Abstract
Helicobacter pylori infection is a risk factor for development of peptic ulcers, and psychological stress (PS) may have a role in the pathogenesis of this condition. However, no interaction between PS and H. pylori infection (HI) has been established in the development of peptic ulcer, because colonization by H. pylori is the first step in the infection of the gastric mucosa, we examined H. pylori colonization of the stomach in BALB/c mice after PS. The mice were subjected to PS in a communication box test, in which they observed other mice experiencing a physical stressor (electrical) before they were inoculated with H. pylori. We found that the H. pylori colonization in the stomach of psychologically stressed mice was significantly greater than in the control mice (P < 0.05), and histological examination showed that the gastric mucosal injury in the stressed mice was more extensive than in the control mice (P < 0.05). To explore the underlying mechanisms, we administered RU486 (a type II glucocorticoid (GC) receptor antagonist) to antagonize the effect of endogenous corticosterone: this treatment decreased colonization by H. pylori in the psychologically stressed mice. We conclude that HI of the stomach of BALB/c mice is enhanced by PS, and the effect may be mediated by GCs.
Introduction
After Helicobacter pylori was identified in human gastric mucosa by Warren and Marshall, this bacterium was found to be associated with gastritis, peptic ulcers, gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma (Ruiz-Bustos et al. Citation2001; Vinette et al. Citation2004). In particular, H. pylori infection (HI) is a major causal factor in peptic ulcer, and recurrence is virtually prevented by eradicating this bacterium (Kim et al. Citation2002). Although there is a high probability of HI in peptic ulcer cases, such infection is not sufficient alone for peptic ulcer to develop: many infected people do not develop ulcers, and some gastric ulcer patients are H. pylori-negative (Aoyama et al. Citation2000; Velin and Michetti Citation2006).
These findings suggest that other factors such as use of non-steroidal anti-inflammatory drugs, stress, diet, or smoking may contribute to the pathogenesis of peptic ulcer (Kurata and Nogawa Citation1997). Among these factors, psychological stress (PS) has been shown to trigger many ulcers and impair the response to treatment (Oh et al. Citation2005); it has been estimated that psychological factors contribute to 30–65% of peptic ulcers (Levenstein Citation2000). A dramatic increase in the incidence of gastric ulcers, especially bleeding ulcers, after the Hanshin–Awaji earthquake in Japan was a strong indicator of the role of PS in these conditions (Aoyama et al. Citation1998; Matsushima et al. Citation1999).
Some studies have shown a synergistic relationship between HI and PS in the formation of ulcers. Animal studies and clinical data have shown that HI affects the development of stress-induced gastric mucosal injury (Aoyama et al. Citation1998; Yamamoto et al. Citation2000). However, a question remains about whether PS can influence HI of the gastric mucosa. In addition there is a question about the mechanism that underlies the synergistic relationship between HI and PS. These questions have not been investigated to date.
It is well known that HI of the gastric mucosa follows a complicated course that depends on various factors, such as the microenvironment of the stomach, the host immune system, and the condition of the microorganism (Shimoyama and Crabtree Citation1998). Many experimental studies have indicated that PS alters the inflammatory and immune response in the gastric mucosa (Jarillo-Luna et al. Citation2008). The mucosal immune system is regulated by the central nervous system through the hypothalamic-pituitary–adrenal (HPA) axis and glucocorticoid (GC) production (Sternberg Citation2006), and PS can affect the HPA axis, increasing GC secretion. We therefore hypothesized that PS may affect the mucosal immune system via this neuroendocrine system, and then influence HI of the gastric mucosa. RU486 is a commonly used antagonist of both the type II GC receptor (GR) and the progesterone receptor, which blocks the effects of GC mediated by GR (Kellie et al. Citation2007). So, we used RU486 to test whether PS affect the H. pylori colonization by increasing GC production.
We examined colonization of the stomachs of BALB/c mice by H. pylori to evaluate the level of infection. Using real-time polymerase chain reaction(PCR) we found that psychologically stressed mice were more heavily colonized by H. pylori than control mice after inoculation with the same amount of the bacterium. Furthermore, we administered the RU486 to mice before exposure to PS to block GC action mediated by GR. This treatment decreased the colonization by H. pylori significantly in comparison to the control psychologically stressed mice.
Materials and methods
Animals
Seventy-two 6-week-old specific-pathogen-free female BALB/c mice were obtained from the Center of Experimental Animals (Third Military Medical University, ChongQing, China). The mice were housed under the following conditions: five per cage; room temperature 23°C; humidity 50%; 12 h alternating light and dark periods (lights on 07:00–19:00 h); and quiet surroundings with minimal disturbance. All mice were fed an autoclaved pellet diet and given sterile water ad libitum. They were allocated to six groups: control (C, n = 12), PS (n = 12), RU486 + PS (RP, n = 12), HI (n = 12), PS + HI (PH, n = 12), RP + HI (RPH, n = 12). After a 1 week equilibration period, these mice were used in the experiments. All animal experiments were approved by the Animal Ethical and Experimental Committee of Third Military Medical University.
PS procedure
Mice in the stress groups (PS, RP, PH, and RPH groups) were stressed using a communication box apparatus. The communication box paradigm originally described by Ogawa and Kuwabara was used as previously reported (Endo et al. Citation2001). Briefly, the box consists of two compartments, A and B (10 × 10 cm each). A total of nine compartments are arranged like a checkerboard and are separated by transparent plastic walls. In the present study, parallel stainless steel electric grids, 3 mm in diameter and located every 10 mm, were set into the floors of all compartments. However, the floors of the B compartments were covered with electrically insulating plastic panels. Mice were individually placed in each compartment and intermittent electrical footshocks shocks (2 mA, 10 s duration, 50 s intershock interval) were delivered through the grid floor by a shock generator (TGDC2-0.5; Tengen Co., Ltd, Shanghai, China). The mice in the A compartments (senders) received the footshocks, while the mice in the B compartments (responders) were exposed to the emotional stress of seeing and hearing the escape behaviour, and/or vocalizations of the sender mice in the adjacent compartments. The responder mice were exposed to emotional stress once a day for half an hour between 9:00 and 12:00; this treatment was repeated for 14 days. Since the sender mice were exposed to the electrical stimuli for only 1 day to avoid tolerance, a different cohort was used each day. The unstressed control mice were placed individually in the compartments of the control isolation box (10 × 10 cm), with no electric grid in the floor and without exposure to the senders, for the same period as the stressed mice. A preliminary study showed that the mice were not injured by the footshock procedure.
Drugs
RU486, a type II GR antagonist (Sigma-Aldrich, Shanghai, China), was dissolved in polyethylene glycol 400 (1 mg/ml; Sigma-Aldrich). Mice in the RP and RPH groups received one subcutaneous injection of this solution (10 mg/kg), while the control mice were given polyethylene glycol, in each case 1 h before each PS episode (Flint and Tinkle Citation2001).
Inoculation with H. pylori
The H. pylori strain B6 (cagA+/vacA+) that was used for inoculation was derived from a Chinese clinical isolate and adapted to colonize the gastric mucosa of BALB/c mice by repeated in vivo passages comprising six isolation–reinoculation cycles. The organism was grown for 72 h at 37°C under microaerobic conditions on blood agar plates made by our laboratory containing antibiotic supplement (5 mg/ml vancomycin, 1250 IU/ml polymyxin B, 2.5 mg/ml trimethoprim, 7.5 mg/ml cephalothin and 1 mg/ml amphotericin B). All the bacteria from the plate were harvested and inoculated into 1 ml of brain heart infusion broth supplemented with 5% fetal bovine serum. A fraction (150 ml) of the broth solution was inoculated again on to a plate as above and incubated under microaerobic conditions for 72 h at 37°C. After harvesting, bacterial suspensions were prepared at a concentration of 1.0 × 109 colony-forming units/ml, using cysteine medium, and used to inoculate the mice. One week after the initiation of stress, the HI, PH, and RPH groups mice were inoculated with 0.2 ml of H. pylori suspension orally by gavage, with a flexible catheter, twice in 1 day. The remaining mice were not inoculated with the bacterium. The mice were housed for 5 weeks under the above-described conditions. Five weeks after inoculation, all the mice were killed under ether anaesthesia to evaluate HI status and histological changes in the stomach.
Behavioral assessments
To assess effectiveness of the PS model, locomotor activity was measured in an open field. The open field box has been described in detail elsewhere (Palermo Neto et al. Citation2001; Romeo et al. Citation2003): a 50 × 50 cm lighted area, surrounded by 50 cm high walls of dark plastic. The floor of the box was divided into twenty-five 10 × 10 cm squares. Tests were conducted under dim light (about 80 l × ). Each mouse was placed in the center of the box and allowed to roam the field for 3 min. Behavioral parameters were recorded: the number of incidences of standing (rearing score) and the distances moved the number of squares stepped over (horizontal activity). The mice of the C and PS groups were used for the tests. The control mice were tested before the stress groups. The stress groups were tested on the 7th and 14th day of PS, immediately after exposure to the stressor.
Serum corticosterone assay
After the open field tests, C, PS and RP mice were used to measure serum corticosterone concentration. A blood sample (20 μl) was collected from the mice by incising the vena caudalis near the tip of the tail with a scalpel. The blood was centrifuged at 5000g at 4°C for 10 min and the serum was diluted with a buffer supplied by the enzyme linked immunosorbent assay (ELISA) kit manufacturer (1:100). Serum corticosterone content was measured by ELISA using a commercially available kit (goat anti-mouse corticosterone ELISA kit, Adlitteram Diagnostic Laboratories Inc., San Diego, CA, USA). ELISA was performed using a 96-well plate, with the wells coated with antibodies. Standards and samples were pipetted into appropriate wells, and a solution of corticosterone labeled with horseradish peroxidase was added to each well. After incubation for 30 min at 37°C, the reaction was terminated by washing the plate with wash solution. Peroxidase activity was determined colorimetrically by adding tetramethylbenzidine solution and incubating for 30 min at room temperature. Stopping solution was added and the optical density of each well was measured at 450 nm using a plate reader (Model 680; Bio-Rad Co. Ltd, Tokyo, Japan). A standard curve was produced using six standard values from which the absorbances of the blanks were subtracted. Results for the samples were read from this standard curve by a computer program. The assay kit sensitivity was 1 ng/ml. The intra- and inter-assay precisions varied between 2.4 and 6.3%, depending on the concentration of sample.
Detection of H. pylori
Infection with H. pylori was assessed by bacterial culture, urease test, H. pylori staining in stomach tissue sections and PCR. Mice that were positive for microbial culture or two of the other three tests were defined as positive for H. pylori. The stomachs were removed from the mice and incised longitudinally from cardia to pylorus. The greater curvature of the stomach was sectioned for haematoxylin–eosin (H–E) staining. The remaining stomach was cut into two parts for H. pylori culture and DNA extraction, respectively.
The colonization by H. pylori was quantitated by real-time quantitative PCR to amplify 16S rDNA of H. pylori and β2-microglobulin gene of mouse in the DNA collected from the stomachs of the HI, PH, and RPH groups 5 weeks after inoculation. Stomachs were homogenized in phosphate buffered solution (PBS) and DNA was extracted using a QIAamp DNA Mini kit (Qiagen Inc., Valencia, CA, USA). For 16S rDNA, the primers used were F: 5′-TTT GTT AGA GAA GAT AAT GAC GGT ATC TAA C-3′ and R: 5′-CAT AGG ATT TCA CAC CTG ACT GAC TAT C-3′, and the TaqMan probe was P: 5′-FAM-CGT GCC AGC AGC CGC GGT-TAMRA-3′. For the β2-microglobulin gene, the primers used were F: 5′-CCT GCA GAG TTA AGC ATG CCA G-3′ and R: 5′-TGC TTG ATC ACA TGT CTC GAT CC-3′, and the TaqMan probe was P: 5′-FAM-TGG CCG AGC CCA AGA CCG TCT AC-TAMRA-3′, purchased from SBS Genetech Ltd, Beijing, China. The assay was run on a Roter-Gene 6000 (Corbett Research Pty Ltd, San Francisco, CA, USA). After a denaturation step at 94°C for 3 min, 35 cycles were carried out for PCR amplification. The cycle profile was 94°C for 15 s, 65°C for 1 min. DNA extracted from H. pylori inocula was used as a positive control, and the negative control included sterile water instead of DNA.
Basic local alignment search tool (BLASTn) analysis of the F and R primers suggests that, in combination, they are specific for H. pylori. Real-time quantitative PCR is a fast and sensitive method for assessing the H. pylori load in infected mice, and the DNA extraction yield by QIAamp DNA Mini kit is greater than with other methods, even when the H. pylori content is low (Roussel et al. Citation2005).
Histopathological examination
Tissue forming the greater curvature of the stomach was fixed in 10% buffered formalin, cut into 2–3 mm wide strips, used for morphological evaluation and sent for pathological examination (Department of Pathology, Southwest Hospital, China). H–E sections were reviewed blind by two observers with experience in human and animal gastric pathology to grade the intensity of grastric inflammation by the scoring system of Sakagami et al. (Citation1996). The scores are as follows: 0, no increase in inflammatory cells; 1, mild infiltration of lymphocytes and/or neutrophils in the lamina propria; 2, uniform infiltration of the lamina propria by lymphocytes, plasma cells, eosinophils and scattered neutrophils; 3, moderately dense infiltration of the lamina propria by lymphocytes and plasma cells and/or moderate numbers of neutrophils in the lamina propria; and 4, dense lymphocyte and plasma cell infiltrates and/or extensive neutrophil infiltrates in the lamina propria.
Statistical analysis
Data from behavioral assessments were analyzed by one-way analysis of variance, LSD tests were used for post hoc analysis. Serum corticosterone was analyzed by analysis of variance, using a repeated measures design, because variance was not equal, data from real-time PCR were analyzed by Mann–Whitney U test. The gastric mucosal injury data were not normally distributed, so were analysed by Kruskal–Wallis tests, followed if significant by paired comparisons with the Mann–Whitney U test. All data are expressed as the mean ± SD. A P value < 0.05 was considered as significant.
Results
Behavioral studies of PS
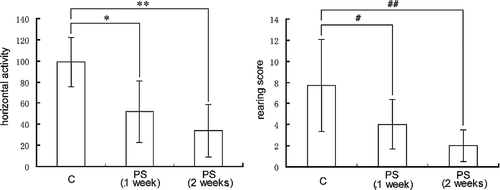
In order to evaluate the PS model, we performed open field tests and serum corticosterone assays to examine the behavioral and endocrine indices. As shown in , both horizontal activity [F(2,27) = 17.710; P < 0.001], and rearing score [F(2,27) = 9.484; P = 0.001] of the mice were significantly decreased on weeks 1 and 2 immediately after PS. LSD tests showed that the horizontal activity in the PS group at 1 and 2 weeks was significantly less (P < 0.001) than in the C group, and rearing score in the PS group at 1 and 2 weeks was significantly less (P < 0.001, P < 0.05, respectively) than in the C group. Neither the horizontal activity, nor the rearing score was different in the PS group between weeks 1 and 2 (P = 0.132 and 0.144, respectively). Hence, with respect to behavior, the open field test data showed that the PS model was effective.
Figure 1 The behavioral indices (rearing score and horizontal activity) of mice in the open field. Groups: C (n = 10), (PS (1 week), n = 10; PS (2 weeks), n = 10). Data are expressed as the mean ± SD. Statistically significant differences are indicated: *P < 0.001, ** P < 0.05, #P < 0.001, ##P < 0.001.
Effect of PS on serum corticosterone concentrations
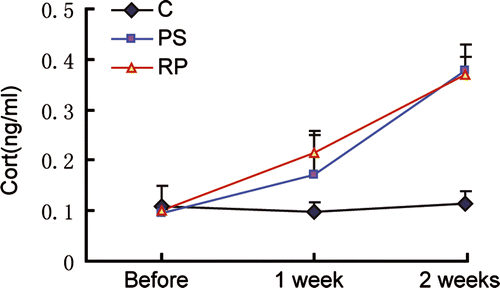
The level of GC production reflects the degree of PS, so we examined the serum corticosterone level of the mice (Choi et al. Citation2006). shows that the serum corticosterone level was significantly greater in the PS and RP groups after stress exposure for 1 and 2 weeks compared to pre-stress, with no significant change over this period in the C group. Before stress, the serum corticosterone levels of the three groups showed no significant difference (P>0.05). Hence serum corticosterone concentration in the PS and RP groups was significantly greater than in the C group at 1 and 2 weeks (P < 0.05), whereas there were no significant difference between the PS and RP groups (P>0.05). In respect of endocrine function, the serum corticosterone data therefore showed that the PS model was effective.
Figure 2 Serum corticosterone concentrations. Groups: C (n = 5), PS (n = 5), and RP (n = 5). Data are expressed as the mean ± SD. Before stress, C, PS and RP did not differ significantly (P>0.05); after 1 and 2 weeks, PS and RP were significantly different from C (P < 0.05). The RP and PS groups did not differ significantly from each other after stress exposure for 1 and 2 weeks (P>0.05).
Colonization of stomach tissue by H. pylori
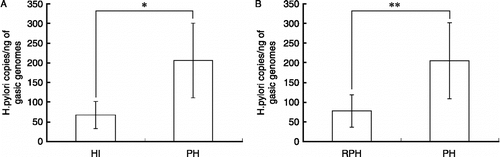
After the PS model was established successfully, we infected the psychological stressed mice with H. pylori. Five weeks after HI, we collected the gastric tissue. The HI rate was 100% in the HI, PH, and RPH groups as confirmed by bacterial culture, the urea test and H. pylori staining in stomach tissue sections. Real-time PCR was performed to quantify 16s rDNA in the H. pylori genome, which is specific to the bacterium; one copy of 16s rDNA represents one H. pylori. shows that the H. pylori copy number in the stomach tissue of the PH group was greater than that in the HI group (P < 0.05, ), and the H. pylori copy number in the RPH group, which were pretreated with RU486 before PS, was significantly lower than that of the PH group (P < 0.05, ). These data show that colonization in the stomach by H. pylori increased after PS and decreased after RU486 administration before stress.
Figure 3 Number of copies of H. pylori in stomach tissue. Groups: infection (HI), PH, and RPH groups; n = 8 per group. HI was detected by real-time PCR Data are expressed as the mean ± SD. The number of H. pylori copies/ng of gastric genome in the PH group was significantly greater than in the HI group (A). The number of H. pylori copies in the RPH group was significantly lower than that of the PH group (B). Statistically significant differences are indicated: *P < 0.05; **P < 0.05.
Gastric mucosal injury caused by H. pylori and PS
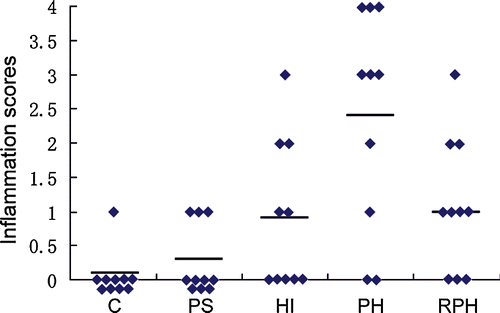
When the mice (C, PS, HI, PH, and RPH; n = 10 per group) were killed at 5 weeks after inoculation with H. pylori, the stomach tissue was sent for pathological examination. The inflammation scores representing the severity of inflammation are depicted in . Data were analyzed by Kruskal–Wallis analysis of variance, which showed significant differences among the five groups [H(4) = 16.875; P = 0.002]. Post hoc comparisons with Mann–Whitney U tests showed that inflammation scores for the PH group were significantly greater than the other groups (all P < 0.05). The scores in the PS group were not significantly different from the C group (P>0.05), but the scores of the HI and RPH groups were significantly greater than the C group (P < 0.05).
Figure 4 Histological grading of inflammation in the stomach. Groups: C, PS, HI, PH, and RPH (n = 10 per group). The scores for the PH group were significantly higher than the other groups (P < 0.05). The scores for the PS group were not significantly different from the C group (P>0.05), and the scores for the HI and RPH groups were significantly higher than the C group (P < 0.05). The horizontal bars indicate the mean scores.
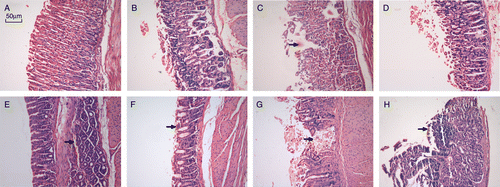
As shown in , there were no differences in the stomach tissue between the Control and PS groups (), and there was slight inflammation in the RPH group (). Gastric mucosal defects were evident in the HI group (), More extensive damage, with the appearance of ulcers in the stomach tissue, was seen in the PH group (). There was also atrophy of gastric mucosal glands, bleeding in the gastric mucosa and formation of folliculus lymphaticus in the PH group (). These assessments showed that PS alone did not significantly affect the histological appearance, but it aggravated the adverse effect of HI.
Figure 5 Representative gastric mucosa histology in the different groups of BALB/c mice. H–E stained sections. (A) Normal gastric mucosa in C group; (B) gastric mucosa in PS group; (C) defects (arrow) evident in HI group; (D) RPH group; (E) bleeding in gastric mucosa (arrow) in PH group; (F) atrophy of gastric mucosal glands (arrow) in PH group; (G) severe defect of stomach tissue, formation of ulcer (ulcer) in PH group; (H) formation of folliculus lymphaticus in gastric mucosa (arrow) in PH group.
Discussion
It is well known that stress has an adverse effect on gastric mucosa damage. Numerous studies have provided evidence that stress could cause peptic ulcer. It has been reported that salt and stress could synergize to worsen H. pylori-induced gastric lesions (Gamboa-Dominguez et al. Citation2007), and the presence of H. pylori caused significant deterioration of stress-induced gastric mucosal lesions (Oh et al. Citation2005). The role of PS in disease and health are of increasing concern since psychological stresssors appear with intensified social competition and a faster tempo of living. Although it seems clear that PS has an important role in the development of peptic ulcer, little has been known about the relationship of between PS and HI in the development of peptic ulcer.
In this study, we used the communication box method to induce PS by exposure to an emotional stressor without direct physical stress; this stressor involves the visual, olfactory, and auditory stimuli that arise from foot-shock-stressed animals (Oishi et al. Citation2003). In previous studies, different types of stress such as foot-shock stress, swimming stress, and immobilization stress were compared (Palma et al. Citation2000; Papale et al. Citation2005). These stresses were induced using different apparatus and time courses, but physical factors are implicitly included. In contrast, the communication box method separates PS from physical stress. We therefore used the communication box method to produce PS in the present study.
In the communication box test, the mice in the PS group are likely to have received emotional responses from the foot-shocked mice, which jumped, struggled, and vocalized during foot-shock exposure. This is considered likely to have induced PS in the PS mice. Indeed, we confirmed this from the behavioral assessments and the serum corticosterone assays. We found that the locomotor activity of mice in an open field decreased significantly after 1 and 2 weeks of the communication box test, and the serum corticosterone level also increased significantly after experience in the communication box over 1 and 2 weeks. These results are in agreement with a previous report (Ishikawa et al. Citation1992). Therefore, we infer that exposure of mice to the communication box as in the present study is psychologically stressful.
We found that the number of H. pylori in the stomach of the PS group mice was significantly higher than that in the HI group (non-stressed) mice, which showed that PS alters the colonization of H. pylori in the mouse gastric mucosa. Infection of the gastric mucosa follows a complicated course related to various factors such as the stomach microenvironment, the host immune system and the condition of the microorganism (Endo et al. Citation2001). It is known that the immune system can be regulated by the neuroendocrine system through the HPA axis and GC production and that PS can stimulate the HPA axis. In the present study, we also found that the serum corticosterone level significantly was significantly increased after PS. Specifically, PS stimulates hypothalamic CRH production leading to an increase in ACTH secretion by the pituitary, resulting in an increase in the synthesis and secretion of GC from the adrenal glands (Gaillard and Al-Damluji Citation1987; Delbende et al. Citation1992). We therefore hypothesized that the altered colonization of the gastric mucosa by H. pylori may be a response to changes in the mucosal immune system due to excess GC produced by PS. The finding that the number of bacteria in the stomach of the RPH group (pre-treated with RU486, a GR antagonist) was decreased significantly compared to the PH group mice supported this proposal. However, contrary to our results, a recent publication showed that endogenous GCs exhibit a gastroprotective effect by strengthening the host defensive factors (Filaretova et al. Citation2007; Kosan et al. Citation2008). However, GCs play a complicated role, including through positive feedback and negative feedback mechanisms. In acute stress, GC may protect the gastric mucosa, but long term high level of GC production, as with repeated exposure to a PS may have a disadvantageous effect on the gastric mucosa and enhance the HI, as seen in the present study.
In summary, the present study is the first to demonstrate that repeated exposure to PS increases the colonization of the gastric mucosa of BALB/c mice by H. pylori and aggravates the mucosal injury caused by this infection, and that this effect may be mediated by excess GC. We will further study the mechanisms of GC induced by PS altering HI.
Acknowledgements
This work was supported by grants from the National Basic Research Program of China (No. 2009CB522606). We would like to thank Professor Min Li (Department of Psychology, Third Military Medical University), Associate Professor Bang-Yun Zhao (Department of Physiology, Third Military Medical University), for the design of experiments and the animal model, and post-graduate Li-Qiang Zhu (Department of Health Statistics, Third Military Medical University), for assistance with statistical analysis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aoyama N, Kinoshita Y, Fujimoto S, Himeno S, Todo A, Kasuga M, Chiba T. 1998. Peptic ulcers after the Hanshin–Awaji earthquake: Increased incidence of bleeding gastric ulcers. Am J Gastroenterol. 93:311–316.
- Aoyama N, Shinoda Y, Matsushima Y, Shirasaka D, Kinoshita Y, Kasuga M, Chiba T. 2000. Helicobacter pylori-negative peptic ulcer in Japan: Which contributes most to peptic ulcer development, Helicobacter pylori, NSAIDS or stress?. J Gastroenterol. 35 Suppl. 12: 33–37.
- Choi EH, Demerjian M, Crumrine D, Brown BE, Mauro T, Elias PM, Feingold KR. 2006. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. Am J Physiol Regul Integr Comp Physiol. 291:R1657–R1662.
- Delbende C, Delarue C, Lefebvre H, Bunel DT, Szafarczyk A, Mocaer E, Kamoun A, Jegou S, Vaudry H. Glucocorticoids, transmitters and stress. Br J Psychiatry Suppl. 199224–35.
- Endo Y, Yamauchi K, Fueta Y, Irie M. 2001. Changes of body temperature and plasma corticosterone level in rats during psychological stress induced by the communication box. Med Sci Monit. 7:1161–1165.
- Filaretova L, Podvigina T, Bagaeva T, Bobryshev P, Takeuchi K. 2007. Gastroprotective role of glucocorticoid hormones. J Pharmacol Sci. 104 3: 195–201.
- Flint MS, Tinkle SS. 2001. C57BL/6 mice are resistant to acute restraint modulation of cutaneous hypersensitivity. Toxicol Sci. 62 2: 250–256.
- Gaillard RC, Al-Damluji S. 1987. Stress and the pituitary–adrenal axis. Baillieres Clin Endocrinol Metab. 1:319–354.
- Gamboa-Dominguez A, Ubbelohde T, Saqui-Salces M, Romano-Mazzoti L, Cervantes M, Dominguez-Fonseca C, de la Luz Estreber M, Ruiz-Palacios GM. 2007. Salt and stress synergize H. pylori-induced gastric lesions, cell proliferation, and p21 expression in Mongolian gerbils. Dig Dis Sci. 52 6: 1517–1526.
- Ishikawa M, Hara C, Ohdo S, Ogawa N. 1992. Plasma corticosterone response of rats with sociopsychological stress in the communication box. Physiol Behav. 52:475–480.
- Jarillo-Luna A, Rivera-Aguilar V, Martinez-Carrillo BE, Barbosa-Cabrera E, Garfias HR, Campos-Rodriguez R. 2008. Effect of restraint stress on the population of intestinal intraepithelial lymphocytes in mice. Brain Behav Immun. 22:265–275.
- Kellie MB, Amy EO, Andrew VP, Alan JT, Elizabeth RW, Fred JK. 2007. Does cortisol acting via the type II glucocorticoid receptor mediate suppression of pulsatile luteinizing hormone secretion in response to psychosocial stress?. Endocrinology. 148 4: 1882–1890.
- Kim YH, Lee JH, Lee SS, Cho EY, Oh YL, Son HJ, Rhee PL, Kim JJ, Koh KC, Paik SW, Rhee JC, Choi KW. 2002. Long-term stress and Helicobacter pylori infection independently induce gastric mucosal lesions in C57BL/6 mice. Scand J Gastroenterol. 37:1259–1264.
- Kosan B, Yuksel O, Ustun I, Koklu S, Topal F, Yilmaz M, Ergul B, Karaahmetoglu S, Eskioglu E, Altiparmak E. 2008. Role of endogenous cortisol on Helicobacter pylori colonization. Clin Biochem. 41 10–11: 917–919.
- Kurata JH, Nogawa AN. 1997. Meta-analysis of risk factors for peptic ulcer. Nonsteroidal antiinflammatory drugs, Helicobacter pylori, and smoking. J Clin Gastroenterol. 24:2–17.
- Levenstein S. 2000. The very model of a modern etiology: A biopsychosocial view of peptic ulcer. Psychosom Med. 62:176–185.
- Matsushima Y, Aoyama N, Fukuda H, Kinoshita Y, Todo A, Himeno S, Fujimoto S, Kasuga M, Nakase H, Chiba T. 1999. Gastric ulcer formation after the Hanshin–Awaji earthquake: A case study of Helicobacter pylori infection and stress-induced gastric ulcers. Helicobacter. 4:94–99.
- Oh TY, Yeo M, Han SU, Cho YK, Kim YB, Chung MH, Kim YS, Cho SW, Hahm KB. 2005. Synergism of Helicobacter pylori infection and stress on the augmentation of gastric mucosal damage and its prevention with alpha-tocopherol. Free Radic Biol Med. 38 11: 1447–1457.
- Oishi K, Nishio N, Konishi K, Shimokawa M, Okuda T, Kuriyama T, Machida K. 2003. Differential effects of physical and psychological stressors on immune functions of rats. Stress. 6:33–40.
- Palermo Neto J, Massoco CO, Favare RC. 2001. Effects of maternal stress on anxiety levels, macrophage activity, and Ehrlich tumor growth. Neurotoxicol Teratol. 23:497–507.
- Palma BD, Suchecki D, Tufik S. 2000. Differential effects of acute cold and footshock on the sleep of rats. Brain Res. 861:97–104.
- Papale LA, Andersen ML, Antunes IB, Alvarenga TA, Tufik S. 2005. Sleep pattern in rats under different stress modalities. Brain Res. 1060:47–54.
- Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. 2003. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav. 43:561–567.
- Roussel Y, Wilks M, Harris A, Mein C, Tabaqchali S. 2005. Evaluation of DNA extraction methods from mouse stomachs for the quantification of H. pylori by real-time PCR. J Microbiol Methods. 62:71–81.
- Ruiz-Bustos E, Ochoa JL, Wadstrom T, Ascencio F. 2001. Isolation and characterisation of putative adhesins from Helicobacter pylori with affinity for heparan sulphate proteoglycan. J Med Microbiol. 50:215–222.
- Sakagami T, Dixon M, O'Rourke J, Howlett R, Alderuccio F, Vella J, Shimoyama T, Lee A. 1996. Atrophic gastric changes inboth Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut. 39:639–648.
- Shimoyama T, Crabtree JE. 1998. Bacterial factors and immune pathogenesis in Helicobacter pylori infection. Gut. 43 Suppl. 1: S2–S5.
- Sternberg EM. 2006. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat Rev Immunol. 6:318–328.
- Velin DM, Michetti P. 2006. Immunology of Helicobacter pylori infection. Digestion. 73:116–123.
- Vinette KM, Gibney KM, Proujansky R, Fawcett PT. 2004. Comparison of PCR and clinical laboratory tests for diagnosing HI in pediatric patients. BMC Microbiol. 4:5.
- Yamamoto N, Sakagami T, Fukuda Y, Koizuka H, Hori K, Sawada Y, Hikasa Y, Tanida N, Shimoyama T. 2000. Influence of Helicobacter pylori infection on development of stress-induced gastric mucosal injury. J Gastroenterol. 35:332–340.