M. (2009). Neonatal LPS injection alters the body weight regulation systems of rats under non-stress and immune stress conditions. Int J Dev Neurosci 28:119–24
- Jasoni CL, Porteous RW, Herbison AE. (2009). Anatomical location of mature GnRH neurons corresponds with their birthday in the developing mouse. Dev Dyn 238:524
- Kalra PS, Edwards TG, Xu B, Jain M, Kalra SP. (1998). The anti-gonadotropic effects of cytokines: the role of neuropeptides. Domest Anim Endocrinol 15:321–32
- Kimura M, Yu WH, Rettori V, McCann SM. (1997). Granulocyte-macrophage colony stimulating factor suppresses LHRH release by inhibition of nitric oxide synthase and stimulation of gamma-aminobutyric acid release. Neuroimmunomodulation 4:237–43
- Kirsten TB, Lippi LL, Bevilacqua E, Bernardi MM. (2013). LPS exposure increases maternal corticosterone levels, causes placental injury and increases IL-1Β levels in adult rat offspring: relevance to autism. PLoS One 8(12):e82244
- Knox AM, Li XF, Kinsey-Jones JS, Wilkinson ES, Wu XQ, Cheng YS, Milligan SR, et al (2009). Neonatal lipopolysaccharide exposure delays puberty and alters hypothalamic Kiss1 and Kiss1r mRNA expression in the female rat. J Neuroendocrinol 21:683–9
- Li X, Shao B, Lin C, O’Byrne KT, Lin Y. (2015). Stress-induced inhibition of LH pulses in female rats: role of GABA in arcuate nucleus. J Mol Endocrinol 55(1):9–19
- Lindzey J, Wetsel WC, Couse JF, Stoker T, Cooper R, Korach KS. (1998). Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotropins in male wild-type and estrogen receptor-alpha knockout mice. Endocrinology 139(10):4092–101
- Low VF, Fiorini Z, Fisher L, Jasoni CL. (2012). Netrin-1 stimulates developing GnRH neurons to extend neurites to the median eminence in a calcium-dependent manner. PLoS One 7(10):e46999
- Luu-The V, Labrie F. (2010). The intracrine sex steroid biosynthesis pathways. Prog Brain Res 181:177–92
- Magni P, Dozio E, Ruscica M, Watanobe H, Cariboni A, Zaninetti R, Motta M, Maggi R. (2007). Leukemia inhibitory factor induces the chemomigration of immortalized gonadotropin-releasing hormone neurons through the independent activation of the Janus kinase/signal transducer and activator of transcription 3 mitogen-activated protein kinase/extracellularly regulated kinase 1/2, and phosphatidylinositol 3-kinase/Akt signaling pathways. Mol Endocrinol 21(5):1163–74
- McCann SM, Kimura M, Karanth S, Yu WH, Mastronardi CA. (2000). The mechanism of action of cytokines to control the release of hypothalamic and pituitary hormones in infection. Ann N Y Acad Sci 917:4–18
- Melnikova VI, Sharova NP, Maslova EV, Voronova SN, Zakharova LA. (2010). Ontogenesis of rat immune system: proteasome expression in different cell populations of the developing thymus. Cell Immunol 266:83–9
- Mouihate A, Mehdawi H. (2015). Toll-like receptor 4-mediated immune stress in pregnant rats activates STAT3 in the fetal brain: role of interleukin-6. Pediatr Res. [Epub ahead of print]. doi:10.1038/pr.2015.86
- Munkhzaya M, Matsuzaki T, Iwasa T, Tungalagsuvd A, Kawami T, Kato T, Kuwahara A, Irahara M. (2015). The suppressive effect of immune stress on LH secretion is absent in the early neonatal period in rats. Int J Dev Neurosci 46:38–43
- Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, et al (2002). Essential role of MD2 in LPS responsiveness and TLR4 distribution. Nat Immunol 3:667–72
- Nemeskeri A, Kurcz M, Halasz B. (1984). Changes in hypophyseal luteinizing hormone (LH) content during fetal and early postnatal life, and capacity of fetal and early postnatal pituitaries to synthesize and release LH in vitro. Neuroendocrinology 38(5):393–6
- Pallarés ME, Adrover E, Baier CJ, Bourguignon NS, Monteleone MC, Brocco MA, González-Calvar SI, Antonelli MC. (2013). Prenatal maternal restraint stress exposure alters the reproductive hormone profile and testis development of the rat male offspring. Stress 16(4):429–40
- Pinilla L, Trimiño E, Garnelo P, Bellido C, Aguilar R, Gaytán F, Aguilar E. (1993). Changes in pituitary secretion during the early postnatal period and anovulatory syndrome induced by neonatal oestrogen or androgen in rats. J Reprod Fertil 97:13–20
- Sharova VS, Izvolskaia MS, Voronova SN, Zakharova LA. (2011). Effect of bacterial endotoxin on migration of gonadotropin-releasing hormone producing neurons in rat embryogenesis. J Dev Biol 42:439–46
- Sharova VS, Izvolskaya MS, Zakharova LA. (2013). Effect of prenatal infection of mice with bacterial endotoxin on the migration of neurons producing gonadotropin-releasing hormone. Dokl Biol Sci 452:273–6
- Sharova VS, Izvolskaia MS, Zakharova LA. (2015). Lipopolysaccharide-induced maternal inflammation affects the gonadotropin-releasing hormone neuron development in fetal mice. Neuroimmunomodulation 22(4):222–32
- Simpson ER, Michael MD, Agarwal VR, Hinshelwood MM, Bulun SE, Zhao Y. (1997). Cytochromes P450 11: expression of the CYP19 (aromatase) gene: an unusual case of alternative promoter usage. FASEB J 11(1):29–36
- Sominsky L, Meehan CL, Walker AK, Bobrovskaya L, McLaughlin EA, Hodgson DM. (2012). Immune regulation of ovarian development:programming by neonatal immune challenge. Horm Behav Behav 62:345–55
- Spencer SJ, Boisse L, Mouihate A, Pittman QJ. (2006). Long term alterations in neuroimmune responses of female rats after neonatal exposure to lipopolysaccharide. Brain Behav Immun 20:325–30
- Straley ME, Togher KL, Nolan AM, Kenny LC, O’Keeffe GW. (2014). LPS alters placental inflammatory and endocrine mediators and inhibits fetal neurite growth in affected offspring during late gestation. Placenta 35:533–8
- Teixeira CV, Silandre D, de Souza Santos AM, Delalande C, Sampaio FJ, Carreau S, da Fonte Ramos C. (2007). Effects of maternal undernutrition during lactation on aromatase, estrogen, and androgen receptors expression in rat testis at weaning. J Endocrinol 192(2):301–11
- Walker FR, Hodyl NA, Hodgson DM. (2009). Neonatal bacterial endotoxin challenge interacts with stress in the adult male rat to modify KLH specific antibody production but not KLH stimulated ex vivo cytokine release. J. Neuroimmunol. 207:57–65
- Wang S, Yan JY, Lo YK, Carvey PM, Ling Z. (2009). Dopaminergic and serotoninergic deficiencies in young adult rats prenatally exposed to the bacterial lipopolysaccharide. Brain Res 1265(10):196–204
- Wang H, Yang LL, Hu YF, Wang BW, Huang YY, Zhang C, Chen YH, Xu DX. (2014). Maternal LPS exposure during pregnancy impairs testicular development, steroidogenesis and spermatogenesis in male offspring. PLoS One 9(9):e106786
- Weisz J, Gunsalus P. (1973). Estrogen levels in immature female rats: true or spurious – ovarian or adrenal? Endocrinology 93:1057–65
- Wu XQ, Li XF, Ye B, Popat N, Milligan SR, Lightman SL, O’Byrne KT. (2011). Neonatal programming by immunological challenge: effects on ovarian function in the adult rat. Reproduction 141(2):241–8
- Yamanaka C, Lebrethon MC, Vandersmissen E, Gerard A, Purnelle G, Lemaitre M, Wilk S, Bourguignon JP. (1999). Early prepubertal ontogeny of pulsatile gonadotropin-releasing hormone (GnRH) secretion: I. Inhibitory autofeedback control through prolyl endopeptidase degradation of GnRH. Endocrinology 140(10):4609–15
- Yano H, Readhead C, Nakashima M, Ren SG, Melmed S. (1998). Pituitary-directed leukemia inhibitory factor transgene causes Cushing’s syndrome: neuro-immune-endocrine modulation of pituitary development. Mol Endocrinol 12(11):1708–20
- Yao L, Kan EM, Lu J, Hao A, Dheen ST, Kaur C, Ling EA. (2013). Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J Neuroinflammation 10:23
- Zakharova LA, Izvolskaia MS. (2012). Interactions between the reproductive and immune systems during ontogenesis: the role of GnRH, sex steroids and immunomediators. In: Kahn SM, editor. “Sex steroids”. Zagreb: InTech. p. 341–70