Abstract
This study investigated whether trait rumination predicts greater increases in salivary cortisol concentration and delayed recovery in response to a standardized, acute laboratory psychosocial stressor (modified Trier Social Stress Test). It also tested whether trait and state rumination predict reactivation of the cortisol response during later verbal recall of the stressor. Fifty-nine undergraduates (31 females; 28 males) completed the stress protocol and returned 2 weeks later for a surprise interview about the first session, conducted in either a supportive or unsupportive context. Participants completed a measure of trait rumination and reported negative thoughts about the stressor in the 2 weeks between sessions (state rumination). Trait rumination was associated with greater reactivity of salivary cortisol level and delayed recovery from the stressor, F(1,310) = 6.77, p < 0.001. It also predicted greater cortisol reactivity when recalling the stressor, but only for males in the unsupportive interview context, F(2,119) = 7.53, p < 0.001. This effect was heightened for males who also scored high on state rumination, F(2,119) = 7.53, p < 0.001. Rumination was not associated with cortisol responses to the interviews in females. The findings indicate that rumination may play a role in prolonging cortisol stress responses through delayed recovery and reactivation and that rumination disposition and the context of stressor recall are important in understanding the rumination–cortisol response association.
Introduction
Psychological stressors can lead to acute physiological changes (Chida and Hamer Citation2008), and when experienced repeatedly or in excess, have been linked to long-term negative health outcomes (Chrousos and Gold Citation1992). This prolonged activation of stress-related physiological systems may occur through several pathways, as outlined by the allostatic load model (McEwen and Seeman Citation2003). One such pathway is through failure to shut off the acute stress response (i.e. delayed recovery of physiological changes in response to stress). A second pathway is through the recurrent experience of stressors that lead to frequent stress response activations, thereby extending physiological activation.
Both of these pathways leading to prolonged activation of the physiological stress response may be initiated by rumination, which can broadly be conceptualized as repetitive, unwanted, and past-oriented thoughts about negative content (Watkins Citation2008). For individuals who ruminate, the physiological effects of an acute stressor may be long lasting due to delayed recovery. In addition, subsequent recall of a stressor may serve to reactivate the physiological stress response later in time. The Perseverative Cognition Hypothesis, Brosschot et al. (Citation2006) addresses this, stating that repetitive, intrusive thoughts may amplify, maintain, or reactivate physiological responses to stressors. Growing evidence originating from the cardiovascular domain supports this model. A variety of rumination measures and experimental manipulations have been consistently and positively associated with persistent elevation, delayed recovery, and/or reactivation of cardiovascular indices (e.g. heart rate, blood pressure; Brosschot et al. Citation2006; Glynn et al. Citation2007).
Whether the Perseverative Cognition Hypothesis also holds true for the hypothalamic–pituitary–adrenal (HPA) axis, however, is not as clear. The HPA axis is a major stress response system critical for survival and adaptation. During acute stress, activation of the HPA axis (and its end-product cortisol) is adaptive. However, extended cortisol exposure resulting from prolonged HPA axis activation can be damaging and it has been associated with disorders such as insulin resistance, atherosclerosis, and hypertension (McEwen and Seeman Citation2003). Despite the important role the HPA axis plays in stress and health, few studies have examined how rumination may contribute to prolonged activation of cortisol secretion. These studies have produced mixed results, including positive, negative, and no associations between rumination and cortisol secretion (Roger and Najarian Citation1998; Young and Nolen-Hoeksema Citation2001; McCullough et al. Citation2007; Zoccola et al. Citation2008; Denson et al. Citation2009).
Several conceptual issues may underlie the inconsistent cortisol findings, including the content of an individual's rumination. For example, some studies have conceptualized rumination as a stress-related trait (i.e. the tendency to ruminate in response to stressful or upsetting events; Roger and Najarian Citation1989), whereas other studies have defined it as a tendency to ruminate on sad mood or depressive symptoms (depressive rumination; Nolen-Hoeksema et al. Citation1993). Stress-related rumination has been associated with increases in salivary and urinary cortisol concentration (Roger and Najarian Citation1998; McCullough et al. Citation2007; Zoccola et al. Citation2008). In contrast, depressive rumination has been associated with blunted cortisol responses to psychological stressors (Zoccola et al. Citation2008) or has had no association with cortisol level (Young and Nolen-Hoeksema Citation2001). Therefore, in studies aiming to understand how rumination may delay recovery of the acute stress response or reactivate it, instruments pertaining to stress-related rumination may be most suitable.
Whether rumination is conceptualized as a trait or a state phenomenon may also explain the inconsistent findings. Some individuals may be more prone to ruminate than others (trait rumination). However, whether an individual engages in event-specific rumination following a stressor (state rumination) may also depend on situational factors. Some circumstances (e.g. trauma and rejection) may challenge deeply held beliefs or threaten fundamental needs (Taylor Citation1983; Janoff-Bulman Citation1992), compelling individuals, regardless of ruminative tendencies, to ruminate. In addition, novel situations, such as experimental laboratory manipulations, may serve to evoke rumination in individuals who otherwise might not. Trait and state rumination measures are not always well correlated (Young and Nolen-Hoeksema Citation2001; Zoccola et al. Citation2008) and have had independent (and sometimes inconsistent) effects on physiological outcomes in a given study (Key et al. Citation2008; Zoccola et al. Citation2008). Therefore, it is important to consider an individual's propensity for rumination as well as his or her ruminative response to a specific situation, when examining the association between rumination and physiological activation.
A number of studies have manipulated rumination in the laboratory by using verbal recall tasks (Neumann et al. Citation2004; Gerin et al. Citation2006; Key et al. Citation2008). In such tasks, participants are typically instructed to identify a recent negative event (e.g. self-defined as stressful, upsetting, or anger inducing) and then to speak about it in front of an experimenter for several minutes (Neumann et al. Citation2004; Gerin et al. Citation2006; Key et al. Citation2008). This combination of stressor recall with public speaking has been used to bring about a stress response while the participant focuses on a rumination-inducing event (Key et al. Citation2008). Following verbal recall of an emotional event and associated reactivity, rumination has delayed the recovery of cardiovascular indices (Gerin et al. Citation2006; Key et al. Citation2008). The social-evaluative nature of these types of public speaking tasks has been shown to be an effective elicitor of physiological stress responses (Al'Absi et al. Citation1997; Dickerson and Kemeny Citation2004), and it is unclear whether the stressful public speaking component (as opposed to the content of the information being recalled) is driving the physiological reactivity in these tasks. Manipulating the interview context would allow examination of whether the nature of the stressor recall experience plays a role in shaping physiological responses for ruminators.
In summary, an acute physiological response to a stressor may become prolonged through delayed recovery or reactivation as a result of rumination or recall. This prolonged activation is well illustrated with cardiovascular indices, but whether this translates to the HPA axis is not clear. Prior investigations suggest a link between rumination and cortisol secretion, but the association may depend on how rumination is conceptualized and assessed. The content of an individual's ruminations as well as their trait ruminative tendency and state ruminative response are important to consider. In addition, whether verbally recalling an acute stressor 2 weeks later can reactivate cortisol responses and whether the cortisol response depends on the nature of the stressor recall experience are not yet known.
The present study had two primary aims. The first was to test whether individual tendency to ruminate moderates the cortisol response to a modified version of the Trier Social Stress Test (TSST; Kirschbaum et al. Citation1993; Yim et al. 2010). Greater trait stress-related rumination was hypothesized to predict greater cortisol reactivity and delayed recovery in response to the stressor. The second aim was to examine whether recall of the stressor 2 weeks later would reactivate cortisol responses, and whether these responses would be contingent upon the interview context of the stressor recall (supportive versus unsupportive interview context) and individual differences in rumination (trait and state stress-related measures). Both greater trait rumination and greater state rumination were hypothesized to predict greater salivary cortisol responses to the stressor recall, and this effect was expected to be more pronounced for those in the unsupportive interview context compared to those in the supportive context.
Materials and methods
Participants
Participants included 59 undergraduate students (31, 52.5%, females; 28 males) from the University of California, Irvine, a subset of whom were part of a larger study examining age and sex differences in physiological and emotional responses to a laboratory stressor and later memory of that stressor (Yim et al. Citation2010; Quas, Yim, Edelstein, Cahill, Rush, unpublished, 2010). The sample was ethnically diverse (45.2% non-Hispanic White, 28.0% Asian, 10.8% Hispanic or Latino/a, 16.0% multi-ethnic or other), with a mean age of 20.1 years (SD = 1.7).
Participants were recruited through the web-based university subject pool and received course extra credit for participation. Individuals were excluded if they indicated behaviors or conditions that could influence the HPA axis (e.g. smoking, chronic or serious health conditions, medication other than oral contraceptives). Because salivary cortisol reactivity is known to differ between menstrual cycle phases (Kirschbaum et al. Citation1999), and the two laboratory sessions were separated in time by 2 weeks, only female participants who reported taking any type of oral contraceptive were included.
Procedures
Laboratory sessions took place in the afternoon (starting between 13:30 and 16:30 h) to control for the pronounced circadian variation in cortisol secretion. The second laboratory visit was scheduled for 2 weeks after the first and began at the same time of day in a different laboratory. Laboratory sessions were conducted between February and December 2006. All procedures were approved by the Institutional Review Board of the University of California, Irvine.
Session one: Psychosocial stressor
Upon arrival to the first session, procedures were described in detail to participants. After giving written informed consent, participants underwent a 20-min rest period during which they provided information about their socio-demographic background and their health (e.g. height, weight, physical activity, and sleep quality). Participants also rated their current emotions with the Positive and Negative Affect Schedule (PANAS; Watson et al. Citation1988). At the end of this period, the first saliva sample was collected (2 min pre-stressor), and then the laboratory stressor task, a modified version of the modified Trier Social Stress Test (TSST-M), was introduced by the experimenter (Yim et al. Citation2010). In the present study, the speech task was increased to 6 min and the mathematics task was shortened to 4 min (in the TSST, both tasks are 5 min in duration). For the speech part of the test, participants were asked to talk about themselves (e.g. personality, why other students would like them) and at least one good and one bad thing about themselves (in the TSST, this part is a mock job interview). After a 2-min introduction and 3-min preparation period, each participant completed a 6-min speech about himself or herself and a 4-min mental arithmetic task in front of a 2-member panel (one male, one female) and a video camera. The panelists took notes and asked standardized questions during the speech. Upon TSST-M completion, participants provided seven additional saliva samples at 1, 10, 20, 30, 45, 60, and 75 min post-stressor termination. After collecting the 1-min post-stressor sample, participants again rated their current emotions with the PANAS (Watson et al. Citation1988) and provided task appraisals by rating several performance-related prompts with a 7-point Likert-type scale (e.g. task difficulty, confidence, overall performance). Afterward, participants filled out additional questionnaires (e.g. trait rumination, details below) and then relaxed or read neutral material (e.g. magazines). At the end of the first session, the experimenter thanked the participant and confirmed the appointment time for the second visit.
Session two: Verbal stressor recall
Participants arrived 2 weeks later to complete the second session. The timing and saliva sampling schedule were identical to the first visit. However, instead of completing the TSST-M following a 20-min baseline and collection of the first saliva sample, a surprise memory interview regarding the first session was conducted. To reduce reminder/anticipatory cues, interviews were conducted in a different building, and neither observer from session one was present. Interviews were conducted by a female research assistant who had not been present during the TSST-M. Participants were randomly assigned to a supportive (N = 30) or unsupportive (N = 29) interview context. In the supportive condition, the interviewer was friendly and engaged in behaviors such as smiling and nodding throughout the interview. In the unsupportive condition, the interviewer was aloof and neutral, maintaining a stern demeanor throughout the interview. In addition, participants in the latter condition were told that their performances were being evaluated by two panelists behind a 2-way mirror. The interview context manipulation was based on similar manipulations used successfully in prior studies of social support and eye-witness testimony (Davis and Bottoms Citation2002). In both conditions, the interview lasted approximately the same amount of time as the TSST-M (mean ± SD = 15.21 ± 3.28 min, range = 10–23 min). Participants were first asked to freely recall what happened during the first session. Next, they were asked a series (55 items) of recognition (yes/no, close-ended or short-answer) questions about details of the prior session (e.g. “How many observers were there when you gave the speech? Was there a video camera in the room? Tell me about the negative event(s) you discussed during the speech. How many saliva samples did you take during the session?”). All interviews were videotaped. Following interview completion, participants provided seven more saliva samples at 1, 10, 20, 30, 45, 60, and 75 min post-interview termination, and during this time, participants completed questionnaires (e.g. current emotions, task appraisals, and trait rumination) and then read neutral material. At the end of the second session, the experimenter thanked and debriefed the participant.
Measures
Trait rumination
Trait rumination was measured with the rehearsal subscale of the revised Emotion Control Questionnaire, which was developed for the purpose of determining vulnerability to stress-related illness (Roger and Najarian Citation1989). During the post-task period of the second session, participants were asked to rate 14 statements and situations as generally “true” (1) or “false” (0) of them. Example items included: “I get ‘worked up’ just thinking about things that have upset me in the past” and “I find it hard to get thoughts about things that have upset me out of my mind.” Items were reverse coded as necessary. All items were summed into one composite score, with higher scores reflecting greater trait rumination. Internal scale reliability for this sample was moderate (Cronbach's α = 0.73).
State rumination
State rumination on the TSST-M was assessed with the 14-item negative thoughts subscale of the Thoughts Questionnaire (Edwards et al. Citation2003), which was administered during the 20-min pre-interview period of the second session. Participants indicated how often they had specific thoughts about the stressor since the first laboratory session, using a 5-point scale, ranging from “never” (0) to “very often” (4). Example items included: “How often did you think about how bad your speech was?” and “How often did you think that you must have looked stupid?” Items were summed for a total state rumination score. A Cronbach's α of 0.93 indicates high reliability.
Salivary cortisol concentration
Saliva samples were collected with Salivette sampling devices (Sarstedt, Nümbrecht, Germany) at eight time points during each session. Participants were not allowed to eat, drink, or smoke once they arrived at the laboratory. At each time point, research assistants instructed the participant to remove the cotton roll from the Salivette device and to place it under his or her tongue or next to his or her cheek for 1 min until saturated. Salivettes were kept at room temperature until completion of each session and then stored at − 70°C until assayed. After thawing for biochemical analysis, samples were centrifuged for 10 min at 2000g and 4°C. The fraction of free cortisol in saliva (salivary cortisol) was determined by a commercially available enzyme-linked immunosorbent assay (IBL-America, Minneapolis, MN, USA). All samples were assayed in duplicate. Interassay and intra-assay coefficients of variance were less than 3.4 and 3.1%, respectively. The assay sensitivity is reported as 0.012 ng/mL.
Statistical analyses
The distribution of cortisol concentrations at each time point was significantly positively skewed, and values were therefore natural log-transformed. All analyses were conducted using transformed values. Multilevel model analyses (SAS 9.2 PROC MIXED), using random coefficients models, were performed to predict salivary cortisol responses to the laboratory tasks. In the present study, two levels were modeled: (1) individual change in cortisol concentration as a function of time and (2) inter-individual differences affecting the cortisol response patterns (e.g. trait rumination and experimental condition). Time effects were computed as linear and quadratic functions and tested in models independently and interactively with other key variables. Modeling time as a quadratic effect provided the best fit in all analyses predicting cortisol responses to the psychosocial stressor during the first session and in analyses including rumination to predict cortisol responses to the stressor recall during the second session. To test whether cortisol responses during the first laboratory session differed by rumination levels, interaction terms consisting of trait rumination scores and time2 were entered in the models. Interactions between trait and state rumination scores and time2 as well as interactions between rumination scores and experimental condition and time2 were examined in analyses, predicting cortisol responses during the second laboratory session. Variables included in interactions were centered on their mean values to minimize collinearity and facilitate a more meaningful interpretation of the results. All analyses containing interactions included the corresponding lower-order terms (e.g. time and trait rumination). To test for differences in cortisol values at a given time point (e.g. baseline, 45 min post-stressor) as a function of trait rumination, time was centered at the specified time point. Statistical tests for differences in the trait rumination intercept at the specified time point are reported.
Random assignment to experimental condition at session two was successful. There were no differences between the two experimental conditions in trait rumination or state rumination, t all < 0.50, p all >0.61, or in cortisol responses to the TSST-M, condition × time2, F(1,315) = 0.46, n.s. As a result, experimental condition was removed from analyses predicting session one cortisol concentration. There were no differences in baseline cortisol concentrations during the second session as a function of experimental condition, t(52) = 0.61, n.s. Potential covariates were screened for inclusion in study analyses. Time of day did not predict cortisol responses at either session and was therefore not included in the final analyses. Length of interview was not associated with cortisol during the second session, F(1,241) = 0.10, n.s. Gender was a significant predictor of cortisol responses: cortisol responses to the TSST-M were more pronounced for males, t(317) = − 7.00, p < 0.0001, than females, t(317) = − 3.66, p < 0.001. In response to the stressor recall, cortisol concentration declined more rapidly for males, t(298) = − 5.12, p < 0.0001, than for females, t(298) = − 2.76, p < 0.01. As such, gender was included as a covariate in analyses predicting cortisol concentration, or analyses were run separately for males and females.
Results
Overall, the TSST-M produced robust salivary cortisol responses in participants during the first session, time2, F(1,56) = 55.47, p < 0.0001. The average cortisol peak occurred at 10 min after stressor termination. The stressor recall during the second session did not elicit increases in cortisol concentration relative to baseline, time2, F(1,295) = 1.73, n.s. However, there were differences in the rate of decline in cortisol concentration between the two interview conditions, condition × time, F(1,352) = 4.72, p < 0.05. Cortisol concentration decreased more rapidly across the session for those in the supportive condition, t(352) = − 7.78, p < 0.0001, than those in the unsupportive interview condition, t(352) = − 4.90, p < 0.0001. shows the mean cortisol concentration at each sampling time point for both study sessions.
Table I. Mean (SD) salivary cortisol values (in nmol/L) at each time point relative to the stressor exposure or stressor recall.
Measures of trait rumination and state rumination during the 2-week period after the stressor were moderately correlated, r = 0.33, p < 0.05.Mean trait and state rumination scores did not differ between males and females, t all < 1.59, p all >0.11. However, gender and trait rumination interacted to predict state rumination, β = 0.37, t(53) = 2.09, p < 0.05. Specifically, higher trait rumination scores predicted higher state rumination scores for females, r = 0.54, p < 0.01. For males, however, there was no association between trait and state rumination, r = 0.06, n.s. Cortisol responses to the TSST-M did not predict state rumination about the stressor over the subsequent 2 weeks, F(1,314) = 0.62, n.s.
Rumination and cortisol responses to the stressor
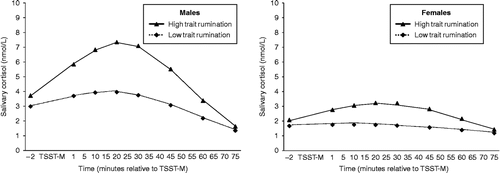
As expected, greater trait rumination was associated with greater cortisol responses to the TSST-M for both males and females, trait rumination × time2, F(1,310) = 6.77, p < 0.01. As shown in , trait rumination predicted greater cortisol reactivity and delayed recovery. Males, however, demonstrated a greater response than females, independent of trait rumination scores, F(1,310) = 32.22, p < 0.001 (Males time2: β = − 0.00042, t(310) = 7.14, p < 0.0001; Females time2: β = − 0.0002, t(310) = 3.67, p < 0.001). Significant differences in cortisol concentration as a function of trait rumination persisted through 45 min post-stressor termination, trait rumination, F(1,310) = 3.90, p < 0.05, indicating delayed recovery for those high on trait rumination. There was no effect of trait rumination on baseline cortisol values, F(1,310) = 0.50, n.s.
Figure 1 The predicted cortisol response to the stressor (TSST-M) for males and females is illustrated. The predicted mean cortisol concentrations at each sampling time point was calculated using unstandardized regression coefficients for subjects scoring one standard deviation above and below the mean trait rumination score. Males: N = 28; Females: N = 31.
Rumination and cortisol responses to the verbal stressor recall task
Trait rumination interacted with experimental condition to predict cortisol responses to the stressor recall in males, trait rumination × condition × time2, F(1,45) = 8.13, p < 0.01, . Specifically, as trait rumination scores increased, cortisol increased during the unsupportive interview, trait rumination × time2, t(99) = − 2.62, p < 0.05. However, trait rumination was unrelated to cortisol in the supportive interview context, trait rumination × time2, t(99) = 1.42, n.s. There was no main effect of trait rumination on cortisol response patterns across both conditions for males, F(1,47) = 0.67, n.s. There was no effect of trait rumination on cortisol response patterns to the stressor recall for females across both or within either experimental condition, F all < 0.53, p all >0.47.
Figure 2 The predicted cortisol response to the stressor recall for males and females is illustrated. The predicted mean cortisol concentrations at sampling time point was deviation above each calculated using unstandardized regression coefficients for subjects scoring one standard and below the mean trait rumination score. Males, unsupportive interview: N = 13; Males, Supportive Interview: N = 15; Females, unsupportive interview: N = 15; Females, Supportive Interview: N = 16.
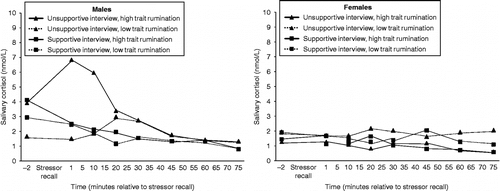
Males who scored high on both trait and state rumination had the most pronounced cortisol responses to the unsupportive interview, trait rumination × state rumination × condition × time2, F(2,119) = 7.53, p < 0.001. No combination of trait and state rumination scores predicted increased cortisol responses to the supportive interview. There were no significant differences in baseline cortisol concentrations as a function of rumination, except for a marginally significant effect of state rumination, predicting elevated baseline cortisol values, r = 0.37, p = 0.06. There were no main or interactive effects of state rumination on cortisol responses to the interview in females, F all < 0.92, p all >0.34.
Discussion
The present study demonstrates an association between trait stress-related rumination and greater reactivity of cortisol secretion as well as delayed recovery in response to a psychosocial stressor for males and females. When verbally recalling the stressor 2 weeks later, salivary cortisol responses depended on interview context and trait rumination in males. Specifically, greater trait rumination scores predicted greater cortisol responses to the unsupportive interview among males. This effect was enhanced for the males who scored highly on both trait and state rumination.
Our findings provide further support for the Perseverative Cognition Hypothesis (Brosschot et al. Citation2006). Rumination was associated with prolonged cortisol activation in the form of delayed recovery to a psychosocial stressor and reactivation (in males) when later recalling that stressor in an unsupportive interview context. The present study corroborates a prior investigation that found a positive association between stress-related trait rumination and urinary cortisol concentration on the morning of a stressful examination (Roger and Najarian Citation1998). In addition, the current study confirms and extends our own past work that linked greater stress-related state rumination during a 10-min rest period after a laboratory speech task to greater cortisol reactivity to that task (Zoccola et al. Citation2008). Notably, these past two studies and the present report defined rumination as a stress-related phenomenon. This conceptualization is significant because two previous studies that used depression-related measures of rumination found no association (Young and Nolen-Hoeksema Citation2001) or a negative association (Zoccola et al. Citation2008) between trait rumination and cortisol responses to acute stressors. Taken together, the results of these studies indicate that rumination may play a role in prolonging the immediate stress response, but that this association may depend upon how rumination is defined and assessed. Those who are prone to stress-related rumination and regularly ruminate on stressors that evoke increased cortisol secretion may be particularly vulnerable to negative health outcomes associated with overexposure to high levels of cortisol.
When verbally recalling the stressor 2 weeks later, cortisol responses were contingent upon the interview context and dispositional rumination. Overall, the interviews did not elicit increases in salivary cortisol level, on average, consistent with previous work suggesting social-evaluative threat is a key element in situations inducing HPA axis responses (Dickerson and Kemeny Citation2004). However, male trait ruminators in the unsupportive interview condition demonstrated increased salivary cortisol level in response to the stressor recall task. This effect was heightened for those who also reported greater state rumination. It is important to note, however, that the trait-by-state interaction for males in the unsupportive interview condition was found in a small subgroup (N = 13), and this finding should be interpreted with caution. Among low trait ruminators, there was no cortisol response in either interview context. The presence of this trait rumination-by-condition interaction, along with an absence of main effects for either trait rumination or interview context, suggests that the combination of a propensity to ruminate on a stressor (i.e. trait rumination) and the presence of a stressor (i.e. unsupportive interview) may be necessary to elicit cortisol responses.
Additionally, it is possible that the interviewer in the supportive interview context buffered the activation of a cortisol stress response for trait ruminators. This interpretation is consistent with previous work showing that a friend's support may attenuate cortisol responses to acute stressors (Kirschbaum et al. Citation1995a; Heinrichs et al. Citation2003). Gender of the interviewer (female) during the stressor recall may have also played a role in the current study. Past work suggests that the provision of social support to a man by a female partner (but not to a woman by a male partner) can reduce cortisol responses to an acute stressor (Kirschbaum et al. Citation1995a). Sample size and study design preclude our ability to statistically test whether these factors (e.g. participant and/or interviewer gender and social support) may have mediated the impact of the trait rumination-by-condition interaction on cortisol responses in this study. Future studies should tease apart the context of the stressor recall interview, and empirically test potential mediators.
Overall, the men had more pronounced salivary cortisol responses to the stressor compared to the women. This is consistent with prior work demonstrating greater salivary cortisol responses to psychological stressors in males than females (Kudielka and Kirschbaum Citation2005), particularly when compared to females using oral contraceptives (Kirschbaum et al. Citation1999). In previous reports, salivary cortisol concentrations in women taking oral contraceptive medication increased by approximately 140 to 173% above baseline values in response to the TSST (Kirschbaum et al. Citation1995b; Kirschbaum et al. Citation1999; Rohleder et al. Citation2003). The percentage increase in cortisol concentration relative to baseline for the women in the present sample was within that range at 148%. Sex differences in brain anatomy and circulating sex hormones may account for these differences in salivary cortisol concentration between men and women and among women (Kudielka and Kirschbaum Citation2005).
The lack of association between rumination and cortisol responses to the stressor recall interview in females is important to note. Use of oral contraceptives by the females, coupled with the absence of an overall increase in salivary cortisol level in response to the stressor recall interview may explain the null association between rumination and cortisol responses to the stressor recall. We cannot exclude, however, that real sex differences exist. Hence, perhaps only male ruminators demonstrate cortisol responses during a stressful recall. Future investigations should examine whether a rumination–recall–cortisol association exists in women who are naturally cycling (i.e. not using oral contraceptives).
High trait and state rumination scores predicted the greatest cortisol responses to the unsupportive interview among males. However, trait and state rumination scores were not correlated among males in this study. Males who reported a tendency for rumination were not necessarily also ruminating in the 2 weeks after the stressor. Females, in contrast, did demonstrate congruence between trait and state measures of rumination. Whether an individual ruminates or not may be determined by both disposition and situation characteristics. It is possible that the TSST-M was a novel situation that overwhelmed habitual coping strategies in some individuals (Wade et al. Citation2008). Hence, a subset of participants may have had greater state rumination on the stressor, irrespective of their individual tendencies to ruminate, and perhaps this occurred more often in males. In addition, another subset of men may have had fewer ruminative thoughts about the TSST-M, consistent with the notion that men may feel less responsible for social interactions (Nolen-Hoeksema and Jackson Citation2001). In light of the lack of association between trait and state measures in males and the interactive effects of trait and state rumination on cortisol responses to the stressor recall, the inclusion of both trait and state measures of rumination may be useful in identifying high-cortisol responders in response to stressors in future studies. It is unclear why we found incongruity between trait and state rumination among men, but not among women, in this study. Future research should directly address these gender differences in the association between the trait and state rumination measures.
This study examined stressor recall as an interactive experience as has been done extensively in the context of cardiovascular reactivity and recovery (Neumann et al. Citation2004; Gerin et al. Citation2006; Key et al. Citation2008). Rumination, however, is generally considered a private cognitive process. The present investigation does not allow us to determine whether those prone to rumination would also demonstrate cortisol reactivity in response to mentally recalling a stressor or non-interactive rumination, because, in the current study, participants recalled the stressor in the presence of an interviewer. Currently, physiological reactivation as a result of mentally recalling a stressor has only been demonstrated with cardiovascular indices (Glynn et al. Citation2002, Citation2007). Whether mental recall is sufficient to reactivate a cortisol response is unclear and should be addressed in future studies. In addition, a 2-week delay between the stressor experience and recall was used in the present study. Future work should consider and/or manipulate length of delay between stressor and recall to determine its potential role in influencing the cortisol response (i.e. perhaps recall of more recent stressors would evoke a more robust response).
In conclusion, the findings from the present study are consistent with the perseverative cognition hypothesis (Brosschot et al. Citation2006), supporting the notion that the acute stress response may become prolonged through delayed recovery and reactivation as a result of rumination and recall. Results also highlight the importance of rumination assessment and stressor recall context in understanding the rumination–cortisol association. The HPA axis is an important regulator of many physiological systems, and persistent activation of the HPA axis has been associated with a number of negative health consequences (Chrousos and Gold Citation1992). Therefore, identifying factors such as rumination, which may contribute to persistent activation of stress-related physiological systems, is important in explicating the stressor–health association.
Acknowledgments
The authors thank Robin Edelstein, Jennifer Piazza, and Cathy Hayakawa for assisting with data collection, Allison Wallin for her assistance in assaying cortisol, and the many undergraduate students who helped with data collection. We also greatly appreciate the time and assistance of the participants, without whom the study would not have been possible. The present study was funded by the National Science Foundation (BCS-0721377) and a Faculty Collaborative Grant from the University of California, Irvine. These funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Notes
* Two additional participants only completed the first session and are not included in the present analyses; one failed to return for the second visit and the second withdrew from the study after the first session.
† Fifty-six participants returned exactly 14 days later and three participants returned between 10 and 16 days later (mean ± SD = 13.95 ± 0.60). Delay between laboratory sessions did not differ as a function of experimental condition, t < 1.58, p>0.12.
‡ Trait rumination intercept tested with time centered at 45 min post-stressor.
References
- Al'Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. 1997. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 34 3: 266–275.
- Brosschot JF, Gerin W, Thayer JF. 2006. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res. 60 2: 113–124.
- Chida Y, Hamer M. 2008. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: A quantitative review of 30 years of investigations. Psychol Bull. 134 6: 829–885.
- Chrousos GP, Gold PW. 1992. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 267 9: 1244–1252.
- Davis SL, Bottoms BL. 2002. Effects of social support on children's eyewitness reports: A test of the underlying mechanism. Law Hum Behav. 26 2: 185–215.
- Denson TF, Fabiansson EC, Creswell JD, Pedersen WC. 2009. Experimental effects of rumination styles on salivary cortisol responses. Motiv Emot. 33 1: 42–48.
- Dickerson SS, Kemeny ME. 2004. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 130 3: 355–391.
- Edwards SL, Rapee RM, Franklin J. 2003. Postevent rumination and recall bias for a social performance event in high and low socially anxious individuals. Cognit Ther Res. 27 6: 603–617.
- Gerin W, Davidson KW, Christenfeld NJS, Goyal T, Schwartz JE. 2006. The role of angry rumination and distraction in blood pressure recovery from emotional arousal. Psychosom Med. 68 1: 64–72.
- Glynn LM, Christenfeld N, Gerin W. 2002. The role of rumination in recovery from reactivity: Cardiovascular consequences of emotional states. Psychosom Med. 64 5: 714–726.
- Glynn LM, Christenfeld N, Gerin W. 2007. Recreating cardiovascular responses with rumination: The effects of a delay between harassment and its recall. Int J Psychophysiol. 66 2: 135–140.
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. 2003. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 54 12: 1389–1398.
- Janoff-Bulman R. 1992. Shattered assumptions: Towards a new psychology of trauma. New York: Free Press.
- Key BL, Campbell TS, Bacon SL, Gerin W. 2008. The influence of trait and state rumination on cardiovascular recovery from a negative emotional stressor. J Behav Med. 31 3: 237–248.
- Kirschbaum C, Pirke KM, Hellhammer DH. 1993. The ‘Trier Social Stress Test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 28 1–2: 76–81.
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom Med. 1995a; 57 1: 23–31.
- Kirschbaum C, Pirke KM, Hellhammer DH. Preliminary evidence for reduced cortisol responsivity to psychological stress in women using oral contraceptive medication. Psychoneuroendocrinology. 1995b; 20 5: 509–514.
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. 1999. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom Med. 61 2: 154–162.
- Kudielka BM, Kirschbaum C. 2005. Sex differences in HPA responses to stress: A review. Biol Psychol. 69 1: 113–132.
- McCullough ME, Orsulak P, Brandon A, Akers L. 2007. Rumination, fear, and cortisol: An in vivo study of interpersonal transgressions. Health Psychol. 26 1: 126–132.
- McEwen BS, Seeman T. 2003. Stress and affect: Applicability of the concepts of allostasis and allostatic load. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. The handbook of affective sciences. Oxford: Oxford University Press. 1117–1138.
- Neumann SA, Waldstein SR, Sellers JJ, Thayer JF, Sorkin JD. 2004. Hostility and distraction have differential influences on cardiovascular recovery from anger recall in women. Health Psychol. 23 6: 631–640.
- Nolen-Hoeksema S, Jackson B. 2001. Mediators of the gender difference in rumination. Psychol Women Q. 25 1: 37–47.
- Nolen-Hoeksema S, Morrow J, Frederickson BL. 1993. Responses styles and the duration of episodes of depressed mood. J Abnorm Psychol. 102 1: 20–28.
- Roger D, Najarian B. 1989. The construction and validation of a new scale for measuring emotional control. Pers Individ Dif. 10 8: 845–853.
- Roger D, Najarian B. 1998. The relationship between emotional rumination and cortisol secretion under stress. Pers Individ Dif. 24 4: 531–538.
- Rohleder N, Wolf JM, Piel M, Kirschbaum C. 2003. Impact of oral contraceptive use on glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Psychoneuroendocrinology. 28:261–273.
- Taylor SE. 1983. Adjustment to threatening events: A theory of cognitive adaptation. Am Psychol. 38 11: 1161–1173.
- Wade NG, Vogel DL, Liao KYH, Goldman DB. 2008. Measuring state-specific rumination: Development of the rumination about an interpersonal offense scale. J Couns Psychol. 55 3: 419–426.
- Watkins ER. 2008. Constructive and unconstructive repetitive thought. Psychol Bull. 134 2: 163–206.
- Watson D, Clark LA, Tellegen A. 1988. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 54 6: 1063–1070.
- Yim IS, Quas JA, Cahill L, Hayakawa C. 2010. Children's and adults’ salivary cortisol response to an identical psychosocial laboratory stressor. Psychoneuroendocrinology. 35 2: 241–248.
- Young EA, Nolen-Hoeksema S. 2001. Effects of ruminations on the saliva cortisol response to a social stressor. Psychoneuroendocrinology. 26 3: 319–329.
- Zoccola PM, Dickerson SS, Zaldivar FP. 2008. Rumination and cortisol responses to laboratory stressors. Psychosom Med. 70 6: 661–667.