Abstract
The aim of the current study was to generate socially conditioned fear in two different strains of rat (Wistar, W and Sprague Dawley, SD) using social conflict, in order to investigate whether the magnitude of the conditioned fear responses in each strain was related to behaviour exhibited prior to or during fear induction (i.e. social conflict). On day one of the study, all intruders were assessed for exploratory activity in a novel environment. Twenty four hours following the novel environment test the locomotor activity of the intruders was assessed, while they underwent a single familiarisation exposure to the arena in which the conflict was subsequently to occur in. Twenty-four hours following familiarisation, intruders underwent either a 10 min social conflict or sham conflict session. One day later we examined the response of the intruders when they were returned to the vacant resident's cage. Upon return to the conflict context, we examined the intruder's ultrasonic distress vocalisations and the extent to which locomotor activity was inhibited. We found that W rats displayed significantly more immobility (i.e. conditioned fear) upon return to context than did SD rats (p < 0.05). Importantly, we observed that the differences in the two strains behaviour upon return to context appeared to be related to their quite different patterns of coping behaviour. The results of the current study indicate that preclinical between-strain comparisons potentially have much to offer in regard to understanding the basis of resilience to social stress.
Introduction
Social conflict is perhaps the most common cause of psychological stress experienced by members of a social species. In humans, social stress is a common precursor to the development of psychopathologies such as depression and anxiety (Takahashi et al. Citation2005). However, like all forms of stress, the impact and associated consequences of social stress can vary greatly from one individual to another. In the case of humans, this variability is often attributed to factors such as differences in personality and coping style (Ginzburg et al. Citation2002; Gill et al. Citation2005; Gil and Caspi Citation2006). Somewhat surprisingly, variability attributable to factors such as these is generally ignored in preclinical studies investigating the consequences of social stress. This is particularly unfortunate as appropriate preclinical studies potentially have much to offer in helping the understanding of the basis of individual differences in vulnerability to psychopathologies elicited by social stress (Pawlak et al. Citation2008).
In a recent study using Sprague Dawley (SD) rats, we investigated the basis of individual differences in the display of stress behaviours upon return to the context of a previous social conflict (i.e. in effect, socially conditioned fear). Importantly, we found that such differences could be predicted on the basis of an individual's pre-existing behavioural traits, specifically novelty seeking behaviour and the coping behaviour deployed during the conflict (Walker et al. Citation2008). However, such studies tend to use rather large groups of animals (Cohen and Zohar Citation2006; Koolhaas et al. Citation2007). Accordingly, this approach can create significant resource challenges for researchers. One alternative approach to investigating the basis of differences in stress vulnerability is to heighten the within-sample contrast, thus allowing the use of more modest sample sizes, by comparing the responses of individuals taken from different strains (Neophytou et al. Citation2000; Pawlak et al. Citation2008; Uchida et al. Citation2008). In this regard it is interesting to note that most previous pre-clinical studies concerning the consequences of social conflict have used W rather than SD rats (CitationHaller et al. 2007, 2006; Ebner et al. Citation2000). Moreover, while there have been no studies that have directly compared W and SD rat responses to social conflict, several studies have demonstrated that they differ considerably in responses to other aversive stimuli, such as predator odour (Rosen et al. Citation2006; Staples and McGregor Citation2006). Accordingly, the aims of the current study were: first, to compare the ability of social conflict to induce conditioned fear in W versus SD rats; second, to determine whether the factors associated with any between-strain differences corresponded to those considered to explain within-strain differences observed in our previous study of SDs (Walker et al. Citation2008). As previously, social conflict was achieved using the resident/intruder paradigm, wherein a naïve male rat (the intruder) is forced to enter the home cage of a larger con-specific male (the resident) that has been trained to attack all such intruders (Miczek and O'Donnell Citation1978; CitationTornatzky and Miczek 1993, 1995; Koolhaas et al. Citation1997; Marini et al. Citation2006). This attack is highly stressful to the intruder and 1 day later a conditioned fear response can be assessed by returning the intruder to the now vacant resident's cage and measuring the intruder's ultrasonic distress vocalisations and the extent of inhibition of its locomotor activity.
Methods
Subjects
Animals used in the present study were W and SD rats obtained from the University of Newcastle central animal house. All studies were approved by the University of Newcastle Animal Care Ethics Committee and performed in accordance with the New South Wales Animal Research Act and the “Australian code of practice and use of animals for scientific purposes”. The rats were maintained in temperature controlled rooms (21 ± 1°C) with food (Y. S. Rat Feeds, Mouse Breeder, Young, NSW, Australia) and water provided ad libitum and were held under a reversed 12 h light cycle with darkness from 02:00 to 14:00 h. All experimental procedures were conducted during the last half of the dark cycle. W (n = 20) and SD (n = 20) males were allocated to one of two separate groups; W (n = 10) and SD (n = 10) intruders exposed to social conflict (SD+, W+), and W (n = 10) and SD (n = 10) sham intruders not exposed to social conflict (SD − , W − ) but otherwise treated identically, including being exposed to a resident's cage albeit without the resident present.
All residents were SD rats, at least 6 months old and weighed between 500 and 700 g. Each male resident was co-housed with a tubally ligated female. These pairs cohabited for at least 6 weeks prior to the commencement of the study in large cages (60 × 30 × 40 cm) referred to as social conflict arenas and previously described in detail (Walker et al. Citation2008). Prior to the commencement of the study all residents were screened to ensure that they would reliably attack intruders with an attack latency of less than 2 min.
All W and SD intruders weighed between 300 and 400 g and were approximately 90 days old. All intruders were group housed (4 per cage) prior to the commencement of the experiment but individually housed thereafter in acrylic cages (40 × 25 × 25 cm) located in rooms separate from those of the residents. Intruders were singly housed for 7 days prior to their entry into the experiment. All rats were moved in and out of the test environment and handled under red light.
Tubal ligation surgery
Tubal ligation was performed on the female rats partnered with the residents in order to prevent them from becoming pregnant during cohabitation. Briefly, rats were anesthetised with intraperitoneal ketamine (0.75 ml/kg) and xylazine (0.5 ml/kg) and the uterine horns were tied and cut at the fimbria. Ligation was preferred to ovariectomy as, other than preventing pregnancy, it allowed the female residents to retain full normal biological activity.
Novel environment exploration test
On day one of the study, all intruders were assessed for exploratory activity in a novel environment over a 1 h period. The novel environment was a 40 × 25 × 25 cm acrylic cage lined with fresh bedding material (Fibercycle, Mudgeeraba, Australia). Exploration of the environment was recorded via an overhead infrared video camera. The total distance travelled was subsequently quantified on a minute by minute basis using an automated tracking program (Dielenberg et al. Citation2006). At the completion of the test session, rats were left in the environment (with food and water) which subsequently became their home cage for the remainder of the experiment.
Pre-conflict arena familiarisation
Twenty-four hours prior to the social or sham conflict the intruder was placed within the annex of the social conflict arena for 10 min with the resident and his female partner having been temporarily removed. Intruders had access to all areas of the social conflict arena. Activity during this 10 min was recorded via an overhead infrared video camera and the total distance travelled subsequently quantified on a minute by minute basis using an automated tracking program.
Social conflict
Twenty-four hours after pre-conflict familiarisation, rats were reintroduced into the same arena for 10 min. Intruders were reintroduced with the male resident present, whereas for sham intruders the male resident was absent. In all cases the intruder rat was attacked by the resident. At the end of the 10 min trial, the intruders were removed and returned to their home cages. Each resident was used in only one conflict per day. Interactions between resident and intruder were recorded via an infrared video camera. In addition to the scoring of resident-intruder interactions, 22 kHz ultrasonic vocalisations were also digitally recorded during the social conflict test using a Mini-3 Bat detector (Ultra Sound Advice, London, UK) coupled to a Sony DV (DCR-DVD100E) video camera. Audio tracks were subsequently demultiplexed and re-encoded as .wav files that were then scored by one trained observer.
A variety of behaviours engaged in by the SD and W intruders during the social conflict were scored: (a) resident initiated fights—aggressive interactions which begin with an approach by the resident; (b) intruder initiated fights—aggressive interactions which begin with an approach by the intruder; (c) upright posture—rigid hind or forelimbs directed at resisting an approach or attack by the resident; (d) exploration—locomotion not directed toward or away from the resident; (e) guarding—positioning within the social conflict arena, usually at doorways, in order to deny the resident access to the intruder's body—especially the intruder's anogenital region; (f) submission—lying on back with limp hind or forelimbs in response to an approach or attack by the resident; (g) immobility—non-responsiveness to contact made by the resident but without the limpness observed during submission; (h) flight—the intruder rapidly moves away from the resident following an instance of guarding, submission, immobility, upright posturing or fighting; (i) walk-away—the intruder slowly moves away from the resident following an instance of guarding, submission, immobility, upright posturing, or fighting. The amount of time spent engaged in, and the frequency of display of each of these behaviours was scored.
Conditioned fear
Twenty-four hours after the social or sham conflict episode, intruders were reintroduced into the same social conflict arena, but with the resident pair absent. Activity was recorded via an overhead infrared video camera for 10 min and the total distance travelled subsequently quantified on a minute-by-minute basis using an automated tracking program. Twenty-two kilohertz ultrasonic vocalisations were digitally recorded and analysed using the procedure described above.
Data analysis
Analyses were performed using SPSS v.15. A series of 2 × 2 ANOVAs were used to assess differences between groups for the following measures: mean distance travelled in novel environment; mean distance travelled in pre-conflict arena familiarisation; mean number of ultrasonic vocalisations during social conflict and conditioned fear; mean percentage change in mobility during the conditioned fear test relative to baseline (distance travelled during the conditioned fear test – distance travelled during the pre-conflict arena familiarisation session/distance travelled during the pre-conflict arena familiarisation session × 100). A series of planned comparisons using four Bonferroni corrected t-tests was used to investigate between and within strain differences in ultrasonic vocalisations during social conflict and conditioned fear. Additionally, post hoc analyses were carried out using Bonferroni-corrected t-tests where appropriate. Levene's test was used to assess violations of equality of variance for each comparison and degrees of freedom were adjusted where appropriate. The duration and frequency of behaviours displayed during social conflict was investigated using a series of independent sample t-tests. Furthermore, all dependent measures were correlated using Pearson's product-moment correlation coefficient (r) for each of the four groups; however no significant or strong correlations were observed between the variables and the results are not reported. Data are shown as group mean ± SEM.
Results
Between strain differences in locomotor activity during the novel environment exploration test and the pre-conflict arena familiarisation session
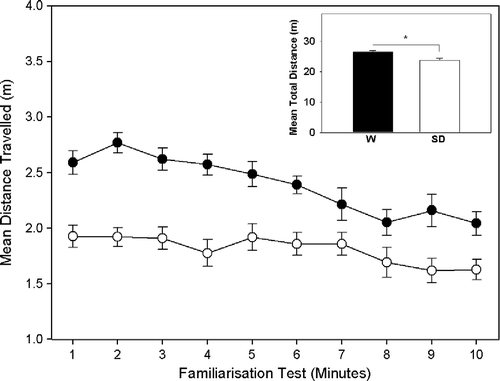
In total, the W rats showed significantly higher levels of locomotor activity compared to the SD rats in both the novel environment exploration test and the familiarisation test (See ) resulting in a highly significant main effect of strain for novel environment, F(1, 39) = 10.91, p = 0.002 and familiarisation, F(1, 39) 35.90, p = 0.001. It should also be noted that no significant main effect for either locomotor activity during novel environment or familiarisation was observed between the social conflict (SC+) and control (SC − ) conditions, therefore it can be concluded that there were no inherent differences in locomotor activity between conflict conditions during the novel environment exploration test and the familiarisation test.
Figure 1 Distance (mean ± SEM) travelled by Wistar (•, n = 20) and Sprague Dawley (○, n = 20) rats in each of the 10 min of a novel environment exploration test. The inset bar graph shows the total distance (mean ± SEM) each strain (W, Wistar; SD, Sprague Dawley) travelled during the test. *p < 0.01.
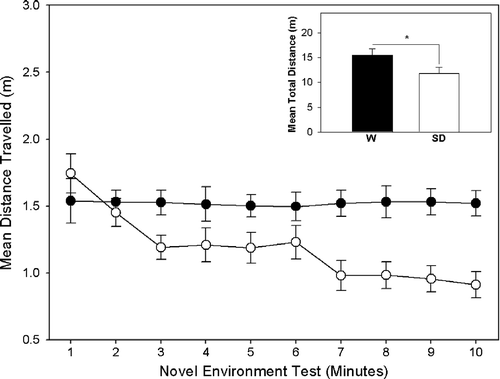
Figure 2 Mean distance travelled by Wistar (n = 20) and Sprague Dawley (n = 20) rats during familiarisation with the resident cage. The line graph represents the mean ( ± SEM) distance travelled by Wistar (•) and Sprague (○) rats in each of the 10 min of exposure. The inset bar graph shows the total mean ( ± SEM) distance each strain (W, Wistar; SD, Sprague Dawley) travelled in the 10 min. *p < 0.01.
Between strain differences in behaviours during social conflict
An independent sample t-test was used to compare behaviours during social conflict between W+ and SD+ rats (). The W+ rats displayed significantly more episodes of intruder initiated fights, upright, walk-away and explore behaviours than the SD+ rats (p < 0.01) and, in the case of the first three behaviours, also spent significantly more time engaged in these behaviours than the SD rats (p < 0.01). Conversely, the SD+ intruders displayed significantly more episodes of submissive (p < 0.05) and immobile (p < 0.01) behaviours than W+ intruders and in the case of immobile behaviour also spent significantly more time engaged in that behaviour than the W+ rats (p < 0.05). With regard to submissive behaviour it was also noted that, while every SD+ intruder displayed at least one episode of submission, only four out of 10 W+ intruders displayed an episode of submission.
Table I. Mean time in seconds and mean number of events with ± SEM in brackets, for each behavioural response recorded during the 10 min social conflict encounter for the Wistar and Sprague Dawley intruder rats.
Between and within strain differences in ultrasonic vocalisations during social conflict
A significant interaction effect was observed between strain and stress condition for ultrasonic vocalisations during social conflict, F(1, 39) = 34.07, p = 0.001. A series of planned comparisons using Bonferroni corrected t-tests was used to investigate between and within strain differences in ultrasonic vocalisations during social conflict. It was found that SD+ intruders made significantly more ultrasonic vocalisations than W+ intruders, t(18) = 7.23, p = 0.001. However, no significant differences were observed between the SD − and W − sham intruders ().
Figure 3 The main graph shows the mean ( ± SEM) number of ultrasonic vocalisations for each of the 10 min of a social conflict encounter. Four groups (n = 10/group) are represented: Wistar (▾) and Sprague Dawley (▿) intruders (W+/SD+) and Wistar (•) and Sprague Dawley (○) sham intruders (W − /SD − ). The inset bar graph shows the total mean ( ± SEM) number of ultrasonic vocalisations during the 10 min of social conflict for each of the four groups: a, represents a significant difference from Wistar intruders (W+) at p < 0.01; b, represents a significant difference from Sprague Dawley intruders (SD+) at p < 0.01.
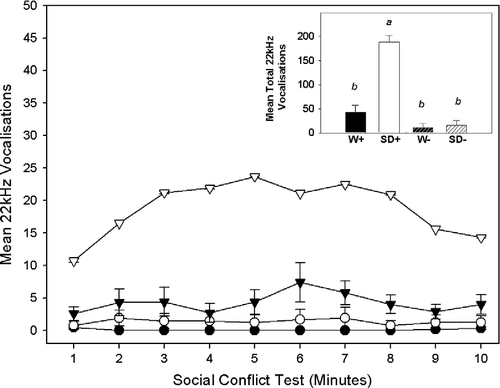
Within strain comparisons revealed that the SD+ intruders made significantly more ultrasonic vocalisations during the social conflict session than did the SD − sham intruders during their corresponding exposure to a vacant resident's cage, t(18) = 10.05, p = 0.001. In contrast, no significant differences in ultrasonic vocalisations during social conflict were observed between the Wistar intruders (W+) and sham intruders (W − ; ).
Between and within strain changes in mobility (relative to baseline) during the conditioned fear test
A significant interaction effect was observed between strains and conditions for changes from baseline mobility during the conditioned fear test F(1, 39) = 5.96, p = 0.02. As shown in , the SD+ intruders exhibited a significantly greater decrease in mobility than W+ intruders during the conditioned fear test, t(11.29) = 4.05, p = 0.002. Notably, a comparison of W intruders that had (n = 4) and had not (n = 6) displayed episodes of submission revealed no significant difference in the extent to which their mobility decreased, relative to baseline, during the conditioned fear test.
Figure 4 The main graph shows the mean ( ± SEM) percentage difference in mobility between the familiarisation (baseline) test and the conditioned fear test for each of the 10 min of the test. Four groups (n = 10/group) are represented: Wistar (▾) and Sprague Dawley (▿) intruders (W+/SD+) and Wistar (•) and Sprague Dawley (○) sham intruders (W − /SD − ). The inset bar graph shows the total mean (+ SEM) percentage difference in mobility during the conditioned fear test, relative to baseline, for each of the four groups. *p < 0.01.
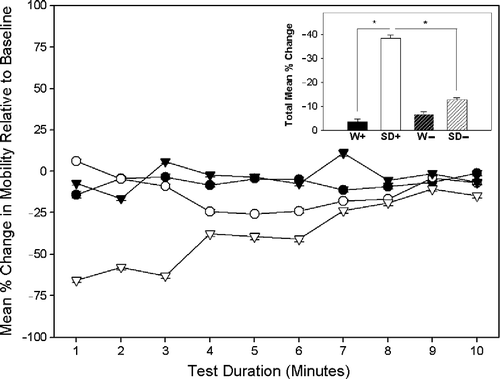
SD+ exhibited a significantly greater decrease in mobility than SD − intruders, t(18) = 2.59, p = 0.018. In contrast, no significant difference in changes from baseline mobility was observed between W+ and W − intruders during the conditioned fear test (). No significant differences were observed between W − and SD − sham intruders.
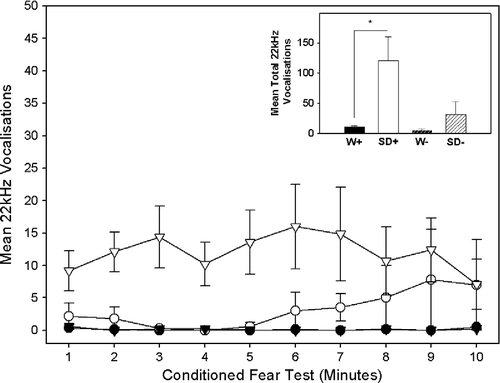
Between and within strain differences in ultrasonic vocalisations during the conditioned fear test
A significant main effect of strain was observed for the number of ultrasonic vocalisations made during the conditioned fear test whereby the SD rats made significantly more ultrasonic vocalisations than the W rats, F(1, 39) = 44.89, p = 0.003. Notably, a post hoc comparison using an independent sample t-test showed that the SD+ intruders made significantly more ultrasonic vocalisations than W+ intruders, t(11.29) = 4.05, p = 0.002 (). In contrast, no significant differences were observed between the SD − and W − sham intruders. No significant differences were observed within either strain between intruders and sham intruders. Also of note, was that a comparison of W intruders (W+) that had (n = 4) and had not (n = 6) displayed episodes of submission revealed no significant difference in the extent to which they emitted ultrasonic vocalisations during the conditioned fear test.
Figure 5 The main graph shows the mean ( ± SEM) number of ultrasonic vocalisations for each of the 10 min of the conditioned fear test. Four groups (n = 10/group) are represented in the main graph: Wistar (▾) and Sprague Dawley (▿) intruders (W+/SD+) and Wistar (•) and Sprague Dawley (○) sham intruders (W − /SD − ). The inset bar graph shows the total mean ( ± SEM) number of ultrasonic vocalisations during the 10 min conditioned fear test for each of the four groups. *p < 0.01.
Discussion
In the present, study it was found that two rat strains, W and SD, showed a striking difference in their display of conditioned fear 24 h after an experience of social conflict. In a previous study of SD rats, evidence was obtained that such differences could best be explained in terms of within-strain variation in behavioural traits that modify susceptibility to the development of conditioned fear (Walker et al. Citation2008). Similar factors seem likely to explain the between-strain differences reported here as, relative to SD rats, W rats displayed higher levels of novelty-seeking behaviour in pre-conflict tests and, during social conflict, displayed very different patterns of coping behaviour.
Pavlovian fear conditioning, which is widely used as a pre-clinical model for anxiety-related disorders, involves pairing an initially neutral stimulus, referred to as the conditioned stimulus (CS) with an innately aversive stimulus referred to as the unconditioned stimulus (US). Subsequently, the CS alone comes to elicit a conditioned response (CR) similar to the fear-like response previously triggered by the US. Although stressors such as foot-shock are often used as the US, it is increasingly common to use ethologically relevant stressors, such as social conflict (Keeney et al. Citation2006). In the present study, social conflict was achieved using the resident/intruder paradigm (Miczek and O'Donnell Citation1978; CitationTornatzky and Miczek 1993, 1995; Koolhaas et al. Citation1997). One day later the extent of the intruder's socially conditioned fear upon return to the cage where the conflict occurred was assessed on the basis of inhibition of mobility and elicitation of ultrasonic distress vocalisations. Compared to the SD+ rats, W+s exhibited much less inhibition of mobility and many fewer ultrasonic vocalisations upon return to context, indicating lower levels of conditioned fear or the SD+ rats. Importantly, these two strains of rats also displayed significant differences in their behaviour, both during the conflict episode and in pre-conflict behavioural testing.
Individuals allocated to the role of intruder in the resident/intruder paradigm display a variety of behaviours in their attempts to cope with the challenge of social conflict, ranging from fighting to outright submission (Albonetti and Farabollini Citation1994; Meerlo et al. Citation1999; Walker et al. Citation2008). Importantly, it has been suggested that coping behaviour can be considered to fall into two categories: “active” coping, characterised by aggression and territorial control, and “passive” coping, characterised by immobility, decreased reactivity, and low aggression (Koolhaas et al. Citation1997). This is a relatively new conceptualisation that suggests an inherent vulnerability to stress differentiates strains along a continuum spanning “active” and “passive” coping style; with the latter more predisposed to psychopathology, previously shown by Walker et al. (Citation2008) for W rats using an individual differences approach. Furthermore a range of physiological correlates associated with a stress response has been found in animals exhibiting a “passive” coping style including higher hypothalamo-pituitary adrenal axis reactivity (Koolhaas et al. Citation1999; Koolhaas Citation2008), higher plasma corticosterone levels (CitationKorte et al. 1992a,b), increased levels of serotonin (Koolhaas et al. Citation2007; Veenema and Neumann Citation2007), humoral immune suppression (Jasnow et al. Citation2001) and decreased cerebral cortical neutrophin expression (Pizarro et al. Citation2004). However, to our knowledge this is the first time that a differential behavioural profile toward a social stressor between two rat strains has been compared and contrasted in a single experiment.
Viewed from this perspective the present data show that, during social conflict, W rats engaged much more frequently than SDs in active coping behaviours, i.e. Fight initiation, upright defence posture and exploration, and much less frequently than SDs in passive coping behaviours, i.e. submission and immobility. The difference between W and SD rats with regard to initiation of fights was particularly notable in this regard. While the frequency with which Ws displayed this behaviour was low compared to most other behaviours, what was striking was that the SDs never displayed this behaviour. Interestingly, other authors working with the social conflict model have shown that Groningen wild-type intruders that initiate attacks against the resident suffer fewer physiological and behavioural disturbances in the ensuing days and weeks than intruders who readily submit (CitationMeerlo et al. 1997, 1999; Stefanski Citation1998). It has been suggested that this outcome is consistent with “active” coping behaviour moderating the stress associated with social conflict to a greater degree than “passive” coping behaviour (Koolhaas et al. Citation1999). Data from the present study appear to go some way in supporting the view that W rats possess a more active coping style whereas the SDs are more characterised as possessing a passive coping style. Consistent with this distinction, SD rats used as intruders in the present study also exhibited significantly more submissive and immobile behaviour during the conflict than did the W intruders. However, it was especially interesting to note that Ws that did submit to the resident during the social conflict episode showed no statistically greater degree of immobility or emission of ultrasonic distress vocalisations during the conditioned fear test than Ws that did not submit. This might suggest that it is the presence of active coping behaviour rather than an absence of passive coping behaviour that is most critical in determining susceptibility to the development of socially conditioned fear. It should be noted that, although we interpret the present data as indicating that SD rats tend to deploy passive coping behaviours to a greater extent than Ws, this does not mean that SD rats can simply be designated “passive copers”. In a recent study involving a large sample of SD rats we showed that within-strain differences social conflict behaviour do occur and, indeed, that they predict differences in the development of socially conditioned fear (Walker et al. Citation2008). The best negative predictor was guarding, i.e. defensive positioning, usually at doorways, to deny the resident access to the intruder's body, and can reasonably be construed as active coping behaviour involving territorial control. It is interesting, however, that the specific social conflict behaviour that may best characterise an actively coping SD rat, i.e. guarding, could differ from what may best characterise an actively coping W rat, e.g. Fights initiated.
In addition to differences in behaviour displayed during social conflict, W and SD rats also differed in their performance in the novel environment and familiarisation tests conducted prior to the social conflict. In both tests W rats exhibited significantly higher levels of locomotor activity than SDs, a difference that would usually be taken to indicate that Ws have a higher propensity for engaging in “novelty-seeking” behaviour (Piazza et al. Citation1993; Kabbaj et al. Citation2000). This is particularly interesting because: (i) it has frequently been shown that animals exhibiting higher levels of novelty-seeking behaviour also tend to exhibit lower levels of anxiety (Kabbaj et al. Citation2000; Pawlak et al. Citation2008); (ii) we have previously shown that differences in novelty seeking predict within-strain differences in coping behaviours displayed by SD rats during social conflict and, independently, their susceptibility to development of socially conditioned fear (Walker et al. Citation2008). This leads us to suggest that perhaps the common understanding of active versus passive coping styles should be extended to include “novelty-seeking” behaviour as a component characteristic, along with those such as territorial control and aggressivity. Accordingly, high levels of novelty seeking might be considered indicative of an active coping style and low levels of novelty seeking indicative of a passive coping style. It must also be acknowledged that SD rats were used as residents during social conflict for both W and SD rats, and this may have contributed in the differential profiles observed between the SD and W rats. However it should also be noted that the W rats not only exhibited significantly greater “active” behaviours during social conflict but also higher levels of pre-conflict mobility during the novel environment test and familiarisation, and cumulatively these results suggest that the difference between the strains most likely reflects a difference in coping style.
Finally, at least two other possible explanations of why W intruders displayed lower levels of socially conditioned fear than SD intruders merit comment: (a) that W intruders were subjected to a less intense US, i.e. social conflict, than SD intruders; (b) that the salience of the CS—in this instance the context of the social conflict—may have been less for W than SD intruders. With regard to the first explanation, it is well recognised that US intensity in a conditioned fear paradigm influences the magnitude of the subsequent CR, a more intense US generating greater conditioned fear (Davis and Astrachan Citation1978; Cordero et al. Citation1998; Pietersen et al. Citation2006). It is notable then that Ws made fewer ultrasonic vocalisations than SD rats during social conflict, suggesting that the experience may indeed have been less aversive for them. However, in our view, it is more pertinent that the frequency and duration of resident-initiated fights was similar for the two strains, suggesting that US intensity was similar. Thus it is our interpretation that while the US may have been less aversive for Ws, perhaps due to the same traits as already discussed, the intensity of the US that the W and SD rats were exposed to was similar. With regard to the second explanation, it is feasible that differences in sensory capacity could result in altered CS salience. Certainly for rodents, olfactory cues would likely be a major factor in recognition of the CS (i.e. the resident's cage) and it is notable that Ws are reported to be less responsive than SD rats to synthetic predator odour (2,4,5-trimethylthiazoline; Rosen et al. Citation2006), potentially suggesting that the olfactory abilities of Ws are inferior to those of SD rats. However, this is not supported by studies of the performance of Ws on olfactory discrimination tasks (Kraemer and Apfelbach Citation2004), nor the observation (Staples and McGregor Citation2006) that natural predator (cat) odour elicits stronger conditioned and unconditioned defensive behaviours in W than in SD rats.
Clinically, it is well understood that there is considerable variability in the vulnerability to stress between individuals. However, this is an issue that needs more attention in pre-clinical studies. At this stage, there is still no consensus in the field as to the most appropriate design to deploy when examining this issue. The results from the current study add to the growing body of experimental work attesting to the potential benefits of exploiting between strain differences to investigate stress vulnerability. With that said, at this point it is not clear whether the between strain approach can be considered as a bona-fide alterative or rather simply represents a complementary approach to within strain investigation of individual differences in resilience and susceptibility.
Acknowledgements
Funding for this research was provided by the National Health and Medical Research Council of Australia as well as the Hunter Medical Research Institute.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Albonetti ME, Farabollini F. 1994. Social stress by repeated defeat: Effects on social behaviour and emotionality. Behav Brain Res. 62:187–193.
- Cohen H, Zohar J. 2006. An animal model of posttraumatic stress disorder: The use of cut-off behavioural criteria. Ann NY Acad Sci. 1032:167–178.
- Cordero MI, Merino JJ, Sandi C. 1998. Correlational relationship between shock intensity and corticosterone secretion on the establishment and subsequent expression of contextual fear conditioning. Behav Neurosci. 112:885–891.
- Davis M, Astrachan DI. 1978. Conditioned fear and startle magnitude: Effects of different footshock or backshock intensities used in training. J Exp Psychol Anim Behav Process. 4:95–103.
- Dielenberg RA, Halasz P, Day TA. 2006. A method for tracking rats in a complex and completely dark environment using computerized video analysis. J Neurosci Methods. 158:279–286.
- Ebner K, Wotjak CT, Landgraf R, Engelman M. 2000. A single social defeat experience selectively stimulates the release of oxytocin, but not vasopressin, within the septal brain area of male rats. Brain Res. 872:87–92.
- Gil S, Caspi Y. 2006. Personality traits, coping style, and perceived threat as predictors of posttraumatic stress disorder after exposure to a terrorist attack: A prospective study. Psychosom Med. 68 6: 904–909.
- Gill JM, Szanton SL, Page GG. 2005. Biological underpinnings of health alterations in women with PTSD: A sex disparity. Biol Res Nurs. 7:44–54.
- Ginzburg K, Solomon Z, Bleich A. 2002. Repressive coping style, acute stress disorder, and posttraumatic stress disorder after myocardial infarction. Psychosom Med. 64:748–757.
- Haller J, Kiem DT, Makara GB. 2007. The physiology of social conflict in rats: What is particularly stressful?. Behav Neurosci. 110:353–359.
- Haller J, Horvath Z, Nikoletta B. 2006. The effect of buspirone on normal and hypoarousal-driven abnormal agression in rats. Prog Neuropsychopharmacol Biol Psychiatry. 31:27–31.
- Jasnow AM, Drazen DL, Huhman KL, Nelson RJ, Demas GE. 2001. Acute and chronic social defeat suppresses humoral immunity of male Syrian hamsters (Mesocricetus auratus). Horm Behav. 40:428–433.
- Kabbaj M, Devine DP, Savage VR, Akil H. 2000. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: Differential expression of stress-related molecules. The Journal of Neuroscience. 20:6983–6988.
- Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. 2006. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol. 18:330–338.
- Koolhaas JM. 2008. Coping style and immunity in animals: Making sense of individual variation. Brain Behav Immun. 22:662–667.
- Koolhaas JM, Meerlo P, De Boer SF, Strubbe JH, Bohus B. 1997. The temporal dynamics of the stress response. Neurosci Biobehav Rev. 21:775–782.
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, Blokhuis HJ. 1999. Coping styles in animals: Current status in behavior and stress-physiology. Neurosci Biobehav Rev. 23:925–935.
- Koolhaas JM, De Boer SF, Buwalda B, Van Reenen CG. 2007. Individual variation in coping with stress: A multidimensional approach of ultimate and proximate mechanisms. Brain Behav Evol. 70:218–226.
- Korte SM, Bouws GA, Koolhaas JM, Bohus B. Neuroendocrine and behavioral responses during conditioned active and passive behavior in the defensive burying/probe avoidance paradigm: Effects of ipsapirone. Physiol Behav. 1992a; 52:355–361.
- Korte SM, Buwalda B, Bouws GAH, Koolhaas JM, Maes FW, Bohus B. Conditioned neuroendocrine and cardiovascular stress responsiveness accompanying behavioral passivity and activity in aged and in young rats. Physiol Behav. 1992b; 51:815–822.
- Kraemer S, Apfelbach R. 2004. Olfactory sensitivity, learning and cognition in young adult and aged male Wistar rats. Physiol Behav. 81:435–442.
- Marini F, Pozzato C, Andreetta V, Jansson B, Arban R, Domenici E, Carboni L. 2006. Single exposure to social defeat increases corticotropin-releasing factor and glucocorticoid receptor mRNA expression in rat hippocampus. Brain Res. 1067:25–35.
- Meerlo P, Overkamp GJ, Koolhaas JM. 1997. Behavioural and physiological consequences of a single social defeat in roman high- and low-avoidance rats. Psychoneuroendocrinology. 22:155–168.
- Meerlo P, Sgoifo A, De Boer SF, Koolhaas JM. 1999. Long-lasting consequences of a social conflict in rats: Behavior during the interaction predicts subsequent changes in daily rhythms of heart rate, temperature, and activity. Behav Neurosci. 113:1283–1290.
- Miczek KA, O'Donnell JM. 1978. Intruder-evoked aggression in isolated and nonisolated mice: Effects of psychomotor stimulants and L-dopa. Psychopharmacology (Berl). 57:47–55.
- Neophytou SI, Graham M, Williams J, Aspley S, Marsden CA, Beckett SR. 2000. Strain differences to the effects of aversive frequency ultrasound on behaviour and brain topography of c-fos expression in the rat. Brain Res. 854:158–164.
- Pawlak CR, Ho YJ, Schwarting RK. 2008. Animal models of human psychopathology based on individual differences in novelty-seeking and anxiety. Neurosci Biobehav Rev. 32 8: 1544–1568.
- Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. 1993. Corticosterone in the range of stress-induced levels possesses reinforcing properties: Implications for sensation-seeking behaviors. Proc Natl Acad Sci USA. 90:11738–11742.
- Pietersen CY, Bosker FJ, Postema F, den Boer JA. 2006. Fear conditioning and shock intensity: The choice between minimizing the stress induced and reducing the number of animals used. Lab Anim. 40:180–185.
- Pizarro JM, Lumley LA, Medina W, Robison CL, Chang WE, Alagappan A, Bah MJ, Dawood MY, Shah JD, Mark B, Kendall N, Smith MA, Saviolakis GA, Meyerhoff JL. 2004. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 1025:10–20.
- Rosen JB, West EA, Donley MP. 2006. Not all rat strains are equal: Differential unconditioned fear responses to the synthetic fox odor 2,4,5-trimethylthiazoline in three outbred rat strains. Behav Neurosci. 120:290–297.
- Staples LG, McGregor IS. 2006. Defensive responses of Wistar and Sprague–Dawley rats to cat odour and TMT. Behav Brain Res. 172:351–354.
- Stefanski V. 1998. Social stress in loser rats: Opposite immunological effects in submissive and subdominant males. Physiol Behav. 63:605–613.
- Takahashi T, Ikeda K, Ishikawa M, Kitamura N, Tsukasaki T, Nakama D, Kameda T. 2005. Anxiety, reactivity, and social stress-induced cortisol elevation in humans. Neuro Endocrinol Lett. 26:351–354.
- Tornatzky W, Miczek KA. 1993. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 53:983–993.
- Tornatzky W, Miczek KA. 1995. Alcohol, anxiolytics and social stress in rats. Psychopharmacology (Berl). 121:135–144.
- Uchida S, Nishida A, Hara K, Kamemoto T, Suetsugi M, Fujimoto M, Watanuki T, Wakabayashi Y, Otsuki K, McEwen BS, Watanabe Y. 2008. Characterization of the vulnerability to repeated stress in Fischer 344 rats: Possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. Eur J Neurosci. 27:2250–2261.
- Veenema AH, Neumann ID. 2007. Neurobiological mechanisms of aggression and stress coping: A comparative study in mouse and rat selection lines. Brain Behav Evol. 70:274–285.
- Walker FR, Hinwood M, Masters L, Dielenberg RA, Day TA. 2008. Individual differences predict susceptibility to conditioned fear arising from psychosocial trauma. J Psychiatr Res. 42:371–383.