Abstract
Stress, if exaggerated, modulates a variety of metabolic pathways and results in development of serious health consequences. The cell membrane sodium–calcium exchanger (NCX) is a major calcium extrusion system and is also modulated by stress. It has been shown previously that mRNA, protein levels and activity of the type 1 NCX (NCX1) in the left ventricle of the rat heart are increased by stressors, such as immobilization or hypoxia. In this study we investigated whether exposure to a subsequent different stressor can affect gene expression, protein level and activity of the NCX1 in rat kidney compared to exposure to only one type of stressor. In these experiments, we used immobilization and cold as the model stressors. We found that cold exposure at 4°C for 24 h, when applied after immobilization repeated seven times, completely abolished the immobilization-induced increase in NCX mRNA level and after 7 days cold exposure the increases in NCX1 protein and activity in rat kidney were also abolished. Permanently increased NCX1 expression can result in imbalance of cellular calcium homeostasis and thus contribute to kidney dysfunction. Based on our results, we conclude that exposure to a cold stressor can have a protective effect on the kidney in rats exposed previously to repeated immobilization stress. This might be explained by differential stimulation of sympathetic neural and adrenal medullary responses by these different stressors.
Introduction
Sodium–calcium exchangers (NCXs) comprise a family of ion counter-transporters that play a prominent role in cellular calcium homeostasis (Lederer et al. Citation1996; Philipson et al. Citation1996). These integral membrane-spanning proteins are generally considered to serve as cellular calcium efflux mechanisms that are driven by the sodium electrochemical gradient. In kidney, the NCX is localized predominantly in the late distal convoluted tubules (Loffing et al. Citation2001), where it contributes to the control of calcium reabsorption (Gesek and Friedman Citation1992; White et al. Citation1996).
In mammals, NCX proteins are encoded by three genes, type 1 NCX (NCX1), type 2 NCX (NCX2), and type 3 NCX (NCX3; Nicoll et al. Citation1996). NCX1 gene transcripts are widely expressed in many tissues, while NCX2 and NCX3 genes transcripts have been found only in skeletal muscle and brain tissue (Quednau et al. Citation1997). NCX1 is found in the heart and kidney, but each expresses one of two isoforms of the NCX1 that differ in the voltage dependence and calcium sensitivity (Ruknudin et al. Citation2000). The only difference between the cardiac and renal NCX1 isoforms is in the alternatively spliced region, which is located near the C-terminal of the putative intracellular loop. If the alternatively spliced region interacts directly or indirectly with a region in the voltage field across the membrane, changes in the alternatively spliced region may affect the voltage dependence of the NCX (Ruknudin et al. Citation2000).
NCX1 expression can be regulated by several agents, especially at the transcriptional level. Retinoic acid upregulates NCX gene expression in the brain and heart (Hudecova et al. Citation2004a), probably via a retinoic acid responsive element, or activator protein 2. Several papers have described adrenergic modulation of NCX1 gene expression, especially in the heart (Menick et al. Citation1996; Reinecke et al. Citation1997; Hudecova et al. Citation2004b,). Administration of 6-hydroxydopamine, which destroys catecholaminergic terminals and produces anatomical and functional noradrenergic denervation, decreases NCX gene expression in the left atrium and ventricle of the rodent heart (Hudecova et al. Citation2004b). Conversely, NCX mRNA levels are up-regulated by 3–5-fold in neonatal feline cardiocytes after α-adrenergic stimulation (Menick et al. Citation1996). Reinecke et al. (Citation1997) have extended these findings, showing that adrenergic stimulation of NCX mRNA level applies also to adult cardiocytes. In addition, NCX1 mRNA levels are significantly increased in skeletal muscle of hyperthyroid compared with euthyroid rats, indicating involvement of thyroid hormones in the transcriptional regulation of the NCX gene in this tissue (Hudecova et al. Citation2004c).
Experience of stress is common, but if exaggerated, may lead to negative consequences and is invoked as an underlying factor in the development of several diseases. Exposure to stressors activates the sympathetic nervous system, the adrenomedullary hormonal system and the hypothalamic-pituitary–adrenocortical axis (Goldstein and Kopin Citation2008). Kvetnansky (Citation2004) has shown that an initial or chronic exposure to a cold environment does not cause any significant changes in plasma levels of adrenaline (secreted by the adrenal medulla), but a significant increase in levels of plasma noradrenaline (from sympathetic nerve terminals) is observed. By contrast, exposure to a single immobilization stress induced increases in both adrenaline and noradrenaline plasma levels. Thus, from this point of view immobilization stress can be considered as a ‘strong’ stressor, which activates both the adrenomedullary and sympathetic neural systems (Kvetnanský et al. Citation1977). Immobilization stress is known to affect some of the calcium transport systems, e.g. inositol 1,4,5-trisphosphate receptors (Krizanova et al. Citation2007). Although a single 2-h exposure of rats to immobilization stress does not affect NCX1 gene expression, repeated immobilization for 2 h each day for 7 days significantly increases NCX1 mRNA, protein levels, and also transport activity in the cardiac left ventricle (Zacikova et al. Citation1999). Exposure to cold, which activates predominantly the sympathetic neural system, does affect NCX1 mRNA and protein levels in the rat left ventricle, even after exposure for 28 days (Hudecova et al. Citation2007).
It is well known that stress causes massive increase in the synthesis and action of catecholamines, which modulate renal glomerular cell functions (Mundel et al. Citation1997; Pavenstadt Citation1998). Elevated levels of noradrenaline can cause acute renal failure and increase intracellular calcium activity. Altered NCX1 function might be involved in pathophysiological states that are associated with changed calcium metabolism (renal hypertension, renal failure). Our previous results showed that some stressors can increase NCX1 mRNA, protein and activity levels also in the kidney. Therefore, we aimed to study, whether exposure to cold as a second, or novel, stressor after previous exposure to a different type of stressor, specifically repeated immobilization, can further affect NCX1 gene expression, protein level and/or activity. Conversely, we also tested whether immobilization used as a novel stressor after cold exposure for 28 days can affect NCX1 mRNA and protein levels.
Materials and methods
Animals
Male Sprague–Dawley rats, 4 months old, weighing approximately 350 g (Charles River Laboratories, Sulzfeld, Germany) were used in the immobilization and cold exposure experiments. Prior to the experiments, rats were housed for 1 week in a controlled environment (22 ± 2°C, 12 h light/dark cycle, lights on at 6.00 a.m., humidity 45–55%). Food and water were available ad libitum. All animal experiments presented were approved by The Ethics Committee of the Institute of Experimental Endocrinology, Slovak Academy of Sciences, Bratislava, Slovakia, under permit No. 2804/07-221/3.
Immobilization and cold exposure
Immobilization stress was performed as described by Kvetnansky and Mikulaj (Citation1970). For these experiments, the legs of each rat were fixed by adhesive tape to a wooden board in the prone position for 2 h. Immobilization experiments started each day at 8.00 a.m. Repeated immobilization stress was achieved by immobilizing rats for seven consecutive days, 2 h daily, rats were killed immediately after the end of the experiment. Exposure to cold was performed in a cold room with a constant temperature of 4°C, standard light conditions and humidity 90% as described previously (Hudecova et al. Citation2007). Food and water in the cold room were also available ad libitum. The whole experiment was designed as follows—rats were divided into seven groups, each group containing 6–8 rats. Groups were used as follows: A—untreated control, B—cold exposure for 1 day (24 h), C—cold for 7 days, D—immobilization (7days) for 2 h+1 day cold, E—immobilization (7days) for 2 h+1 day at room temperature, F—immobilization (7days) for 2 h+7 days cold, G—immobilization (7days) for 2 h+7 days at room temperature. All groups of rats were decapitated on the same day immediately after the end of the experiment.
The term ‘novel stressor’ was used for a stressor that was used subsequently, after the application of a previous, different stressor. Therefore, for experimental groups D and F cold was a novel stressor, since it was used after the exposure to immobilization stress. In the last experiment, immobilization was used as a novel stressor, since it was applied after the 28-days exposure to cold. After the end of the experiment, rats were decapitated, both kidneys were removed, cleaned from fat, and each kidney was wrapped and frozen separately in liquid nitrogen. Experiments were performed on the right kidney.
RNA preparation and quantification of relative mRNA levels by reverse transcriptase polymerase chain reaction (PCR)
Total RNA was isolated with TRI Reagent (MRC Ltd, Cincinnati, OH, USA). Briefly, tissue samples were homogenized with a tissue homogenizer (Biospec Products, Inc., Bartlesville, OK, USA) in TRI Reagent and after 5 min the homogenate was extracted with chloroform. RNAs in the aqueous phase were precipitated with isopropanol. The RNA pellet was washed with 75% ethanol and stored in 96% ethanol at − 70°C. The purity and concentration of isolated RNA were checked with a GeneQuant Pro spectrophotometer (Amersham Biosciences, Buckinghampshire, UK) at 260 nm, 260 nm/280 nm and 230 nm/260 nm, respectively. Reverse transcription was performed using 1.5 μg of total RNAs and Ready-To-Go You-Prime First-Strand Beads (GE Healthcare-Life Sciences, Uppsala, Sweden) with pd(N)6 primer. PCR specific for the type of NCX1 mRNA (GI 451751) was carried out afterwards with the following primers: NCX1a: 5′-AGG CGG CTT CTC TTT TAC-3′, NCX1b: 5′-CGA CTT CCA AAA CCA GAC-3′. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; GI 56187) was used as a housekeeper gene control for semi-quantitative evaluation of PCR. The following primers for GAPDH were used: GA1: 5′-AGA TCC ACA ACG GAT ACA TT-3′ and GA2: 5′-TCC CTC AAG ATT GTC AGC AA-3′. PCR specific for NCX1 was started by initial denaturation at 94°C and was followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 48°C for 1 min and polymerization at 72°C for 1 min. PCR specific for GAPDH started by initial denaturation at 94°C and was followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min and polymerization at 72°C for 1 min. PCRs were terminated by final polymerization at 72°C for 7 min. All PCR products were analyzed on 2% agarose gels. Intensity of individual bands was evaluated by measuring the optical density per mm2 and compared to GAPDH mRNA.
Western blot analysis
NCX protein was determined in crude membrane fraction from the rat kidney. Protein concentration was determined according to Lowry et al. (Citation1951). Twenty micrograms of protein extract from each sample was separated by electrophoresis on 10% sodium dodecyl sulfate polyacrylamide gels and proteins were transferred to Hybond ECL membrane using semidry blotting (Owl, Inc., Woburn, MA, USA). Membrane was blocked in 5% non-fat dry milk diluted in Tris-buffered saline–Tween 20 and then incubated with the mouse monoclonal antibody to NCX1 (Abcam, Cambridge, UK), diluted 1:1000. This antibody recognizes an epitope between 371–525 amino acids on the intracellular side of the plasma membrane and reacts with human, rabbit, rat, dog, and guinea pig NCX1; the antibody recognizes a 120 kDa protein. Horseradish peroxidase-linked secondary antibody and chemiluminescence were used for visualization. Optical density of individual bands was quantified using PCBAS 2.0 software.
Calcium transport activity
Crude membrane fractions were isolated as described previously (Krizanova et al. Citation1990).
Two hundred micrograms of each crude membrane fraction was suspended in 100 μl of buffer A—20 mM Tris–HCl pH 7.0, 0.3 M sucrose and 160 mM NaCl. Fluorescent probe Rhod-5N-tripotassium salt (Molecular Probes, Junction City, OR, USA) was added to a final concentration of 2 μM. Membrane vesicles were prepared by three cycles of freeze/thaw procedure in liquid nitrogen. Vesicles were washed in buffer A and after final centrifugation suspended in 200 μl of 20 mM Tris–HCl pH 7.0, 0.3 M sucrose and 160 mM NaCl or 160 mM KCl. CaCl2 was added immediately before measurement to a final concentration of 0.5 mM. Fluorescent signal was measured in a 96-well plate in a fluorescence reader (BioTek Instruments GmbH, Bad Friedrichshall, Germany), where excitation at 545 nm and emission at 575 nm were used. Calcium transport was calculated as a difference between samples without calcium and with calcium. NCX activity was obtained as a difference between samples with NaCl and KCl. Ionomycin (5 μM; Calbiochem, San Diego, CA, USA) was used to normalize fluorescence values to the internal volume of vesicles.
Statistical analysis
Each value represents the mean for 6–8 rats. Results are presented as group means ± SEM. Statistically significant differences among groups were determined by one-way analysis of variance (ANOVA); p < 0.05 was considered to be significant. For multiple comparisons, an adjusted t-test with p values corrected by the Bonferroni method was used (StatSoft, Tulsa, OK, USA).
Results
Cold exposure for 1 day (24 h) had no effect on NCX1 mRNA (, left) or protein (, right) levels in the right rat kidney compared to controls. Repeated immobilization stress for 7 days, 2 h daily, significantly increased both NCX1 mRNA and protein levels. When the cold exposure for 1 day was applied after seven consecutive daily immobilizations, the NCX1 mRNA level decreased almost to control values (, left), while protein levels remained still significantly increased (, right). NCX1 activity in vesicles prepared from kidney membrane fractions was measured using the fluorescent dye Rhod-5N-tripotassium salt (). No significant difference was observed for NCX1 activity in the kidney between control and cold-exposed groups (once for 24 h) of rats. Immobilization stress (seven times, 2 h daily) significantly increased NCX activity. However, when cold exposure for 1 day was applied as a novel stressor after the repeated immobilization stress, a significant decrease in NCX activity occurred ()
Figure 1 Effect of cold and immobilization alone or consecutively on NCX1 mRNA (left) and protein (right) levels in the kidney. UC—untreated control, 1DC—exposure to 4°C for 1 day, 7IMO1RT—daily exposure to immobilization for 7 days, 2 h per day with subsequent rest for 1 day at room temperature, 7IMO1DC—daily immobilization for 7 days, 2 h per day with subsequent exposure to cold for 1 day. Values are group mean ± SEM; n, 6–8 rats per group. One-way ANOVA: p = 1.191 × 10− 5, F = 27.3625 (left), p = 3.938 × 10− 5, F = 21.6354 (right). *p < 0.05 and **p < 0.01 vs. UC; ++p < 0.01 vs. 7IMO1RT.
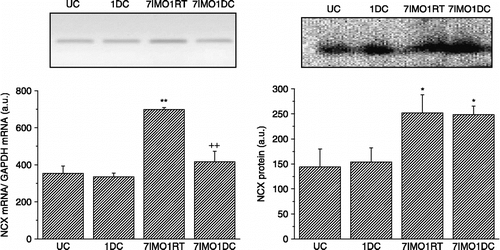
Figure 2 Effect of cold and immobilization alone or consecutively on NCX1 activity in the kidney. UC—untreated control, 1DC—exposure to 4°C for 1 day, 7IMO1RT—daily exposure to immobilization for 7 days, 2 h per day with subsequent rest for 1 day at room temperature, 7IMO1DC—daily immobilization for 7 days, 2 h per day with subsequent exposure to cold for 1 day. Values are group mean ± SEM; n, 6–8 rats per group. One-way ANOVA: p = 4.943 × 10− 8, F = 74.7025. *p < 0.05 vs. UC.
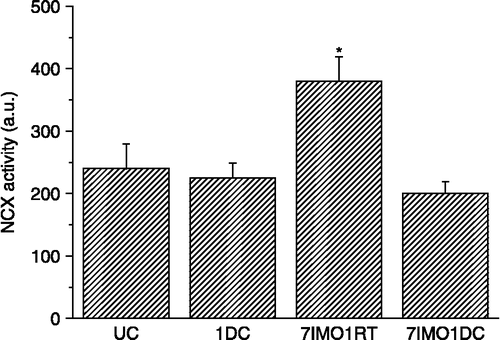
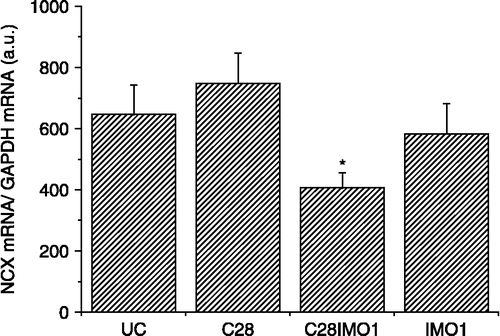
Repeated cold exposure did not significantly increase NCX1 mRNA (, left) or protein levels (, right). When cold exposure for 7 days was applied after the seven times repeated immobilization stress, kidney NCX1 mRNA (, left) and protein levels (, right) were significantly decreased compared to the group exposed only to repeated IMO. NCX1 activity corresponded to the data for mRNA and protein (). When 7-days cold was applied as a novel stressor after seven times repeated immobilization, a decrease in the NCX activity compared to rats stressed only by repeated immobilization was observed. Although 28-days cold and also single immobilization did not show any significant effect on the NCX1 mRNA compared to non-stressed rats, the combination of both of these stressors significant decreased NCX1 mRNA level ().
Figure 3 Effect of cold and immobilization alone or consecutively on NCX1mRNA (left) and protein (right) levels. UC—untreated control, 7DC—exposure to 4°C for seven consecutive days, 7IMO7RT—daily exposure to immobilization for 7 days, 2 h per day with subsequent rest for 7 days at room temperature, 7IMO7DC—daily exposure to immobilization for 7 days, 2 h per day with subsequent exposure to cold for seven consecutive days. Values are displayed as mean ± SEM; n, 6–8 rats per group. One-way ANOVA: p = 8.425 × 10− 4, F = 11.244 (left), p = 4.662 10− 14, F = 793.3528 (right) **p < 0.01 vs. UC; +p < 0.05 vs. 7IMO7RT.
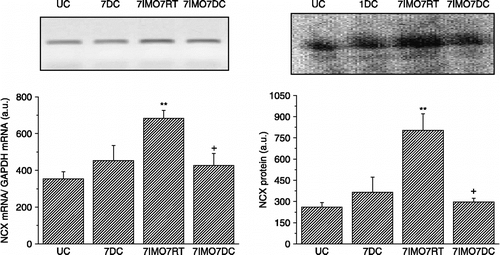
Figure 4 Effect of cold and immobilization alone or consecutively on NCX1 activity in the kidney. UC—untreated control, 7DC—exposure to 4°C for 7 days, 7IMO7RT—exposure to daily immobilization for 7 days, 2 h per day with subsequent rest for 7 days at room temperature, 7IMO7DC—daily immobilization for 7 days, 2 h per day with subsequent exposure to cold for 7 days. Values are group mean ± SEM; n, 6–8 rats per group. One-way ANOVA: p = 2.446 × 10− 9, F = 126.078. **p < 0.01 vs. UC.
Figure 5 Effect of long-term cold exposure, and a single immobilization alone or after long-term cold on NCX1 mRNA levels in the kidney. UC—untreated control, C28—exposure to 4°C for 28 consecutive days, IMO1—exposure to immobilization on 1 day for 2 h, C28IMO1—exposure to cold for 28 consecutive days with subsequent exposure to a single immobilization for 2 h. Values are mean ± SEM; n, 6–8 rats per group. One-way ANOVA: p = 3.243 × 10− 5, F = 22.4902. *p < 0.05 vs. UC.
Discussion
In this study, we have shown that exposure to cold as another and different stressor after repeated immobilization, significantly altered renal NCX1 mRNA and protein level and activity, compared to response to repeated immobilization alone. Specifically, a single cold exposure applied after the repeated immobilization, completely abolished the immobilization induced increase in NCX1 mRNA level, and after prolonged cold exposure also reduced the immobilization-induced increase in NCX1 protein. We propose that after the exposure to immobilization, NCX1 activity increased first, and later NCX1 mRNA and protein level started to increase as well. After repeated immobilization the activity was still increased, as now were the mRNA levels and subsequently (but not immediately) also protein levels. Exposure to cold after repeated immobilization decreased the immobilization-induced increased NCX1 activity, with decreased NCX1 mRNA levels as well. It seems that a decrease in protein levels takes longer, and therefore was observed only after the repeated cold exposure ().
It has previously been shown that different stressors activate NCX in various tissues and species. Although a single immobilization did not affect NCX mRNA, protein and activity in the rat heart (Zacikova et al. Citation1999) and kidney (data not shown), repeated immobilization for 7 days resulted in increased levels of NCX mRNA, protein and activity in the rat left ventricle and also in the kidney. When rats were exposed to cold for 1, 7 and 28 days, no changes in NCX1 mRNA and protein were observed in the left ventricle of the rat heart compared to control (Hudecova et al. Citation2007). Interestingly, when crayfish was subjected to cold acclimation (4°C), increases in NCX mRNA and protein levels were observed in the tail and kidney (Wheatly et al. Citation2007). Hypoxia increases NCX1 mRNA level in the left ventricle and cardiomyocytes, but not in murine cerebellum (Hudecova et al. Citation2007).
When animals or humans are exposed to the same stressor daily for many days, there is the potential based upon prior experience to mobilize physiological systems to minimize the effort required for maintenance and homeostasis. Thus, habituation of sympathetic and adrenal medullary and other neuroendocrine responses would allow for conservation of energy by dampening responses to a familiar and highly predictable situation. When chronically stressed animals are exposed to a novel stressor, three outcomes are possible. First, the physiological response could be similar to that of controls exposed to the same stressor, with prior stress history having no proactive effects on later reaction to the novel condition. Second, the physiological response could be reduced compared to that of controls, suggesting cross-tolerance between stressors. Specifically, prior experience with one stressor would result in a reduced physiological response to a novel stressor. Finally, the physiological response could be greater compared to that of controls, indicating sensitization to the novel stressor (Konarska et al. Citation1989).
NCX1 is not affected by cold alone (Hudecova et al. Citation2007) or by single exposure to immobilization (Zacikova et al. Citation1999). Nevertheless, immobilization repeated daily for 7 days resulted in more than 3-fold increased levels of NCX1 mRNA and protein (Zacikova et al. Citation1999). From the results presented here, when cold was used as a novel stressor after repeated immobilization it attenuated the increased NCX1 gene expression and protein level induced by the immobilization. Vice versa, when immobilization was used as a novel stressor, reduced NCX1gene expression was observed in the kidney of rats exposed to cold for 7 days.
Circulating levels of the catecholamines adrenaline and noradrenaline are rapidly increased during immobilization stress (Kvetnansky et al. Citation1978). Also, rats exposed to long-term cold show an exaggerated response of plasma catecholamines and also catecholamine synthesizing enzymes in the adrenal medulla. Catecholamines activate both α- and β-adrenergic receptor pathways. NCX expression level is up-regulated by the activation of the α-adrenergic signal transduction pathway in adult ventricular myocytes (Reinecke et al. Citation1997). Also, NCX upregulation can markedly alter the consequences of β-adrenergic receptor stimulation in cardiomyocytes (Sato et al. Citation2004). Thus, the immobilization-induced increase in NCX1 gene expression might be due to increased adrenergic regulation (Hudecova et al. Citation2004b). Rats exposed to long-term immobilization and subsequently to cold show increased level of plasma noradrenaline, but plasma adrenaline is not significantly changed (Dronjak et al. Citation2004). Based on these results, we might speculate that desensitization of adrenergic modulation plays a crucial role in NCX1 gene expression, when cold was applied as a novel stressor.
Chronic renal failure (CRF) results in diminished physical activity and has been shown to be an independent risk factor for cardiovascular disease (CVD) in a number of recent epidemiological studies. CRF is associated with an increase in calcium content of the myocardium (Zhang et al. Citation1994). There are several possible explanations for the association of chronic kidney diseases with CVD. Reduced renal function is associated with a high prevalence of traditional CVD risk factors, such as hypertension, diabetes, dyslipidemia, and left ventricular hypertrophy. In addition, reduced renal function may be associated with increased levels of non-traditional risk factors, such as inflammation and oxidative stress.
In the kidney, NCX contributes mainly to the control of calcium reabsorption (Gesek and Friedman Citation1992; White et al. Citation1996). More precise studies localized renal NCX in the basolateral membrane along the distal convoluted tubule and in the collecting duct (Loffing et al. Citation2001; Biner et al. Citation2002). It is supposed that NCX and Ca2+-ATPase act as major calcium extrusion systems at the basolateral plasma membrane of renal epithelial cells (Loffing et al. Citation2001). Permanently increased stress-induced levels of the NCX in the kidney may lead to imbalance in calcium homeostasis and to development of a pathophysiological state.
In summary, we found that exposure to cold applied after repeated immobilization stress abolished the immobilization-induced increase in NCX1 mRNA and protein levels and activity. Although the physiological relevance of this observation remains to be elucidated we suggest that the protective effect cold exposure on renal calcium homeostasis following repeated immobilization stress might reflect different influences of differential activation of the sympathetic and adrenomedullary systems by these two types of stressor.
Acknowledgements
This work was supported by grants APVV 51-0397-07, APVV 0148-06, VEGA 2/7123, and VEGA 2/6012.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Biner HL, Arpin-Bott MP, Loffing J, Wang X, Knepper M, Hebert SC, Kaissling B. 2002. Human cortical distal nephron: Distribution of electrolyte and water transport pathways. J Am Soc Nephrol. 13:836–847.
- Dronjak S, Jezova D, Kvetnansky R. 2004. Different effects of novel stressors on sympathoadrenal system activation in rats exposed to long-term immobilization. Ann N Y Acad Sci. 1018:113–123.
- Gesek FA, Friedman PA. 1992. Mechanism of calcium transport stimulated by chlorothiazide in mouse distal convoluted tubule cells. J Clin Invest. 90:429–438.
- Goldstein DS, Kopin IJ. 2008. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: A meta-analysis. Endocr Regul. 42:111–119.
- Hudecova S, Stefanik P, Macejova D, Brtko J, Krizanova O. Retinoic acid increased expression of the Na+/Ca2+ exchanger in the heart and brain. Gen Physiol Biophys. 2004a; 23:417–422.
- Hudecova S, Tillinger A, Mravec B, Kvetnansky R, Krizanova O. Effect of 6-hydroxydopamine on the gene expression of Na+/Ca2+ exchanger in the rat heart. Gen Physiol Biophys. 2004b; 23:307–313.
- Hudecova S, Vadaszova A, Soukup T, Krizanova O. Effect of thyroid hormones on the gene expression of calcium transport systems in rat muscles. Life Sci. 2004c; 75:923–931.
- Hudecova S, Kubovcakova L, Kvetnansky R, Kopacek J, Pastorekova S, Novakova M, Knezl V, Tarabova B, Lacinova L, Sulova Z, Breier A, Jurkovicova D, Krizanova O. 2007. Modulation of expression of Na+/Ca2+ exchanger in heart of rat and mouse under stress. Acta Physiol (Oxf). 190:127–136.
- Konarska M, Stewart RE, McCarty R. 1989. Sensitization of sympathetic-adrenal medullary responses to a novel stressor in chronically stressed laboratory rats. Physiol Behav. 46:129–135.
- Krizanova O, Novotova M, Zachar J. 1990. Characterization of DHP binding protein in crayfish striated muscle. FEBS Lett. 267:311–315.
- Krizanova O, Myslivecek J, Tillinger A, Jurkovicova D, Kubovcakova L. 2007. Adrenergic and calcium modulation of the heart in stress: From molecular biology to function. Stress. 10:173–184.
- Kvetnansky R. 2004. Stressor specificity and effect of prior experience on catecholamine biosynthetic enzyme phenylethanolamine N-methyltransferase. Ann NY Acad Sci. 1032:117–129.
- Kvetnansky R, Mikulaj L. 1970. Adrenal and urinary catecholamines in rats during adaptation to repeated immobilization stress. Endocrinology. 87:738–743.
- Kvetnanský R, Palkovits M, Mitro A, Torda T, Mikulaj L. 1977. Catecholamines in individual hypothalamic nuclei of acutely and repeatedly stressed rats. Neuroendocrinology. 23:257–267.
- Kvetnansky R, Sun CL, Lake CR, Thoa N, Torda T, Kopin IJ. 1978. Effect of handling and forced immobilization on rat plasma levels of epinephrine, norepinephrine, and dopamine-beta-hydroxylase. Endocrinology. 103:1868–1874.
- Lederer WJ, He S, Luo S, duBell W, Kofuji P, Kieval R, Neubauer CF, Ruknudin A, Cheng H, Cannell MB, Rogers TB, Schulze DH. 1996. The molecular biology of the Na(+)–Ca2+ exchanger and its functional roles in heart, smooth muscle cells, neurons, glia, lymphocytes, and nonexcitable cells. Ann NY Acad Sci. 779:7–17.
- Loffing J, Loffing-Cueni D, Valderrabano V, Kläusli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B. 2001. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol. 281:F1021–F1027.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275.
- Menick DR, Barnes KV, Thacker UF, Dawson MM, McDermott DE, Rozich JD, Kent RL, Cooper G4th. 1996. The exchanger and cardiac hypertrophy. Ann NY Acad Sci. 779:489–501.
- Mundel P, Reiser J, Borja AZM, Pavenstadt H, Davidson GR, Kriz W, Zeller R. 1997. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 236:248–258.
- Nicoll DA, Quednau BD, Qui Z, Xia YR, Lusis AJ, Philipson KD. 1996. Cloning of a third mammalian Na+–Ca2+ exchanger, NCX3. J Biol Chem. 271:24914–24921.
- Pavenstadt H. 1998. New aspects concerning the role of catecholamines in the pathogenesis of glomerular diseases. Nephrol Dial Transplant. 13:1916–1919.
- Philipson KD, Nicoll DA, Matsuoka S, Hryshko LV, Levitsky DO, Weiss JN. 1996. Molecular regulation of the Na(+)–Ca2+ exchanger. Ann NY Acad Sci. 779:20–28.
- Quednau BD, Nicoll DA, Philipson KD. 1997. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am J Physiol. 272:C1250–C1261.
- Reinecke H, Vetter R, Drexler H. 1997. Effects of alpha-adrenergic stimulation on the sarcolemmal Na+/Ca(2+)-exchanger in adult rat ventricular cardiocytes. Cardiovasc Res. 36:216–222.
- Ruknudin A, He S, Lederer WJ, Schulze DH. 2000. Functional differences between cardiac and renal isoforms of the rat Na+–Ca2+ exchanger NCX1 expressed in Xenopus oocytes. J Physiol. 529:599–610.
- Sato M, Gong H, Terracciano CM, Ranu H, Harding SE. 2004. Loss of beta-adrenoceptor response in myocytes overexpressing the Na+/Ca(2+)-exchanger. J Mol Cell Cardiol. 36:43–48.
- Wheatly MG, Gao Y, Stiner LM, Whalen DR, Nade M, Vigo F, Golshani AE. 2007. Roles of NCX and PMCA in basolateral calcium export associated with mineralization cycles and cold acclimation in crayfish. Ann NY Acad Sci. 1099:190–192.
- White KE, Gesek FA, Friedman PA. 1996. Structural and functional analysis of Na+/Ca2+ exchange in distal convoluted tubule cells. Am J Physiol. 271:F560–F570.
- Zacikova L, Kvetnansky R, Krizanova O. 1999. Increased expression of the Na(+)/Ca(2+) exchanger in the rat heart after immobilization stress is not induced by cortisol. FEBS Lett. 457:423–428.
- Zhang YB, Smogorzewski M, Ni Z, Massry SG. 1994. Altered cytosolic calcium homeostasis in rat cardiac myocytes in CRF. Kidney Int. 45:1113–1119.