Abstract
Several clinical and preclinical studies have indicated that hippocampal shrinkage and decreased neurogenesis are implicated in the pathology of depression. Recent animal studies have shown, however, that the development of depression-related symptoms may take place through neurogenesis-independent pathways. To evaluate whether the stress-induced morphological changes in the hippocampal formation are causally related to the development of anhedonia-like symptoms, we combined the chronic mild stress (CMS) rat model of depression with stereological estimations of the number of proliferating progenitors, the total number of granule cells, and the volume of the ventral hippocampal formation (VHF). First, we found that stress-susceptible and stress-resilient animals, as categorized according to the behavioral read-out, both have a decrease in hippocampal cell proliferation. Our results also indicated that the anhedonia-like state in CMS rats develops prior to maximal suppression of cell proliferation, but correlates with a reduction in the total number of granule cells in the VHF. Furthermore, recovery from depression-related symptoms correlated with re-establishment of proliferation rates, but not with the total number of granule cells. Notably, decreases in the number of granule cells occurred independently of the induction of an anhedonia-like phenotype. There were no stress-induced changes in the volume of the VHF. We conclude that cell proliferation and a reduction in the total number of granule cells in the VHF are triggered by chronic stress, but do not associate with development of an anhedonia-like state in rats.
Introduction
Chronic stress has been causally related to the development and the progression of depressive disorders (Kendler et al. Citation1999, Citation2001). However, the pathological changes associated with major depression are not well understood. The hippocampal formation has been identified as one of the brain regions altered in depressed patients and in experimental animal models of major depression. It has been shown that the extent of cognitive impairment and reductions in hippocampal volume correlate with the duration of this illness (Bremner et al. Citation2000; Sheline et al. Citation2003). Hippocampal volume changes associated with post-traumatic stress disorder can also be prevented or reversed by chronic antidepressant treatment (Vermetten et al. Citation2003). From the studies of different animal models of depression, there is abundant evidence that stress suppresses the genesis of hippocampal granule cells (Czeh et al. Citation2001; van der Hart et al. Citation2002; Santarelli et al. Citation2003; Alonso et al. Citation2004; Jayatissa et al. Citation2006). This effect has been shown to be reversed by treatment strategies used for the management of major depression (Malberg and Duman Citation2003; Fuchs et al. Citation2004b; Jayatissa et al. Citation2006; Perera et al. Citation2007; Revesz et al. Citation2008). The neurogenic theory of depression was proposed on the bases of these observations (Duman Citation2004). A casual relationship between the reduction in hippocampal neurogenesis and depression has never been demonstrated. In a recent clinical study, indices of adult neurogenesis were unaltered in hippocampal regions of specimens from patients suffering from depression and furthermore, antidepressant treatment did not modulate this neural stem cell proliferation (Reif et al. Citation2006). However, there is accumulating evidence that decreased hippocampal neurogenesis is a risk factor rather than an actual causal factor in the development of depression (Mineur et al. Citation2007; Holick et al. Citation2008; Surget et al. Citation2008).
The chronic mild stress (CMS) model is a well-validated animal model of major depression (Willner et al. Citation1992; Willner Citation1997, Citation2005). The rationale for the CMS model is that, in a subgroup of vulnerable animals, chronic stress decreases responsiveness to rewards which indicates an anhedonia-like state. The usual way to assess the stress-induced development of an anhedonia-like behavior in rats, is to monitor the time-dependent decrease in intake of, or preference for sweet solution (Willner et al. Citation1987). In the CMS model, a subgroup of rats does not develop an anhedonia-associated decrease in consumption of sweet solutions, although exposed to stress. This CMS-resilient group displays different behavioral and molecular profiles from the CMS-sensitive group, which develops an anhedonia-like behavior (Bergstrom et al. Citation2007, Citation2008; Bisgaard et al. Citation2007).
Previously, we reported that CMS reduces the number of granule cells in the ventral part of the hippocampal formation (VHF) in rats (Jayatissa et al. Citation2006, Citation2008). In the present study, we explored whether the stress-induced morphological changes in the VHF represent a primary factor, or an epiphenomenon, in the development of depression-related behavior in rats. To this end, we have investigated changes in the proliferation of neural progenitors and in the total number of granule cells in the VHF. In addition, we have investigated whether CMS results in a reduced volume of the ventral part of the granule cell layer (GCL) and Ammon's horn. Thus, we have tested the following two hypotheses:
| 1 | CMS induces anhedonia-like behavior and diminishes the number of granule cells in the VHF in a time-dependent manner. | ||||
| 2 | Anhedonia-like behavior correlates with a reduction in the number of granule cells in the VHF. | ||||
The results from our study indicate that the reduction of the GCL in the VHF is caused by chronic exposure to stress, but does not correlate with the induction of an anhedonia-like phenotype. In addition, stress did not cause a reduction in the volume of the ventral part of the GCL or Ammon's horn. It is concluded that decreased neurogenesis constitutes an important risk factor in individuals predisposed to the development of major depression, but is not a causal factor in itself.
Materials and methods
Animals
Male Wistar rats (Taconic, Denmark), which were 6–7 weeks old and weighing about 200 g when adaptation to sucrose consumption was initiated, and approximately 350 g at the start of the stress procedure were used in the study. The rats were housed individually, except when grouping was applied as a stress parameter. Food and water were available ad libitum, except when food or/and water deprivation were used as part of the stress protocol. The standard 12-h light/dark cycle, lights on/off at 06.00/18.00 h was only changed as part of the stress regime. Room temperature (RT) was 22°C.
Sucrose consumption test
After 1 week of acclimatization rats were trained to consume a palatable sucrose solution (1.5%). The training lasted 5 weeks. The sucrose test was made twice a week during the first 3 weeks and once a week during the last 2 weeks of training. The rats were deprived of food and water for 14 h prior to the test. The test consisted of exposure to a single bottle, containing a 1.5% sucrose solution, for 1 h. During the stress period, the sucrose consumption test was performed every Friday between 8.00 and 9.00 a.m.
The experimental design
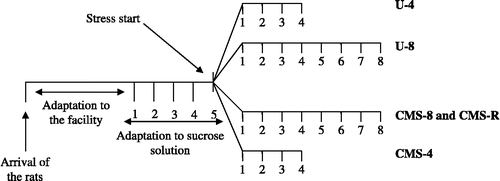
On the basis of sucrose consumption during the three final baseline tests, the rats were divided into four matched groups: U-4, U-8, CMS-4, and CMS-8 groups. Description of the experimental groups is presented in . The U-4 and U-8 groups were placed in a separate room and left unchallenged. They were food and water deprived for 14 h prior to the sucrose consumption test, but otherwise had free access to food and water. The rest of the groups were exposed to 4- or 8 weeks of chronic stress. Stressors were administered during both light and dark hours.
Table I. Description of the experimental groups of rats used in the present study.
During the exposure to stress, the CMS group segregated into two experimental categories: stress-susceptible (CMS-4 or -8) and stress-resilient (CMS-R) groups (). This segregation lasted throughout the experiment. Stress-susceptible rats reduced sucrose intake by 40% or more in response to stress, while stress resilient rats remained at the baseline level.
The experimental design is presented in .
Figure 1 Experimental design. Experimental design and groups compared for the following hypothesis: CMS induces anhedonia-like behavior and diminishes hippocampal cell proliferation and the total number of granule cells in a time-dependent manner. U-4, unchallenged 4 weeks (n = 10); U-8, unchallenged 8 weeks (n = 8); CMS-8, chronic mild stress 8 weeks (n = 8); CMS-R, chronic mild stress resilience (n = 8); and CMS-4, chronic mild stress 4 weeks (n = 10).
CMS protocol
The stress protocol has been described previously (Jayatissa et al. Citation2006). The timetable was fixed for each week. Briefly, the stress protocol consisted of a single period of intermittent illumination, stroboscopic light, grouping, food or water deprivation; two periods of soiled cage and no stress; three periods of 45° box tilting. Each of the stressors lasted from 10 to 14 h.
Bromodeoxyuridine (BrdU) administration
Four injections of BrdU (100 mg/kg dissolved in phosphate-buffered saline (PBS) with a final concentration of 20 mg/ml, pH 7.4) were administered at 2 h intervals during 1 day (Cameron and McKay Citation2001). The rats were killed 16 h after the last injection.
Tissue processing
After an overdose of sodium pentobarbital (60 mg/ml) the rats were transcardially perfused with 100 ml 0.9% saline, followed by 200 ml of ice cold 4% paraformaldehyde (pH 7.2–7.4). The brains were then removed and post-fixed overnight in the same solution at 4°C. The brains were transferred to a solution consisting of 30% sucrose (diluted in phosphate buffer, pH 7.0–7.4) supplemented with 10% sodium azide and stored at 4°C until they sank. The brains were then divided into the right and left hemispheres and sectioned horizontally along the entire dorso-ventral axis (i.e. − 8.82 to − 3.10 mm below bregma referring to the dorso-ventral coordinates; Paxinos and Watson Citation1998) on a cryostat (Leica). The dorsal hippocampal formation was defined as − 3.10 to − 4.28 mm and the ventral part from − 4.60 to − 8.82 mm relative to bregma. The 40 μm sections (approximately 150 sections per brain) were collected in series of every sixth (approximately 20 sections per brain) and stored in cryoprotectant antifreeze solution (25% ethylene glycol and 25% glycerin in a 0.05 M phosphate buffer) at − 20°C. Prior to staining, the sections were separated into those from the dorsal and ventral parts of hippocampus as described previously (Jayatissa et al. Citation2008). The ventral sections from the left brain hemisphere were used in this study.
BrdU/neuronal nuclei (NeuN) fluorescence immunostaining
The staining procedure was carried out on free-floating sections. The sections were rinsed (3 × 10 min) in 0.02 M potassium PBS (KPBS) and incubated in 1 M HCl for 30 min at 65°C. They were then rinsed in blocking solution A [5% normal donkey serum (NDS), and 5% normal horse serum (NHS) in 0.25% Triton-KPBS] for 1 h at RT and incubated with 1:100 anti-BrdU (Oxford Biotechnology, Kidlington, Oxford, UK, OBT0030) and 1:100 anti-NeuN (Chemicon, Temecula, CA, USA, MAB377) in blocking solution A for 48 h at 4°C. After rinsing in 0.25% Triton-KPBS (2 × 10 min) and in blocking solution B (2% NDS and 2% NHS in 0.25% Triton-KPBS; 1 × 10 min) the sections were incubated with 1:200 Cy3 donkey anti-rat (Jackson Immunoresearch, West Grove, PA, USA, Jackson 712-165-153) and with 1:200 biotinylated donkey anti-mouse (Jackson Immunoresearch) antibodies in blocking solution B for 2 h in the dark at RT. Sections were washed in 0.25% Triton-KPBS (3 × 10 min) and incubated for 2 h with 1:200 Alexa-488-conjugated streptavidin (Molecular Probes, Eugene, OR, USA, S11223) in the dark at RT. They were then rinsed in KPBS (3 × 10 min) and stored in KPBS at 4°C. The sections were then mounted on Superfrost Plus glass slides (Microm International, Randburg, Germany), air-dried and coverslipped with a glycerol-based mounting medium. The NeuN staining was carried out in order to visualize the GCL.
May Grunwald–Giemsa staining
For the estimation of the total neuron number, sections parallel to those stained for BrdU/NeuN were stained accordingly to the May Grunwald–Giemsa protocol. Briefly, the free-floating sections were mounted on microscope glass slides. The mounted sections were first placed in methanol for 5 min; then in May Grunwald stain (Bie & Bernsten cat. no. LAB00300.0500, DK) for 7 min; in Giemsa Azur–Eosin–Methylblue stain for microscopy (working solution 1:100 Giemsa stain in phosphate buffer; Bie & Bernsten cat. no. 1.09204.0500, DK) for 20 min. They were then washed twice for 1 min in phosphate buffer pH 6.5 (Bie & Bernsten cat. no. 310706-028, DK). The sections were air-dried and coverslipped with a glycerol-based mounting medium.
Data quantification
Every sixth section throughout the ventral hippocampus ( − 4.60 to − 8.82 mm below bregma referring to the dorso-ventral coordinates; Paxinos and Watson Citation1998) was analyzed by an investigator who was an unaware of the identity of the rat from which the material was derived. The BrdU positive cells were counted in the GCL, including subgranular zone of the dentate gyrus, using a Zeiss Axiovert 200 M fluorescence microscope (Carl Zeiss International, Oberkochen, Germany) with 40 × objective (Zeiss EC plan neofluar). The total number of BrdU-labeled cells per dentate gyrus was estimated by multiplying the number of the cells counted in every sixth section by six.
The total number of neurons in the GCL of each rat was estimated with the optical fractionator method (West et al. Citation1991) using the Stereo Investigator software, version 8 (MBF Bioscience, Williston, VT, USA) and an Axioplan 2 (Zeiss) microscope. The NeuN in the GCL were counted with a sampling scheme consisting of the following parameters: the area associated with the steps (astep) in the xy-plane between disector samples was 225 × 225 μm2; the counting frame (aframe) area was 12 × 12 μm2; the height of the optical disector was 10 μm; the top of the optical dissector sample was placed at a fixed distance of 3 μm from the top of the section. The GCL was outlined under a 10 × objective (Apochrom 20 × 0.75), and the counting was performed using a 100 × objective (Plan-Neufluar 100 × 1.3). The mean thickness of the sections, t, was 21.36 μm.
The total number of neurons in the GCL was estimated with the following formula:where N is the total number of neurons in the subdivision; ΣQ− , the number of neurons actually counted per individual; t, the thickness of the section; h, the height of the disector; asf, an area sampling fraction (aframe/astep); and ssf, the section sampling fraction, in this case 1/6.
Determination of the volume of the vGCL and the ventral Ammon's horn
The volumes of the GCL and the Ammon's horn of the left hemisphere of the hippocampal formation were estimated in the same sections as those used to estimate the total number of neurons. The volume was estimated using Stereo-Investigator Software version 8 (MBF Bioscience). The area of the grid associated with each point, A/P, was 50 μm2 for the GCL and 300 μm2 for the Ammon's horn. The volume was estimated according to the Cavalieri principle with the unbiased point counting method (Gundersen et al. Citation1988):where ΣP is the sum of points inside the traced borders of the GCL; A/P, the area associated with each point; and T, the thickness of the section (40 μm) multiplied by six.
Statistics
The sucrose intake data were analyzed by two-way ANOVA for repeated measures, followed by analysis of significance between groups using Bonferroni post hoc test.
The differences in the number of proliferating cells, the total number of granule cells, and the volume of the GCL and the Ammon's horn were analyzed by one-way ANOVA followed by Bonferroni post hoc test. The statistical significance was set at p < 0.05. The coefficients of error (CE) of the individual estimates of cell numbers were calculated with the quadratic approximation formula (Gundersen et al. Citation1999; Slomianka and West Citation2005). The group means of the CE of the individual estimates is presented for the estimates of the total cell number and the volume for each group. The coefficient of variation (CV) of the means of the estimates of cell numbers and volumes of the whole experimental groups was calculated as standard deviation/mean.
Results
CMS models time-dependent progression of anhedonia-like symptoms in rats
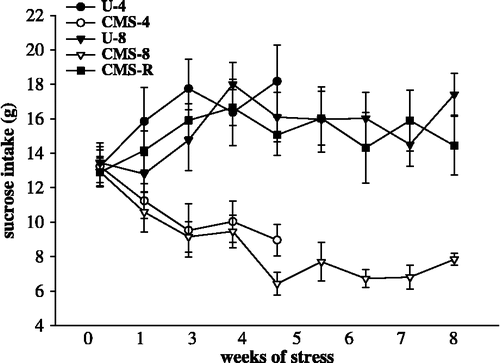
There were no significant differences in sucrose baseline values between all groups tested (p>0.05). Data analysis of the group of rats exposed to 4 weeks of stress (CMS-4) and the unchallenged controls (U-4) revealed a significant group by time interaction (F(4,72) = 7.92; p < 0.0001). Likewise, there was a significant group by time interaction (F(16,216) = 4.48; p < 0.0001) in the data analysis of rats exposed to 8 weeks of stress (CMS-8, CMS-R) and the unchallenged controls (U-8).
Bonferroni post hoc tests revealed a significantly diminished sucrose intake in the CMS-4 group compared to the U-4 group. The sucrose intake of the CMS-8 was significantly diminished compared to the U-8, however, no significant differences were found comparing the CMS-R group and the U-8 group. Weekly group comparisons are shown in and data on sucrose intake in .
Table II. Significant differences in sucrose intake between the experimental groups.
Figure 2 Sucrose intake during the experiment. Influence of 4- and 8 weeks of chronic stress on sucrose consumption. The data were analyzed by two-way ANOVA for repeated measures, followed by Bonferroni post hoc group comparisons (). U-4, unchallenged 4 weeks (n = 10); CMS-4, chronic mild stress 4 weeks (n = 10); U-8, unchallenged 8 weeks (n = 8); CMS-8, chronic mild stress 8 weeks (n = 8), and CMS-R, chronic mild stress resilience (n = 8). Data are mean ± SEM.
CMS causes a time dependent suppression of cell proliferation in the vGCL
One-way ANOVA revealed significant differences among the four compared groups: U-4, CMS-4, U-8, and CMS-8 (F(3,29) = 4.06; p = 0.016; ).
Figure 3 Cell proliferation in the hippocampal vGCL in the experimental groups. Cell proliferation in the vGCL after exposure to 4- and 8 weeks of CMS. Data were analyzed by one-way ANOVA followed by Bonferroni post hoc test. The statistical significance was set at *p < 0.05. U-4, unchallenged 4 weeks (n = 10); U-8, unchallenged 8 weeks (n = 8); CMS-4, chronic mild stress 4 weeks (n = 10); CMS-8, chronic mild stress 8 weeks (n = 8); and CMS-R, chronic mild stress resilience (n = 8). Data are mean ± SEM.
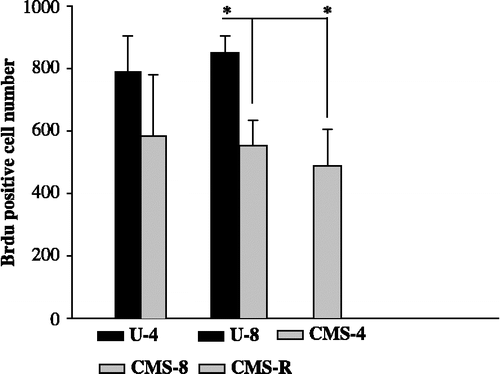
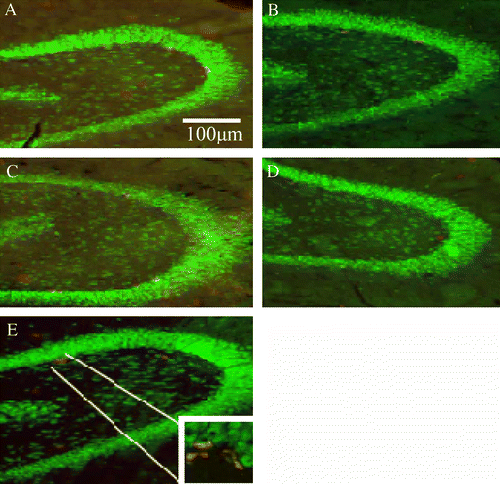
Figure 4 BrdU positive cells in the GCL of the VHF. (A) U-4, unchallenged 4 weeks, (B) CMS-4, chronic mild stress 4 weeks, (C) U-8, unchallenged 8 weeks, (D) CMS-8, chronic mild stress 8 weeks group, and (E) CMS-R, chronic mild stress resilience group. BrdU positive cells or cell clusters appear in red, the mature neurons appear in green. Scale bar: 100 μm.
After 4 weeks of exposure to CMS there was a non-significant 26% decrease in the number of proliferating cells in the GCL of the VHF (p = 0.086; ). After four additional weeks of exposure to chronic stress there was an additional decrease in cell proliferation rates (to 34.9%). This resulted in a significantly lower number of BrdU positive cells in the CMS-8 group compared to the U-8 group (p = 0.046; ).
Suppression of cell proliferation is a result of stress exposure and does not associate with anhedonia-like behavior in rats
One-way ANOVA revealed significant differences among the three compared groups: U-8, CMS-8, and CMS-R (F(2,20) = 9.36; p = 0.0001; ).
The number of proliferating cells was significantly reduced by CMS in the CMS-8 group, when compared to the unchallenged group (U-8; p = 0.007). The number of proliferating cells in the vGCL in the resilience group (CMS-R) was comparable to that in the CMS-8 group and significantly lower than in the unchallenged group (U-8; p = 0.002; ).
Four weeks of CMS efficiently reduces the total number of granule cells in the vGCL
One-way ANOVA revealed significant differences in the total number of granule cells among the compared groups: U-4, CMS-4, U-8, and CMS-8 (F(3,37) = 5.13; p = 0.0045; ). The total number of neurons in the CMS-4 group was significantly smaller than that of the U-4 group (p = 0.037), and significantly smaller in the CMS-8 group than in the U-8 group (p = 0.036). There was no further decrease in the total number of granule cells in the CMS-8 group when compared to the CMS-4 group ( and ).
Figure 5 The total cell number in the vGCL in the experimental groups. Total cell number in the vGCL after exposure to 4- and 8 weeks of CMS. Data were analyzed by one-way ANOVA followed by Bonferroni post hoc test. The statistical significance was set at p < 0.05, *p < 0.05, **p < 0.01. U-4, unchallenged 4 weeks (n = 10); U-8, unchallenged 8 weeks (n = 8); CMS-4, chronic mild stress 4 weeks (n = 10); CMS-8, chronic mild stress 8 weeks (n = 8); and CMS-R, chronic mild stress resilience (n = 8). Data are mean ± SEM.
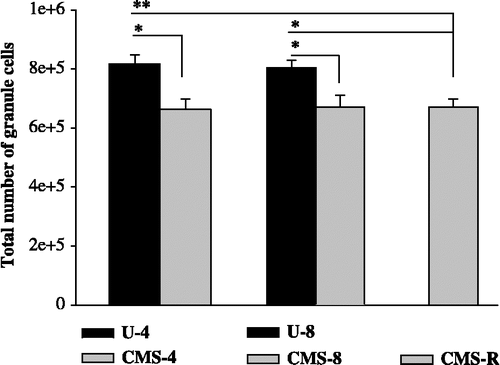
Table III. CE and CV values for estimates of the total number of granule cells in the ventral part of the hippocampal formation.
Lower total number of granule cells results from exposure to chronic stress and does not correlate with anhedonia-like behavior in rats
One-way ANOVA revealed significant differences among the compared groups: U-8, CMS-8, and CMS-R (F(2,28) = 7.53; p = 0.0024; ).
The total number of neurons was significantly reduced by CMS in the CMS-8 group, when compared to the unchallenged group (U-8; p = 0.031). The total number of granule cells in the ventral dentate gyrus in the resilience group (CMS-R) was comparable to that in the CMS-8 group and significantly lower than in the unchallenged group (U-8; p = 0.003; and ).
CMS does not reduce the volume of the vGCL nor the entire ventral Ammon's horn
Neither 4 nor 8 weeks of CMS reduced the volume of the vGCL or the volume of the ventral Ammon's horn ().
Table IV. Changes in volume of the vGCL and the ventral Ammon's horn among the experimental groups.
Discussion
The aim of the present study was to investigate whether the stress-induced suppression of hippocampal cell proliferation and a reduction in the total number of granule cells are related to the anhedonia-related behavior in rats subjected to the CMS model of depression. We have investigated a number of different structural parameters to examine whether the stress-induced morphological changes in the vGCL of the hippocampal formation can be causally linked to development of anhedonia-related symptoms in rats, or whether they are epiphenomena.
We have investigated the time-relation between development of anhedonia-like behavior and a reduction in the number of granule cells in the vGCL and whether the morphological changes in the vGCL occur only in rats that are susceptible to the development of an anhedonia-like phenotype.
The CMS paradigm
When using animal models to investigate the pathology of depression, it is important to keep in mind that in different models different symptoms of this disease are modeled. As a consequence using different animal models to study a specific hypothesis can provide diverging results. For that reason, it is important to confirm major conclusions in different animal models. It is also important that the animal model used to investigate the disease pathology should model one of the main and several of the minor symptoms of major depression. Accordingly, the CMS paradigm, which models anhedonia, is an appropriate choice for investigating the pathology of depression.
CMS is one of the best validated rodent models of human depression (Willner et al. Citation1992). The theoretical rationale for this model is that, in a subgroup of susceptible animals, stress gradually decreases the individual's responsiveness to rewards and thereby indicates the presence of an anhedonia-like state. The diminished sensitivity of rats to rewards can be measured as either a decrease in consumption of, or preference for, sweet substances (Willner et al. Citation1987), food pellets, and amphetamine (Papp et al. Citation1991), or as an increased threshold for ventral tegmental self-stimulation (Moreau et al. Citation1992). In addition, CMS results in the appearance of other symptoms of depression, such as decreases in sexual, aggressive, and investigative behaviors (D'Aquila et al. Citation1994), in locomotor activity (Willner Citation1997), and changes in sleep architecture (Cheeta et al. Citation1997; Gronli et al. Citation2004). In rat groups exposed to CMS, there is always a subgroup comprising about 30% of rats, that are resilient to the stress effects and do not develop an anhedonia-related decrease in sucrose consumption (Bergstrom et al. Citation2007, Citation2008; Bisgaard et al. Citation2007). In addition, all of the different classes of antidepressants mediate recovery from stress-induced anhedonia (Willner Citation1997). In our hands, CMS also models the treatment resistance phenomenon, as about 50% of the treated animals do not respond to antidepressant therapy (Jayatissa et al. Citation2006, Citation2008; Bergstrom et al. Citation2007; Bisgaard et al. Citation2007). This segregation into drug responders and non-responders mimics clinical drug refractoriness (Bondolfi et al. Citation1996).
CMS-induced changes in hippocampal cytogenesis
In our previous studies, we have shown that a CMS-induced decline in the proliferation of the progenitor cell in the vGCL is followed by both a suppression of cell differentiation, and a reduction in total number of granule cells in the vGCL (Jayatissa et al. Citation2006, Citation2008). These changes can be reversed by the chronic administration of an antidepressant and correlated with the anhedonia-like state of the CMS exposed rats. Our data are in agreement with numerous other studies, which show a decrease in different stages of neurogenesis in response to CMS and its reversal by chronic antidepressant treatment (Alonso et al. Citation2004; Zhou et al. Citation2007; Silva et al. Citation2008; Wang et al. Citation2008).
In our present study, we have used stereological methods to estimate the number of proliferating cells, to assess the initial level of neurogenesis, and the total number of granule cells. We have also estimated the volume of the vGCL and of the ventral Ammon's horn to study the effects of stress on possible volume changes in the VHF.
The stereological investigations described here were carried out on the left ventral part of the hippocampal formation. The left hippocampal formation was chosen in view of clinical studies, that have shown reductions in the volume of the left hippocampus in depressed human subjects (Frodl et al. Citation2008; Kronmuller et al. Citation2008a, Citation2008b). The choice of the ventral part of the hippocampal formation is based on our previous studies, which show that the hippocampal cellular changes that occur after exposure to CMS are confined to the vGCL (Jayatissa et al. Citation2006, Citation2008).
In order to challenge the theory of a causal relationship between diminished hippocampal neurogenesis and depression-related behavior in rats, we tested two hypotheses.
Hypothesis 1. CMS induces anhedonia-like behavior and diminishes hippocampal cell proliferation and the total number of granule cells in a time-dependent manner.
In this longitudinal study, we have focused on a temporal correlation between the development of a stress-induced anhedonia-like profile in rats and both the maximal suppression of cell proliferation and reduction of the total number of granule cells in the vGCL. As expected, 4 weeks of exposure to CMS caused a significant decrease in sucrose intake, which persisted for the subsequent 4 weeks of stress exposure ( and ). However, the maximal reduction in the number of proliferating cells in the vGCL was only evident after 8 weeks of exposure to the stress regime (). By contrast, the reduction in the total number of granule cells was maximal after 4 weeks of stress and did not decrease further during the 8 week stress regime ( and ).
These results indicate that the onset of anhedonia-associated behavior in rats does not depend on the reduction in proliferation rates in the vGCL. Because the maximal reduction of the total number of cells was already evident after 4 weeks of CMS, these results indicate that mechanisms other than the suppression of cell proliferation are involved in a stress-induced loss of granule cells in the vGCL.
These data are consistent with the results from previous investigations correlating cell proliferation rates and helpless behavior in rats. The learned helplessness behavior in rats is present already 24 h after exposure to inescapable foot shock and before the maximal suppression of cell proliferation in the GCL (Vollmayr et al. Citation2003). Moreover, in the same study it was shown that reduction in the number of proliferating cells in the subgranular zone does not predispose to helpless behavior.
Hypothesis 2. Anhedonia-like behavior correlates with reduction in the number of granule cells in the VHF.
In the second part of this study, we have tested whether the suppression of neurogenesis occurs only in animals in which susceptibility to chronic stress is manifested by development of anhedonia-associated behavior, or whether it is a general phenomenon resulting from exposure to chronic stress.
As expected, the group of rats exposed to CMS segregated into two subgroups. One group developed anhedonia-related symptoms during the 8 weeks stress exposure (CMS-8) and another group was resilient to the effects of CMS (CMS-R; and ). In both of these groups, there was a significantly lower number of proliferating cells in the vGCL (), together with a reduction in the total number of granule cells (). These results indicate that stress reduces the level of hippocampal cytogenesis and the total number of cells in the vGCL independently of the anhedonia-like status of the animals.
These results are in agreement with the findings made with use of the learned helplessness model of depression, in which diminished cell proliferation was found in the hippocampus of all stressed rats, including those that did not developed helpless behavior after foot shock stress (Vollmayr et al. Citation2003). It has also been shown that both inescapable shock, which induces helpless behavior, and escapable shock, which does not induce helpless behavior, result in a reduction in number of proliferating cells in the GCL of the hippocampus (Malberg and Duman Citation2003).
Another strategy to test the influence of diminished cell proliferation on the progression of depression-related symptoms in rats involves the elimination of neurogenesis combined with behavioral testing. Several observations from these studies indicate that there is no causal link between neurogenesis and depression-related behavior. For example, elimination of neurogenesis in mice did not alter novelty suppressed feeding or grooming scores (Santarelli et al. Citation2003; Holick et al. Citation2008; Surget et al. Citation2008). Moreover, we have shown that blocking of neurogenesis, with the cell proliferation blocker methylazoxymethanol, did not induce an anhedonia-like behavior (data not shown).
Hippocampal volume after exposure to CMS
We have reported previously that there are no changes in volumes of the GCL in rats exposed to the CMS procedure (Jayatissa et al. Citation2008). In order to investigate whether changes in hippocampal volume could take place in regions outside the GCL, we estimated the volume of both the vGCL and of the entire ventral Ammon's horn. We confirmed the results from our previous investigation showing that, in spite of reduction in the total number of granule cells, there were no volume changes in the vGCL. Moreover, we showed that CMS does not affect the volume of the entire Ammon's horn ().
Our results indicate that stress-induced reduction in the total number of granule cells is not sufficient to reduce volume of the vGCL and that chronic stress does not affect the volume of the ventral Ammon's horn. A reduction of the total number of granule cells, without changes in the volume of the dentate gyrus, has been found in the olfactory bulbectomy model of depression (Jaako-Movits and Zharkovsky Citation2005), and in mice exposed to maternal separation (Fabricius et al. Citation2008).
However, a reduction in hippocampal volume was found in a tree shrew stress model of depression (Fuchs et al. Citation2004a; Czeh et al. Citation2005), and a reduction in the volume of the GCL was detected in the chronic corticosterone exposure model (Murray et al. Citation2008). Therefore, it seems that the volumetric changes in the hippocampal formation depend on the kind of stressors applied, and are sensitive to high levels of corticosterone.
In summary, the results from the present study indicate that regulation of adult hippocampal neurogenesis by stress is not the only factor involved in the development of depression-associated behavior in rodents. However, it is possible that a stress-induced reduction in neurogenesis and in the total number of granule cells results in depression-like behavior only when it is combined with either a particular genetic predisposition or additional environmental insults. In this case, a constitutive low level of hippocampal neurogenesis can be an important factor that predisposes an individual to depressive episodes.
Acknowledgements
We thank Phine Katrine Kjær Wiborg and Stine Dhiin for skillful technical assistance. This work was supported by the Lundbeck Foundation. All the procedures involving animals were approved by Danish National Committee for Ethics in Animal Experimentation (2002/561-575).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrie P. 2004. Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry. 9:278–286 224.
- Bergstrom A, Jayatissa MN, Thykjaer T, Wiborg O. 2007. Molecular pathways associated with stress resilience and drug resistance in the chronic mild stress rat model of depression: A gene expression study. J Mol Neurosci. 33:201–215.
- Bergstrom A, Jayatissa MN, Mork A, Wiborg O. 2008. Stress sensitivity and resilience in the chronic mild stress rat model of depression; an in situ hybridization study. Brain Res. 1196:41–52.
- Bisgaard CF, Jayatissa MN, Enghild JJ, Sanchez C, Artemychyn R, Wiborg O. 2007. Proteomic investigation of the ventral rat hippocampus links DRP-2 to escitalopram treatment resistance and SNAP to stress resilience in the chronic mild stress model of depression. J Mol Neurosci. 32:132–144.
- Bondolfi G, Chautems C, Rochat B, Bertschy G, Baumann P. 1996. Non-response to citalopram in depressive patients: Pharmacokinetic and clinical consequences of a fluvoxamine augmentation. Psychopharmacology (Berl). 128:421–425.
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. 2000. Hippocampal volume reduction in major depression. Am J Psychiatry. 157:115–118.
- Cameron HA, McKay RD. 2001. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 435:406–417.
- Cheeta S, Ruigt G, van Proosdij J, Willner P. 1997. Changes in sleep architecture following chronic mild stress. Biol Psychiatry. 41:419–427.
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. 2001. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 98:12796–12801.
- Czeh B, Pudovkina O, van der Hart MG, Simon M, Heilbronner U, Michaelis T, Watanabe T, Frahm J, Fuchs E. 2005. Examining SLV-323, a novel NK1 receptor antagonist, in a chronic psychosocial stress model for depression. Psychopharmacology (Berl). 180:548–557.
- D'Aquila PS, Brain P, Willner P. 1994. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav. 56:861–867.
- Duman RS. 2004. Depression: A case of neuronal life and death?. Biol Psychiatry. 56:140–145.
- Fabricius K, Wortwein G, Pakkenberg B. 2008. The impact of maternal separation on adult mouse behaviour and on the total neuron number in the mouse hippocampus. Brain Struct Funct. 212:403–416.
- Frodl TS, Koutsouleris N, Bottlender R, Born C, Jager M, Scupin I, Maximilian Reiser MD, Hans-Jürgen Möller MD, Eva M, Meisenzahl MD. 2008. Depression-related variation in brain morphology over 3 years: Effects of stress?. Arch Gen Psychiatry. 65:1156–1165.
- Fuchs E, Czeh B, Flugge G. Examining novel concepts of the pathophysiology of depression in the chronic psychosocial stress paradigm in tree shrews. Behav Pharmacol. 2004a; 15:315–325.
- Fuchs E, Czeh B, Kole MH, Michaelis T, Lucassen PJ. Alterations of neuroplasticity in depression: The hippocampus and beyond. Eur Neuropsychopharmacol. 2004b; 14 Suppl.5: S481–S490.
- Gronli J, Murison R, Bjorvatn B, Sorensen E, Portas CM, Ursin R. 2004. Chronic mild stress affects sucrose intake and sleep in rats. Behav Brain Res. 150:139–147.
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A. 1988. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 96:379–394.
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. 1999. The efficiency of systematic sampling in stereology-reconsidered. J Microsc. 193:199–211.
- Holick KA, Lee DC, Hen R, Dulawa SC. 2008. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 33:406–417.
- Jaako-Movits K, Zharkovsky A. 2005. Impaired fear memory and decreased hippocampal neurogenesis following olfactory bulbectomy in rats. Eur J Neurosci. 22:2871–2878.
- Jayatissa MN, Bisgaard C, Tingstrom A, Papp M, Wiborg O. 2006. Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology. 31:2395–2404.
- Jayatissa MN, Bisgaard CF, West MJ, Wiborg O. 2008. The number of granule cells in rat hippocampus is reduced after chronic mild stress and re-established after chronic escitalopram treatment. Neuropharmacology. 54:530–541.
- Kendler KS, Karkowski LM, Prescott CA. 1999. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 156:837–841.
- Kendler KS, Thornton LM, Gardner CO. 2001. Genetic risk, number of previous depressive episodes, and stressful life events in predicting onset of major depression. Am J Psychiatry. 158:582–586.
- Kronmuller KT, Pantel J, Gotz B, Kohler S, Victor D, Mundt C, Magnotta VA, Giesel F, Essig M, Schröder J. Life events and hippocampal volume in first-episode major depression. J Affect Disord. 2008a; 110:241–247.
- Kronmuller KT, Pantel J, Kohler S, Victor D, Giesel F, Magnotta VA, Mundt C, Essig M, Schröder J. Hippocampal volume and 2-year outcome in depression. Br J Psychiatry. 2008b; 192:472–473.
- Malberg JE, Duman RS. 2003. Cell proliferation in adult hippocampus is decreased by inescapable stress: Reversal by fluoxetine treatment. Neuropsychopharmacology. 28:1562–1571.
- Mineur YS, Belzung C, Crusio WE. 2007. Functional implications of decreases in neurogenesis following chronic mild stress in mice. Neuroscience. 150:251–259.
- Moreau JL, Jenck F, Martin JR, Mortas P, Haefely WE. 1992. Antidepressant treatment prevents chronic unpredictable mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behavior in rats. Eur Neuropsychopharmacol. 2:43–49.
- Murray F, Smith DW, Hutson PH. 2008. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur J Pharmacol. 583:115–127.
- Papp M, Willner P, Muscat R. 1991. An animal model of anhedonia: Attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl). 104:255–259.
- Paxinos G, Watson C. 1998. The rat brain in stereotaxic coordinates. Orlando, FL: Academic Press.
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, Rosoklija G, Sackeim HA, Dwork AJ. 2007. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 27:4894–4901.
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. 2006. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 11:514–522.
- Revesz D, Tjernstrom M, Ben Menachem E, Thorlin T. 2008. Effects of vagus nerve stimulation on rat hippocampal progenitor proliferation. Exp Neurol.. 214:259–265.
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. 2003. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 301:805–809.
- Sheline YI, Gado MH, Kraemer HC. 2003. Untreated depression and hippocampal volume loss. Am J Psychiatry. 160:1516–1518.
- Silva R, Mesquita AR, Bessa J, Sousa JC, Sotiropoulos I, Leao P, Almeida OF, Sousa N. 2008. Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: The role of glycogen-synthase-kinase-3beta. Neuroscience. 152:656–669.
- Slomianka L, West MJ. 2005. Estimators of the precision of stereological estimates: An example based on the CA1 pyramidal cell layer of rats. Neuroscience. 136:757–767.
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. 2008. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 64:293–301.
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. 2003. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 54:693–702.
- Vollmayr B, Simonis C, Weber S, Gass P, Henn F. 2003. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry. 54:1035–1040.
- Wang SH, Zhang ZJ, Guo YJ, Teng GJ, Chen BA. 2008. Hippocampal neurogenesis and behavioural studies on adult ischemic rat response to chronic mild stress. Behav Brain Res. 189:9–16.
- West MJ, Slomianka L, Gundersen HJ. 1991. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 231:482–497.
- Willner P. 1997. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology (Berl). 134:319–329.
- Willner P. 2005. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 52:90–110.
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. 1987. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant 1. Psychopharmacology (Berl). 93:358–364.
- Willner P, Muscat R, Papp M. 1992. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci Biobehav Rev. 16:525–534.
- Zhou QG, Hu Y, Hua Y, Hu M, Luo CX, Han X, Zhu XJ, Wang B, Xu JS, Zhu DY. 2007. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J Neurochem. 103:1843–1854.
- van der Hart MG, Czeh B, de Biurrun G, Michaelis T, Watanabe T, Natt O, Frahm J, Fuchs E. 2002. Substance P receptor antagonist and clomipramine prevent stress-induced alterations in cerebral metabolites, cytogenesis in the dentate gyrus and hippocampal volume. Mol Psychiatry. 7:933–941.