Abstract
Brain natriuretic peptide (BNP), a cardiac peptide, has been implicated in the regulation of hypothalamic-pituitary–adrenocortical (HPA) responses to psychological stressors. The influence of academic stress on circulating concentration of the N-terminal fragment of BNP precursor (NT-proBNP), and in relation to the stress hormone (cortisol) response was studied in 170 college students undergoing major examinations. Just prior to the examination, we measured self-estimated stress level, systolic, and diastolic blood pressure (SBP, DBP), heart rate (HR), plasma levels of cortisol, and NT-proBNP. These parameters were compared to the participants' baseline measurements, taken at the same hour of a different ‘control day’, without a major examination to induce stress. Hemodynamic variables (SBP, DBP, and HR) increased on the examination day compared with baseline values (p < 0.001). Circulating cortisol concentration increased before examinations (+42%, p < 0.001). The response to stress was marked by a significant decrease in plasma NT-proBNP concentration ( − 40%, p < 0.001). We found in males a significant interaction between the cortisol elevation with examination stress and the NT-proBNP reduction (p = 0.02). In response to academic stress, the plasma cortisol elevation was accompanied by a marked reduction in plasma NT-proBNP level. These data may indicate that mental stress entails an interface between the HPA axis and the peripheral natriuretic peptide system, leading to reciprocating changes in circulating levels of the corresponding hormones.
Introduction
Psychological stress is common in our society. Students usually face different kinds of psychological stressors associated with the pressures of academic success, social problems, financial issues, and an uncertain future (Fish and Nies Citation1996). The brain evokes physiological responses to this kind of stress that ultimately result in activation of the hypothalamic-pituitary–adrenocortical (HPA) system and culminates in the release of the stress hormone cortisol from the adrenal cortex (Owens and Nemeroff Citation1991; Whitnall Citation1993). The nature and magnitude of the biological changes may be dependent on the severity and duration of the stressor as well as the coping styles used to face it (Kiecolt-Glaser et al. Citation1992). In this regard, psychological stressors, which do not constitute a direct physical threat to homeostasis, usually produce a more limited response (Kiecolt-Glaser et al. Citation1992). A wealth of data suggests that HPA responses to these stressor subtypes are regulated by distinct brain structures (Herman and Cullinan Citation1997; Herman et al. Citation2003).
Brain natriuretic peptide (BNP), first isolated from porcine brain (Sudoh et al. Citation1988), is a second member of the natriuretic peptide family which is known to control cardiovascular and body fluid homeostasis. BNP is released predominantly from the ventricular myocardium as a response to cardiac ventricular dilatation and pressure overload (Levin et al. Citation1998; Boomsma and van den Meiracker Citation2001). Increased circulating levels of BNP and N-terminal pro-BNP (NT-proBNP), a cleaved fragment of BNP precursor (amino acids 1–76), have been shown to be of prognostic value in patients with heart failure or coronary artery disease (Gardner et al. Citation2003; Schnabel et al. Citation2006; Burke and Cotts Citation2007; Clerico et al. Citation2007). Although predominantly produced by the heart, BNP has also been found in neurons of discrete brain regions where its expression was shown to be 13 times higher than that of atrial natriuretic peptide (ANP; Ueda et al. Citation1988). In animal models, endogenous ANP release from the hypothalamus during stress acts to suppress adrenocorticotropic hormone (ACTH) secretion (Antoni et al. Citation1992; Franci et al. Citation1992). This suggests that central BNP may also take part in the neuromodulatory system that controls emotional behavior and the stress hormone cascade (Imura et al. Citation1992). In the last decade, considerable evidence has emerged that indicates a role for BNP in the regulation of HPA responses to different stress paradigms (Gardi et al. Citation1997; Jászberényi et al. Citation2000; Wiedemann et al. Citation2000).
The few studies that have investigated the effects of natriuretic peptides in modulating HPA activity were mostly done on animal models, and examined the role of ANP and C-type natriuretic peptide (CNP). While there are data in the medical literature on BNP response to acute physical stress (Friedl et al. Citation1998; Ohba et al. Citation2001; Siegel et al. Citation2001; Herrmann et al. Citation2003; Niessner et al. Citation2003; Leers et al. Citation2006; Banfi et al. Citation2008), few data are available on systemic BNP responses to acute psychological stress. Moreover, it has been proposed that the cardiac endocrine response may be regulated differently by several types of stressors (De Bold et al. Citation1996; McGrath and de Bold Citation2005). Because natriuretic peptides have powerful physiological effects on hemodynamic, body fluid, and electrolyte homeostasis and given the inhibitory action they share on neurohormonal and immunological systems, it is of interest to clarify their role in responding to acute mental stress. We hypothesized, based on the inhibitory role of the natriuretic peptides in stress-induced HPA activation, that BNP would suppress ACTH release and hence affect cortisol secretion. The aim of the present study was therefore to investigate the response of circulating BNP to a mental stressor, specifically an academic final examination, in healthy college students. We also evaluated the possible relationship between circulating BNP and plasma cortisol concentrations.
Materials and methods
Subjects
We recruited 170 healthy physical education college students (age 25 ± 4 years) of both genders. Demographic data are presented in . All students were nonsmokers, had no history of or treatment for coronary artery disease, diabetes mellitus, hypertension, or hypercholesterolemia and were not receiving any medical treatment. The study was approved by the Institution Review Board (Helsinki Committee) of the ‘Hillel-Yafe’ Medical Center, and all participants gave written informed consent before inclusion in the study.
Table I. Subjects' physical characteristics.
Experimental protocol
Students were studied twice: the first time (examination day) 15–30 min before a major university examination, and the second time (rest day), in a period away from study routines (up to 90 days following the examination day). Tests were performed at the same hour of the day (morning hours) in order to control for diurnal variation in values of cortisol, NT-proBNP, heart rate (HR), and blood pressure. Students were asked to indicate how stressed they felt on a 10-point scale just prior to entry to the written test. The score of the questionnaire ranged from 1 = not stressed at all, to 10 = extremely stressed. Systolic and diastolic arterial blood pressures (SBP and DBP, respectively), and HR recordings over 1 min were measured just before the start of the examination, using the worldwide standard Riva Rocci/Korotkoff technique with a mercury sphygmomanometer and an oscilloscope and peripheral pulse measurements over 1 min. Blood samples for analysis of cortisol and NT-proBNP concentrations were drawn from the antecubital brachial vein using ethylenediamine-tetraacetic acid-containing tubes. The samples were then spun at 3000 rpm for 10 min at 0°C. The plasma was then extracted and frozen in aliquots at − 70°C until analysis. NT-proBNP was measured by an electrochemiluminescence assay using the automated assay of Roche Diagnostics, Mannheim, Germany (Elecsys proBNP). The sensitivity of the assay was 5 ng/l. Intra- and interassay coefficients of variance at 175 ng/l were 2.7 and 3.2%, respectively. There were no cross-reactions with other hormones or pharmaceutical drugs. Plasma cortisol was measured with the Elecsys cortisol electro-chemiluminescence immunoassay (Roche Diagnostics) reagents. Assay sensitivity was 0.5 nmol/l. Intra- and interassay coefficients of variance at 208 nmol/l were 1.3 and 1.6%, respectively. Both assays were run on an automated analyzer (Elecsys 2010 Roche Diagnostics, Indianapolis, IN, USA).
Statistical analysis
The SPSS statistical package version 13.0 was used to perform all statistical evaluations (SSPS, Inc., Chicago, IL, USA). Normally distributed, continuous data, unless otherwise stated, are expressed as mean values ( ± SD). Non-normally distributed continuous data are expressed as medians (25th and 75th percentile). Normality was tested by the Kolmogorov–Smirnov test and the differences between male and females by Student's t-test for independent samples. As concentrations of NT-proBNP were not normally distributed, the Mann–Whitney test was used for comparison between sexes. Multivariate analysis using ANOVA for repeated measures was performed to analyze endocrine (BNP and cortisol) and hemodynamic (HR and blood pressure) responses to stressors. Included in the model were: age, gender, and body mass index (BMI). NT-proBNP values were log-transformed before entering the ANOVA. Spearman's correlation analysis was used to estimate the statistical link between selected parameters, treating data from the two experimental days. All p values and confidence intervals are two-sided, and the level of significance was set at p < 0.05.
Results
In the analysis we compared hormonal and hemodynamic variables of the study group in response to stress (examination day) and at baseline (rest day). Since gender had a significant impact on cortisol and NT-proBNP values, we analyzed the data for males (n = 70) and females (n = 95) separately. Results of the multivariate analysis using ANOVA for repeated measures, adjusted for age and BMI are shown in . Generally, and as expected, hemodynamic variables (SBP, DBP, and HR) significantly increased on the examination stress day compared with baseline values (control day). In males, SBP was greater than in females on both days (p < 0.0001; F = 1.04, 1.25, DF = 74, 94, for stress and rest day, respectively), while there was no significant difference in DBP values. Baseline HR was similar between sexes, but was higher on the examination day (p = 0.01; F = 1.24, DF = 74, 94).
Table II. Subjects' hormonal and hemodynamic variables at rest and in response to stress.
Plasma cortisol concentration, taken as an average for all students, was greater on the examination day than on the control day (+42%; , p = 0.003 and p < 0.001 for males and females, respectively). The plasma NT-proBNP concentration, however, was significantly less on the examination day than on the control day ( − 40%; , p = 0.02 and p < 0.001 for males and females, respectively). On the examination and control days, cortisol levels were greater in females than in males (, p = 0.0008; F = 3.32, DF = 74, 94, and p < 0.0001; F = 2.04, DF = 74, 94, for cortisol level on the examination stress day and on the control day, respectively). Similarly, control day concentrations of NT-proBNP and the corresponding values before examinations were greater in female students compared to male students (, p < 0.0001; F = 4.87 and 3.40, DF = 74, 94, for stress and rest day, respectively). Males showed a significant inverse relationship between the percentage change in circulating cortisol and NT-proBNP from control to examination day (Spearman's r = − 0.43, p = 0.01, n = 70). In the female group, this inverse relationship was not statistically significant (Spearman's r = − 0.15, p = 0.27, n = 95).
The median and inter-quartile ranges of the stress scores on examination days were 5 (2–7) and 6 (4–8) for males and females, respectively. The differences between sexes were statistically significant (p < 0.0001). In both males and females circulating NT-proBNP concentrations did not significantly correlate with self-reported stress level (Spearman's r = − 0.09, p = 0.46, n = 70; Spearman's r = − 0.08, p = 0.44, n = 95, for males and females, respectively). Females demonstrated a significant positive correlation between circulating cortisol concentration and the subjective stress measure on the examination day (Spearman's r = 0.28, p = 0.02; n = 95).
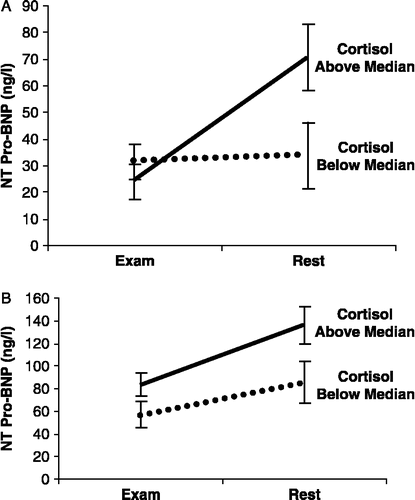
In order to evaluate the interaction between cortisol and NT-proBNP concentrations, we divided the population into two groups (based on the median value of absolute change in cortisol level). We repeated the ANOVA for repeated measure of NT-proBNP levels. In this model, we also included the parameter of cortisol level change below/above median in addition to the prior parameters of age and BMI. The results of this model demonstrated in males a significant interaction between the change in plasma cortisol (below median and above median) and the change in NT-proBNP concentrations between the examination and the rest day (, p for interaction = 0.02). Male students with the above median difference in cortisol between examination stress and rest had lower stress day levels and higher rest levels of NT-proBNP than students with below median differences. In females (), no interaction was found: female students with the above median difference in cortisol between examination stress and rest had higher stress day levels and higher rest levels of NT-proBNP than students with below median differences.
Figure 1 The change in circulating concentrations of NT-proBNP from rest to stress in relation to the change in circulating cortisol concentrations. The change in circulating concentrations of NT-proBNP from rest to stress in relation to the change in circulating cortisol concentrations. Male (n = 70 (A)) and female (n = 95 (B)) students were divided into two groups according to their absolute change in circulating levels of cortisol (below median and above median). Multivariate analysis using ANOVA for repeated measures revealed in males a significant interaction (p = 0.02): male students with the above median difference in cortisol between stress and rest have lower stress-day level and higher rest-day concentrations of NT-proBNP than students with below median differences. In females (B), no interaction was found: female students with the above median difference in cortisol between stress and rest have higher stress-day and higher rest-day concentrations of NT-proBNP than students with below median differences. The error bars indicate s.e.m.
Discussion
The main finding of this study is that the stress response of undergraduate students to a common real life mental stressor as represented by anticipation of a major written university examination is marked by a reduction in circulating concentration of natriuretic peptide, NT-ProBNP. The students in the present study were exposed to a period of prolonged study and work hours, and faced the challenge of academic success in anticipation of the major test. The presence of stress before examination (as indicated by the self-perceived stress scores) was confirmed by the objective evidence of a stress induced rise in circulating cortisol level and correspondiing changes in HR, SBP, and DBP.
Stressful life events have long been hypothesized to stimulate specific neuroendocrine activity, with elevated cortisol secretion associated with acute stress. Studies have reported increased cortisol levels during anticipation of stressful events such as academic examinations, either oral or written (Malarkey et al. Citation1995; Lacey et al. Citation2000; Martinek et al. Citation2003; CitationNg et al. 2003a,b; Krahwinkel et al. Citation2004; Harl et al. Citation2006; Weekes et al. Citation2006; Schoofs et al. Citation2007). The higher level of circulating cortisol in our students on the examination day clearly indicates activation of the HPA axis. Moreover, we, like others (Ross et al. Citation2001; Lucini et al. Citation2002; Pramanik et al. Citation2005), observed a significant elevation in SBP, DBP, and HR in response to examination stress. Of note, in our female group circulating cortisol levels correlated significantly with self-reported stress level, although in previous studies no evidence was found to suggest a relationship between psychological measures of stress and hormonal levels (CitationNg et al. 2003a,b; Martinek et al. Citation2003; Weekes et al. Citation2006).
Keeping these subjective and objective parameters of stress in mind, the main goal of our study was to analyze the response of circulating NT-ProBNP to a common trigger of life stress. To the best of our knowledge, this report is the first to describe the NT-ProBNP profile of healthy students in response to an academic stressor. It should be noted that probably two separate cardiac endocrine systems exist, one in the atrium where ANP and its related peptides are preferentially produced and the other in the ventricle, predominantly secreting BNP and its related peptides. It has been also suggested that the endocrine response of the heart varies depending on whether the stimulus is acute, sub-acute, or chronic (De Bold et al. Citation1996; McGrath and de Bold Citation2005). The normal ventricular myocardium produces only a limited amount of BNP in response to an acute stimulation while chronic cardiac dysfunction induces the secretion of a greater amount of BNP than ANP, probably because BNP is produced mainly by the ventricular myocardium, which has greater mass. Moreover, in healthy subjects most circulating ANP and BNP derive from the atria (Clerico et al. Citation2006). These data explain why BNP is considered to be a biomarker of cardiac disease while ANP is a better index of acute overload (Clerico and Emdin Citation2004; Clerico et al. Citation2005). Since circulating ANP levels are known to be more affected by rapid cardiovascular hemodynamic changes such as body position, and given that our study population consisted of healthy college students, we preferred to measure BNP-related peptides.
Although BNP may be considered a more reliable index of the activation status of the cardiac natriuretic system than NT-proBNP, as measured here, the plasma concentration in healthy individuals is very low compared to the NT-proBNP level which is also more stable and demonstrates a wide range (Clerico and Emdin Citation2004; Clerico et al. Citation2005). Since the present study was designed to investigate differences in stress-induced BNP responses of healthy college students in whom baseline BNP levels are low or undetectable, we preferred to assay NT-proBNP, which has a wider range of values. This enabled better quantification of the possible differences between stress and baseline levels and to improve comparison between individuals.
Previous studies on animal models demonstrated that the HPA system is inhibited at all regulatory levels by ANP, while CNP stimulates the release of cortisol (Wiedemann et al. Citation2000). Moreover, preclinical and clinical data point to the anxiolytic effects induced by ANP (Ströhle and Holsboer Citation2003). As scattered as the data regarding these natriuretic peptides are, even fewer data are available concerning BNP activity in the human brain and its elicited neuromodulatory effects during mental stress. We found that in subjects exposed to mental stress, the mean circulating NT-proBNP level evidently declined. These findings corroborate previous data from animal models, showing that BNP inhibits the stress-induced rise in plasma cortisol (Jászberényi et al. Citation2000). Interestingly, we found in males a significant interaction between the amount of change in cortisol and the change in NT-proBNP between rest and stress: male students with the above median difference in cortisol between stress and rest had a lower stress-day level and higher rest levels of NT-proBNP than students with below median differences.
The endocrine system of the heart is complex and probably responds differently to different types of stressors, e.g. it has been proposed that the cardiac endocrine response to pressure or volume load varies depending on whether the stimulus is acute, sub-acute or chronic (De Bold et al. Citation1996; McGrath and de Bold Citation2005). Likewise, the magnitude of the stress response may be dependent on the severity and duration of the stressor. In this regard, psychological stressors, which do not constitute a direct physical threat to homeostasis, usually produce a more limited response (Kiecolt-Glaser et al. Citation1992). Thus, it is conceivable that an acute psychological stressor such as a major examination may affect the cardiac endocrine function by different mechanisms than other physiological or pathological stressors, such as physical activity or fluid immersion. Many previous physiological and clinical studies reported on an activation of the autonomic system, which induced significant hemodynamic variations, including increased blood pressure and HR and resulted in increase levels of ANP and BNP, especially when stress was prolonged enough to affect cardiac performance or effective circulating plasma volume. We found a fall in levels of circulating NT-proBNP before an ‘academic stress’. This effect of psychological stress is different from that reported after physical activities, even those which may represent both physical and psychological stress as they are associated with increased venous return and higher left ventricular pressures, resulting consequently in increased secretion of BNP (Friedl et al. Citation1998; Ohba et al. Citation2001; Siegel et al. Citation2001; Herrmann et al. Citation2003; Niessner et al. Citation2003; Leers et al. Citation2006; Banfi et al. Citation2008). It should be noted however that there is a distinction between BNP in the brain and circulating BNP. Antoni et al. (Citation1992) have shown that in response to acute stressful stimuli ANP is released from the hypothalamo-hypophyseal pathway, acts directly to suppress pituitary ACTH secretion and thus constitutes a hypothalamic corticotrophin-release inhibiting pathway. These data support the concept that the cardiac (peripheral) natriuretic hormone systems and the hypothalamic (central) natriuretic hormone systems are separate. Accordingly, circulating NT-proBNP levels predominantly reflect expression of the cardiac source. Of note, in this study both variables were measured simultaneously, therefore we cannot conclude whether the NT-proBNP response precedes the cortisol response or vice versa, nor can we preclude the involvement of other dominant factors in determining circulating the NT-proBNP response. Thus, although, our results may support the possibility that central mechanisms involving HPA regulation in response to stress cause a reduction in the levels of NT-proBNP which leads to increased secretion of cortisol, the nature of these possible mechanisms is beyond the scope of our current investigation and more studies are needed to evaluate this hypothesis.
We found circulating NT-proBNP to be higher in female students compared to male students on both experimental days. Several studies suggest that age and gender are significantly and independently linked to circulating natriuretic peptide levels (Prontera et al. Citation2004; Di Serio et al. Citation2005; Hess et al. Citation2005; Hadzović-Dzuvo et al. Citation2007). With regard to gender, the data support higher NT-proBNP levels in females than in males (Prontera et al. Citation2004; Hess et al. Citation2005), which is consistent with our findings. We, like others, also noted that circulating cortisol levels induced by the academic examinations were gender dependent (Kirschbaum et al. Citation1999, Lacey et al. Citation2000; Ross et al. Citation2001; Zeller et al. Citation2004; Weekes et al. Citation2006).
Limitations
We used a single question for the evaluation of the subjective stress. While an in-depth questionnaire may be useful compared to a single question, it could be argued that this may impose additional stress on the students. Of note, since we took only one blood sample before the examination we cannot describe whether the low NT-proBNP is an acute response, or reflects a more chronic change. Due to the university examinations schedule, we had to design our study such that all subjects were studied first on the examination day and only later on the rest day. We are aware of the possible limitation that some of the differences reported for examination stress may have been due to the subjects' lack of familiarity with the laboratory setting. Finally, since the present study was not designed to explore a functional mechanism, our findings do not necessarily imply a causal relation and further studies are needed to investigate mechanistically the physiological relevance of increased circulating NT-proBNP in response to psychological stress.
Conclusions
In healthy undergraduate college students who are exposed to the mental stress of a major written academic examination, the increase in plasma cortisol level is accompanied by a reduction in plasma NT-proBNP level. These data suggest that mental stress involves an interface between the HPA axis and the peripheral natriuretic peptides system, leading to reciprocating changes in circulating plasma levels of the corresponding hormones.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Antoni FA, Hunter EF, Lowry PJ, Noble JM, Seckl JR. 1992. Atriopeptin: An endogenous corticotrophin-release inhibiting hormone. Endocrinology. 130:1753–1755.
- Banfi G, D'Eril GM, Barassi A, Lippi G. 2008. N-Terminal proB-type natriuretic peptide (NT-proBNP) concentrations in elite rugby players at rest and after active and passive recovery following strenuous training sessions. Clin Chem Lab Med. 46:247–249.
- Boomsma F, van den Meiracker AH. 2001. Plasma A- and B-type natriuretic peptides: Physiology, methodology and clinical use. Cardiovasc Res. 51:442–549.
- Burke MA, Cotts WG. 2007. Interpretation of B-type natriuretic peptide in cardiac disease and other comorbid conditions. Heart Fail Rev. 12:23–36.
- Clerico A, Emdin M. 2004. Diagnostic accuracy and prognostic relevance of the measurement of cardiac natriuretic peptides: A review. Clin Chem. 50:35–50.
- Clerico A, Prontera C, Emdin M, Passino C, Storti S, Poletti R, Zyw L, Zucchelli GC. 2005. Analytical performance and diagnostic accuracy of immunometric assays for the measurement of plasma B-type natriuretic peptide (BNP) and N-terminal proBNP. Clin Chem. 51:445–447.
- Clerico A, Recchia FA, Passino C, Emdin M. 2006. Cardiac endocrine function is an essential component of the homeostatic regulation network: Physiological and clinical implications. Am J Physiol Heart Circ. 290:H17–H29.
- Clerico A, Fontana M, Zyw L, Passino C, Emdin M. 2007. Comparison of the diagnostic accuracy of brain natriuretic peptide (BNP) and the N-terminal part of the propeptide of BNP immunoassays in chronic and acute heart failure: A systematic review. Clin Chem. 53:813–822.
- De Bold AJ, Bruneau BG, Kuroski de Bold MK. 1996. Mechanical and neuroendocrine regulation of the endocrine heart. Cardiovasc Res. 31:7–18.
- Di Serio F, Ruggieri V, Varraso L, De Sario R, Mastrorilli A, Pansini N. 2005. Analytical evaluation of the Dade Behring dimension R × L automated N-terminal proBNP (NT-proBNP) method and comparison with the Roche Elecsys 2010. Clin Chem Lab Med. 43:1263–1273.
- Fish C, Nies MA. 1996. Health promotion needs of students in a college environment. Public Health Nurs. 13:104–111.
- Franci CR, Anselmo-Franci JA, McCann SM. 1992. The role of endogenous atrial natriuretic peptide in resting and stress-induced release of corticotrophin, prolactin, growth hormone, and thyroid-stimulating hormone. Proc Natl Acad Sci USA. 89:11391–11395.
- Friedl W, Mair J, Pichler M, Paulweber B, Sandhofer F, Puschendorf B. 1998. Insertion/deletion polymorphism in the angiotensin-converting enzyme gene is associated with atrial natriuretic peptide activity after exercise. Clin Chim Acta. 274:199–211.
- Gardi J, Bíró E, Vecsernyés M, Julesz J, Nyári T, Tóth G, Telegdy G. 1997. The effects of brain and C-type natriuretic peptides on corticotropin-releasing factor in brain of rats. Life Sci. 60:2111–2117.
- Gardner RS, Ozalp F, Murday AJ, Robb SD, McDonagh TA. 2003. N-Terminal pro-brain natriuretic peptide. A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 24:1735–1743.
- Hadzović-Dzuvo A, Kucukalić-Selimović E, Nakas-Ićindić E, Zaćiragić A, Drazeta Z. 2007. N-Terminal pro-brain natriuretic peptide (NT-proBNP) serum concentrations in apparently healthy Bosnian women. Bosn J Basic Med Sci. 7:307–310.
- Harl B, Weisshuhn S, Kerschbaum HH. 2006. Cortisol titer increases with novelty of academic oral examinations. Neuro Endocrinol. 27:669–674.
- Herman JP, Cullinan WE. 1997. Neurocircuitry of stress: Central control of the hypothalamo-pituitary–adrenocortical axis. Trends Neurosci. 20:78–84.
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. 2003. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary–adrenocortical responsiveness. Front Neuroendocrinol. 24:151–180.
- Herrmann M, Scharhag J, Miclea M, Urhausen A, Herrmann W, Kindermann W. 2003. Post-race kinetics of cardiac troponin T and I and N-terminal probrain natriuretic peptide in marathon runners. Clin Chem. 49:831–834.
- Hess G, Runkel S, Zdunek D, Hitzler WE. 2005. N-Terminal pro-brain natriuretic peptide (NT-proBNP) in healthy blood donors and in patients from general practitioners with and without a diagnosis of cardiac disease. Clin Lab. 51:167–172.
- Imura H, Nakao K, Itoh H. 1992. The natriuretic peptide system in the brain: Implications in the central control of cardiovascular and neuroendocrine functions. Front Neuroendocrinol. 13:217–249.
- Jászberényi M, Bujdosó E, Telegdy G. 2000. Effects of brain natriuretic peptide on pituitary–adrenal activation in rats. Life Sci. 66:1655–1661.
- Kiecolt-Glaser JK, Cacioppo JT, Malarkey WB, Glaser R. 1992. Acute psychological stressors and short-term immune changes: What, why, for whom, and to what extent?. Psychosom Med. 54:680–685.
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. 1999. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom Med. 61:154–162.
- Krahwinkel T, Nastali S, Azrak B, Willershausen B. 2004. The effect of examination stress conditions on the cortisol content of saliva—a study of students from clinical semesters. Eur J Med Res. 9:256–260.
- Lacey K, Zaharia MD, Griffiths J, Ravindran AV, Merali Z, Anisman H. 2000. A prospective study of neuroendocrine and immune alterations associated with the stress of an oral academic examination among graduate students. Psychoneuroendocrinology. 25:339–356.
- Leers MP, Schepers R, Baumgarten R. 2006. Effects of a long-distance run on cardiac markers in healthy athletes. Clin Chem Lab Med. 44:999–1003.
- Levin ER, Gardner DG, Samson WK. 1998. Natriuretic peptides. N Engl J Med. 339:321–328.
- Lucini D, Norbiato G, Clerici M, Pagani M. 2002. Hemodynamic and autonomic adjustments to real life stress conditions in humans. Hypertension. 39:184–188.
- Malarkey WB, Pearl DK, Demers LM, Kiecolt-Glaser JK, Glaser R. 1995. Influence of academic stress and season on 24-hour mean concentrations of ACTH, cortisol, and beta-endorphin. Psychoneuroendocrinology. 20:499–508.
- Martinek L, Oberascher-Holzinger K, Weishuhn S, Klimesch W, Kerschbaum HH. 2003. Anticipated academic examinations induce distinct cortisol responses in adolescent pupils. Neuro Endocrinol. 24:449–453.
- McGrath M, de Bold AJ. 2005. Determinants of natriuretic peptide gene expression. Peptides. 26:933–943.
- Niessner A, Ziegler S, Slany J, Billensteiner E, Woloszczuk W, Geyer G. 2003. Increases in plasma levels of atrial and brain natriuretic peptides after running a marathon: Are their effects partly counterbalanced by adrenocortical steroids?. Eur J Endocrinol. 149:555–559.
- Ng V, Koh D, Chia SE. Examination stress, salivary cortisol, and academic performance. Psychol Rep. 2003a; 93:1133–1134.
- Ng V, Koh D, Mok BY, Chia SE, Lim LP. Salivary biomarkers associated with academic assessment stress among dental undergraduates. J Dent Educ. 2003b; 67:1091–1094.
- Ohba H, Takada H, Musha H, Nagashima J, Mori N, Awaya T, Omiya K, Murayama M. 2001. Effects of prolonged strenuous exercise on plasma levels of atrial natriuretic peptide and brain natriuretic peptide in healthy men. Am Heart J. 141:751–758.
- Owens MJ, Nemeroff CB. 1991. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 43:425–473.
- Pramanik T, Ghosh A, Chapagain G. 2005. Effect of examination stress on the alteration of blood pressure in young normotensives. Blood Press Monit. 10:149–150.
- Prontera C, Emdin M, Zucchelli GC, Ripoli A, Passino C, Clerico A. 2004. Analytical performance and diagnostic accuracy of a fully-automated electrochemiluminescent assay for the N-terminal fragment of the pro-peptide of brain natriuretic peptide in patients with cardiomyopathy: Comparison with immunoradiometric assay methods for brain natriuretic peptide and atrial natriuretic peptide. Clin Chem Lab Med. 42:37–44.
- Ross AE, Flaa A, Høieggen A, Reims H, Eide IK, Kjeldsen SE. 2001. Gender specific sympathetic and hemorrheological responses to mental stress in healthy young subjects. Scand Cardiovasc J. 35:307–312.
- Schnabel R, Lubos E, Rupprecht HJ, Espinola-Klein C, Bickel C, Lackner KJ, Cambien F, Tiret L, Münzel T, Blankenberg S. 2006. B-type natriuretic peptide and the risk of cardiovascular events and death in patients with stable angina: Results from the AtheroGene study. J Am Coll Cardiol. 47:552–558.
- Schoofs D, Hartmann R, Wolf OT. Neuroendocrine stress responses to an oral academic examination: No strong influence of sex, repeated participation and personality traits. Stress. 2007 Jul 16 (Epub ahead of print).
- Siegel AJ, Lewandrowski EL, Chun KY, Sholar MB, Fischman AJ, Lewandrowski KB. 2001. Changes in cardiac markers including B-natriuretic peptide in runners after the Boston marathon. Am J Cardiol. 88:920–923.
- Ströhle A, Holsboer F. 2003. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 36:S207–S214.
- Sudoh T, Kangawa K, Minamino N, Matsuo H. 1988. A new natriuretic peptide in porcine brain. Nature. 332:78–81.
- Ueda S, Minamino N, Sudoh T, Kangawa K, Matsuo H. 1988. Regional distribution of immunoreactive brain natriuretic peptide in porcine brain and spinal cord. Biochem Biophys Res Commun. 155:733–739.
- Weekes N, Lewis R, Patel F, Garrison-Jakel J, Berger DE, Lupien SJ. 2006. Examination stress as an ecological inducer of cortisol and psychological responses to stress in undergraduate students. Stress. 9:199–206.
- Whitnall MH. 1993. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 40:573–629.
- Wiedemann K, Jahn H, Kellner M. 2000. Effects of natriuretic peptides upon hypothalamo-pituitary–adrenocortical system activity and anxiety behaviour. Exp Clin Endocrinol Diabetes. 108:5–13.
- Zeller A, Handschin D, Gyr N, Martina B, Battegay E. 2004. Blood pressure and heart rate of students undergoing a medical licensing examination. Blood Press. 13:20–24.