Abstract
While both the adrenal medulla and sympathetic nervous system are important in mediating the catecholaminergic response to stress, there are crucial differences in the mechanism. Stress elevates tyrosine hydroxylase (TH) protein and mRNA levels in both the adrenal medulla and sympathetic ganglia. In the adrenal medulla, transcription of the TH gene is rapidly induced with immobilization (IMO) stress. Here, we examine whether IMO also increases TH transcription in the superior cervical ganglia (SCG). Quantitative real-time reverse transcription polymerase chain reaction was used to determine the changes in TH mRNA and in transcripts containing intron 2. As expected in the adrenal medulla following repeated IMO TH mRNA and intron containing transcripts were elevated about 5-fold. In the SCG, a significant increase in TH mRNA was observed following repeated 2 h IMO for 2 or 6 days, but not with single IMO. The intron 2 containing transcripts were elevated about 50% above controls with even single IMO, and were at similarly elevated levels after the 2nd or 6th repeated daily IMO. The results indicate, for the first time, that transcriptional mechanisms are involved in mediating the IMO stress triggered elevation in TH gene expression in the SCG.
Introduction
Originally the sympathetic nervous system and the adrenal medulla were considered as comprising a unitary sympathoadrenal system mediating the “fight or flight” response to stress (Cannon Citation1929). Early studies showed that a variety of stressors increase activity of tyrosine hydroxylase (TH) the major rate limiting enzyme in catecholamine biosynthesis in both the adrenal medulla and sympathetic ganglia (Thoenen et al. Citation1969; Kvetnansky et al. Citation1970; Thoenen Citation1970; Kvetnansky et al. Citation1971). The elevation of TH activity triggered in response to cold and immobilization (IMO) stress is associated with increased TH immunoreactive protein and mRNA levels (Sabban and Kvetnansky Citation2001).
However, it has become clear in recent years that the adrenal medulla and sympathetic nervous system can be regulated differently. The magnitude of the changes triggered by stress and the time course of changes in expression of TH and dopamine β-hydroxylase (DBH) genes differ between these tissues (Sabban et al. Citation2004). In addition, the involvement of the hypothalamo-pituitary–adrenal (HPA) axis in the stress-triggered increase in TH and DBH gene expression appears to differ between the adrenal medulla and sympathetic ganglia. Activation of the HPA axis and specifically the elevation of adrenocortiotropin hormone (ACTH) secretion may play a crucial, perhaps direct, role in the regulation of TH and DBH in sympathetic ganglia (Nankova et al. Citation1996; Nankova et al. Citation2003). Injection of rats with ACTH for 7 days triggers a marked elevation of TH and DBH mRNA levels in rat superior cervical ganglia (SCG), and TH mRNA levels in the SCG of the ACTH-treated animals were as high as observed after a single IMO stress. In contrast, these ACTH injections had little effect on TH and DBH mRNA levels in the adrenal medulla (Nankova et al. Citation1996). In line with these results the elevation of TH gene expression in the SCG was observed in adrenalectomized rats further suggesting a direct effect of ACTH (Serova et al. Citation2008).
Transcription is an important pathway for regulation of gene expression of CA biosynthetic enzymes in the adrenal medulla in response to stress (Osterhout et al. Citation1997; Nankova et al. Citation1999). However, the mechanism in sympathetic ganglia is less clear. It has been difficult to determine definitely whether or not the stress triggered elevation in TH mRNA levels is due to decreased turnover rate or to increased transcription.
Detection of changes in intron-containing transcripts can be used to determine whether changes observed in mRNA levels are mediated by transcription. RNase protection and in situ hybridization with intron 2 specific probe were shown to provide a sensitive indication of changes in TH transcription rate (Chang et al. Citation2000). Semiquantitative real-time reverse transcription polymerase chain reaction (RT-PCR) measurement of changes in TH RNA primary transcripts that express intron 2 sequences in the adrenal medulla and in cell culture led to results that were comparable with those attained with nuclear run-on assays of transcription (Sun et al. Citation2004).
In this study, we use determination of changes in TH transcripts expressing intron 2 to examine the effect of IMO stress on changes in transcription in the adrenal medulla and SCG. The results indicate that IMO triggers increased transcription in SCG, but the elevation is much smaller than observed in the adrenal medulla.
Methods
Animal procedures
All animal procedures were approved by the Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals. Male Sprague–Dawley rats (275–300 g) obtained from Taconic Farms (Germantown, NY, USA) were housed four per cage at 24°C in a humidity controlled room, on a 12 h light/dark cycle (lights on at 6 a.m. and off at 6 p.m.) in an isolated and sound proof room to minimize background stress. Food and water were available ad libitum. IMO stress was accomplished by taping the forelimbs and hind limbs of the rat in a prone position with surgical tape to metal mounts attached to a board as previously described (Kvetnansky and Mikulaj Citation1970; Sabban et al. Citation2006). Rats (seven per group) were immobilized for 2 h (1 × IMO), 2 h daily for 2 days (2 × IMO), or 2 h daily for 6 days (6 × IMO). Rats were killed immediately after stress. Control rats were not subjected to stress. All animal manipulations were performed between 7 a.m. and 1 p.m.
Animals were euthanized by decapitation without anaesthetic and both adrenal medulla and SCG were dissected (Sabban et al. Citation2006). Tissue samples were frozen immediately in liquid nitrogen and kept at − 80°C.
RNA isolation and quantitative RT-PCR
Total RNA was isolated by using RNA-STAT 60 (Tel-Test, Friendswoods, TX, USA) and treated with RNase-free DNase (Ambion, Austin, TX, USA) to remove the genomic DNA contamination according to the manufacturer's protocol. The concentration of RNA was measured with a Quan-it™ Ribo-Green® RNA assay kit (Molecular Probes, Eugene, OR, USA). RT reactions were performed in 5 μl PCR mixture (1 × AMV buffer, 10 mM dNTP, 8 units RNAse inhibitor, 1.25 units AMV, 10 μM reverse primer, and 300 ng of template RNA). Reverse transcription was carried out at 42°C for 1 h followed by 10 min at 85°C.
Real-time quantitative PCR was performed as described previously (Cheng et al. Citation2005) by using LightCycler® 1.2 System (Roche Applied Science, Indiana, USA) with LightCycler® FastStart DNA Master SYBR Green I (Roche Applied Science). The primers for amplification of intron 2 containing transcripts (Sun et al. Citation2004) and TH mRNA with position from NC-005100 were as follows. Note the TH reverse primer spans two exons.
The denaturation program (95°C for 7 min) was followed by a four-segment amplification and quantification program repeated 37 times (95°C for 3 s; 54°C for 3 s for TH mRNA or 59°C for 3 s for TH intron 2; 72°C for 18 s; 85°C for 0 s for a single fluorescence measurement) and a melting curve program (70–99°C) and, finally, a cooling program down to 40°C. The threshold cycle was determined using the Fit Points method to provide optimal standard curve values (0.98–1.0). For quantification assays, a standard curve was used and produced by amplification of several 10-fold dilutions of linearized plasmids containing TH cDNA and TH intron 2 amplicons. The results of real-time PCR were normalized to the amount of total RNA used in the PCR reaction.
Statistical analyses
Statistical significance was determined by Student's t-test for experiments with two groups or by performing an analysis of variance followed by Tukey post-test examination for experiments with more than two groups. A level of p < 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism and InStat Programs (GraphPad Software, Inc., San Diego, CA, USA).
Results
RT-PCR for intron-2 containing transcripts
RNA was used in a broad range of concentrations for RT-PCR with the primers for intron 2 of the TH gene. The analysis was found to be linear over a wide range of concentrations, from 102 to 108 copies. Melting curve analysis revealed that there was a single product with a melting point at about 86°C. No amplification was obtained when the RT step was omitted.
Comparison to standards revealed that basal TH mRNA levels in the rat adrenal medulla were mean ± SE of (2 ± 0.9) × 106 copies/ng RNA and (6 ± 2.6) × 105 copies/ng RNA for transcripts with intron 2. In the SCG, basal TH mRNA levels were (2.5 ± 0.7) × 105 copies/ng RNA and (1.8 ± 0.2) × 104 copies/ng RNA for transcripts with intron 2.
Effect of IMO stress on TH mRNA levels and intron 2 transcripts in adrenal medulla and in the SCG
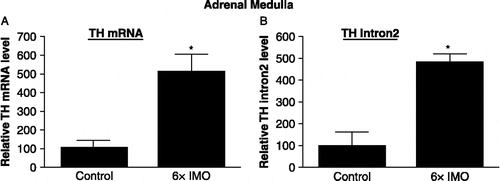
Changes in total TH mRNA levels and in transcripts containing intron 2 after treatment of samples with DNase were detected in adrenal medulla of rats exposed to IMO stress for six consecutive days (6 × IMO) and euthanized immediately thereafter. As shown in , following repeated IMO stress (6 × IMO), there was about a 5-fold increase in TH mRNA levels in the adrenal medulla. There was also a comparable, about 5-fold increase in transcripts containing intron 2 of the TH gene ().
Figure 1 Effect of repeated immobilization (IMO) stress on TH mRNA levels and transcripts containing intron 2 in the adrenal medulla. The relative levels of TH mRNA and transcripts containing intron 2 were determined by RT-PCR in adrenal medulla from controls and rats exposed to 2 h IMO daily for 6 days (6 × IMO). Data are the mean ± S.E. values of TH mRNA or intron 2 levels relative to those in the control samples. The control levels were taken as 100. * p ≤ 0.05 compared to control.
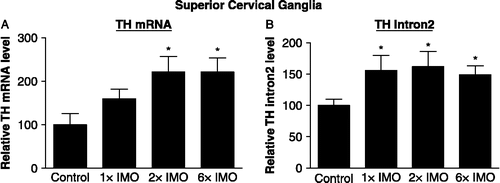
Total RNA was also isolated from the SCG of rats subjected to IMO stress for 2 h once (1 × IMO) or for 2 h daily on two (2 × IMO) or six (6 × IMO) consecutive days and euthanized immediately afterwards and used to determine levels of TH mRNA (). With a single IMO the increase by about 50% in TH mRNA levels was not significantly different from the levels in control rats. TH mRNA levels in the SCG were significantly elevated in the rats exposed to IMO stress repeatedly for 2 or 6 days, and were about double basal levels. The levels of RNA transcripts containing intron 2 in the same samples are shown in . Even a single exposure to IMO elevated transcripts with intron 2 by about 50%. A similar increase was observed following two and six daily exposures to IMO.
Figure 2 Effect of immobilization (IMO) stress on TH mRNA levels and transcripts containing intron 2 in the superior cervical ganglia. The relative levels of TH mRNA and transcripts containing intron 2 were determined by RT-PCR in the SCG from controls and rats exposed to 2 h IMO daily for 1 (1 × IM), 2 (2 × IMO) or 6 (6 × IMO) consecutive days. Data are the mean ± S.E. values of TH mRNA or intron 2 levels relative to those in the control samples. The control levels were taken as 100. * p ≤ 0.05 compared to control.
Discussion
The results of this study show, for the first time, that IMO stress not only elevates TH mRNA levels but also significantly raises TH transcription in the SCG. The levels of RNA transcribed from the TH gene, with intron 2 not yet spliced out, were elevated following single as well as repeated IMO stress. The magnitude of transcriptional activation was much smaller in the SCG than in the adrenal medulla.
Our results for the adrenal medulla are consistent with the previously published report (Sun et al. Citation2004). The authors found about a 5-fold increase in TH mRNA and in intron 2 containing transcripts after a week of daily exposure to 2 h IMO. This finding is also consistent with the changes based on run-on assays of transcription following IMO daily for two or seven consecutive days (Nankova et al. Citation1999). Use of transgeneic mice with the TH promoter directing chloramphenicol acetyl transferase reporter activity demonstrated a similar effect, with TH reporter activity substantially elevated in the adrenal medulla with one, two, three and seven daily exposures to IMO stress (Osterhout et al. Citation1997).
However, to our knowledge this is the first study to determine that the IMO triggered elevation of TH mRNA is associated with increased TH transcripts with unspliced introns in the SCG. This likely reflects increased transcription rate, as it correlates with other measures of transcription in the adrenal medulla, as indicated above. However, we cannot rule out the possibility that the elevated level of transcript containing intron 2 might also reflect a stress triggered reduction in the rate of removal or splicing of this intron in the SCG. Run-on assays of transcription have been difficult to perform in the SCG, as it is harder to disrupt this tissue under conditions which preserve the nuclei intact. Previously, differences were also observed in involvement of AP-1 factors in the adrenal medulla and SCG. AP-1 is involved in trans-synaptic induction of TH gene expression by reserpine in the adrenal medulla but not in the SCG (Trocme et al. Citation1997). In this regard, c-Jun-N-terminal kinase (JNK) is activated in the adrenal medulla at several time intervals of single and repeated IMO, while no statistically significant changes in JNK activity were observed in the SCG under the same conditions (Nankova et al. Citation1998).
The SCG also was found to differ from the adrenal medulla in terms of the transcription factors which bind a proximal cAMP/Ca response element (CRE/CaRE) in the TH gene promoter following reserpine treatment. Reserpine led to enhanced binding of repressor ICER in the adrenal medulla, while in the SCG it enhances the binding of CREM factors to the CRE/CaRE motif on the TH promoter (Trocme et al. Citation2001). Our laboratory found increases in two complexes which bound to the CRE/CaRE motif of the TH promoter in electrophoretic mobility shift assays with SCG of rats exposed to IMO stress. At least one of the complexes contains CREB, or CREB immunoreactive protein (Sabban et al. Citation2004). We speculate that induction of CREB or other CRE binding protein may be involved in the slow IMO-triggered activation of TH transcription detected in the SCG.
In the adrenal medulla, following repeated stress the elevation in transcription, as determined by transcripts containing intron 2, and in mRNA levels are nearly identical at about 5-fold higher than in the controls. However, in the SCG with repeated stress the 2-fold increase in mRNA is somewhat greater than the observed increase in TH transcription, suggesting that TH mRNA stability might also be regulated by the stress. Alternatively, differences at the time of sampling may be due to different kinetics of changes in transcription and subsequent increase in TH mRNA.
The magnitude of the elevation in transcription observed in the SCG is only about 50% under all the conditions. This increase is not yet reflected in significant changes in TH mRNA following a single 2 h IMO. Interestingly, the time course of changes in TH mRNA levels in stellate ganglia following IMO revealed that while there was no significant change immediately after 2 h of IMO, there was a prolonged steady increase in TH mRNA level for at least a day after cessation of the stress (Micutkova et al. Citation2003; Kvetnansky et al. Citation2004). This would suggest that a prolonged sustained elevation of transcription may be responsible for chronic or sustained sympathetic activation, and consequently play an important role in disorders such as cardiovascular disease, metabolic syndrome or decreased immunity exacerbated by stress.
Acknowledgements
This work was supported by NIH grant NS44218.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cannon W. 1929. Oranization of physiological homeostasis. Physiol Rev. 9:399–431.
- Chang MS, Hahn MK, Sved AF, Zigmond MJ, Austin MC, Sherman TG. 2000. Analysis of tyrosine hydroxylase gene transcription using an intron specific probe. J Neurosci Methods. 94:177–185.
- Cheng SY, Glazkova D, Serova L, Sabban EL. 2005. Effect of prolonged nicotine infusion on response of rat catecholamine biosynthetic enzymes to restraint and cold stress. Pharmacol Biochem Behav. 82:559–568.
- Kvetnansky R, Mikulaj L. 1970. Adrenal and urinary catecholamines in rats during adaptation to repeated immobilization stress. Endocrinology. 87:738–743.
- Kvetnansky R, Weise VK, Kopin IJ. 1970. Elevation of adrenal tyrosine hydroxylase and phenylethanolamine-N-methyl transferase by repeated immobilization of rats. Endocrinology. 87:744–749.
- Kvetnansky R, Gewirtz GP, Weise VK, Kopin IJ. 1971. Catecholamine-synthesizing enzymes in the rat adrenal gland during exposure to cold. Am J Physiol. 220:928–931.
- Kvetnansky R, Micutkova L, Rychkova N, Kubovcakova L, Mravec B, Filipenko M, Sabban EL, Krizanova O. 2004. Quantitative evaluation of catecholamine enzymes gene expression in adrenal medulla and sympathetic ganglia of stressed rats. Ann N Y Acad Sci. 1018:356–369.
- Micutkova L, Rychkova N, Sabban EL, Krizanova O, Kvetnansky R. 2003. Quantitation of changes in gene expression of norepinephrine biosynthetic enzymes in rat stellate ganglia induced by stress. Neurochem Int. 43:235–242.
- Nankova B, Kvetnansky R, Hiremagalur B, Sabban B, Rusnak M, Sabban EL. 1996. Immobilization stress elevates gene expression for catecholamine biosynthetic enzymes and some neuropeptides in rat sympathetic ganglia: Effects of adrenocorticotropin and glucocorticoids. Endocrinology. 137:5597–5604.
- Nankova BB, Fuchs SY, Serova LI, Ronai Z, Wild D, Sabban EL. 1998. Selective in vivo stimulation of stress-activated protein kinase in different rat tissues by immobilization stress. Stress. 2:289–298.
- Nankova BB, Tank AW, Sabban EL. 1999. Transient or sustained transcriptional activation of the genes encoding rat adrenomedullary catecholamine biosynthetic enzymes by different durations of immobilization stress. Neuroscience. 94:803–808.
- Nankova BB, Kvetnansky R, Sabban EL. 2003. Adrenocorticotropic hormone (MC-2) receptor mRNA is expressed in rat sympathetic ganglia and up-regulated by stress. Neurosci Lett. 344:149–152.
- Osterhout CA, Chikaraishi DM, Tank AW. 1997. Induction of tyrosine hydroxylase protein and a transgene containing tyrosine hydroxylase 5′ flanking sequences by stress in mouse adrenal gland. J Neurochem. 68:1071–1077.
- Sabban EL, Kvetnansky R. 2001. Stress-triggered activation of gene expression in catecholaminergic systems: Dynamics of transcriptional events. Trends Neurosci. 24:91–98.
- Sabban EL, Nankova BB, Serova LI, Kvetnansky R, Liu X. 2004. Molecular regulation of gene expression of catecholamine biosynthetic enzymes by stress: Sympathetic ganglia versus adrenal medulla. Ann N Y Acad Sci. 1018:370–377.
- Sabban EL, Liu X, Serova L, Gueorguiev V, Kvetnansky R. 2006. Stress triggered changes in gene expression in adrenal medulla: Transcriptional responses to acute and chronic stress. Cell Mol Neurobiol. 26:845–856.
- Serova LI, Gueorguiev V, Cheng SY, Sabban EL. 2008. Adrenocorticotropic hormone elevates gene expression for catecholamine biosynthesis in rat superior cervical ganglia and locus coeruleus by an adrenal independent mechanism. Neuroscience. 153:1380–1389.
- Sun B, Chen X, Xu L, Sterling C, Tank AW. 2004. Chronic nicotine treatment leads to induction of tyrosine hydroxylase in locus ceruleus neurons: The role of transcriptional activation. Mol Pharmacol. 66:1011–1021.
- Thoenen H. 1970. Induction of tyrosine hydroxylase in peripheral and central adrenergic neurones by cold-exposure of rats. Nature. 228:861–862.
- Thoenen H, Mueller RA, Axelrod J. 1969. Trans-synaptic induction of adrenal tyrosine hydroxylase. J Pharmacol Exp Ther. 169:249–254.
- Trocme C, Mallet J, Biguet NF. 1997. AP-1 mediates trans-synaptic induction of tyrosine hydroxylase gene expression in adrenal medulla but not in superior cervical ganglia. J Neurosci Res. 48:489–498.
- Trocme C, Ravassard P, Sassone-Corsi P, Mallet J, Biguet NF. 2001. CREM and ICER are differentially implicated in trans-synaptic induction of tyrosine hydroxylase gene expression in adrenal medulla and sympathetic ganglia of rat. J Neurosci Res. 65:91–99.