Abstract
Plasma brain-derived neurotrophic factor (BDNF) levels are associated with several neural disorders. Previously, we reported that BDNF is produced from salivary glands under acute immobilization stress. Additionally, salivary glands are the origin of plasma BDNF during stress; however, the association between the expression of BDNF by the salivary glands under chronic stress conditions is not known. In the present study, we investigated whether plasma BDNF levels in chronic stress depend on the salivary glands. Expression of BDNF mRNA and protein were identified in the submandibular glands when male rats were exposed to chronic restraint stress (12 h daily for 22 days). Chronic stress significantly increased plasma BDNF concentration, as well as adrenocorticotropic hormone and corticosterone levels, but was not altered under chronic stress in bilaterally sialoadenectomized rats. Since chronic stress increases plasma BDNF levels in the sialoadenectomized rat model, the plasma BDNF level was not dependent on BDNF from the salivary glands. Although the salivary glands were the source of plasma BDNF in acute stress conditions in our previous study, it seems that that the increased BDNF expression in the salivary glands in chronic stress does not contribute importantly to the increased circulating BDNF level. The increased plasma BDNF levels may play important roles in homeostasis under stress conditions.
| Abbreviations | ||
| ACTH | = | adrenocorticotropic hormone |
| BDNF | = | brain-derived neurotrophic factor |
| EGF | = | epidermal growth factor |
| GC | = | glucocorticoids |
| HRP | = | horseradish peroxidase |
| NGF | = | nerve growth factor |
| NT | = | neurotrophin |
Introduction
The salivary glands comprise the major parotid, submandibular, and sublingual glands, and numerous minor salivary glands in the oral cavity (Hand Citation1980). Their primary role is to secrete saliva, which is involved in food digestion, promotion of mastication, and antimicrobial activity; however, the salivary glands may play other important roles as they produce a variety of biologically active substances. Cell growth factors, such as epidermal growth factor (EGF) and nerve growth factor (NGF), in particular, are produced in the rat submandibular gland (CitationCohen 1960,1962). Mouse salivary gland tissue expresses a high level of NGF (CitationCohen 1960,1962), which is massively released from salivary glands into the bloodstream during fighting (Aloe et al. Citation1986; De Simone et al. Citation1990), and plasma EGF level is reduced after damage to the major salivary glands (Hwang et al. Citation1991). Hence, the salivary glands may play an important role in systemic health (Tsukinoki et al. Citation2005).
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin (NT) family that includes NGF, NT-3, and NT-4 (Lewin and Barde Citation1996). BDNF is the most abundant NT in the central nervous system, and is closely involved in neural cell survival and maintenance, and in neural transmission (Lewin and Barde Citation1996). In the hippocampus in particular, the expression of BDNF varies depending on stress (Givalois et al. Citation2004), exercise (Adlard and Cotman Citation2004), and learning (Egan et al. Citation2003), and BDNF plays an important role in facilitating the formation of neural networks. BDNF is also found elsewhere, such as in the lachrymal glands (Ghinelli et al. Citation2003), lymphocytes (Sobue et al. Citation1998), and vascular endothelial cells (Nakahashi et al. Citation2000). Moreover, rat salivary glands increase their expression of BDNF under acute stress conditions (Tsukinoki et al. Citation2006). Furthermore, the plasma level of BDNF is increased by acute immobilization stress and the submandibular glands contribute to this (Tsukinoki et al. Citation2007). Indeed, the salivary glands are considered to be a primary source of plasma BDNF, the concentration of which is greater in intact rats than in sialoadenectomized rats (Tsukinoki et al. Citation2007). These results suggest that BDNF produced by the salivary glands may affect various organs in acute stress. However, changes in BDNF level in the salivary glands during chronic stress have not been reported, even though the roles of BDNF in chronic stress have been widely studied.
Decreased circulating BDNF level has been found in depression (Karege et al. Citation2005) and schizophrenia (Tan et al. Citation2005), and alteration of BDNF content in the central nervous system has been reported to play an important role in the pathogenesis of neural diseases related to chronic stress (Lommatzsch et al. Citation2005). Because plasma BDNF levels increase with the use of psychotropic agents (Shimizu et al. Citation2003), the BDNF content in serum and plasma may have clinical significance in the evaluation of mental disorders. Furthermore, chronic stress, an important onset factor for mental disorders, decreases BDNF content in the rat hippocampus (Ueyama et al. Citation1997), but few studies have measured blood BDNF levels during chronic stress (Joachim et al. Citation2008).
The aim of this study was to determine whether chronic stress affects the expression of BDNF in the salivary gland of rats. In addition, we sought association between alteration of plasma BDNF level in chronic stress and the salivary glands.
Materials and methods
Animals
Sprague–Dawley male rats, aged 5 weeks, each weighing 150–170 g (Japan SLC, Shizuoka, Japan), were used in this study. They were housed in groups of six rats per cage in a room maintained under standardized conditions of 12 h light/dark cycle (lights on at 7:00 am) and temperature (22 ± 3°C). Rats had free access to food pellets and tap water. Body weights of rats were recorded every 2 days in the morning after being taken out of restraining cages from the beginning to the end of the experimental period.
Restraint and sialoadenectomy procedure
Restraint stress was involved enclosing each rat in a flexible wire mesh (5 × 5 mm) shaped to fit its body. Restrained rats were able to move a little and had no access to food or water during the restraint; controls rats also had no access to food or water during this time. Because rats are nocturnal, they were restrained from 7:00 pm to 7:00 am (12 h) each 24 h for the 22-day experimental period. After release from the restrainer, rats were returned to their home cages each morning and had access to food and water ad libitum, as did the controls (Nakajima et al. Citation2006).
The major salivary glands were removed bilaterally (sialoadenectomy) or a sham operation performed, in 3-week-old male rats under sodium pentobarbital anaesthesia (65 mg/kg, i.p.; Hwang et al. Citation1991). A 2 cm skin incision in the neck was made, the major salivary glands were isolated from the surrounding subcutaneous fat tissue, a ligature was placed around the supplying vessels and the salivary glands were then removed, bilaterally. Two weeks after surgery, the repeated restraint stress and handling procedure began. All experiments were performed with 12 rats per experimental group. At the end of the experiment, all rats were deeply anaesthetized with sodium pentobarbital (65 mg/kg, i.p.) for blood and tissue collection. To avoid diurnal variations in BDNF expression, all rats were killed between 7:00 and 11:00 am; the stressed rats were anaesthetized immediately after the end of the last restraint session. The experimental protocol used in this study was reviewed and approved by the Ethics Committee on Animal Experiments of Kanagawa Dental College, and was carried out with adherence to the Guidelines for Animal Experimentation of Kanagawa Dental College.
Blood sampling
At the end of the experiment on the 23rd day all rats were deeply anaesthetized with sodium pentobarbital, as above, and blood samples were collected between 7:00 and 11:00 am by cardiac puncture into Venoject® tubes containing EDTA (Terumo, Tokyo, Japan). The tubes were immediately placed on ice and then centrifuged (2000 rpm, 15 min, 4°C). Plasma was stored at − 20°C prior to radioimmunoassay.
RNA extraction and cDNA synthesis
After blood sampling, the anaesthetized intact rats were killed by decapitation and submandibular glands were removed. Total RNA isolation from the submandibular glands was performed using the ISOGEN reagent (Nippon Gene, Toyama, Japan) in accordance with the manufacturer's instructions. The RNA product was resuspended in 20 μl diethyl pyrocarbonate-treated water. The quality of RNA was judged from the pattern of ribosomal RNA after electrophoresis through a 1.5% agarose gel containing ethidium bromide and visualization by UV illumination. RNA concentrations were determined by absorbance readings at 260 nm with a SmartSpec Plus spectrophotometer (Bio-Rad, Tokyo, Japan). RNA was stored at − 80°C until use. Total RNA was reverse transcribed at 50°C for 30 min, 99°C for 5 min, and 5°C for 5 min using a single-strand cDNA synthesis kit (Roche Diagnostics Ltd, Lewes, UK) according to the manufacturer's instructions. Following the reverse transcription reaction, the cDNA products were stored at − 20°C until use.
Real-time PCR analysis
Real-time PCR was performed using a LightCycler (Roche) according to the manufacturer's instructions. Reactions were performed in a 20 μl volume (BDNF: 0.3 μM of each primer and 4 mM MgCl2; NT-3: 0.5 μM of each primer and 3 mM MgCl2). Reactions with Taq DNA polymerase, nucleotides, and buffer for BDNF were performed with LightCycler-DNA Master SYBR Green I mix (Roche). Oligonucleotide primers designed to amplify rat BDNF were specific for the coding region of exon 5. BDNF-specific primers were 5′-CAGGGGCATAGACAAAAG-3′ (forward) and 5′-CTTCCCCTTTTAATGGTC-3′ (reverse; BDNF PCR product: 167 bp; CitationTsukinoki et al. 2006,2007; Lee et al. Citation2008) as designed and synthesized by Nippon Gene Laboratory. Real-time PCR for amplification of the rat β-actin housekeeping gene was performed using a LightCycler Primer/Probe set, 5′-CCTGTATGCCTCTGGTCGTA-3′ (forward) and 5′-CCATCTCTTGCTCGAAGTCT-3′ (reverse; β-actin PCR product: 260 bp), following the manufacturer's instructions (Nihon Gene Research Labs Inc., Sendai, Japan). Denaturation was performed at 95°C for 10 min, after which Segment 1 (95°C for 10 s), Segment 2 (60°C for 10 s), and Segment 3 (72°C for 10 s) were repeated for 40 cycles. We performed melting analysis and agarose gel electrophoresis to confirm the specificity of the PCR products obtained using each primer pair. Gene expression is given in terms of the ratio of the copy number of BDNF mRNA to β-actin mRNA for each sample.
Tissue preparation for immunohistochemistry
The rats were killed under deep anaesthesia as above, between 7:00 and 11:00 am; rats in the chronic stress groups were killed immediately after the last restraint session. Resected rat submandibular gland tissue samples were fixed in 10% buffered formaldehyde (pH 7.4) for 24 h and embedded in paraffin and serial 3-μm sections were cut and stained with hematoxylin and eosin and processed for immunohistochemistry. Immunohistochemical analysis was performed using Simple stain MAX-PO (Nichirei, Tokyo, Japan). Slides were pre-incubated in 3% H2O2 for 5 min. Sections were then incubated with anti-human BDNF monoclonal antibody (1:100, Techne, Minneapolis, MN, USA) for 1 h at room temperature. After washing with PBS, sections were reacted with secondary antibody, horseradish peroxidase (HRP)-labeled anti-rabbit IgG with amino acid polymer (Nichirei), for 30 min at room temperature. Color was developed using 0.02% 3,3′-diaminobenzidine-tetrahydrochloride containing 0.0003% H2O2 in Tris-buffered saline for 5 min, and sections were then counterstained with hematoxylin. To provide negative controls, non-immunized rabbit or mouse IgG was used instead of the primary antibody.
Measurement of plasma adrenocorticotropic hormone and corticosterone
adrenocorticotropic hormone (ACTH) was assayed using a radioimmunoassay kit (ELISA-ACTH, CIS; Atomic Energy Laboratory of Biochemical Products, Gif sur Yvette, France), following the manufacturer's instructions. ACTH concentrations are reported in pg/ml. A COAT-A-COUNT Rat Corticosterone kit (Siemens Medical Solutions Diagnostics, New York, NY, USA) was used for measurement of plasma corticosterone concentration (ng/ml).
Submandibular gland protein extraction and ELISA analysis
Submandibular gland tissue samples were homogenized in ice-cold lysis buffer containing 137 mM NaCl, 20 mM Tris–HCl (pH 8.0), 1% NP40, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 1 μg/ml leupetin, and 0.5 mM sodium vanadate. The tissue homogenate solutions were centrifuged at 14,000g for 5 min at 4°C. The supernatants were collected and used for quantification of total protein and NT levels. Total protein concentrations were determined by the Bradford method using absorbance readings at 595 nm with a SmartSpec Plus spectrophotometer (Bio-Rad).
BDNF concentrations were assayed in the submandibular gland and in plasma using an ELISA kit (Promega, Co., Madison, WI, USA). Briefly, standard 96-well flat-bottom NUNC-ImmunoMaxisorp ELISA plates were incubated with the corresponding captured antibody, which binds the NT of interest, overnight at 4°C. The plates were blocked by incubation for 1 h at room temperature with a 1 × blocking and sample buffer. Serial dilutions of a known amount of BDNF ranging from 0 to 500 pg/ml were performed in duplicate for standard curve determination. Wells containing the standard curve samples and supernatants of submandibular gland tissue homogenates and plasma were incubated at room temperature for 6 or 2 h, as specified by the protocol. They were then incubated with a second specific antibody overnight at 4°C or for 2 h at room temperature. A species-specific antibody conjugated to HRP was used for a tertiary reaction for 2.5 or 1 h at room temperature following this incubation step. TMB One Solution was used to develop color in the wells. This reaction was terminated with 1 M hydrochloric acid at a specific time (10–15 min) at room temperature, and absorbance was then recorded at 450 nm in a plate reader within 30 min of stopping the reaction. BDNF values were determined by comparison with the regression line for each BDNF standard. Using these kits, BDNF can be quantified the range of 0–500 pg/ml. For the BDNF assay kit, cross-reactivity with other neurotrophic proteins is < 2–3%.
Statistical analysis
Statistical analyses were carried out using the SPSS (Version 13.0; SPSS Inc., Chicago, IL, USA) statistics program. All statistical analyses were carried out using one-way ANOVA followed by Tukey's post-test to assess differences among groups. All data are expressed as the group mean ± SEM. A probability level of 0.05 or less was accepted as significant.
Results
Plasma concentrations of ACTH and corticosterone: chronic stress with intact salivary glands
ACTH concentrations were greater in chronically stressed rats, intact or with sham sialoadenectomy, than in control non-stressed rats, with no differences between the intact and the sham-operated stressed groups, or between the intact and sham-operated controls (F(3,44) = 29.07; p < 0.001, ANOVA; ). Similarly, plasma corticosterone concentrations were greater in chronically stressed rats, intact or with sham sialoadenectomy, than in controls, with no differences between the intact and the sham-operated stressed groups, or between the intact and sham-operated controls (F(3,44) = 72.81; p < 0.001, ANOVA; ).
Figure 1 Plasma ACTH and corticosterone concentrations: chronic stress with intact salivary glands. (a) Plasma ACTH; (b) plasma corticosterone concentrations, in terminal cardiac puncture blood samples. Control: no stress; Chronic stress: after daily 12 h restraint stress for 22 days; Sham: sham sialoadenectomy. Values are mean ± SEM; n = 12 rats in each group. **p < 0.01, ANOVA/Tukey's.
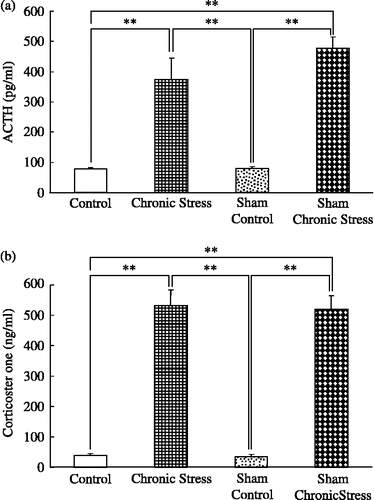
Quantitative analysis of BDNF mRNA in the submandibular gland and chronic stress
Melting curve analysis demonstrated a single fluorescent peak representing the Tm of BDNF mRNA in all samples, except for the negative control sample (data not shown). In addition, a single band was observed following agarose gel electrophoresis (data not shown). These findings confirmed that the PCR product was BDNF mRNA. BDNF mRNA/β-actin mRNA ratios were greater in chronically stressed rats, intact or with sham sialoadenectomy, than in controls, with no differences between the intact and the sham-operated stressed groups, or between the intact and sham-operated controls (F(3,44) = 41.8; p < 0.001, ANOVA; ).
Figure 2 BDNF mRNA quantification in rat submandibular glands and chronic stress. Data are BDNF/β-actin mRNA ratios. Control: no stress; Chronic stress: after daily 12 h restraint stress for 22 days; Sham: sham sialoadenectomy. Values are mean ± SEM; n = 12 rats in each group. **p < 0.01, ANOVA/Tukey's.
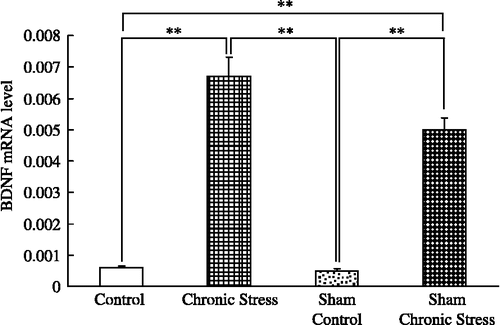
Submandibular gland BDNF content and chronic stress
Submandibular gland BDNF concentrations were greater in chronically stressed rats, intact or with sham sialoadenectomy, than in controls, with no differences between the intact and the sham-operated stressed groups, or between the intact and sham-operated controls (F(3,44) = 17.94; p < 0.001, ANOVA; ).
Figure 3 Submandibular gland extract BDNF content and chronic stress. Data are BDNF concentrations in tissue extracts. Control: no stress; Chronic stress: after daily 12 h restraint stress for 22 days; Sham: sham sialoadenectomy. Values are mean ± SEM; n = 12 rats in each group. **p < 0.01, ANOVA/Tukey's.
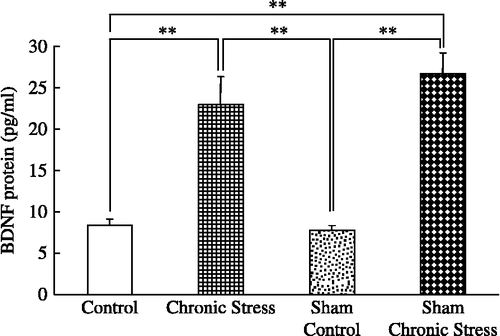
Plasma BDNF concentrations and chronic stress
Plasma BDNF concentrations were greater in chronically stressed rats, intact or with sham sialoadenectomy, than in controls, with no differences between the intact and the sham-operated stressed groups, or between the intact and sham-operated controls (F(3,44) = 13.15; p < 0.001, ANOVA; ).
Figure 4 Plasma BDNF concentrations and chronic stress. Data are plasma BDNF concentrations in terminal cardiac puncture blood samples. Control: no stress; Chronic stress: after daily 12 h restraint stress for 22 days; Sham: sham sialoadenectomy. Values are mean ± SEM; n = 12 rats in each group. **p < 0.01, ANOVA/Tukey's.
BDNF immunohistochemistry in submandibular gland
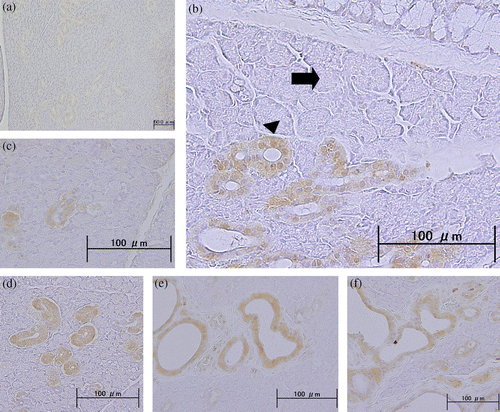
Rat brain sections were used as a positive control for BDNF immunohistochemistry, and showed high levels of BDNF expression in neuronal cells (data not shown). Non-stressed submandibular gland tissue from unstressed rats revealed little to no expression of BDNF in various duct-type cells (). Granular duct cells weakly expressed BDNF protein with the exception of duct-type cells. Intense BDNF expression was observed in various duct-type cells of the chronic-stress group; however, BDNF expression was not consistently observed in acinar cells or myoepithelial cells in the chronic-stress group (). Ductal epithelia, including intercalated (), striated (), interlobular, intralobular (), and excretory ducts (), displayed intense immunoexpression.
Figure 5 BDNF immunohistochemistry in submandibular gland. Photomicrographs show the immunohistochemical localization of BDNF protein, identified with an anti-BDNF monoclonal antibody in paraffin-embedded sections of submandibular gland from: (a) a non-stressed rat: only faint staining was observed; (b)–(f) rats after daily 12 h restraint stress for 22 days: BDNF protein was observed in duct cells, and there was no obvious BDNF expression in acinar cells (arrow) or myoepithelial cells (arrowhead; b). BDNF protein was immunolocalized in the intercalated (c), striated (d), interlobular, intralobular (e), and excretory ducts (f). Scale bars = (a) 500 μm; (b)–(f) 100 μm.
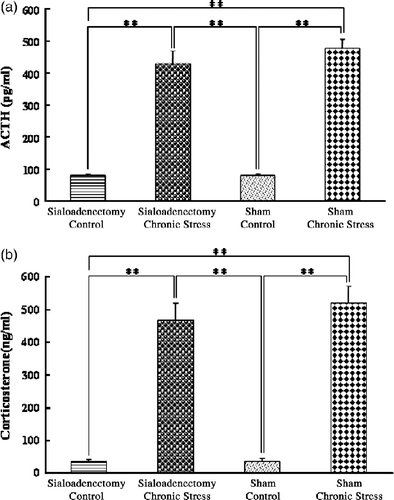
Plasma concentrations of ACTH and corticosterone: chronic stress and sialoadenectomy
Plasma ACTH concentrations were greater in chronically stressed rats, with sialoadenectomy or sham-operated, than in controls, with no differences between the sialoadenectomized and the sham-operated stressed groups, or between the sialoadenectomized and sham-operated controls (F(3,44) = 46.6; p < 0.001, ANOVA; ). Plasma corticosterone concentrations were greater in chronically stressed rats, with sialoadenectomy or sham-operated, than in controls, with no differences between the sialoadenectomized and the sham-operated stressed groups, or between the sialoadenectomized and sham-operated controls (F(3,44) = 75.23; p < 0.001, ANOVA; ).
Figure 6 Plasma concentrations of ACTH and corticosterone: chronic stress and sialoadenectomy. (a) Plasma ACTH; (b) Plasma corticosterone concentrations, in terminal cardiac puncture blood samples. Control: no stress; Chronic stress: after daily 12 h restraint stress for 22 days; Sialoadenectomy: removal of submandibular salivary glands; Sham: sham sialoadenectomy. Values are mean ± SEM; n = 12 rats in each group. **p < 0.01, ANOVA/Tukey's. Note: the data for the Sham groups are the same as in .
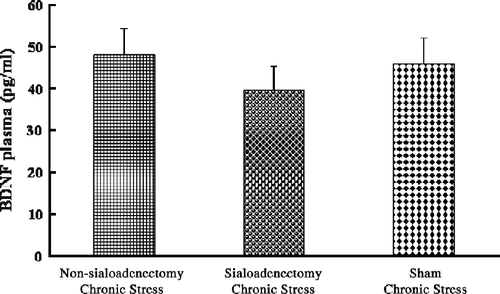
Plasma BDNF concentrations: chronic stress and sialoadenectomy
Plasma BDNF concentrations were not different among the three chronic stress groups: in particular there was no significant effect of sialoadenectomy (F(2,33) = 0.51; p = 0.6, ANOVA; ).
Figure 7 Plasma BDNF concentrations: chronic stress and sialoadenectomy. Data are terminal plasma BDNF concentrations in cardiac puncture blood samples. Chronic stress: after daily 12 h restraint stress for 22 days; Non-sialoadenectomy: no surgery; Sialoadenectomy: removal of submandibular salivary glands before chronic stress; Sham: sham sialoadenectomy before stress. Values are mean ± SEM; n = 12 rats in each group. There were no significant differences among groups (p>0.05).
Discussion
Using multiple techniques, we demonstrated increased expression of BDNF mRNA and protein in rat submandibular gland tissue following repeated restraint stress. Tsukinoki et al. (Citation2006) found that the expression of BDNF in the rat submandibular gland is modulated by conditions of acute stress or non-stress. It has been reported that the NGF level is also altered by acute stress in this gland (Aloe et al. Citation1986). The present study indicates that BDNF expression in the submandibular gland is up-regulated by a chronic stressor.
Chronic immobilization stress decreases mRNA levels of BDNF in rat hippocampus (Ueyama et al. Citation1997) and can cause damage and atrophy in this brain region, which expresses high levels of receptors for glucocorticoids (GC; Duman and Monreggia Citation2006). It has been reported that the mechanisms underlying the down-regulation of BDNF are associated with high GC content of the blood induced by chronic stress (Schaaf et al. Citation1998). However, our results showed that the increased circulating GC did not decrease the production of BDNF in salivary glands during repeated restraint. In addition, atrophy was not evident in the duct type cells of the salivary glands after chronic stress. The salivary glands are predominantly under control by autonomic innervation rather than hormones, which regulates secretion of saliva (Takakura et al. Citation2003). Although brain levels of BDNF are markedly affected by stressful events (Smith et al. Citation1995), stress-induced changes in BDNF levels may be tissue-specific, as indicated by the present study. Whether the increased salivary gland expression of BDNF in the present study was induced by the autonomic innervation, requires further investigation.
In the second part of this study, we examined whether the plasma level of BDNF under repeated stress is related to salivary gland BDNF production. When rats were exposed to stress for 3 weeks, plasma BDNF levels in the chronic stress group were significantly higher than those in the control group. We previously found enhanced plasma BDNF levels in rats after acute stress (Tsukinoki et al. Citation2006). The present study indicates that chronic stress also increases plasma BDNF. To date, there has been little investigation into changes in plasma BDNF using experimental models which are characterized by physical stress (Alleva and Francia Citation2008). In clinical studies, blood BDNF levels have been found to be lower in persons with schizophrenia (Tan et al. Citation2005) or depression (Karege et al. Citation2005). Because plasma BDNF levels increase with the use of psychotropic agents (Shimizu et al. Citation2003), the BDNF content in serum and plasma may reflect the clinical stage of such mental disorder. Interestingly, elevation of BDNF levels by exogenous BDNF contributes to the protection of neural cells in the rat hippocampus (Radecki et al. Citation2005). Since BDNF can cross the blood–brain barrier (Pan et al. Citation1998), circulating BDNF is likely to contribute to protecting neural cells and maintaining their function. Hence an increase in plasma BDNF level may be an important neuroprotective response under chronic stress.
Salivary glands have been shown, in studies on sialoadenectomized rats, to be the source of increased plasma BDNF level during acute immobilization stress (Tsukinoki et al. Citation2007). However, in the present study no significant difference was found in plasma BDNF levels between sialoadenectomized rats and intact rats under chronic stress. Hence, although BDNF mRNA and protein levels increased in the submandibular gland during chronic stress, this did not contribute significantly to the increased plasma BDNF level. Expression of BDNF has been investigated in several other organs, including the heart (Timmusk et al. Citation1993), lung (Timmusk et al. Citation1993), liver (Cassiman et al. Citation2001), pancreas (Hanyu et al. Citation2003), and spleen (Schuhmann et al. Citation2005). At the cellular level, vascular endothelial cells have been considered to be an important source of BDNF (Nakahashi et al. Citation2000). These previous reports and our findings suggest that some organs, including salivary glands, may release BDNF into the circulation differently under conditions of chronic compared with acute stress. Further studies are needed to understand the source(s) and biological significance of plasma BDNF during chronic stress.
Acknowledgements
This research was supported in part by a Grant-in Aid for Young Scientists (Start-up, #1989023), Scientific Research (B, #20390467), Research Institute on Occlusive Medicine of Kanagawa Dental College, and Open Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adlard PA, Cotman CW. 2004. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 124:985–992.
- Alleva E, Francia N. Psychiatric vulnerability: Suggestions from animal models and role of neurotrophins. Neurosci Biobehav Rev. 2008 (Epub ahead of print).
- Aloe L, Alleva E, Bohm A, Levi-Montalcini R. 1986. Aggresive behavior induces release of nerve growth factor from mouse salivary gland into the bloodstream. PNAS. 83:6184–6187.
- Cassiman D, Denef C, Desmet VJ, Roskams T. 2001. Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology. 33:148–158.
- Chohen S. 1960. Purification of a nerve-growth promoting protein from the mouse salivary gland and its neuro-cytotoxic antiserum. PNAS. 46:302–311.
- Chohen S. 1962. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the newly born animal. J Biol Chem. 237:1555–1562.
- De Simone R, Alleva E, Tirassa P, Aloe L. 1990. Nerve growth factor released into the bloodstream following intraspecific fighting induces mast cell degranulation in adult mice. Brain Behav Immun. 4:74–81.
- Duman RS, Monreggia LM. 2006. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 59:1116–1127.
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. 2003. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 112:257–269.
- Ghinelli E, Johansson J, Rios JD, Chen LL, Zoukhri D, Hodges RR, Dartt DA. 2003. Presence and localization of neurotrophins and neurotrophin receptors in rat lacrimal gland. Invest Opthalmol Vis Sci. 44:3352–3357.
- Givalois L, Arancibia S, Alonso G, Tapia-Arancibia L. 2004. Expression of brain-derived neurotrophic factor and its receptors in the median eminence cells with sensitivity to stress. Endocrinology. 145:4737–4747.
- Hand AR. 1980. Salivary glands. In: Bhaskar SN, editors. Orban's oral histology and embryology. St Louis, MO: Mosby336–370.
- Hanyu O, Yamatani K, Ikarashi T, Soda S, Maruyama S, Kamimura T, Kaneko S, Hirayama S, Suzuki K, Nakagawa O, Nawa H, Aizawa Y. 2003. Brain-derived neurotrophic factor modulates glucagon secretion from pancreatic alpha cells: Its contribution to glucose metabolism. Diabetes Obes Metab. 5:27–37.
- Hwang DL, Wang S, Chen RC, Lev-Ran A. 1991. Trauma, especially of the submandibular glands, causes release of epidermal growth factor into bloodstream in mice. Regul Pept. 34:133–139.
- Joachim RA, Noga O, Sagach V, Hanf G, Fliege H, Kocalevent RD, Peters EM, Klapp F. 2008. Correlation between immune and neuronal parameters and stress perception in allergic asthmatics. Clin Exp Allergy. 22:283–290.
- Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. 2005. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 57:1068–1072.
- Lee T, Saruta J, Sasaguri K, Sato S, Tsukinoki K. 2008. Allowing animals to bite reverses the effects of immobilization stress on hippocampal neurotrophin expression. Brain Res. 1195:43–49.
- Lewin GR, Barde YA. 1996. Physiology of the neurotrophins. Annu Rev Neurosci. 19:289–317.
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. 2005. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 26:115–123.
- Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. 2000. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 470:113–117.
- Nakajima K, Hamada N, Takahashi Y, Sasaguri K, Tsukinoki K, Umemoto T, Sato S. 2006. Restraint stress enhances alveolar bone loss in an experimental rat model. J Periodontal Res. 41:527–534.
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. 1998. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 37:1553–1561.
- Radecki DT, Brown LM, Martinez J, Teyler TJ. 2005. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus. 15:246–253.
- Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E. 1998. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 813:112–120.
- Schuhmann B, Dietrich A, Sel S, Hahn C, Klingenspor M, Lommatzsch M, Gudermann T, Braun A, Renz H, Nockher WA. 2005. A role for brain-derived neurotrophic factor in B cell development. J Neuroimmunol. 163:15–23.
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M. 2003. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 54:70–75.
- Smith MA, Makino S, Kvetnansky R, Post RM. 1995. Stress and glucocorticoids affect the expression od brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 15:1768–1777.
- Sobue G, Yamamoto M, Doyu M, Li M, Yasuda T, Mitsuma T. 1998. Expression of mRNAs for neurotrophins (NGF, BDNF, and NT-3) and their receptors (p75 NGFR, trk, trkB, and trkC) in human peripheral neuropathies. Neurochem Res. 23:821–829.
- Takakura AC, Moreira TS, Laitano SC, De Luca LAJr, Renzi A, Menani JV. 2003. Central muscarinic receptors signal pilocarpine-induced salivation. J Dent Res. 82:993–997.
- Tan XL, Zhou DF, Zhang XY. 2005. Decreased plasma brain-derived neurotrophic factor levels in schizophrenic patients with tardive dyskinesia: Association with dyskinetic movements. Schizoph Res. 74:263–270.
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. 1993. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 10:475–489.
- Tsukinoki K, Yasuda M, Miyoshi Y, Mori Y, Ootsuru M, Saruta J, Sato S, Kaneko A, Watanabe Y, Osamura Y. 2005. Role of hepatocyte growth factor and c-Met receptor in neoplastic conditions of salivary glands. Acta Histochem Cytochem. 38:25–30.
- Tsukinoki K, Saruta J, Sasaguri K, Miyoshi Y, Jinbu Y, Kusama M, Sato S, Watanabe Y. 2006. Immobilization stress induces BDNF in rat submandibular glands. J Dent Res. 85:844–848.
- Tsukinoki K, Saruta J, Muto N, Sasaguri K, Sato S, Tan-Ishii N, Watanabe Y. 2007. Submandibular glands contribute to increases in plasma BDNF levels. J Dent Res. 86:260–264.
- Ueyama T, Kawai Y, Nemoto K, Sekimoto M, Toné S, Senba E. 1997. Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. Neurosci Res. 28:103–110.