Abstract
A short (S) variant, compared to a long (L) variant, of the promoter region of the serotonin transporter gene-linked polymorphic region (5HTTLPR) has been related to emotional hyper-reactivity. We tested whether the 5HTTLPR could modulate acute stress responses in the brain and, the cardiovascular and neuroendocrine systems. Ten Japanese male participants carrying double copies of the S alleles and 10 Japanese males carrying S and L alleles conducted a mental arithmetic task, and their regional cerebral blood flow by 15O positron emission tomography and cardiovascular and neuroendocrine parameters were measured. During the acute stress task, the participants with the SS alleles showed stronger reactivity in blood pressure and secretion of epinephrine, compared to the participants with the SL and LL alleles. Furthermore, the SS carriers showed greater activation in stress-related brain regions such as the hypothalamus, cerebellum, midbrain, and pulvinar compared to the SL and LL carriers during the acute stress task. The present findings indicated that the S allele of the 5HTTLPR is associated with greater brain and physiological reactivity to acute stress in Japanese men.
Introduction
Acute stressors stimulate the sympathetic and adrenomedullary (SAM) systems and the hypothalamic–pituitary–adrenal (HPA) axis, leading to physiological responses which can be interpreted as recruitment of energy supply to cope against a real or perceived threat to survival. Thus, stress reactivity is essential for environmental adaptation, and conversely, dysfunction of this reactivity can be a cause of physiological and psychiatric disorders (McEwen Citation1998; Charmandari et al. Citation2005). One major genetic factor determining inter-individual differences in the stress reactivity is polymorphism of the serotonin (5-hydroxytryptamine, 5HT) transporter (5HTT).
5HTT plays a critical role in regulating 5HT levels in the brain by transporting 5HT from the extracellular space into 5HT neurons. The human 5HTT gene is encoded on the chromosome 17q11.1-q12 (Ramamoorthy et al. Citation1993) and has a polymorphism in the 5′-flanking promoter region termed the serotonin transporter gene-linked polymorphic region (5HTTLPR) (Heils et al. Citation1995). In lymphoblast cell lines containing the promoter sequence (long (L) or short (S) form of 5HTTLPR), the promoter activity of the 5HTT gene is dependent on these allelic variants (Heils et al. Citation1996; Heinz et al. Citation2000). Transcriptional activity of the L allele is more than twice that of the S allele (Collier et al. Citation1996). Thus, the S promoter allelic variant is linked to the lower expression of 5HTT mRNA, resulting in lower serotonin reuptake, when compared to the L allelic variant (Lesch et al. Citation1996; Bradley et al. Citation2005).
In animal studies, rhesus macaques carrying an S allele of 5HTTLPR exhibited exaggerated behavioral and neuroendocrine responses to acute stress and abnormalities in 5HT metabolism (Champoux et al. Citation2002; Barr et al. Citation2004). Accordingly, 5HTT knockout mice showed facilitated catecholamine responses to brief and mild stressors when compared to wild-type controls (Tjurmina et al. Citation2002; Li et al. Citation2004). However, in human studies, findings are mixed. Individuals with one or two copies of the S allele exhibited higher rates of depression and suicidality as a function of exposure to increasing levels of stressful events than did individuals with two copies of the L allele (Caspi et al. Citation2003). The S allele carriers also showed enhanced secretion of cortisol in response to psychological acute stressors (Gotlib et al. Citation2008). The S allele was also associated with greater heart rate (HR) reactivity to laboratory stress in women (McCaffery et al. Citation2003). However, CitationWilliams et al. (2001, 2008) reported opposite results that the L allele was associated with enhanced cardiovascular responses to mental stress. To date, there has been no direct test for the effects of 5HTTLPR on reactivity of the SAM system and of the HPA axis to acute stress challenges.
One research strategy to examine further the above described mixed findings about the involvement of 5HTTLPR in the SAM and HPA stress responses might be to integrate measurement of such responses and brain function. Though no direct test of association of the 5HTTLPR with brain activation under acute stress has been reported yet, other evidence suggests a possible linkage between the 5HTTLPR and stress reactivity. Neuroimaging studies found that the S allele carriers showed greater activation in the amygdala and other emotion-related brain regions when facial expressions of fear were presented (CitationHariri et al. 2002, 2005). Furthermore, the S allele carriers showed uncoupling in the cingulate and amygdala circuit, which is critical for emotion regulation (Heinz et al. Citation2005; Pezawas et al. Citation2005).
Based on such previous findings, we inferred that 5HTTLPR might explain variations in the functional association of brain and peripheral physiological reactivity to an acute stressor, specifically, that S allele carriers would show greater activation in stress-related brain regions and more prominent enhancement of physiological responses. We examined this hypothesis by simultaneous measurement of 15O–water positron emission tomography (PET) and cardiovascular (HR; SBP, systolic blood pressure; and DBP, diastolic blood pressure), and neuroendocrine (plasma epinephrine, norepinephrine, and adrenocorticotropic hormone (ACTH) concentrations) indices during a typical experimental acute stress task; mental arithmetic with time pressure (Isowa et al. Citation2004; Kimura et al. Citation2005; Ohira et al. Citation2008). We chose PET as a neuroimaging technique because of its convenience for simultaneous recording of physiological indices compared to other techniques, such as functional magnetic resonance imaging.
Most previous studies using Caucasian samples compared the S allele carriers (SS and SL) with the LL allele carriers, or reported dose-dependent effects of the S allele. However, the distribution of 5HTT genotypes is different between ethnic groups. The frequencies of the L allele are over 70% in Africans, 50–60% in Caucasians, and less than 30% in Asians (Williams et al. Citation2001; Mizuno et al. Citation2006). Considering this, we compared Japanese male SS and SL + LL allele carriers in the present study.
Methods
Participants
Ten participants with the SS allele for 5HTTLPR and 10 participants with the SL (n = 8) or LL allele (n = 2) participated in the present study. All the participants were male, right-handed, Japanese, and undergraduate or graduate students of Nagoya University (age (mean ± standard error); SS: 21.5 ± 0.45 years, SL + LL: 22.7 ± 1.02 years). The participants were healthy, had no past history of psychiatric or neurological illness, and were not taking any medications. Only men were studied to avoid influences of the menstrual cycle on any physiological stress responses in women (Kudielka and Kirschbaum Citation2004). All participants gave written informed consent in accordance with the Declaration of Helsinki. The present study was approved by the Human Studies Committee of Aichi Medical University and the Ethics Committee of Kizawa Memorial Hospital.
Genotyping
Genomic DNA was extracted with a DNA Extractor WB-Rapid Kit (Wako Inc., Osaka, Japan) from fresh or frozen blood samples collected from the participants. Polymerase chain reactions (PCR) were performed using the primers, 5′-GGCGTTGCCGCTCTGAATGC and 5′-GAGGGACTGAGCTGGACAACCAC, in a total volume of 25 μl solution containing 100 ng genomic DNA, 0.4 mM dNTPs, 0.2 μM of each primer, 1.25 U of Takara LA Taq polymerase (Takara Bio Inc., Otsu, Japan), and GC buffer I (Takara Bio Inc., Ohtsu-shi, Japan). Initial denaturation at 95°C for 5 min was followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 61°C for 30 s, and extension at 72°C for 1 min. The PCR products were then analyzed in a 2% agarose gel stained with ethidium bromide. The amplification product for the L allele was 528 bp, and for the S allele was 484 bp (Lesch et al. Citation1996). By this procedure, individuals carrying double copies of the S alleles (SS genotype), individuals carrying the S and L alleles (SL genotype), and individuals carrying double copies of the L alleles (LL genotype) were identified. In the present study we compared stress responses in the SS (n = 10) genotype and the genotypes because the proportion of the LL genotype is small in the Asian population (Mizuno et al. Citation2006).Footnote1
Procedures
Participants were instructed to eat a light breakfast on the morning of the experiment, and caffeine-containing beverages were not allowed. Participants suffering from an infectious illness within 2 weeks of the experiment were rescheduled.
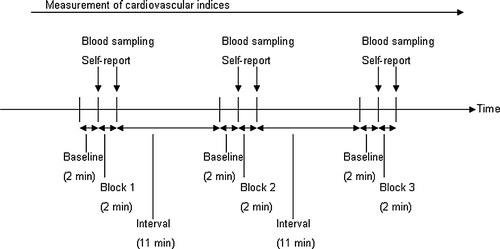
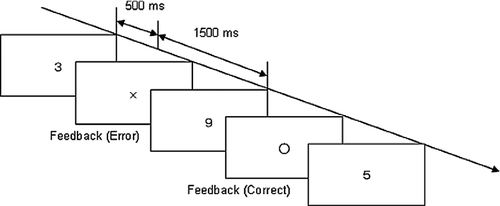
Participants performed the acute stress task in three blocks, each lasting for 2 min. Just before each block, there was a 2 min baseline period. In addition, there was an 11 min interval between a block and the next baseline period. In the acute stress task, the participants were told to add the currently displayed number (from 2 to 9) to the next one shown on a PC monitor, and to orally report the sum of the numbers (). In cases in which the sum equaled a number of two figures, participants were asked only to report the last figure of the number. Each number was displayed for 500 ms, and followed by a 1500 ms interval before the next was displayed. In each trial, feedback about correctness/error for each answer was given by displaying visual symbols such as a circle or a cross. To maintain motivation, participants were instructed to obtain more than 90% correct answers.
Figure 1 The mental arithmetic task used as an acute stressor. When number ‘9’ in this figure is presented, a participant adds ‘9’ and ‘3’, the previous number, and orally reports “2”, the last figure of the sum. If the participant's answer is correct, feedback signal ‘O’ is presented, if the answer is incorrect, feedback signal ‘ × ’ is presented.
Just before and immediately at the end of each test block, participants were required to rate the strength of stress experienced on a percentage scale (0%, not stressful at all; 100%, extremely stressful). The participants received a PET scan of 60 s in each block. Just before the start of each block and just after the end of each block, blood samples (6 ml each) were taken using a heparinized 22-gauge butterfly catheter placed in the antecubital vein of the right forearm to measure neuroendocrine indices (). Cardiovascular indices were continuously recorded throughout the experimental session. All the participants were paid 15,000 yens for participation.
Measurement of cardiovascular and neuroendocrine data
Cardiodynamic activity was recorded using electrocardiography (ECG) and non-invasive finger blood pressure (FINAP) measurements. To determine HR, ECG was recorded using a MP 100 system (BIOPAC Systems Inc., Goleta, CA, USA). SBP and DBP were recorded using the finger cuff of a Portapres Model 2 (TNO Biomedical Instrumentation Inc., Amsterdam, The Netherlands) attached to the third finger of the non-dominant arm of each participant. Analyses of ECGs and FINAP waveforms were performed using the AcqKnowledge software for the MP 100 system. Mean values of HR, SBP, and DBP were determined for 2 min, just before the task as baseline, and during 2 min of the task in each block.
Blood samples for plasma catecholamine and ACTH were anticoagulated with ethylenediamine tetra-acetate, chilled, and centrifuged; the plasma was then removed and frozen at − 80°C until analysis. Plasma epinephrine and norepinephrine were determined by high performance liquid chromatography. Alumina was used for extraction; the recovery rate for all amines, evaluated with a dihydroxybenzylamine standard, was between 60 and 70%. The intra-assay coefficient of variation was less than 5%, and the inter-assay variations were less than 6% for the measurement of epinephrine and norepinephrine. ACTH was assayed in triplicates using an immunoradiometric assay (Mitsubishi Chemical Inc., Tokyo, Japan). The intra-assay coefficient of variation was less than 6%, and inter-assay variations were less than 7% for measurement of ACTH.
Neuroimaging by PET
During each block, distribution of regional cerebral blood flow (rCBF) was measured with a General Electric ADVANCE NXi PET scanner operated in a high-sensitivity three-dimensional mode. A venous catheter for administering the tracer was inserted in an antecubital fossa vein in the left forearm. After the participant's head was positioned in the inflatable plastic head-holder that prevented possible head movements, a 10 min transmission scan using a rotating 68germanium pin source was completed. In each block, following a 370 MBq bolus injection of H215O over 30 s, scanning was started and continued for 60 s. Initiation of bolus injection was time-locked to the start of presentation of the stimulus, and presentation of the stimulus lasted until 30 s after termination of scanning. The integrated radioactivity accumulated during 60 s of scanning was used as the index of rCBF. Three scans were acquired per subject, and the interval between successive scans was 15 min in order to allow for radioactive levels to return to baseline level. A Hanning filter was used to reconstruct images into 35 planes with 4.5 mm thickness and a resolution of 2 mm × 2 mm (full width half maximum).
SPM 99 (Friston et al. Citation1995) implemented in Matlab (version 5.3, Mathworks, Sherborn, MA, USA) was used for spatial pre-processing and statistical analyses. Images were initially realigned using sinc-interpolation to remove artifacts before being transformed into a standard stereotactic space. Images were corrected for whole brain global blood flow by proportional scaling, and smoothed using a Gaussian kernel to a final in-plane resolution of 10 mm at full width at half maximum. Differences within genotypes (SS vs. SL + LL) were the primary focus of the present study, and thus, two patterns of subtraction analyses of images were conducted to reveal significant increases of rCBF. The effects at each voxel were estimated using a general linear model; SS − SL + LL, and SL + LL − SS. Voxel values for each contrast yielded a statistical parametric map of the t statistic (SPM t), and were subsequently transformed to a unit normal distribution (SPM Z). Conventional peak voxel-value significance thresholds were set at p < 0.001 (uncorrected), and cluster significance thresholds were set at 5 voxel.
Statistical analyses
Rate of accurate answers in the mental arithmetic task as task performance in each block was subjected to a two-way mixed (Genotype (SS vs. SL + LL: a between participant factor) × Block (1, 2, 3: a within participant factor)) analysis of variance (ANOVA). Data for task performance of two participants in the SS group and two participants in the SL + LL group were missed because of a technical problem. For subjective stress data, change in the value was calculated by subtracting the score before the task from the score after the task in each block for each participant. Such change values were analyzed using two-way mixed (Genotype (SS vs. SL + LL: a between participant factor) × Block (1, 2, 3: a within participant factor)) ANOVAs. For cardiovascular data, mean values of HR, SBP, and DBP were calculated during baseline and task periods, and subjected to three-way mixed (Genotype (SS vs. SL + LL: a between participant factor) × Block (1, 2, 3: a within participant factor) × Period (baseline vs. task: a within participant factor)) ANOVAs. For neuroendocrine data, values for concentrations of epinephrine, norepinephrine, and ACTH at baseline and after task at each block were subjected to three-way mixed (Genotype (SS vs. SL + LL: a between participant factor) × Block (1, 2, 3: a within participant factor) × Period (baseline vs. task: a within participant factor)) ANOVAs. The Greenhouse-Geisser epsilon correction factor was used where appropriate. In cases where significant effects were found in the ANOVAs, post hoc analyses using Tukey tests (p < 0.05) were conducted to examine which combinations of data points differed significantly.
Results
Self-report and behavioral data
Changes in subjective stress values and accuracy in the mental arithmetic task in both groups are shown in . ANOVAs revealed no significant effects, suggesting that subjective perceived stress and task performance did not differ between the 5HTTLPR genotypes.
Table I. 5HTTLPR genotype and cardiovascular and neuroendocrine parameters before and after stressor for each block.
Cardiovascular and neuroendocrine data
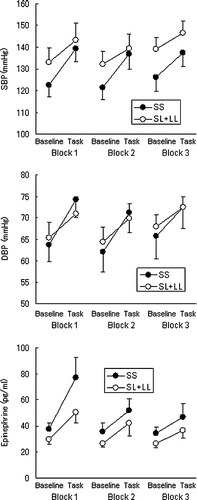
Cardiovascular and neuroendocrine data are shown for both groups in . For cardiovascular indices, significant interactions between Genotype and Period were observed in SBP (F (1, 18) = 4.56, p < 0.05, ) and DBP (F (1, 18) = 5.04, p < 0.05,
). These significant interactions indicate that the SS genotype showed greater reactivity in SBP and DBP to acute stress (). In addition, interactions between Block and Period (SBP: F (2, 36) = 3.53, p < 0.05,
; DBP: F (2, 36) = 4.28, p < 0.05,
) and the main effects of Period (SBP: F (1, 18) = 61.27, p < 0.01,
; DBP: F (1, 18) = 75.41, p < 0.01,
) were significant for both indices. Tukey tests (p < 0.05) revealed that both the SS and SL + LL genotypes showed significant elevation of blood pressure during the acute stress task in all blocks. In HR, the ANOVA revealed a significant interaction between Block and Period (F (2, 36) = 8.84, p < 0.01,
) and a main effect of Period (F (1, 18) = 40.59, p < 0.01,
), indicating that HR significantly increased during the task in both groups, especially in the first block. However, no significant effect of Genotype was observed. Furthermore, analyses of the changes in SBP, SBP, and HR from baseline to task showed no significant effects of Genotype (data not shown). For neuroendocrine indices, a significant interaction between Genotype, Block, and Period was observed for epinephrine (F (2, 36) = 3.30, p < 0.05,
). An interaction between Block and Period (F (2, 36) = 12.19, p < 0.01,
) and main effects of Block (F (2, 36) = 11.33, p < 0.01,
) and Period (F (1, 18) = 14.86, p < 0.01,
) were also significant. Additional analyses using Tukey tests (p < 0.05) revealed that concentrations of epinephrine were significantly increased after the task in both groups in all blocks. Furthermore, plasma epinephrine concentration in the SS carriers was greater than that of the SL + LL carriers after the task in the first block, whereas plasma epinephrine concentration was not different between the two groups at baseline in the first block (). No significant effects were found for norepinephrine or ACTH. We further compared the SS carriers (n = 10) and SL carriers (n = 10; without the LL carriers) for all indices using the same statistical methods described. The SL group indices were not distinguishable from values in the SL + LL group. Statistical tests for comparisons between the SS and SL groups showed similar results as comparisons of the SS and SL + LL groups, although interactions of Genotype and Period in SBP and DBP only showed trends to significance (SBP: F (1, 16) = 3.58, p < 0.08,
; DBP: F (1, 16) = 3.84, p < 0.07,
), which may reflect decreased statistical power. A previous report indicated that SL and LL groups have similar physiological responses to acute stress (Gotlib et al. Citation2008). Furthermore, the majority of the Japanese population comprises SS and SL carriers. Hence, we inferred that comparisons between the SS and SL + LL groups are meaningful.
Figure 3 Responses of arterial blood pressures and plasma epinephrine concentration. Data are plotted against blocks (1, 2 and 3) during which the arithmetic task was performed and period within each block (baseline vs. task. SBP, systolic blood pressure; DBP, diastolic blood pressure. See for statistical analysis.
PET data
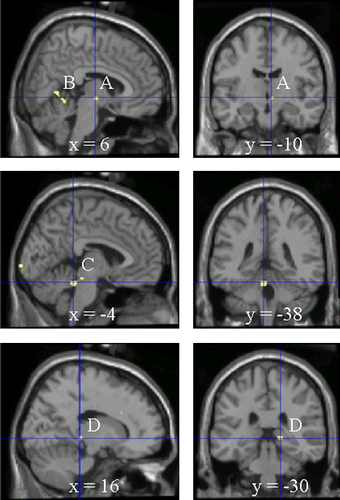
Results of subtraction analyses are summarized in . Subtraction of the SS from the SL + LL group data revealed a significant increase in rCBF in the cerebellum, midbrain, hypothalamus, and pulvinar (). Additionally, significant activation was also found in the putamen, loci in the occipital areas (BA19), and in the hippocampus. No significant increase in rCBF was observed in the reversed pattern of subtraction ((SL + LL) − SS).
Table II. Significant increases in rCBF during arithmetic task, by b PET and subtraction of SS from SL + LL 5HTTLPR genotype values.
Figure 4 Differences in activated brain regions between 5HTTLPR genotypes during the arithmetic task. Activated brain regions were identified by PET and by subtraction of SS from SL + LL values (p < 0.001, uncorrected). A, hypothalamus; B, cerebellum; C, midbrain; D, pulvinar. See for statistical analysis.
Discussion
The present study revealed that human carriers of homogeneous S alleles for the 5HTTLPR showed a tendency for greater reactivity of blood pressure to an acute stressor when compared to the SL and LL carriers (). Epinephrine reactivity, which reflects SAM activity, was greater in the SS carriers than in the SL + LL carriers, in the initial stage (Block 1) but not in later stages (Blocks 2 and 3) of the stress task (). Additionally, although HR increased during each block, a difference in HR reactivity between 5HTTLPR genotypes was not statistically significant. Hence, taken together, the enduring greater reactivity for BP shown in the SS carriers may be attributed not only to β-adrenergic cardiac effects, but also to α-adrenergic vascular effects. An explanation for modulation of sympathetic activity by the central serotonin system has come from animal studies. Stimulation of 5HT1A receptors attenuates neuroendocrine stress responses mediated by hypothalamic responses in mice (Li et al. Citation2004), and hippocampal norepinephrine levels and the c-Fos immunoreactivity of the rat locus coeruleus (Rioja et al. Citation2007). Given reduced serotonin neurotransmitter function via increased firing rates of serotonin neurons (Barton et al. Citation2008) and thus down-regulation of 5HT1A receptors (Hariri and Holmes Citation2006) in the SS carriers, the findings in animal studies lead us to infer activation in the hypothalamus, hippocampus, and midbrain, and in turn, enhanced sympathetic responses during exposure to acute stress in the SS carriers of 5HTTLPR in the present study.
We measured concentrations of epinephrine and norepinephrine in venous blood samples, which do not necessarily reflect precisely sympathetic activity (Esler et al. Citation1989; Thompson et al. Citation1995). Thus, the hypothesis about enhanced sympathetic activation in the SS carriers cannot be tested directly. Furthermore, considering plasma kinetics of catecholamines, the duration of the experimental blocks in this study (2 min) might be relatively short to evaluate stress reactivity of catecholamines. The duration of the blocks was determined for the neuroimaging technique using 15O − PET, because the half life of 15O is 2 min. This experimental procedure might not have imposed a high stress load, so the epinephrine responses of the participants habituated rapidly (; ). The same experimental procedure robustly elicited catecholamine responses to stress previously (Ohira et al. Citation2008), but a more prolonged and intense stressor would seem desirable to evaluate reactivity in the SAM system.
Carriers of the SS alleles for 5HTTLPR, compared to carriers of the L allele, indicated more activation in the hypothalamus, which is the central regulator of the SAM and HPA systems. Although a limitation in the spatial resolution of PET imaging prevented the detailed identification of which nuclei in the hypothalamus were involved, the activated area was located in a dorsomedial part of the hypothalamus, which is considered a crucial mediator of autonomic acute stress responses (Horiuchi et al. Citation2006). The SS carriers showed more activation than the L carriers also in the cerebellum, which is another important component in a central autonomic network (Spyer Citation1999). The cerebellum has mutual neural pathways with several nuclei of the hypothalamus including the dorsomedial nucleus and with the brainstem, and is deeply involved in autonomic modulation of cardiovascular and respiratory functions (Zhu et al. Citation2006). Furthermore, greater involvement of the midbrain in the SS carriers than in the L carriers may reflect that both ascending influences on the cortex and descending influences on autonomic activity originate within this region. The peak of activation in the SS carriers when compared to other genotypes was in the dorsal portion of the midbrain adjacent to the cerebellum. Here, the parabrachial nucleus which projects directly to efferent autonomic tracts (Korte et al. Citation1992; Baffi and Palkovits Citation2000) or the locus coeruleus which contains central norepinephrine neurons (Sands et al. Citation2000; Charney Citation2004), are plausible sites for regulation of acute stress-induced physiological responses. Importantly, involvement of those brain regions where activation was observed in the SS carriers in the present study have been reported in previous functional neuroimaging studies using psychological acute stressors such as mental arithmetic tasks, Stroop tasks, a public speech, viewing emotional pictures, and social isolation (CitationCritchley et al. 2000, 2005; Coan et al. Citation2006; Ohira et al. Citation2006; Eisenberger et al. Citation2007; Ohira et al. Citation2008; Pruessner et al. Citation2008).On the basis of these findings, it is suggested that the stress-related neural network was more recruited in the SS carriers when exposed to an acute stressor, which might enhance their sympathetic responses. Such a conclusion is contrary to previous studies reporting greater sympathetic reactivity in the L carriers than in the S carriers (CitationWilliams et al. 2001, 2008), and this contrast requires further investigation.
We found activation in several other brain regions in the SS carriers; the pulvinar, putamen, hippocampus. The pulvinar is a large thalamic nucleus that is involved in multimodal sensory processing (Jones Citation1985). It conveys visual emotional signals directly to limbic areas such as the amygdala and anterior cingulate cortex (Jones and Burton Citation1976; Romanski et al. Citation1997). Importantly, individuals with the SS alleles of 5HTTLPR have more pulvinar neurons, so might have enhanced subcortical input of emotionally relevant stimuli (Young et al. Citation2007). Activation of the pulvinar in the SS carriers observed in the present study seems to support these previous findings. Additionally, both the putamen and hippocampus are considered to be the parts of a neural network processing negative affect (Gray Citation1988; Davis et al. Citation1997; Calder et al. Citation2000; Lange et al. Citation2005), thus activation in these regions in the SS carriers may reflect negative affect related to perception of the task. Contrary to previous studies (CitationHariri et al. 2002, 2005; Heinz et al. Citation2005; Pezawas et al. Citation2005), amygdalar activation was not shown in the SS carriers in the present study. One explanation may be that the task in the present study demanded effort, but was not emotional and threatening, unlike presentation of aversive pictures used by others.
The effects of 5HTTLPR genotype on cardiovascular, epinephrine, and brain responses might be caused by differences in cognitive ability rather than stress reactivity. Namely, the SS carriers might have found the mental arithmetic task harder than the SL + LL carriers resulting in greater reactivity. Indeed, recent studies of animals (Izquierdo et al. Citation2007; Vallender et al. Citation2008) and humans (Fiedorowicz et al. Citation2007; Jollant et al. Citation2007) have shown that the S allele compared to the L allele is associated with reduced higher cognitive function. However, another study has shown no differences for basic intelligence between human 5HTTLPR genotypes (Gotlib et al. Citation2008). Nonetheless, the mental arithmetic task used in the present study was rather simple, and the subjective rating of perceived stress and the task performance by the SS carriers were not different from those of the SL and LL carriers, indicating similar difficulty in performing the task among the genotypes. Furthermore, the brain areas activated in the significant comparison (SS minus SL + LL) were subcortical stress-related regions and not regions involved in higher cognitive function.
The HPA (ACTH) responses to the acute stressor did not differ between the 5HTTLPR genotypes. This result, evidently inconsistent with a previous finding (Gotlib et al. Citation2008), might relate to differences in the stress tasks. A human stress protocol generally elicits cardiovascular responses, but without an audience, as in the present study, may not activate the HPA axis (Kirschbaum et al. Citation2001). These phenomena have been interpreted as greater SAM system sensitivity to a stressor demanding effort, and HPA axis sensitivity to threatening and uncontrollable stressors (Henry Citation1992).
The present study has several limitations. Firstly, the sample size was small for a genetic study, although we found statistically significant differences for adrenaline secretion and brain activation between the 5HTTLPR genotypes, replication with a larger sample would be important. However, Roiser et al. (Citation2006), compared 15 individuals with the SS genotype and 15 with the LL genotype for 5HTTLPR, and reported robust significant differences in behavioral indices in a reaction time task. Neural and physiological responses, rather than behavioral and psychological responses, are usually sensitive to 5HTTLPR genotype (Hariri and Holmes Citation2006); the significant effects of the genotype with PET imaging, cardiovascular, and neuroendocrine parameters in this study are consistent with this pattern. Secondly, complex interactions between 5HTTLPR genotypes, gender, and ethnicity have been reported. For example, compared to white persons, African Americans showed opposite effects of 5HHLPR genotype on their traits; the L allele was associated with higher levels of negative affect (Gelernter et al. Citation1998). Further, while Japanese male SS carriers show higher levels of trait anxiety than SL and LL carriers, the association of 5HTTLPR genotype and anxiety was reversed in Japanese females (Mizuno et al. Citation2006). Additionally, a gender and ethnicity interaction has been reported for association between 5HTTLPR genotype and cerebrospinal fluid level of 5-hydroxyindoleacetic acid (Williams et al. Citation2003). Thus, whether our findings are specific to Japanese males, or apply to other populations remains to be determined.
In conclusion, despite the limitations, the present study indicates that the S allele for 5HTTLPR is associated with greater reactivity in specific brain regions and in peripheral sympathetic responses to an acute laboratory stress in Japanese men.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research of the Japan Society for the Promotion of Science (No. 16330136) and by a Health and Labour Sciences Research Grant on Research on Occupational Safety and Health from Japan Ministry of Health, Labour, and Welfare (No. H17-RODO-5). The authors thank Dr Shuichi Ueno of Tokushima University for his technical advice. Portions of the present study were presented at the 13th Annual Meeting of the Organization for Human Brain Mapping (Chicago, June 2007). The experiment reported in this article complies with the current law of Japan. The 12th author (SF) contributed to this study equally as the first author (HO).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Notes
1. Recently it has been shown that the L allele includes an adenine (A) –guanine (G) single nucleotide polymorphism in the repeated structure (Nakamura et al. Citation2000). Unlike the LA allele, the LG allele leads to low 5HTT expression, almost equivalent to that of the S allele (Hu et al. Citation2006). This might cause a wide variation in stress-related 5HTT functions in L allele carriers. However, we chose to conduct main analyses by classifying participants into three genetic groups based on their S and L alleles, because of the small number of participants. Nevertheless, we conducted supplementary analyses for effects of this SNP on each index of psychological, behavioral, physiological, and neuroimaging responses. To distinguish between the LA and LG fragments, the PCR product was digested with MspI (Nippon Gene Inc., Tokyo, Japan) and the resulting polymorphic fragments were separated on a 2% agarose gel (LA: 340, 127, and 62 bp; LG: 174, 166, 127, and 62 bp). It was shown that the SL + LL group in this study comprised 5 SLA participants, 3 SLG participants, 1 LALA participant, and 1 LGLG participant. With the small statistical power, no significant effect of the SNP on any dependent variable was observed.
References
- Baffi JS, Palkovits M. 2000. Fine topography of brain areas activated by cold stress. A fos immunohistochemical study in rats. Neuroendocrinology. 72:102–113.
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. 2004. Rearing condition and rh5-HTTLPR interact to influence limbic–hypothalamic–pituitary–adrenal axis response to stress in infant macaques. Biol Psychiatry. 55:733–738.
- Barton DA, Esler MD, Dawood T, Lambert EA, Haikerwal D, Brenchley C, Socratous F, Hastings J, Guo L, Wiesner G, Kaye DM, Bayles R, Schlaich MP, Lambert GW. 2008. Elevated brain serotonin turnover in patients with depression: Effect of genotype and therapy. Arch Gen Psychiatry. 65:38–46.
- Bradley SL, Dodelzon K, Sandhu HK, Philibert RA. 2005. Relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. Am J Med Genet. 136:58–61.
- Calder AJ, Keane J, Manes F, Antoun N, Young AW. 2000. Impaired recognition and experience of disgust following brain injury. Nat Neurosci. 3:1077–1078.
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. 2003. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 301:386–389.
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. 2002. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 7:1058–1063.
- Charmandari E, Tsigos C, Chrousos G. 2005. Endocrinology of the stress response. Annu Rev Physiol. 67:259–284.
- Charney DS. 2004. Psychobiological mechanisms of resilience and vulnerability: Implications for successful adaptation to extreme stress. Am J Psychiatry. 161:195–216.
- Coan JA, Schaefer HS, Davidson RJ. 2006. Lending a hand: Social regulation of the neural response to threat. Psychol Sci. 17:1032–1039.
- Collier DA, Stöber G, Li T, Heils A, Catalano M, Di Bella D. 1996. A novel functional polymorphism within the promoter of the serotonin transporter gene: Possible role in susceptibility to affective disorders. Mol Psychiatry. 1:453–460.
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. 2000. Cerebral correlates of autonomic cardiovascular arousal: A functional neuroimaging investigation in humans. J Physiol. 523:259–270.
- Critchley HD, Taggart P, Sutton PM, Holdright DR, Batchvarov V, Hnatkova K, Malik M, Dolan RJ. 2005. Mental stress and sudden cardiac death: Asymmetric midbrain activity as a linking mechanism. Brain. 128:75–85.
- Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. 1997. Functional MRI of pain-and attention-related activations in the human cingulate cortex. J Neurophysiol. 77:3370–3380.
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. 2007. Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage. 35:1601–1612.
- Esler M, Jennings G, Lambert G. 1989. Measurement of overall and cardiac norepinephrine release into plasma during cognitive challenge. Psychoneuroendocrinology. 14:477–481.
- Fiedorowicz JG, Moser DJ, Hynes SM, Beglinger LJ, Schultz SK, Ellingrod VL. 2007. LA allelic heterozygosity of the 5HTTLPR polymorphism is associated with higher cognitive function and lower interpersonal sensitivity. Psychiatr Genet. 17:3–4.
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. 1995. SPMs in functional imaging: A general linear approach. Hum Brain Mapp. 1:214–220.
- Gelernter J, Kranzler H, Coccaro EF, Siever LJ, New AS. 1998. Serotonin transporter protein gene polymorphism and personality measures in African American and European American participants. Am J Psychiatry. 155:1332–1338.
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. 2008. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 63:847–851.
- Gray JA. 1988. The neuropsychological basis of anxiety: Handbook of anxiety disorder. New York: Pergamon Press. 10–40.
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. 2005. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 62:146–152.
- Hariri AR, Holmes A. 2006. Genetics of emotional regulation: The role of the serotonin transporter in neural function. Trends Cogn Sci. 10:182–191.
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. 2002. Serotonin transporter genetic variation and the response of the human amygdala. Science. 297:400–403.
- Heils A, Teufel A, Petri S, Seemann M, Bengel D, Balling U, Riederer P, Lesch KP. 1995. Functional promoter and polyadenylation site mapping of the human serotonin (5-HT) transporter gene. J Neural Transm Gen Sect. 102:247–254.
- Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP. 1996. Allelic variation of human serotonin transporter gene expression. J Neurochem. 66:2621–2624.
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger DR. 2000. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 47:643–649.
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grüsser SM, Flor H, Schumann G, Mann K, Büchel C. 2005. Amygdala–prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 8:20–21.
- Henry JP. 1992. Biological basis of the stress response. Integr Physiol Behav Sci. 27:66–83.
- Horiuchi J, McDowall LM, Dampney RA. 2006. Differential control of cardiac and sympathetic vasomotor activity from the dorsomedial hypothalamus. Clin Exp Pharmacol Physiol. 33:1265–1268.
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. 2006. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 78:815–826.
- Isowa T, Ohira H, Murashima S. 2004. Reactivity of immune, endocrine and cardiovascular parameters to active and passive acute stress. Biol Psychol. 65:101–120.
- Izquierdo A, Newman TK, Higley JD, Murray EA. 2007. Genetic modulation of cognitive flexibility and socioemotional behavior in rhesus monkeys. Proc Natl Acad Sci USA. 104:14128–14133.
- Jollant F, Buresi C, Guillaume S, Jaussent I, Bellivier F, Leboyer M, Castelnau D, Malafosse A, Courtet P. 2007. The influence of four serotonin-related genes on decision-making in suicide attempters. Am J Med Genet B Neuropsychiatr Genet. 144B:615–624.
- Jones EG. 1985. The thalamus. New York: Plenum Press.
- Jones EG, Burton H. 1976. A projection from the medial pulvinar to the amygdala in primates. Brain Res. 104:142–147.
- Kimura K, Isowa T, Ohira H, Murashima S. 2005. Temporal variation of acute stress responses in sympathetic nervous and immune systems. Biol Psychol. 70:131–139.
- Kirschbaum C, Ebrecht M, Hellhammer DH. 2001. Similar cortisol responses to the TSST and to a modified Stroop test-two laboratory stress protocols for studies of intervention-induced changes in HPA responsiveness. Psychosom Med. 63:161.
- Korte SM, Buwalda B, De Kloet ER, Bohus B. 1992. Adrenaline release by the 5-HT1A receptor agonist 8-OH-DPAT is partly responsible for pituitary activation. Eur J Pharmacol. 309:281–286.
- Kudielka BM, Kirschbaum C. 2004. Sex differences in HPA axis responses to stress: A review. Biol Psychol. 69:113–132.
- Lange C, Kracht L, Herholz K, Sachsse U, Irle E. 2005. Reduced glucose metabolism in temporo-parietal cortices of women with borderline personality disorder. Psychiatry Res. 139:115–126.
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. 1996. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 274:1527–1531.
- Li Q, Holmes A, Ma L, Van de Kar LD, Garcia F, Murphy DL. 2004. Medial hypothalamic 5-hydroxytryptamine (5-HT)1A receptors regulate neuroendocrine responses to stress and exploratory locomotor activity: Application of recombinant adenovirus containing 5-HT1A sequences. J Neurosci. 24:10868–10877.
- McCaffery JM, Bleil M, Pogue-Geile MF, Ferrell RE, Manuck SB. 2003. Allelic variation in the serotonin transporter gene-linked polymorphic region (5-HTTLPR) and cardiovascular reactivity in young adult male and female twins of European–American descent. Psychosom Med. 65:721–728.
- McEwen BS. 1998. Stress, adaptation, and disease: Allostasis and allostatic load. Ann NY Acad Sci. 840:33–44.
- Mizuno T, Aoki M, Shimada Y, Inoue M, Nakaya K, Takahashi T, Itoyama Y, Kanazawa M, Utsumi A, Endo Y, Nomura T, Hiratsuka M, Mizugaki M, Goto J, Hongo M, Fukudo S. 2006. Gender difference in association between polymorphism of serotonin transporter gene regulatory region and anxiety. J Psychosom Res. 60:91–97.
- Nakamura M, Ueno S, Sano A, Tanabe H. 2000. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 5:32–38.
- Ohira H, Nomura M, Ichikawa N, Isowa T, Iidaka T, Sato A, Fukuyama S, Nakajima T, Yamada J. 2006. Association of neural and physiological responses during voluntary emotion suppression. NeuroImage. 29:721–733.
- Ohira H, Isowa T, Nomura M, Ichikawa N, Kimura K, Miyakoshi M, Iidaka T, Fukuyama S, Nakajima T, Yamada J. 2008. Imaging brain and immune association accompanying cognitive appraisal of an acute stressor. NeuroImage. 39:500–514.
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 2005. 5-HTTLPR polymorphism impacts human cingulate–amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci. 8:828–834.
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. 2008. Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging study. Biol Psychiatry. 63:234–240.
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. 1993. Antidepressant- and cocaine-sensitive human serotonin transporter: Molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci USA. 90:2542–2546.
- Rioja J, Santín LJ, López-Barroso D, Doña A, Ulzurrun E, Aguirre JA. 2007. 5-HT1A receptor activation counteracted the effect of acute immobilization of noradrenergic neurons in the rat locus coeruleus. Neurosci. Lett.. 412:84–88.
- Roiser JP, Blackwell AD, Cools R, Clark L, Rubinsztein DC, Robbins TW, Sahakian BJ. 2006. Serotonin transporter polymorphism mediates vulnerability to loss of incentive motivation following acute tryptophan depletion. Neuropsychopharmacology. 31:2264–2272.
- Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS. 1997. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol. 379:313–332.
- Sands SA, Strong R, Corbitt J, Morilak DA. 2000. Effects of acute restraint stress on tyrosine hydroxylase mRNA expression in locus coeruleus of Wistar and Wistar-Kyoto rats. Brain Res Mol Brain Res. 75:1–7.
- Spyer KM. 1999. Central nervous control of the cardiovascular system. In: Mathias CJBannister R. Autonomic failure: A textbook of clinical disorders of the autonomic nervous system. Oxford: Oxford University Press. 45–55.
- Thompson JM, O'Callaghan CJ, Kingwell BA, Lambert GW, Jennings GL, Esler MD. 1995. Total norepinephrine spillover, muscle sympathetic nerve activity and heart-rate spectral analysis in a patient with dopamine beta-hydroxylase deficiency. J Auton Nerv Syst. 55:198–206.
- Tjurmina OA, Armando I, Saavedra JM, Goldstein DS, Murphy DL. 2002. Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology. 143:4520–4526.
- Vallender EJ, Lynch L, Novak MA, Miller GM. Polymorphisms in the 3′ UTR of the serotonin transporter are associated with cognitive flexibility in rhesus macaques. Am J Med Genet B Neuropsychiatr Genet. 2008 [Epub ahead of print].
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. 2001. Central nervous system serotonin function and cardiovascular responses to stress. Psychosom Med. 63:300–305.
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. 2003. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 28:533–541.
- Williams RB, Marchuk DA, Siegler IC, Barefoot JC, Helms MJ, Brummett BH, Surwit RS, Lane JD, Kuhn CM, Gadde KM, Ashley-Koch A, Svenson IK, Schanberg SM. 2008. Childhood socioeconomic status and serotonin transporter gene polymorphism enhance cardiovascular reactivity to mental stress. Psychosom Med. 70:32–39.
- Young KA, Holcomb LA, Bonkale WL, Hicks PB, Yazdani U, German DC. 2007. 5HTTLPR polymorphism and enlargement of the pulvinar: Unlocking the backdoor to the limbic system. Biol Psychiatry. 61:813–818.
- Zhu JN, Yung WH, Kwok-Chong Chow B, Chan YS, Wang JJ. 2006. The cerebellar-hypothalamic circuits: Potential pathways underlying cerebellar involvement in somatic-visceral integration. Brain Res Rev. 52:93–106.