Abstract
Salivary cortisol measurement has proved useful for the non-invasive study of the hypothalamic–pituitary–adrenocortical axis, and salivary α-amylase has been suggested as a comparable marker for the sympathetic system. Despite some studies showing an increase in salivary α-amylase after challenges that stimulate the sympathetic nervous system, questions remain about interpretation. The aims of this study were to explore the stability of salivary α-amylase, its diurnal profile, response to the cold hand test, and correlation with cortisol. Salivary α-amylase was stable following 5 days at room temperature, and five freeze-thaw cycles. Its diurnal profile was opposite to that of cortisol. There was no salivary α-amylase response to the cold hand stress test, in the morning (11am) or afternoon (3pm), unlike cortisol which showed a response in the afternoon in the same samples. There was no correlation between salivary α-amylase and cortisol at any time. In conclusion, salivary α-amylase is stable to a range of conditions. Its diurnal pattern is compatible with sympathetic stimulation. Lack of response to the cold hand test suggests that secretion of salivary alpha-amylase is controlled by mechanisms more complex than sympathetic regulation alone.
Introduction
Measurement of salivary cortisol has proved useful in a range of human studies of the function of the hypothalamic–pituitary–adrenocortical (HPA) axis in health and disease (e.g. Chandola et al. Citation2008). Salivary cortisol exhibits a marked diurnal profile, with a post-awakening rise, followed by declining levels across the day (Pruessner et al. Citation1997). The acute cortisol stress response has been well characterised. Increases are consistently reported in response to pharmacological (Schlotz et al. Citation2008), psychosocial and physical stressors, (Kammerer et al. Citation2002; McRae et al. Citation2006). Cortisol in saliva is stable and can be stored for up to 1 week at room temperature, and at least a year at − 20°C (Garde and Hansen Citation2005).
More recently, salivary α-amylase has been proposed as a comparable surrogate non-invasive marker of sympathetic activity (Rohleder et al. Citation2004). This enzyme, involved in starch digestion, is secreted into the oral cavity from the acinar cells of the parotid gland, under autonomic control (Nater et al. Citation2006; Proctor and Carpenter Citation2007). Many psychological and psycho-physiological stimuli associated with sympathetic activation have been shown to increase salivary α-amylase activity (Rohleder et al. Citation2004; Citationvan Stegeren et al. 2008). Pharmacological studies have shown that output is increased by yohimbine and decreased by β-blockade, providing strong evidence for its control by sympathetic activity (Ehlert et al. Citation2006; van Stegeren et al. Citation2006).
Comparisons between changes in salivary α-amylase and cortisol measurements have largely focused on acute psychological stress tests, such as the Trier Social Stress Test (TSST; Rohleder et al. Citation2004; Gordis et al. Citation2008). The α-amylase response occurs more rapidly, peaking 5–10 min post-stress, while the cortisol response is generally observed at 20 min post-task (Rohleder et al. Citation2004). A report of a positive correlation between the two markers (Grillon et al. Citation2007) contrasts with a study indicating no relationship (Nater et al. Citation2006), while others suggest a dissociation of these markers during stress in some individuals is of interest (Gordis et al. Citation2008).
Several questions remain concerning salivary α-amylase measurement as a tool in stress research. To be useful as a clinical marker, it needs to be physically robust; no data on this issue have so far been published. Other conditions that may affect its activity, such as laboratory handling and sample processing, have not been investigated. Finally the salivary α-amylase response to simple physical stress, such as the cold hand (pressor) test, which can be easily used in a range of conditions, has not been fully established (Citationvan Stegeren et al. 2008). The cold hand test is a non-invasive acute physical stress test which has been shown to elicit a salivary and plasma cortisol (Kammerer et al. Citation2002; McRae et al. Citation2006), plasma noradrenaline, and blood pressure response (Jones et al Citation1996; Flaa et al. Citation2008).
In this study, we have examined the stability of salivary α-amylase, the diurnal profiles of salivary α-amylase activity and cortisol concentration, together with the α-amylase and cortisol responses to the cold hand test, including effects of the time of testing responses.
Materials and methods
Participants
Twenty-one participants, (mean age = 24.4 years, SD = 8.0 years, all female, non-pregnant) were recruited in Switzerland, as a control sample for a study of HPA axis function in pregnancy (Kammerer et al. Citation2009). All participants were free from somatic and psychiatric disorder, were non-smokers and not taking illicit drugs or prescribed medication. The protocol was approved by the Ethics committee of the Canton of Zurich, Switzerland, and written informed consent was obtained from all participants.
Saliva samples were collected using Salivettes (Sarstedt, Germany). Subjects placed the cotton swab in their mouths for approximately 2 min or until saturated, before replacing the swabs in the collection tube. Subjects were asked to abstain from eating and drinking (except water) for 30 min, and from caffeine or physical exertion for an hour prior to sampling. Additional saliva samples for use in our stability analyses were provided from randomly selected participants (n = 10) taking part in the diurnal sampling or cold hand test protocol.
Stability of α-amylase
To determine the effect of different pre-freezer treatments, saliva samples were centrifuged and then placed in a commercial freezer at − 20°C (off swab treatment) or placed directly in the freezer without centrifuging (frozen on swab treatment; n = 10). A subset of these samples (n = 5) were re-frozen for a further 24 h, thawed at room temperature for approximately 20 min until liquid and re-assayed. This process was repeated for a total of 120 h, totalling five full freeze-thaw cycles and five assays.
To investigate the effects of storage at room temperature on salivary α-amylase five participants collected approximately 2 ml of saliva by passive drool into a 20 ml universal container. These samples were then absorbed onto threee cotton swabs from three Salivettes for each participant. The first sample for each individual was frozen immediately, while the second and third samples were left at room temperature for 3 and 5 days, respectively.
Diurnal variation
Subjects were asked to perform diurnal sampling on two week-days, as close as possible to day 10 of their menstrual cycle. Home record forms were issued to each participant to record time of waking and sampling. They were asked to collect samples on waking (mean wake time: 07:08 h SD: 58 min), 30 min post-waking (mean: 07:38 h, SD: 58 min) then at 3 (10:22 h, SD: 54 min) and 12 h post-waking (mean 19:11 h, SD: 135 min). All samples were refrigerated once taken, collected and returned to the laboratory in cool-packs and then stored at − 20°C until assayed.
Cold hand test
To investigate the α-amylase and cortisol response to the cold hand test a subsample completed the cold hand test (n = 10, mean age = 25.3 years, SD = 3.7 years). To evaluate the effect of time of testing, the cold hand test protocol was carried out on two separate occasions; once at 11.00 h and once at 15.00 h. These tests were carried out in a random order and on different days.
Upon arrival, participants read for a 15-min period of relaxation before baseline saliva collection. Participants then placed their non-dominant hand in the ice cold water (4°C) for 60 s. The second salivary sample was taken at 5 min following the cold hand test and then 10, 20 and 30 min post-test. Subjects were alone throughout the experiment, and only supervised during the stress test and saliva sampling. Conversation between the subject and the tester was kept to a minimum. Subjects were also asked to refrain from excessive movement or posture changes during the experiment in order to limit the effect of movement and orthostatic factors on sympathetic activity.
Biochemical measures
All assays were carried out using commercially available kits (Salimetrics, State College, PA, USA). On the day of assay samples were thawed for 30 min and centrifuged for 15 min at 1200g. For salivary α-amylase activity, a kinetic enzyme assay was carried out. The final assay consists of 0.5% saliva in diluent, incubated with 320 μl of chromogenic substrate (2-chloro-p-nitrophenol linked with maltotriose). Enzyme activity over 2 min results in a colour change that can be measured at 405 nm. The inter- and intra-assay coefficients of variance were 10.9 and 4.05% respectively. Salivary cortisol was measured using an immunoassay. This assay is based on the competition for binding sites between cortisol in saliva, with horseradish peroxidase labelled cortisol. The inter- and intra-assay coefficients of variance were 7.9 and 8.9%, respectively.
Statistical analysis
All biochemical data were tested for normality. Both α-amylase and cortisol showed a skewed distribution and normalised by ln transformation. All statistical analyses were therefore carried out using parametric tests with ln transformed data with SPSS version 15 (Chicago, IL, USA).
Results
Stability of α-amylase
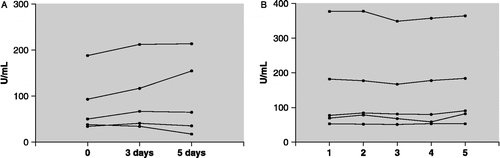
Salivary α-amylase was found to be generally highly stable (). Saliva samples, stored for up to 5 days at room temperature showed no significant change in activity (F(1, 4) = 0.45, n = 5, ns, repeated measures one-way ANOVA; ). Similarly, multiple freeze-thaw cycles did not affect the activity (F(4, 16) = 2.66, n = 5, ns, repeated measures one-way ANOVA; ). Additionally, freezing salivary samples collected on a cotton swab had no effect on α-amylase activity, when compared with samples frozen directly (t = − 0.19, n = 10, ns).
Diurnal profiles
shows the diurnal profiles of salivary α-amylase activity and cortisol concentration. Salivary α-amylase showed a distinctive diurnal profile (F(3, 80) = 3.13, n = 21, p < 0.05, one-way ANOVA), opposite to that of cortisol. Salivary α-amylase demonstrated a reduction in activity 30 min after waking. By 3 h post-waking α-amylase activity had increased above waking levels, and continued to rise until 12 h post-waking. In contrast, salivary cortisol concentration showed a positive awakening response, peaking at 30 min post-waking, followed by a steady decline over the course of the day, (F(3, 84) = 16.4, n = 21, p < 0.001, one-way ANOVA).
Figure 2 The diurnal profile (mean ± SEM) of salivary α-amylase activity and cortisol concentration. There was a significant effect of time for both salivary α-amylase (p < 0.05, n = 21) and cortisol (p < 0.001, n = 21).
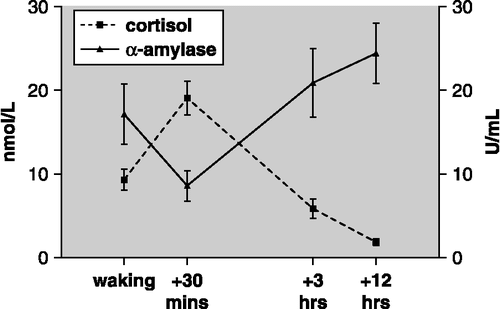
There was no correlation (Pearson) between α-amylase activity and cortisol concentration at any of the time points.
The cold hand test
We found that salivary α-amylase showed no significant response to the cold hand test in the morning or the afternoon (). By contrast, there was a significant time-of-day effect for the cortisol response to the cold hand test (). Cortisol reactivity was marked in the afternoon (15.00 h, F(4, 36) = 4.62, n = 10, p < 0.01, one-way ANOVA) but absent in the morning (11.00 h, F(4, 36) = 1.63, n = 10, p = 0.19, one-way ANOVA).
Figure 3 The time of testing effect on salivary α-amylase and cortisol reactivity (mean ± SEM) to the cold hand test. BL = baseline, times (m) are min after hand immersion in cold water for 60 s. Cold hand test was at 11.00 h (am) or 15.00 h (pm). Salivary α-amylase am and pm both p = ns for stressor effect. Salivary cortisol, BL versus post-stressor: am p = ns, n = 10; pm, p < 0.01 n = 10.
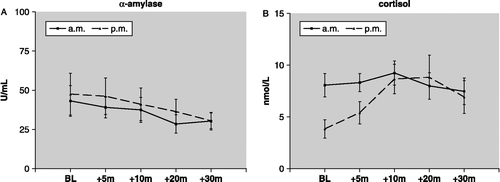
There was no correlation between α-amylase and cortisol levels at any time point during the cold hand test.
Discussion
Our data indicate that α-amylase activity is a stable salivary marker with a distinctive diurnal pattern, with opposite phase to that of cortisol. In contrast to cortisol, the cold hand test did not elicit a significant increase in salivary α-amylase activity in either morning or afternoon.
We found α-amylase activity was not significantly affected by storage at room temperature for up to 5 days. Thus saliva may be returned by post without affecting activity. The stability of α-amylase following five freeze-thaw cycles facilitates multiple analyses of samples, and shows that archived samples may be assayed for α-amylase.
The diurnal profile of α-amylase activity observed here replicates the earlier studies which found a decrease upon waking and rising levels throughout the day (Rohleder et al Citation2004; Nater et al Citation2007). Another study has found little change across the day but does report significantly higher α-amylase activity in the evening (Yamaguchi et al. Citation2006). The unchanged morning levels reported in that study may be due to the absence of a 30-min post-waking sample (Yamaguchi et al. Citation2006).
It is of interest to consider the regulation of the diurnal pattern of salivary α-amylase. The pattern we report is similar to the previously reported urinary noradrenaline profile (Hansen et al. Citation2001), with a morning dip followed by a rise evident in both. One well controlled study also found a decline in plasma noradrenaline level on waking, in contrast to adrenaline level, which increased (Dodt et al. Citation1997). The diurnal pattern of salivary α-amylase activity is thus compatible with the hypothesis that basal levels of α-amylase are an index of noradrenergic sympathetic, although not of adrenergic activity.
While the cortisol diurnal profile has been described to be altered in a number of pathological conditions, similar studies with α-amylase levels are only just beginning. Increased salivary α-amylase output has recently been reported in generalised social anxiety disorder (Van Veen et al. 2008). Conversely chronic stress in asthmatic children was associated with lower basal α-amylase levels (Wolf et al. Citation2008). These studies suggest psychological stress or anxiety may be associated with altered diurnal patterns of α-amylase.
The cold hand test failed to elicit a consistent α-amylase response, in either the morning or afternoon. The lack of an α-amylase response contrasts with previous work employing psycho-social stressors, which have found a clear response during late-morning/afternoon testing (Gordis et al. Citation2008). Our data appear to contrast with the one other study describing the α-amylase response to the cold hand test. However, Van Stegeren et al. (Citation2008) measured the response to the cold hand test following a psychological stressor. Salivary α-amylase levels did not increase but failed to return to baseline. Unlike our study, the salivary α-amylase response to the cold hand test alone was not measured (van Stegeren et al. Citation2008). This study does suggest that a psychological stressor may be a more potent stimulator of salivary α-amylase giving rise to a marked increase in levels (van Stegeren et al. Citation2008).
The possibility that our stressor lacked potency is unlikely, as a robust cortisol response was observed in the afternoon. It is likely that the greater cortisol response observed in the afternoon is due to a lower baseline at that time. This is similar to a previous study using psychological stressors during pregnancy (de Weerth et al. Citation2007), but contrasts with another paper indicating no time of day effect in healthy non-pregnant individuals (Kudielka et al. Citation2004). It is noteworthy that unlike our study, Kudielka et al. (Citation2004) used a psychological stressor (TSST) and did not test the same individuals across days, instead a composite sample was drawn from five independent studies carried out at different times of the day (Kudielka et al. Citation2004).
Previous studies have shown a significant plasma noradrenaline response to the cold hand test indicative of sympathetic activation (Willemsen et al. Citation1998; Flaa et al. Citation2008). Despite this we found no change in salivary α-amylase activity in response to the cold hand test. A possible explanation is the selective activation of α-adrenergic responses elicited by the cold hand test (Willemsen et al Citation1998; Flaa et al. Citation2008). Psychological stressors can result in greater β-adrenergic activation (Willemsen et al. Citation1998), which may involve the β-adrenoceptors of the parotid gland, necessary for the release of salivary α-amylase (van Stegeren et al. Citation2006). The lack of response we observed suggests the salivary α-amylase response is stressor specific.
Another factor to consider is the action of the parasympathetic system. Animal work indicates that parasympathetic activity is involved in regulation of both flow rate and protein content of saliva (Proctor and Carpenter Citation2007). A recent study in humans suggests the heart rate response to the cold hand test is complex, in some cases involving co-activation of sympathetic and parasympathetic systems (Mourot et al. Citation2008). Speculatively, the absence of a salivary α-amylase response as we report, might reflect more complex control, possibly including the parasympathetic system. Both autonomic systems may act together to modulate the cardiac, and potentially the salivary α-amylase, response to acute stress (Mourot et al. Citation2008). This possibility is yet to be investigated. Recently, increases in α-amylase activity have been found to be independent of saliva flow rate (Rohleder et al. Citation2006). However, the role of the parasympathetic system in the control of salivary α-amylase output is yet to be fully determined. Pharmacological blockade studies, targeting the parasympathetic system, may help to clarify the specificity of salivary α-amylase as a marker of sympathetic activity.
The current study has certain limitations. Our sample was small and consisted of females only. The data concerning the stability of α-amylase are not expected to be affected by such factors. Also gender has not been found to affect salivary diurnal α-amylase activity (Nater et al. Citation2007), nor responsivity to a combined psychological and cold hand task (van Stegeren et al. Citation2008).
Future studies would benefit from the parallel use of other indices of sympathetic activity.
In conclusion, our data show that salivary α-amylase activity is a stable biomarker with a marked diurnal profile. Its diurnal pattern is consistent with control by sympathetic activity. However we found that a potentially useful physical stressor, considered to be associated with sympathetic activation, failed to elicit a consistent salivary α-amylase response despite a significant increase in salivary cortisol concentration. These findings suggest the salivary α-amylase response to acute stress may be dependent on the nature of the stressor investigated. Further work is required to clarify the regulation of salivary α-amylase, and particularly the role of the parasympathetic system. Even though there is good evidence that salivary α-amylase can be increased by sympathetic activity, importantly, it may also be subject to other influence, and be more sensitive to some types of stress than to others.
Acknowledgements
The authors are most grateful to the participants of this study. They would like to thank Diana Adams, Imperial College London, for technical assistance and support throughout this study. They thank Dr Maureen Marks, Institute of Psychiatry, King's College London, for statistical advice.
The authors are grateful to Eli Lilly Suisse (SA) and Pfizer Schweiz (AG) for Independent Medical Research Grants to author MK which enabled data collection.
Declaration of interest The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chandola T, Britton A, Brunner E, Hemingway H, Malik M, Kumari M, Badrick E, Kivimaki M, Marmot M. Work stress and coronary heart disease: What are the mechanisms?. Eur Heart J. 2008640–648.
- de Weerth C, wied CCG-d, Jansen LMC, Buitelaar JK. 2007. Cardiovascular and cortisol responses to a psychological stressor during pregnancy. Acta Obstetr Gynecol Scand. 86:1181–1192.
- Dodt C, Breckling U, Derad I, Fehm HL, Born J. 1997. Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension. 30:71–76.
- Ehlert U, Erni K, Hebisch G, Nater U. 2006. Salivary alpha-amylase levels after yohimbine challenge in healthy men. J Clin Endocrinol Metab. 91:5130–5133.
- Flaa A, Eide IK, Kjeldsen SE, Rostrup M. 2008. Sympathoadrenal stress reactivity is a predictor of future blood pressure: An 18-year follow-up study. Hypertension. 52:336–341.
- Garde AH, Hansen AM. 2005. Long-term stability of salivary cortisol. Scand J Clin Lab Invest. 65:433–436.
- Gordis EB, Granger DA, Susman EJ, Trickett PK. 2008. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Horm Behav. 53:96–103.
- Grillon C, Duncko R, Covington MF, Kopperman L, Kling MA. 2007. Acute stress potentiates anxiety in humans. Biol Psychiatr. 62:1183–1186.
- Hansen AM, Garde AH, Skovgaard LT, Christensen JM. 2001. Seasonal and biological variation of urinary epinephrine, norepinephrine, and cortisol in healthy women. Clin Chim Acta. 309:25–35.
- Jones PP, Spraul M, Matt KS, Seals DR, Skinner JS, Ravussin E. 1996. Gender does not influence sympathetic neural reactivity to stress in healthy humans. Am J Physiol Heart Circ Physiol. 270:H350–H357.
- Kammerer M, Adams D, Castelberg Bv B, Glover V. 2002. Pregnant women become insensitive to cold stress. BMC Pregnancy Childbirth. 2:8.
- Kammerer M, Pinard C, Taylor A, von Castleberg B, Künzli H, Glover V. Symptoms associated with the DSM IV diagnosis of depression in pregnancy and post partum. Arch Womens Ment Health. 2009 in press.
- Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. 2004. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 29:983–992.
- McRae AL, Saladin ME, Brady KT, Upadhyaya H, Back SE, Timmerman MA. 2006. Stress reactivity: Biological and subjective responses to the cold pressor and trier social stressors. Hum Psychopharmacol. 21:377–385.
- Mourot L, Bouhaddi M, Boussuges A, Regnard J. Autonomic cardiac control during the cold pressor test in normal subjects. Physiol Res. 2008.
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, Ehlert U. 2006. Stress-induced changes in human salivary alpha-amylase activity—associations with adrenergic activity. Psychoneuroendocrinology. 31:49–58.
- Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. 2007. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 32:392–401.
- Proctor GB, Carpenter GH. 2007. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 133:3–18.
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. 1997. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sci. 61:2539–2549.
- Rohleder N, Nater UM, Wolf JM, Ehlert U, Kirschbaum C. 2004. Psychosocial stress-induced activation of salivary alpha-amylase: An indicator of sympathetic activity?. Ann NY Acad Sci. 1032:258–263.
- Rohleder N, Wolf JM, Maldonado EF, Kirschbaum C. 2006. The psychosocial stress-induced increase in salivary alpha-amylase is independent of saliva flow rate. Psychophysiology. 43:645–652.
- Schlotz W, Kumsta R, Layes I, Entringer S, Jones A, Wust S. 2008. Covariance between psychological and endocrine responses to pharmacological challenge and psychosocial stress: A question of timing. Psychosom Med. 70:787–796.
- van Stegeren A, Rohleder N, Everaerd W, Wolf OT. 2006. Salivary alpha amylase as marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology. 31:137–141.
- van Stegeren AH, Wolf OT, Kindt M. 2008. Salivary alpha amylase and cortisol responses to different stress tasks: Impact of sex. Int J Psychophysiol. 69:33–40.
- van Veen JF, van Vliet IM, DeRijk RH, van Pelt J, Mertens B, Zitman FG. 2008. Elevated alpha-amylase but not cortisol in generalized social anxiety disorder. Psychoneuroendocrinology. 33:1313–1321.
- Willemsen G, Ring C, Carroll D, Evans P, Clow A, Hucklebridge F. 1998. Secretory immunoglobulin A and cardiovascular reactions to mental arithmetic and cold pressor. Psychophysiology. 35:252–259.
- Wolf JM, Nicholls E, Chen E.. 2008. Chronic stress, salivary cortisol, and [alpha]-amylase in children with asthma and healthy children. Biol Psychol. 78:20–28.
- Yamaguchi M, Deguchi M, Miyazaki Y. 2006. The effects of exercise in forest and urban environments on sympathetic nervous activity of normal young adults. J Int Med Res. 34:152–159.