Abstract
Gain of “antimicrobial resistance” and “adaptive virulence” has been the dominant view of Pseudomonas aeruginosa (Pa) in cystic fibrosis (CF) in the progressively damaged host airway over the course of this chronic infection. However, the pathogenic effects of CF airway-adapted Pa strains are notably reduced. We propose that CF Pa and other bacterial cohabitants undergo host adaptation which resembles the changes found in bacterial symbionts in animal hosts. Development of clonally selected and intraspecific isogenic Pa strains which display divergent colony morphology, growth rate, auxotrophy, and antibiotic susceptibility in vitro suggests an adaptive sequence of infective exploitation-parasitism-symbiotic evolution driven by host defenses. Most importantly, the emergence of CF pseudomonal auxotrophy is frequently associated with a few specific amino acids. The selective retention or loss of specific amino acid biosynthesis in CF-adapted Pa reflects bacterium-host symbiosis and coevolution during chronic infection, not nutrient availability. This principle also argues against the long-standing concept of dietary availability leading to evolution of essential amino acid requirements in humans. A novel model of pseudomonal adaptation through multicellular bacterial syntrophy is proposed to explain early events in bacterial gene decay and decreased (not increased) virulence due to symbiotic response to host defense.
Introduction
Pseudomonas aeruginosa (Pa) is a gamma-proteobacterium species which is highly diversified in its nutritional niches, and it is largely associated with organic and moist matter in soil (Green et al., Citation1974; Regnath et al., Citation2004). This organism also finds a habitat in domestic water devices such as water faucets, shower heads, and swimming pools (Barben et al., Citation2005). Its environmental associations are broad and far reaching as it has a highly adaptable genome with a highly efficient and versatile regulatory network (Goldberg et al., Citation2008; Roy et al., Citation2010). It rarely causes disease in healthy persons, though it is a significant opportunistic pathogen which may cause systemic infection in patients with compromised host defense mechanisms (Lyczak et al., Citation2002; Mathee et al., Citation2008; Murray et al., Citation2007; Smith et al., Citation2006; Strausbaugh & Davis, Citation2007).
Chronic pulmonary Pa infection is particularly common among patients with cystic fibrosis (CF), who, like patients with burn injuries, have lost the integrity of a physical barrier to infection. CF is a genetically inherited condition in which the transmembrane conductance regulator (CFTR) is defective in chloride channel regulation, chiefly affecting airway epithelial cells after birth (O'Sullivan & Freedman, Citation2009). Pathophysiologic manifestations of CFTR deficiency may begin to affect organ systems in utero, including pancreatic blockage leading to autodigestion of host tissue and a higher rate of meconium ileus (10–15%) as a consequence of reduced water and bicarbonate excretion in the gut (Flume & Van Devanter, Citation2012). However, CF host inflammatory response in the gastrointestinal tract is less severe after birth because the human gut has evolved to acquire beneficial microbiota. Furthermore, intestinal transit is largely dependent on peristalsis (not ciliary activity), and may improve postnatally with compensatory changes in muscular tone or other physiological adaptations. Shortly after birth, the nonfunctional CFTR in CF significantly impairs airway mucociliary function in individuals who otherwise show normal humoral and cellular immunity with no evidence of increased susceptibility to viral infection or malignancy (Abraham et al., Citation1996; Dakin et al., Citation2002; Noah et al., Citation1997; Sheldon et al., Citation1993). Although tissue destruction and toxin production have long been viewed as characteristic traits of pseudomonal virulence, Pa is an environmentally ubiquitous saprophyte. Therefore, it is not very different from the vast number of environmental organisms that have not otherwise become part of the human microbiota during evolution. In other words, humans are not the “definitive host” of this type of microbe.
The single-cell and free-living saprophytic lifestyle of Pa isolates is determined by and has evolved from a nutritionally competitive polymicrobial environment, and this bacterial lifestyle is minimally affected by the non-CF host during short-term pseudomonal interactions, in which acute and fulminant infection is inevitable (Furukawa et al., Citation2006). On the contrary, isolates of Pa from chronically infected CF airways have been recognized for their greater capacity to build a complex bacterial conglomerate referred to as bacterial syntrophy, which is an advanced form of biofilm (Qin et al., Citation2012). In contrast to their wild-type free-living counterparts, the isogenic Pa strains within a syntrophy have lost their single-cell replicative mode of living, essential for transmission or recolonization of a new host (Head & Yu, Citation2004; Hogardt & Heesemann, Citation2010; Mena et al., Citation2007; Qin et al., Citation2012). Pseudomonal strain-specific long term success and intraclonal divergence within a host airway space (or cavity) suggest the underlying importance of bacterial multicellularity en route to symbiosis.
Symbiosis refers to persistent or long-term interactions between organisms (Dale & Moran, Citation2006, Moran, Citation2006). Nearly 80 years ago, long before the full spectrum of symbiosis was defined, K. F. Meyer wrote an analysis entitled “Latent Infections” and asserted “symbiosis must be regarded as a condition of life balancing between two extremes: complete immunity and deadly infective diseases” (Meyer, Citation1936). Based on microbiological studies, the traditional interpretation of Pa associated with chronic airway infected CF patients has been dominated by such concepts as “multi-resistance” or “pan-resistance” to antimicrobial agents and a gain of “biofilm or cell-associated virulence” (Bragonzi et al., Citation2009; Cigana et al., Citation2011; Head & Yu, Citation2004; Hogardt & Heesemann, Citation2010; Lee et al., Citation2006). However, this is paradoxically unsupported by the in vitro properties of CF Pa isolates in which accumulation of mutations in virulence regulators, as well as virulence genes and drug resistant determinants, have been attributed to a “hyper-mutable” state (Bragonzi et al., Citation2009; Cigana et al., Citation2011; Hoiby et al., Citation2010; Smith et al., Citation2006; Livermore, Citation2002; Mena et al., Citation2008; Oliver & Mena, Citation2010). This analysis focuses on the evolution of pseudomonal syntrophy during niche specialization in response to polarized airway epithelia in the CF host.
Infective exploitation, parasitism, and symbiosis
Environmental microbes may by chance come in contact with a new nutrient-abundant environment, such as a human or animal host. This bacterial invasion is an infection from the host perspective. Microbial infection may be eliminated quickly by host defenses and/or antimicrobial treatment, or host demise may result with no long-term benefit to either the host or the organism. Alternatively, microbial organisms may establish long-term residence in a given anatomic site, and thus define a novel microbe-host relationship (Blaser & Kirschner, Citation2007; Hewlett, Citation1916; Hoiby et al., Citation2010; Qin et al., Citation2012). Chronic bacterial infection is often a form of parasitism with no net benefit to the host, a relationship promoted by a persistent nutrient abundant host environment. Microbial parasitism, commensalism, and mutualism all have been regarded as forms of symbiosis (Moran et al., Citation2008). Each of these three forms of symbiosis can be found in microbe-human interactions, and they often overlap in a manner which may be referred to as chronic, persistent, or recurrent infection (Hewlett, Citation1916; Meyer, Citation1936). Pseudomonal colonization and continual airway destruction in patients with CF represents an extreme example of such an overlap of infective exploitation and symbiosis.
Clinical characteristics of pseudomonal infection in the airway of CF patients
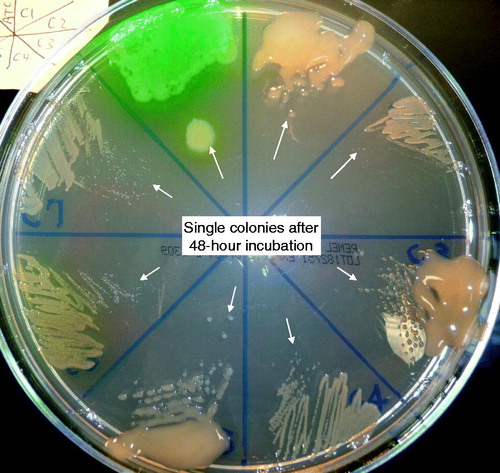
Pa and other Gram-negative bacteria have long been considered common pathogens associated with CF airway infections (O'Sullivan & Freedman, Citation2009). Displacement of the natural upper airway microbes (Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus) by Pa is a hallmark of CF pulmonary disease progression (O'Sullivan & Freedman, Citation2009). Deep airway infections due to S. aureus and other oral organisms have also been shown to contribute to the microbial burden and inflammation that causes a decline in respiratory function (Flume & Van Devanter, Citation2012; Foweraker, Citation2009; Wolter et al., Citation2013). However, 60–85% of the CF population is colonized by Pa (FitzSimmons, Citation1993; Mahadeva et al., Citation1998). By age 3, more than 95% of these children have culture or serologic evidence of Pa infection (Burns et al., Citation2001). With the exception of nosocomial spread, Pa isolates from a newly infected CF patient are polyclonal. Upon initial isolation, colonizing bacterial strains are indistinguishable from their environmental counterparts in terms of their quantifiable in vitro colonial morphology and antimicrobial susceptibility patterns (Burns et al., Citation2001). After a period of 9–10 years on average, or sometimes after as little as a few months, a hyper-mucoid Pa may make its first appearance along with other divergent colony types (Burns et al., Citation1998; Jelsbak et al., Citation2007; Li et al., Citation2005). This pseudomonal divergent (or heterogeneous) in vitro growth pattern is indicative of emergence of bacterial multicellularity ().
Figure 1. Colony size comparison depicting bacterial growth rate. Comparison of growth properties of 7 isogenic Pa isolates from a single patient (PA-C1 to -C7) on Mueller Hinton agar after a full 48-hour incubation in 35 °C ambient air: from the green wild-type PA-ATCC 27853 on top left in clock-wise sequence: PA-C1 to PA-C7 (Qin et al., Citation2012).
Pseudomonal pulmonary infection in CF is chronic and is localized inside airway compartments only. Pseudomonal infection is very different in the setting of CF versus non-CF in terms of disease process and resolution. In contrast to pseudomonal chronic infections similar to or typically seen in CF, an invasive Pa infection involves bacterial attachment, colonization, local infection, and bloodstream dissemination or systemic disease (Pollack, Citation2000). Pseudomonal colonization and adherence to the host airway mucosal surface without entering the blood stream is most evident in the setting of CF (Valderrey et al., Citation2010). Pa never gives rise to blood stream infection in CF patients, even in those who are affected by a high bacterial burden and/or undergoing pulmonary exacerbations (Williams et al., Citation2010). Pseudomonal CF airway tissue tropism and its host airway containment constitute an extreme example of a chronic infection that may last over a CF patient’s lifetime that is approaching 30–40 years (http://www.cff.org/). The nature of this long-term bacteria–host association clearly depends on the decrease of Pa virulence as it adapts to the CF host airway environment (Cigana et al., Citation2011; Lee et al., Citation2006; Moran, Citation2006; Smith et al., Citation2006; Qin et al., Citation2012).
CF-airway-niche-specialized Pa does not transmit from host to host. It is well recognized that Pa in CF patients is acquired largely from the immediate environment, rather than patient-to-patient transmission (Oliver & Mena, Citation2010, Macia et al., Citation2005, Pirnay et al., Citation2009). Longitudinal bacterial culture following patient disease progression suggests that a gradual decrease in bacterial diversity is associated with an increase in patient age and advanced pulmonary disease (Cox et al., Citation2010). Transmission of CF airway adapted strains, for example, a hyper-mucoid Pa, has never been reported (Aaron et al., Citation2004, Citation2010; Feliziani et al., Citation2010; Furukawa et al., Citation2006; Hogardt et al., Citation2007; Luzar & Montie, Citation1985; Oliver & Mena, Citation2010).
Nearly a half century of pseudomonal microbiology studies have shown that CF airway adapted Pa strains isolated from chronically infected patients i) are highly clonal in individual patients over time and are largely patient unique; ii) show divergent but overall retarded autologous replication in vitro (); and iii) persist over a given patient’s lifetime. In truth, repeat acquisition of clonally unrelated wild-type Pa strains, after laboratory detection of a CF airway-adapted Pa, has rarely been reported (Bragonzi et al., Citation2009; Smith et al., Citation2006; Qin et al., Citation2012).
Pseudomonal niche specialization in the CF host airway: a process of diminishing pathogenic and deleterious effects
The development of patient-specific and functionally divergent Pa isogenies is often first noted as variation of colony morphology in vitro (). Other emerging features associated with the adapting CF Pa are more perplexing and include: i) the evolution of slow-growing auxotrophic strains from initially rapidly-growing prototrophic strains; ii) the rise of mutations in virulence determinants from highly virulent wild-type strains; and iii) the emergence of antibiotic hyper-susceptible strains from antibiotic resistant parental strains ( and ) (Agarwal et al., Citation2005; Barth et al., Citation1998; Feary et al., Citation1969; Haussler et al., Citation1999; Hoboth et al., Citation2009; Hogardt et al., Citation2007; Luzar & Montie Citation1985; Luzar et al., Citation1985; Qin et al., Citation2012; Starkey et al., Citation2009; Taylor et al., Citation1992; Thomas et al., Citation2000; Yang et al., Citation2008). Moreover, the prototrophic and auxotrophic isogenic strains together show high density growth both in vitro and in vivo, and display variable growth properties and antimicrobial susceptibilities, irrespective of their specific nutrient requirements (Palmer et al., Citation2007; Qin et al., Citation2012). It is clear that host defense plays a significant role in “domestication” of a strictly uniclonal Pa syntrophy in situ (Qin et al., Citation2012).
Figure 2. Determination of pseudomonal methionine auxotrophy in isogenic Pa strains from a single patient. Methionine (a 6 mm sterile absorbent filter disc in the center contains methionine) biosynthetic deficiency is based on bacterial growth requirement on M9 minimal salts agar containing 1% glucose as the sole carbon source (X. Qin, unpublished data).
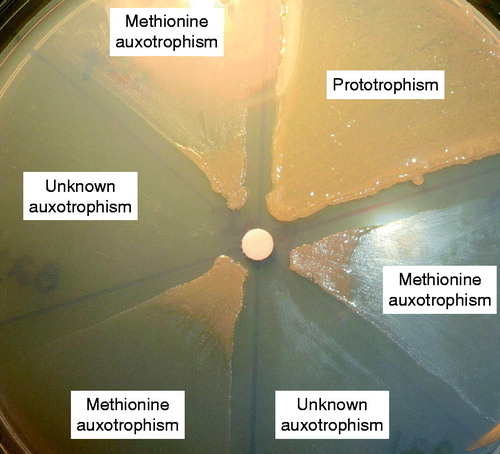
Pseudomonal isogenic divergence is driven by clonal selection and virulence counter-selection in the host airway. Genomic profiling of Pa strains isolated from airway secretions of chronically infected CF patients has suggested these organisms undergo a vigorous clonal selection process with secondary emergence of phenotypically divergent isogenic strains ( and ) (Aaron et al., Citation2004; Cigana et al., Citation2011; Ciofu et al., Citation2010; Smith et al., Citation2006; Head & Yu, Citation2004; Hogardt et al., Citation2007; Huse et al.; Citation2010; Oliver & Mena, Citation2010; Oliver et al., Citation2002). Strains that arise from a founder clone in the same airway show phenotypic dissociation (2–6 phenotypes per specimen) and carry quorum-sensing lasR mutations, as well as noncytotoxic variants with lesions in T3SS (type three secretion system) ExoS and PopD. Most importantly, the accumulation of loss-of-function mutations in DNA mismatch repair genes, such as mutS, mutL, and uvrD, has been recognized for its effect on niche-adapted Pa strains with relaxed DNA replication fidelity, and collectively labeled as “anti-mutator deficient” properties (Barth & Pitt, Citation1995a; Dacheux et al., Citation2000, Citation2001; Feliziani et al., Citation2010; Furukawa et al., Citation2006; Heurlier et al., Citation2005; Hogardt et al., Citation2007; Irvin et al., Citation1981; Oberhardt et al., Citation2010; Rau et al., Citation2010; Roy-Burman et al., Citation2001; Smith et al., Citation2006; Thomas et al., Citation2000; Vendeville et al., Citation2005; Zierdt & Schmidt Citation1964). Recently, terms such as “cheater cells” and “easy riders” have been used to describe this functional heterogeneity and isogenic interdependency (Xavier et al., Citation2011).
CF Pa is known to lose motility, pigmentation, and hemolysis over the course of chronic infection. Consequently, each isogenic Pa strain within the clonal conglomerate loses its ability to cause acute infection, regardless of host immune status (Lore et al., Citation2012; Luzar & Montie, Citation1985; Luzar et al., Citation1985; Qin et al., Citation2012). Hoffmann and colleagues were first to demonstrate that mucoid or non-mucoid CF Pa isolates show greatly decreased production of proteases, chitinase, and elastase (Hoffmann et al., Citation2005). Although airway-adapted Pa strains tend to promote prolonged lung infection in a CF mouse model, their ability to cause animal lethality is significantly reduced as compared to that of wild-type Pa (Hoffmann et al., Citation2005). Lore and colleagues have repeatedly confirmed that CF airway-niche adapted Pa shows reduced virulence and inflicts less lung tissue infiltration when tested in various animal hosts (Bragonzi et al., Citation2009; Lore et al., Citation2012). Many of these findings, however, have not to date been recognized as host imprints on pseudomonal allopatric (a disjunct removal from its natural environment) speciation once enclosed inside the CF airway.
Accumulation of quorum sensing deficient Pa reflects development of bacterial syntrophy and implies symbiotic adaptation
Conventional diagnostic microbiology is based on the isolation and examination of free-living bacterial isolates in pure culture that bears little resemblance to the microbial associations with the host airway in vivo (). Chronic infections due to bacterial persistence in the form of biofilm have gained recognition over the last two decades (Hoiby et al., Citation2001, Citation2010; May et al., Citation1991). The theory of pseudomonal biofilm assembly on the mucosal surface of CF airway has begun to address bacterial lifestyle changes that include differential gene expression, isogenic divergence, and organized cell-to-cell communication (Boucher, Citation2004; Mowat et al., Citation2011; Oliver, Citation2010; Oliver & Mena, Citation2010; Whiteley et al., Citation2001; Wu et al., Citation2001). However, few studies have directly addressed the question of intraclonal diversity, and even fewer have grappled with the confounding fact that heightened bacterial virulence is associated with bacteria in planktonic growth state (Fux et al., Citation2005; Head & Yu, Citation2004; Huse et al., Citation2010; Manos et al., Citation2008; Palmer, Citation2005).
The quorum sensing (QS) molecule N-acyl-homoserine lactone (HSL) produced by Pa is fundamental to bacterial communication and cooperative activity (Diggle et al., Citation2007; Williams & Camara, Citation2009; Williams et al., Citation2007). HSL production and regulatory function is bacterial density dependent, and serves to organize pseudomonal activity in initiation of colonization and biofilm formation in the CF airway (Atkinson & Williams, Citation2009; Heurlier et al., Citation2006; Hoiby et al., Citation2011; Vendeville et al., Citation2005; Waters & Bassler, Citation2005). One study analyzed diseased human airways both in mechanically ventilated patients and CF patients (Kohler et al., Citation2009). As early as 9 days post colonization in acutely ill patients, subpopulations of clinical Pa isolates defective in QS emerged with mutations in the autoinducer lasR (Kohler et al., Citation2009; Winstanley & Fothergill, Citation2009). QS-deficient lasR mutants have been detected in a significant proportion (30–63%) of CF Pa isolates (Feliziani et al., Citation2010; Hoffman et al., Citation2009). Moreover, isolates of Pa defective in lasR, lasI, and/or rhlI have been shown to be much less virulent than QS-positive strains in animal models (Willcox et al., Citation2008; Wu et al., Citation2001). Once more, such intraclonal de-regulation and isogenic role differentiation support the concept of pseudomonal multicellularity during symbiotic adaptation.
Concurrent host pulmonary dysfunction despite serial isolation of progressively less virulent CF Pa continues to be a paradox in the field of pseudomonal research (Smith et al., Citation2006). With systematic analysis of serial isolates from CF patients; Ciofu and colleagues correlated occurrence of mutations in the anti-mutator genes mutS and mutL with mutations in mucA and lasR which result in a phenotypic switch to mucoid/non-mucoid colonies and QS loss in CF Pa (Ciofu et al., Citation2010). Two important findings were highlighted in this study. First, isolates with phenotypes including non-mutator, weak mutator, and strong mutator phenotypes were found in time-elapsed longitudinal samples, suggesting the co-existence of isogenic subpopulations in the same patient. Second, DNA coding sequences of particular genes prone to mutability were common to all patient isolates. However, the exact nucleotide mutations identified in the anti-mutator genes were very different from clone to clone, and from CF patient to CF patient (Qin et al., Citation2012). Hogardt and colleagues also monitored serial pseudomonal isolates with clone-specific mutS mutations unique to each patient. This study confirmed that non-mutator and mutator strains coexist in the respiratory tract for prolonged periods in advanced CF. The study also confirmed that mutator strains tend to be the predominant isolates in end-stage CF disease and show startling loss of virulence associated phenotypic traits, including reduced cytotoxicity, amino acid auxotrophy, and inability to survive in tap water (Hogardt et al., Citation2007). Therefore, the in situ development of isogenic strains appears to be a general phenomenon with mutations affecting the same set of genes, but the specific coding sequence alterations are independent events in clonally unique strains associated with the individual CF host.
Established pseudomonal syntrophy exhibits inter-clonal antagonistic properties to prevent wild-type Pa from colonizing the same host airway
Once a clonally selected Pa syntrophy is established in the host airway, the same host airway becomes immune to subsequent super-colonization by wild-type Pa strains from the living environment of the host (Bragonzi et al., Citation2009; Burns et al., Citation2001; Smith et al., Citation2006; Qin et al., Citation2012). Not by coincidence, in an ancient bipartite symbiotic system, the various actinomycetous bacteria (Pseudonocardia) associated with Acromyrmex leaf-cutting ants have shown both intraspecific (or sympatric) diversity for antimicrobial metabolite production and antagonistic activity against allopatric “intruder” strains, or even unrelated microbes with varying degrees of inhibitory activity (Poulsen et al., Citation2007). Similar inter-clonal growth inhibition between Pa strains has been observed in vitro. For example, the growth of a wild-type strain PA14 is clearly inhibited by an auxotrophic and poorly growing CF strain PA-C4 (). Moreover, the nutrient cross-feeding patterns are distinctly different, continuous between isogenic strains (), or discontinuous (see a “Zone of growth inhibition” in ) between non-isogenic strains. The latter suggests that either or both strains (PA-B4 and PA-A8) may produce an inhibitory substance(s) that recognizes clonal differences (). These antagonistic growth patterns observed in clonally unrelated prototroph-auxotroph Pa pairs further conform the common knowledge that a single dominant Pa clone and its radiating isogenic derivatives are often found in the same host lifetime (Bragonzi et al., Citation2009; Qin et al., Citation2012; Smith et al., Citation2006). With parities in nature, a host-niche-adapted Pa may provide host protection against subsequent wild-type Pa colonization, a form of host benefit (Qin et al., Citation2012).
Figure 3. Patterns of growth complementation by cross feeding between prototrophic and auxotrophic Pa isolates that are isogenic or non-isogenic according to their clonality. The prototropic strains are labeled as “Feeders”. Panel (A), an example of growth inhibition between the wild-type PA14 (feeder) and an auxotrophic CF strain PA-C4. Panel (B), a characteristic growth complementation between two isogenic CF Pa strains. The radiating satellite growth of an auxotrophic PA-A8 around the feeder strain PA-A3 is continuous. Panel (C), a characteristic growth complementation between two non-isogenic CF Pa strains. The satellite growth of an auxotrophic CF strain PA-A8 around a non-isogenic feeder CF strain PA-B4 is discontinuous. A zone of growth inhibition is apparent between the auxotrophic PA-A8 and the feeder PA-B4 (Qin et al., Citation2012).

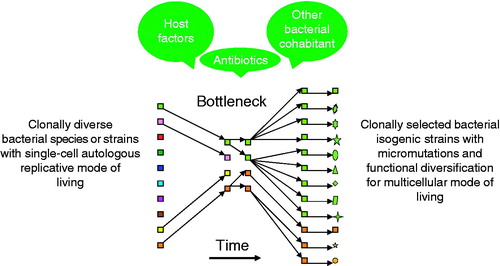
CF airway-adapted Pa, which may be considered as a human airway “extremophile”, undergoes allopatric speciation that is physically separated and phylogenetically removed from its brethren in the natural soil environment. The rise and fall of a new bacterial species has recently been well summarized by K. Georgiades and D. Raoult, “specialists regularly escape from bacterial complexes to colonize a niche and, therefore, earn species name” (Georgiades & Raoult, Citation2010). A CF niche-specific Pa syntrophy has thereby earned its mode of living by overcoming an evolutionary bottleneck consisting of host defenses, antibiotic pressure, and other microbial cohabitants ().
Figure 4. Factors contributing to the selection of pseudomonal syntrophy. Host defense, presence of other species of bacteria, and antimicrobial treatment play a role in selection of a successfully adapted P. aeruginosa (in green, and another coexisting bacterial species in orange), a multicellular clonal conglomerate with divergent phenotypes and isogenic mosaicism (same color in various shapes), symbiotic to the diseased host airway.
CF airway adapted Pa is taking identifiable steps towards symbiosis
Chronic bacterial infection in human hosts is commonly confined to a specific anatomic site. This physical confinement allows evolution of mutuality between species to occur, although this state is frequently initiated by a “virulent” destructive process. Prokaryote-animal symbiosis has been considered a source of evolutionary innovation that is very widespread in nature and often results in improved fitness of the host (Dale & Moran, Citation2006; Moran et al., Citation2008). The phenotypic and genotypic characteristics associated with some specific bacterial symbionts are listed in . These include naturally occurring examples of bacterial symbionts which are considered evolutionarily “mature”, as they harbor genomic adaptive changes that have evolved over millennia and exhibit strong associations with their hosts, while seemingly inflicting no harm on the host.
Table 1. Comparison of genome and functional features of obligate and facultative bacterial symbionts with those of P. aeruginosa isolates adapted in CF airways (many facts adopted from Moran et al., Citation2008).
Symbiotic bacteria studied to date are found to colonize specific tissue(s) or particular organ system(s) in animal hosts in rather low or fixed numbers and are collectively called bacteriomes () (Moran et al., Citation2008; Ruby, Citation2008). There are no sharply defined boundaries which separate categories of symbiosis, but symbionts can be either obligate or facultative based on the mode of living and transmission () (Moran et al., Citation2008). While obligate symbionts are generally vertically transmissible and are unable to live apart from their hosts, facultative symbionts show tissue tropism but are able to colonize the host at high infective densities, and some can spread horizontally or by means of repeat infection when a new host generation arises. Many symbionts characteristically have evolved to retain a stable minimal genome with very few mobile elements, or genes such as mutS, mutL, and recA which govern DNA mismatch repair and recombination (Moran, Citation2007; Munson et al., Citation1991). Patterns of gene loss and gene decay in facultative symbionts are postulated to be associated with relaxed DNA mismatch repair. Inactivation of mutS and mutL is associated with a rise of pseudogenes which can be for example as high as 62% of the predicted coding sequences in Sodalis glossinidius (a gamma-proteobacterium) in the Tsetse fly () (Belda et al., Citation2010). In view of genotypic degenerative patterns documented in animal symbionts, it is not a coincidence that genes necessary for the pseudomonal DNA mismatch repair system are among the first to be lost (Ciofu et al., Citation2005).
Host defense mechanisms select for pseudomonal syntrophy in the diseased airway
CF host airways are by no means “little more than soft, thin-walled flasks of culture media” (Levin & Antia, Citation2001). Both innate and acquired immunity against microbial intrusion into the lungs of CF patients are largely intact, despite the hindrance of high fluid viscosity and mucociliary dysfunction. Although the pulmonary system appears essentially normal at birth, airway obstruction due to mucus buildup can be associated with chemotaxis of neutrophilic inflammation in infants with CF whose upper airway has yet to be populated by normal microbiota (Flume & Van Devanter, Citation2012). In the first year or two of life, increasing airway inflammation is indicative of the CF host’s response to initial bacterial colonization, and this is likely stimulated by acquired bacterial oral flora such as S. pneumoniae, H. influenzae and S. aureus which traverse the homeostatic upper airway microbial boundaries and descend into the deep airways (Goldberg et al., Citation2008; O'Sullivan & Freedman, Citation2009). This immune response includes the mobilization of alveolar macrophages and neutrophils, as well as the secretion of airway surface fluid containing lysozyme, lactoferrin, secretory leukocyte protease inhibitor, complements, surfactant proteins, and a myriad of proinflammatory cytokines (Bals et al., Citation1999; Ricciardolo et al., Citation2004). This strong inflammatory response also contributes to airway fluid viscosity secondary to tissue destruction, as does the emergence of “hyper-mucoid” Pa. Nevertheless, with systemic immunologic functions intact, the CF host is fully capable of keeping bacterial opportunists contained within the airway space.
Inactivation of specific amino acid biosynthesis in CF Pa suggests bacterial-host airway symbiosis
The concept of bacterial symbiosis in CF airway infection is strongly supported by the long-standing observation that pseudomonal auxotrophic strains can be found in 33–86% CF patients (Barth & Pitt, Citation1996; Haussler et al., Citation1999; Hoboth et al., Citation2009; Hogardt et al., Citation2007; Oberhardt et al., Citation2010; Taylor et al., Citation1992; Thomas et al., Citation2000). This metabolic divergence associated with CF Pa demonstrates that lifestyle changes are not limited to bacteria-to-bacteria interactions, but also bacteria-to-host associations. Auxotrophic Pa strains show nutritional deficiencies with an unusually high frequency for methionine (in 40% of patients) and arginine () (Pitt et al., Citation2003; Qin et al., Citation2012). It is unlikely a mere coincidence that these two metabolic deficiencies are also common in beneficial insect symbionts (Moran et al., Citation2008). Moreover, CF Pa auxotrophy for several specific amino acids, such as methionine, valine, leucine, isoleucine, histidine and tryptophan, matches a number of “essential amino acids” required by the human host. The original opinion which still persists in many quarters, the “high amino-acid content of sputum from cystic fibrosis patients promotes growth of auxotrophic Pa”, is now open to question (Barth & Pitt, Citation1995a, Citationb, Citation1996; Hoboth et al., Citation2009; Palmer et al., Citation2005; Son et al., Citation2007; Thomas et al., Citation2000). Without host selection and/or antibiotic pressure, evolution of bacterial auxotrophy can never be reproduced in vitro in rich media. In truth, the outstanding pattern of CF Pa auxotrophy concurs with the plausible prediction of bacterial adaptation in chronic infection by a way of metabolic “humanization” or immunological evasion (Cigana et al., Citation2011; Hewlett, Citation1916).
Host counter-selection of bacterial virulence is indicated by methionine auxotrophy in CF Pa
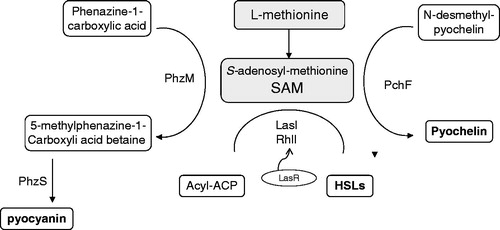
Elimination of methionine biosynthesis in CF Pa can not be randomly selected for by the nutrient abundance in the host airway, but instead clearly reflects a bacterial symbiotic response which promotes bacterial survival in the acquired host (Qin et al., Citation2012). Biosynthesis of at least three biologically active pseudomonal non-peptide compounds requires methionine as the precursor molecule (). These three metabolites, namely, the QS molecule HSL, pyocyanin, and pyochelin, are intimately involved in bacterial cell-to-cell communication, metabolic regulation, and cytotoxic events demonstrated in both human and animal hosts (Heurlier et al., Citation2006; Mavrodi et al., Citation2001; Patel & Walsh, Citation2001). HSL molecules have been considered to play a significant role in modulating a wide range of metabolic and competitive (rather than ‘virulent’) functions in pseudomonal species in their natural soil environment, and regulate up to 6–10% of the pseudomonal chromosomal genes (Heurlier et al., Citation2006). HSLs influence airway inflammation indirectly through “virulence” factor production which includes biosynthesis of pyocyanin (a reducing agent with antibacterial and cytotoxic properties) and pyochelin (one of the siderophores for iron chelation in Pa). Host counter-selection of QS molecules may therefore explain the significant accumulation of lasR mutations in CF Pa (Williams et al., Citation2007). As methionine and S-adenosyl-methionine (SAM) are directly involved in the biosynthesis of HSLs, pyocyanin and pyochelin (), it is conceivable that methionine auxotrophy may be a direct result of bacterial symbiotic adaptation to gain host tolerance.
Figure 5. l-Methionine as a precursor molecule for the biosynthesis of various important non-peptide compounds in P. aeruginosa. S-adenosyl-methionine (SAM): a common precursor molecule associated with the biosynthesis of homoserine lactone (HSL), pyocyanin, and pyochelin. ACP, acyl-carrier protein; LasI, N-(3-oxododecanoyl)-l-homoserine lactone (3OC12-HSL) synthase; RhlI, N-butyryl-l-homoserine lactone (C4-HSL) synthase. PhzM, a SAM dependent methyltransferase (Heurlier et al., Citation2006). PhzS, flavin dependent mono-oxygenase (Mavrodi et al., Citation2001). PchF, a methyltransferanse of a four enzyme assembly line for the non-ribosomal peptide siderophore pyochelin (Patel & Walsh, Citation2001).
The production of QS molecules and their induction of cytotoxic compounds appear to be necessary only during the early stages of bacterial exploitation of a new territory of nutrient abundance. In addition, pseudomonal QS molecules may also be necessary if other microbial competitors are present (Duan et al., Citation2003; Geng & Belas, Citation2010). In contrast, the same molecular “artillery” produced in synchronicity can be counterproductive to bacterial differentiation into a functional hierarchy with highly specialized syntrophic roles in a multicellular conglomerate. Thus, the production of QS and its induction of cytotoxic compounds are in conflict with long-term pseudomonal nutrient benefit in CF airway, and are therefore actively selected against by the host.
The loss of methionine (an essential amino acid) biosynthesis in the vast majority of vertebrate animal hosts including humans may not occur by chance when co-evolution of a host and its microbiota, such as Enterobacteriaceae in the gut, is taken into consideration (Payne & Loomis, Citation2006). In contrast to CF pseudomonal “humanization” in the process of chronic infection, species of Enterobacteriaceae in the lumen of the human gut are of gamma-proteobacterium origin. Enterobacteriaceae, in association with a distinctly polymicrobial and symbiotic ecology, have clearly preserved their prototrophic free-living lifestyle. As a part of the human gut microbiota which contributes to host health, Enterobacteriaceae are vertically transmitted from mother to child after birth (Kau et al., Citation2011; Ley et al., Citation2006, Citation2008). Species of Enterobacteriaceae are able to produce cognate QS molecules from methionine, which without doubt play a significant role in regulation of the complex luminal human gut microbiota. Moreover, the universally preserved protein translational start codon AUG accepts only a methionine derivative, N-formylmethionine (fMet), for the initiation of nascent peptide chains in all bacteria (Laursen et al., Citation2005). SAM is the direct donor of the methyl moiety which forms fMet-tRNA in bacteria. fMet is not used in eukaryotes for cytosolic protein synthesis. Therefore, the retention or loss of methionine biosynthesis associated with microbial cohabitants versus their human host is highly suggestive of microbe-host symbiotic interaction and coevolution.
CF Pa evasion of host defenses by inactivation of arginine biosynthesis
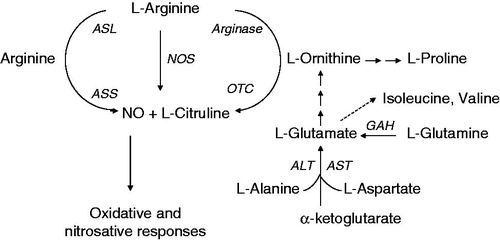
Arginine auxotrophy is directly beneficial to the adapting organism in allowing evasion of a harsh mechanism of host defense, as l-arginine is the common substrate for nitric oxide (NO) synthase and arginase () (Grasemann et al., Citation2006a; Luiking et al., Citation2004; Munder, Citation2009; Ricciardolo et al., Citation2004). NO produced by airway epithelia is also involved in bronchomotor tone, ciliary beating, and regulation of inflammatory and immune responses (Bals et al., Citation1999; Nappi, Citation2001; Ricciardolo et al., Citation2004). One of the highly studied but underappreciated features of airway immunodeficiency is the decreased concentration of exhaled nitric oxide (NO) in patients with CF (Grasemann et al., Citation1997). NO is believed to be an ancient innate immune mechanism in both invertebrates and vertebrates which has broad antiviral and antibacterial properties. Oxides of nitrogen inhibit the growth of, or show killing activity against a wide range of microbes in vitro (Fang, Citation1997). In mammals, production of NO from l-arginine de novo contributes to first line host defense against infection, which is commonly stimulated by proinflammatory cytokines, microbial lipopolysaccharides and lipoteichoic acid (Fang, Citation1997). High arginase and low l-arginine plasma levels are found in CF patients (Grasemann et al., Citation2006b). The depletion of NO in airways and reduced l-arginine in plasma is thus directly associated with the bacterial burden in CF airways. In the CF airway environment, increased arginase activity results in reduced l-arginine availability for NO production, thereby contributing to microbial latency and pulmonary obstruction (Fang, Citation1997; Grasemann et al., Citation2005a, Citationb). Grasemann et al. showed that antibiotic treatment and inhaled l-arginine are able to improve NO output and pulmonary function in patients with CF (Grasemann, Citation2010; Grasemann et al., Citation1997). Therefore, outsourcing of arginine biosynthesis during adaptation is not simply a passive response to the nutrient abundance in host airway.
Figure 6. l-Arginine is a common substrate for nitric oxide (NO) synthases (NOS) and arginase. The other key enzymes involved in arginine/ornithine and NO synthesis are ornithine carbamoyltransferase (OTC), argininosuccinate synthase (ASS), argininosuccinate lyase (ASL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and glutamine aminohydrolase (GAH).
Bacterial auxotrophy for l-arginine is also found in human skin colonizing bacteria such as Staphylococcus spp. Emmett and colleagues studied arginine auxotrophy in populations of staphylococci (e.g. S. aureus, S epidermidis, S. hominis, S. intermedius) isolated from the skin of human and other mammals (Emmett & Kloos, Citation1975, Citation1979). In addition, the growth requirements for valine and proline, whose biosynthesis can also be linked to arginine, were also found to be common in these staphylococci (). These authors suggested that arginine dependency of these cutaneous organisms “could serve as a homeostatic mechanism enabling the adaptation” and “the advanced development of auxotypes with multiple requirements would tend to “lock” a population into a niche, from which it could not easily be successfully established elsewhere”. This remarkable conclusion was made nearly 35 years ago, long before the importance of the human microbiota, its evolutionary heredity and the maintenance of its integrity, was fully appreciated.
The elimination of methionine and arginine biosynthesis in CF Pa is directly beneficial to the survival of the organism, but shows little benefit to the host. However, such parasitic adaptation may be the first step towards symbiosis. Auxotrophy for arginine and/or methionine is also consistent with the inactivation of biosynthesis of these molecules found in many insect symbionts (e.g. Buchnera aphidicola, Wigglesworthia glossinidia, Sodalis glossinidius, Candidatus Blochmannia, Candidatus Carsonell) that share ancestral gamma-proteobacterium lineage with Pa. Perhaps for these same reasons, these insect symbionts have long since disposed of biosynthesis of these amino acids and their derivatives (Moran et al., Citation2008). The antiquity of NO-based innate immunity has led this author to believe that arginine, an immediate NO generating molecule, has been preserved in all vertebrate hosts and some invertebrate hosts as one of the non-essential amino acids (Nappi, Citation2001). Conversely, arginine biosynthesis in symbiotic bacteria has frequently been selected against during the development of bacterial symbiosis in response to host oxidative or nitrosative attacks.
Auxotrophy associated with bacterial small-colony-variants in other chronic infections: a sign of bacterial endosymbiosis
Bacterial small-colony-variants (SCV) isolates of S. aureus, S. maltophilia, and B. cepacia have frequently been found in the airway secretions of CF patients (Anderson et al., Citation2007; Haussler et al., Citation1999, Citation2003a, Citationb; Proctor et al., Citation2006; Wolter et al., Citation2013). Moreover, many examples of bacterial SCV associated chronic infections other than CF suggest translocation of bacterial commensals to otherwise “sterile” anatomic environments, even in immunocompetent hosts who have received adequate antimicrobial treatment. Under antimicrobial pressure and displaced from their natural environment, free-living bacteria undergo metabolic transformation, notably exhibiting retarded growth rate and resulting in the SCV phenotype in vitro. Bacterial (frequently S. aureus and Escherichia coli) SCVs are able to cause persistent infections, such as chronic bone and joint infections and chronic urinary tract infections, respectively (Anderson et al., Citation2007; Borderon & Horodniceanu, Citation1978; Proctor et al., Citation2006; von Eiff et al., Citation2006).
Bacterial SCVs often show electron transport chain impairment due to auxotrophy for heme or menaquinone. Once more, the selective loss of component(s) essential for a functional electron transport chain in bacterial SCVs is not due to nutrient availability in host tissue. In truth, bacterial SCVs are selected to affirm an intracellular parasitic lifestyle, which is supported by host mitochondrial oxidative respiration. This is no different from bacterial endosymbiosis, with which the parasitic organism is able to evade host defensins, other competing microbial species, and the inhibitory activities of many antimicrobial agents that do not penetrate the host cell membrane. The characteristic inactivation of specific metabolic pathways in bacterial SCVs associated with chronic clinical infections other than CF is widespread but underappreciated (Proctor et al., Citation2006).
Pseudomonal syntrophy: a pathway to symbiotic genome reduction
Pseudomonal tissue tropism and its long-term containment within the CF airway bears striking similarities to examples of animal symbionts that are restricted to specialized host cells. Pseudogene accumulation and isogenic divergence are early signs of host-dependent genomic purification processes () (Giles et al., Citation2009; Qin et al., Citation2012). This model of early phase bacterium-host symbiosis is able to provide critical information regarding a pathway by which characteristic genome reduction (or minimal genome) can be achieved (McCutcheon & Moran, Citation2012). Without generalization, one practical path to gene loss and genome reduction devoid of lethality to the evolving symbiont may begin with bacterial syntrophy, which supports a gradual loss of function and loss of genetic coding materials, essential for a single-cell-replicative mode of living. CF pseudomonal isogenic mosaicism at the genetic coding level further supports the conception of a relentless genomic purification process via multicellularity (Qin et al., Citation2012).
The role of antimicrobials in preemptive therapy and in selection of pseudomonal clonal divergence
Chronic airway infections due to Pa and other Gram-negative environmental organisms are the major cause of the increased morbidity and mortality observed in patients with CF (Hoiby & Koch, Citation2000; Nixon et al., Citation2001). Antimicrobial treatment in modern medicine has demonstrated great success in the management of pulmonary infections and has prolonged the lives of patients with CF (Goldberg et al., Citation2008; O'Sullivan & Freedman, Citation2009; Starner & McCray, Citation2005). Antimicrobial strategies include preemptive efforts for delaying colonization of Pa, as well as the control of the bacterial burden post colonization (Doring, Citation2010; Doring et al., Citation2000; Gibson et al., Citation2003a, Citationb; Rosenfeld et al., Citation2003; Starner & McCray, Citation2005). The modality of drug administration has included both parenteral and inhaled routes in order to achieve high drug concentrations without adverse toxicity (Doring et al., Citation2000; Gibson et al., Citation2003a, Citationb). The lifetime treatment strategies used to combat focal airway infection have had a profound impact on types and mechanisms of resistance identified in CF “pathogens” (Piddock, Citation2006; Poole, Citation2001; Qin et al., Citation2012). Pseudomonal isogenic divergence both in its nutritional requirements and in its antimicrobial susceptibility patterns is therefore additional evidence of bacterial syntrophy through which both symbiotic alignment with the host and drug resistance can be achieved with energetic efficiency () (Lane & Martin, Citation2010; Qin et al., Citation2012).
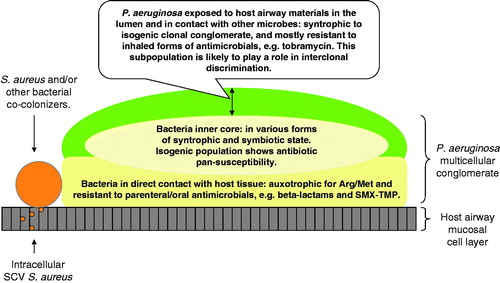
Figure 7. A diagram of CF airway-adapted P. aeruginosa: a multicellular syntrophy composed of host-specialized and functionally-divergent isogenic strains within a colonial architecture (Qin et al., Citation2012).
Lack of vertical transmission limits the extent of CF pseudomonal symbiosis
There has been a complete absence of any report on highly adapted CF Pa capable of spread among CF hosts or dissemination into immunocompromised hosts other than CF (Hogardt & Heesemann, Citation2010; Hogardt et al., Citation2007; Oliver & Mena, Citation2010). Oliver and colleagues concluded that “transmission of mutator strains between CF patients has never been demonstrated” (Oliver & Mena, Citation2010). In addition, patients affected by CF constitute a very small fraction of the human population (1/2500 in Caucasians, American Lung Association, State of Lung Disease in diverse communities 2010, http://www.lung.org/finding-cures/our-research/solddc-index.html). Confined in space and time, pseudomonal symbiosis is destined to abort prematurely with the demise of the host. Thus, the kind of host benefits which may be seen in long-term symbiotic relationships can never be achieved.
Host defense, bacterial co-infection, and antimicrobials participate in selection of pseudomonal syntrophy
Selective forces driving CF Pa into symbiosis are not without adverse clinical implications. It is evident that adapted Pa isogenic strains from chronic CF airway secretions are no longer “fully armed” free-living bacteria “suitable” for the nutrient competitive environment in soil. This CF airway-adapted pseudomonal conglomerate (which sometimes may be found together with other bacterial species) forms a bacterial “consortium” driven by colonial success and multipartite symbiosis under tremendous host and antimicrobial selective pressure (). Courses of antimicrobial treatment that frequently may be subinhibitory against these tightly packed bacteria may thus serve as a double-edged sword. One edge of this antimicrobial sword pressures the microbes into an adaptive multicellular resistant syntrophy. Conversely, antimicrobial agents may at the same time act as signaling molecules, characteristically operating in the polymicrobial world. These active microbial sensing molecules only serve to transmit erroneous messages for the maintenance of Pa’s armamentaria, necessary for nutrient competition with other microbes (Duan et al., Citation2003; Montanari et al., Citation2007; Ratcliff & Denison, Citation2011; Yim et al., Citation2007). With the overall attenuation of virulence en route to symbiosis, CF Pa survival within the highly organized bacterial conglomerate can still be maintained by hyper-resistant and hyper-toxic subpopulations, as they are captive to antimicrobial pressure from the medical treatment, as well as from the inhibitory signaling molecules produced by other microbial cohabitants in the interest of nutrient competition (). A selective evolutionary bottleneck is therefore proposed, wherein host defense, antimicrobial pressure, and the coexistence of other competing bacteria join forces to promote Pa adaptation ().
Prospects for treatment
Evolution of invertebrate and vertebrate species rarely occurs in a sterile space in nature. Microbial association with complex animal species has not only shaped the evolution of species, but has also played a pivotal role in both the health and sickness of host species (Kau et al., Citation2011; Racey et al., Citation2010). The evolutionary outcome of retention and loss of specific amino acid biosynthesis in animal hosts cannot be explained solely by the relative abundance of dietary nutrients. Interactions with microbes almost certainly play a role in the evolution of our species pertaining to specific nutrient requirements, for example, essential amino acids.
Despite the unattainabilty of beneficial symbiosis, specialized CF Pa syntrophy shows signs of host protection from repeat colonization by wild-type Pa strains ubiquitous in nature (Duan et al., Citation2003; Jacques et al., Citation1998). As N.A. Moran stated in her summary of “symbiosis”, “any microbe that forms chronic infections in an individual host or in a host lineage may evolve to conserve or even to benefit its host, as this will help to maintain its immediate ecological resources” (Moran, Citation2006). However, the host airway damage in CF patients elicited by Pa during the course of symbiotic adaptation is irreversible and certainly can not be repaired by the divergently attenuated Pa. Consequently, the resulting Pa conglomerate is composed of isogenic strains with varying degrees of symbiotic adaptation. Although some are seemingly less virulent, these Pa strains are still far removed from a final state of mutuality with the host (Cigana et al., Citation2011; Hogardt et al., Citation2007; Hoiby et al., Citation2010; Haussler et al., Citation2003a). Nonetheless, CF pseudomonal allopatric speciation towards an airway symbiont deserves attention. The concepts of medical intervention in prevention of infectious disease have lately been expanded to include not only vaccination for host immunological preparation, but also organic reconstitution of healthy human microbiota using fecal transplants (Brandt & Aroniadis, Citation2013; Damman et al., Citation2012; Lemon et al., Citation2012). In well-documented environmental studies, the diversity and population ecology of antagonistic Streptomyces in soil have been shown to co-evolve which has implications for plant disease suppression (Kinkel et al., Citation2011, Citation2012). Has nature thus provided us with possible novel therapeutic strategies for CF?
Progressive attenuation of pseudomonal virulence in the CF airway is observed in the course of the natural infection in all patients. However, injury to the host airways is an invariable part of the disease, due to the gradual nature of the attenuation process. As attenuated strains of Pa evolve, they become niche-specialized antagonists to wild-type Pa. These interclonal antagonistic properties might be exploited therapeutically. In order to mitigate or bypass the highly pathogenic processes associated with the early course of natural infection, perhaps an attenuated “probiotic” symbiont could be introduced prior to initial infection, thereby preventing colonization by the virulent wild-type organism. Alternatively, with current advances in technology and the promise of self-replicating synthetic bacterial cell construction, bacterial candidates modeled after beneficial endosymbionts might be used as CFTR gene delivery vectors to treat the underlying molecular defect (Gibson et al., Citation2010; Tangney & Gahan, Citation2010).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Glossary
Syntrophy is a nutritional term. It involves cross-feeding or transfer of compounds between species, such that neither species grows well in the absence of the other, and both species benefit from the presence of the other. This term has been expanded to include interdependence between organisms to facilitate defenses against predators or inhibitory substances, such as antibiotics.
Prototrophy is used to characterize an organism that is able to synthesize all organic compounds for growth from simple sugars or inorganic compounds.
Auxotrophy is used to characterize an organism that is unable to synthesize a particular organic compound(s) required for growth from simple sugars or inorganic compounds.
Sympatric is used to characterize biological populations of species that exist in the same geographic or physical area and regularly encounter each other.
Allopatric is used to characterize biological populations of the same species that are separated by a geographic or physical barrier.
Acknowledgements
I would like to thank Dr. Michael A. McNutt (Peking University School of Medicine, Beijing, PRC) for our reciprocal interests in learning English and Chinese and for our shared passion in medicine and evolutionary biology. Dr. McNutt deserves special thanks for his sustained support of multiple iterations of manuscript edits in professional English style. I would like to thank Dr. Raj P. Kapur (Seattle Children s Hospital and University of Washington School of Medicine, Seattle, WA) for his critical review of my manuscript and intellectual interactions with me along the process as I was formulating the scientific concept of this manuscript. I would like to thank Dr. Joe Rutledge (Seattle Children s Hospital and University of Washington School of Medicine, Seattle, WA) for his encouragement and support of my scientific pursuits. I would also like to take this opportunity to thank Dr. Harry W. Taber (Wadsworth Center, New York State of Public Health, Albany, NY) for his graduate mentorship early in my career with the emphasis on the spirit of a “free thinker”.
References
- Aaron SD, Ramotar K, Ferris W, et al. (2004). Adult cystic fibrosis exacerbations and new strains of Pseudomonas aeruginosa. Am J Respir Crit Care Med 169:811–5
- Aaron SD, Vandemheen KL, Ramotar K, et al. (2010). Infection with transmissible strains of Pseudomonas aeruginosa and clinical outcomes in adults with cystic fibrosis. JAMA 304:2145–53
- Abraham EH, Vos P, Kahn J, et al. (1996). Cystic fibrosis hetero- and homozygosity is associated with inhibition of breast cancer growth. Nat Med 2:593–6
- Agarwal G, Kapil A, Kabra SK, et al. (2005). Characterization of Pseudomonas aeruginosa isolated from chronically infected children with cystic fibrosis in India. BMC Microbiol 5:43
- Anderson SW, Stapp JR, Burns JL, Qin X. (2007). Characterization of small-colony-variant Stenotrophomonas maltophilia isolated from the sputum specimens of five patients with cystic fibrosis. J Clin Microbiol 45:529–35
- Atkinson S, Williams P. (2009). Quorum sensing and social networking in the microbial world. J R Soc Interface 6:959–78
- Bals R, Weiner DJ, Wilson JM. (1999). The innate immune system in cystic fibrosis lung disease. J Clin Invest 103:303–7
- Barben J, Hafen G, Schmid J. (2005). Pseudomonas aeruginosa in public swimming pools and bathroom water of patients with cystic fibrosis. J Cyst Fibros 4:227–31
- Barth AL, Pitt TL. (1995a). Auxotrophic variants of Pseudomonas aeruginosa are selected from prototrophic wild-type strains in respiratory infections in patients with cystic fibrosis. J Clin Microbiol 33:37–40
- Barth AL, Pitt TL. (1995b). Auxotrophy of Burkholderia (Pseudomonas) cepacia from cystic fibrosis patients. J Clin Microbiol 33:2192–4
- Barth AL, Pitt TL. (1996). The high amino-acid content of sputum from cystic fibrosis patients promotes growth of auxotrophic Pseudomonas aeruginosa. J Med Microbiol 45:110–19
- Barth AL, Woodford N, Pitt TL. (1998). Complementation of methionine auxotrophs of Pseudomonas aeruginosa from cystic fibrosis. Curr Microbiol 36:190–5
- Belda E, Moya A, Bentley S, Silva FJ. (2010). Mobile genetic element proliferation and gene inactivation impact over the genome structure and metabolic capabilities of Sodalis glossinidius, the secondary endosymbiont of tsetse flies. BMC Genomics 11:449
- Blaser MJ, Kirschner D. (2007). The equilibria that allow bacterial persistence in human hosts. Nature 449:843–9
- Borderon E, Horodniceanu T. (1978). Metabolically deficient dwarf-colony mutants of Escherichia coli: deficiency and resistance to antibiotics of strains isolated from urine culture. J Clin Microbiol 8:629–34
- Boucher RC. (2004). New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J 23:146–58
- Bragonzi A, Paroni M, Nonis A, et al. (2009). Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am J Respir Crit Care Med 180:138–45
- Brandt LJ, Aroniadis OC. (2013). An overview of fecal microbiota transplantation: techniques, indications, and outcomes. Gastrointest Endos 78:240–9
- Burns JL, Emerson J, Stapp JR, et al. (1998). Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis 27:158–63
- Burns JL, Gibson RL, McNamara S, et al. (2001). Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis 183:444–52
- Cigana C, Lore NI, Bernardini ML, Bragonzi A. (2011). Dampening Host Sensing and Avoiding Recognition in Pseudomonas aeruginosa Pneumonia. J Biomed Biotechnol 2011:852513(1–10)
- Ciofu O, Riis B, Pressler T, et al. (2005). Occurrence of hypermutable Pseudomonas aeruginosa in cystic fibrosis patients is associated with the oxidative stress caused by chronic lung inflammation. Antimicrob Agents Chemother 49:2276–82
- Ciofu O, Mandsberg LF, Bjarnsholt T, et al. (2010). Genetic adaptation of Pseudomonas aeruginosa during chronic lung infection of patients with cystic fibrosis: strong and weak mutators with heterogeneous genetic backgrounds emerge in mucA and/or lasR mutants. Microbiology 156:1108–19
- Cox MJ, Allgaier M, Taylor B, et al. (2010). Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS One 5:e11044
- Dacheux D, Toussaint B, Richard M, et al. (2000). Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent, but ExoU-independent, oncosis of macrophages and polymorphonuclear neutrophils. Infect Immun 68:2916–24
- Dacheux D, Attree I, Toussaint B. (2001). Expression of ExsA in trans confers type III secretion system-dependent cytotoxicity on noncytotoxic Pseudomonas aeruginosa cystic fibrosis isolates. Infect Immun 69:538–42
- Dakin CJ, Numa AH, Wang H, et al. (2002). Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 165:904–10
- Dale C, Moran NA. (2006). Molecular interactions between bacterial symbionts and their hosts. Cell 126:453–65
- Damman CJ, Miller SI, Surawicz CM, Zisman TL. (2012). The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol 107:1452–9
- Diggle SP, Crusz SA, Camara M. (2007). Quorum sensing. Curr Biol 17:R907–10
- Doring G, Conway SP, Heijerman HG, et al. (2000). Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur Respir J 16:749–67
- Doring G. (2010). Prevention of Pseudomonas aeruginosa infection in cystic fibrosis patients. Int J Med Microbiol 300:573–7
- Duan K, Dammel C, Stein J, et al. (2003). Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol Microbiol 50:1477–91
- Emmett M, Kloos WE. (1975). Amino acid requirements of staphylococci isolated from human skin. Can J Microbiol 21:729–33
- Emmett M, Kloos WE. (1979). The nature of arginine auxotrophy in cutaneous populations of staphylococci. J Gen Microbiol 110:305–14
- Fang FC. (1997). Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest 99:2818–25
- Feary TW, Williams B, Calhoun DH, Walker TA. (1969). An analysis of arginine requiring mutants in Pseudomonas aeruginosa. Genetics 62:673–86
- Feliziani S, Lujan AM, Moyano AJ, et al. (2010). Mucoidy, quorum sensing, mismatch repair and antibiotic resistance in Pseudomonas aeruginosa from cystic fibrosis chronic airways infections. PLoS One 5:e12669(1–12)
- FitzSimmons SC. (1993). The changing epidemiology of cystic fibrosis. J Pediatr 122:1–9
- Flume PA, Van Devanter DR. (2012). State of progress in treating cystic fibrosis respiratory disease. BMC Med 10:88
- Foweraker J. (2009). Recent advances in the microbiology of respiratory tract infection in cystic fibrosis. Br Med Bull 89:93–110
- Furukawa S, Kuchma SL, O'Toole GA. (2006). Keeping their options open: acute versus persistent infections. J Bacteriol 188:1211–7
- Fux CA, Shirtliff M, Stoodley P, Costerton JW. (2005). Can laboratory reference strains mirror “real-world” pathogenesis? Trends Microbiol 13:58–63
- Geng H, Belas R. (2010). Molecular mechanisms underlying roseobacter-phytoplankton symbioses. Current Opin Biotechnol 21:332–8
- Georgiades K, Raoult D. (2010). Defining pathogenic bacterial species in the genomic era. Frontiers Microbiol 1:151(1–13)
- Gibson RL, Burns JL, Ramsey BW. (2003a). Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 168:918–51
- Gibson RL, Emerson J, McNamara S, et al. (2003b). Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am J Respir Crit Care Med 167:841–9
- Gibson DG, Glass JI, Lartigue C, et al. (2010). Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329:52–6
- Giles TN, Fisher DJ, Graham DE. (2009). Independent inactivation of arginine decarboxylase genes by nonsense and missense mutations led to pseudogene formation in Chlamydia trachomatis serovar L2 and D strains. BMC Evol Biol 9:166
- Goldberg JB, Hancock RE, Parales RE, et al. (2008). Pseudomonas 2007. J Bacteriol 190:2649–62
- Grasemann H, Michler E, Wallot M, Ratjen F. (1997). Decreased concentration of exhaled nitric oxide (NO) in patients with cystic fibrosis. Pediatr Pulmonol 24:173–7
- Grasemann H, Grasemann C, Kurtz F, et al. (2005a). Oral l-arginine supplementation in cystic fibrosis patients: a placebo-controlled study. Eur Respir J 25:62–8
- Grasemann H, Schwiertz R, Matthiesen S, et al. (2005b). Increased arginase activity in cystic fibrosis airways. Am J Respir Crit Care Med 172:1523–8
- Grasemann H, Kurtz F, Ratjen F. (2006a). Inhaled l-arginine improves exhaled nitric oxide and pulmonary function in patients with cystic fibrosis. Am J Respir Crit Care Med 174:208–12
- Grasemann H, Schwiertz R, Grasemann C, et al. (2006b). Decreased systemic bioavailability of l-arginine in patients with cystic fibrosis. Respir Res 7:87(1–7)
- Grasemann H. (2010). l-Arginine deficiency in cystic fibrosis lung disease. Open Nitric Oxide J 2:37–40
- Green SK, Schroth MN, Cho JJ, et al. (1974). Agricultural plants and soil as a reservoir for Pseudomonas aeruginosa. Appl Microbiol 28:987–91
- Haussler S, Tummler B, Weissbrodt H, et al. (1999). Small-colony variants of Pseudomonas aeruginosa in cystic fibrosis. Clin Infect Dis 29:621–5
- Haussler S, Lehmann C, Breselge C, et al. (2003a). Fatal outcome of lung transplantation in cystic fibrosis patients due to small-colony variants of the Burkholderia cepacia complex. Eur J Clin Microbiol Infect Dis 22:249–53
- Haussler S, Ziegler I, Lottel A, et al. (2003b). Highly adherent small-colony variants of Pseudomonas aeruginosa in cystic fibrosis lung infection. J Med Microbiol 52:295–301
- Head NE, Yu H. (2004). Cross-sectional analysis of clinical and environmental isolates of Pseudomonas aeruginosa: biofilm formation, virulence, and genome diversity. Infect Immun 72:133–44
- Heurlier K, Denervaud V, Haenni M, et al. (2005). Quorum-sensing-negative (lasR) mutants of Pseudomonas aeruginosa avoid cell lysis and death. J Bacteriol 187:4875–83
- Heurlier K, Denervaud V, Haas D. (2006). Impact of quorum sensing on fitness of Pseudomonas aeruginosa. Int J Med Microbiol 296:93–102
- Hewlett AW. (1916). Monographic medicine, Vol. 1. New York and London: Appleton and Company
- Hoboth C, Hoffmann R, Eichner A, et al. (2009). Dynamics of adaptive microevolution of hypermutable Pseudomonas aeruginosa during chronic pulmonary infection in patients with cystic fibrosis. J Infect Dis 200:118–30
- Hoffman LR, Kulasekara HD, Emerson J, et al. (2009). Pseudomonas aeruginosa lasR mutants are associated with cystic fibrosis lung disease progression. J Cyst Fibros 8:66–70
- Hoffmann N, Rasmussen TB, Jensen PO, et al. (2005). Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infect Immun 73:2504–14
- Hogardt M, Heesemann J. (2010). Adaptation of Pseudomonas aeruginosa during persistence in the cystic fibrosis lung. Int J Med Microbiol 300:557–62
- Hogardt M, Hoboth C, Schmoldt S, et al. (2007). Stage-specific adaptation of hypermutable Pseudomonas aeruginosa isolates during chronic pulmonary infection in patients with cystic fibrosis. J Infect Dis 195:70–80
- Hoiby N, Koch C. (2000). Maintenance treatment of chronic pseudomonas aeruginosa infection in cystic fibrosis. Thorax 55:349–50
- Hoiby N, Ciofu O, Bjarnsholt T. (2010). Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol 5:1663–74
- Hoiby N, Ciofu O, Johansen HK, et al. (2011). The clinical impact of bacterial biofilms. Int J Oral Sci 3:55–65
- Hoiby N, Krogh Johansen H, Moser C, et al. (2001). Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect 3:23–35
- Huse HK, Kwon T, Zlosnik JE, et al. (2010). Parallel evolution in Pseudomonas aeruginosa over 39 000 generations in vivo. MBio 1:e00199–10
- Irvin RT, Govan JW, Fyfe JA, Costerton JW. (1981). Heterogeneity of antibiotic resistance in mucoid isolates of Pseudomonas aeruginosa obtained from cystic fibrosis patients: role of outer membrane proteins. Antimicrob Agents Chemother 19:1056–63
- Jacques I, Derelle J, Weber M, Vidailhet M. (1998). Pulmonary evolution of cystic fibrosis patients colonized by Pseudomonas aeruginosa and/or Burkholderia cepacia. Eur J Pediatr 157:427–31
- Jelsbak L, Johansen HK, Frost AL, et al. (2007). Molecular epidemiology and dynamics of Pseudomonas aeruginosa populations in lungs of cystic fibrosis patients. Infect Immun 75:2214–24
- Kau AL, Ahern PP, Griffin NW, et al. (2011). Human nutrition, the gut microbiome and the immune system. Nature 474:327–36
- Kinkel LL, Bakker MG, Schlatter DC. (2011). A coevolutionary framework for managing disease-suppressive soils. Ann Rev Phytopathol 49:47–67
- Kinkel LL, Schlatter DC, Bakker MG, Arenz BE. (2012). Streptomyces competition and co-evolution in relation to plant disease suppression. Res Microbiol 163:490–9
- Kohler T, Buckling A, van Delden C. (2009). Cooperation and virulence of clinical Pseudomonas aeruginosa populations. Proc Natl Acad Sci USA 106:6339–44
- Lane N, Martin W. (2010). The energetics of genome complexity. Nature 467:929–34
- Laursen BS, Sorensen HP, Mortensen KK, Sperling-Petersen HU. (2005). Initiation of protein synthesis in bacteria. Microbiol Mol Biol Rev 69:101–23
- Lee DG, Urbach JM, Wu G, et al. (2006). Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol 7:R90
- Lemon KP, Armitage GC, Relman DA, Fischbach MA. (2012). Microbiota-targeted therapies: an ecological perspective. Science translational medicine 4:137rv135
- Levin BR, Antia R. (2001). Why we don't get sick: the within-host population dynamics of bacterial infections. Science 292:1112–5
- Ley RE, Peterson DA, Gordon JI. (2006). Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124:837–48
- Ley RE, Hamady M, Lozupone C, et al. (2008). Evolution of mammals and their gut microbes. Science 320:1647–51
- Li Z, Kosorok MR, Farrell PM, et al. (2005). Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 293:581–8
- Livermore DM. (2002). Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis 34:634–40
- Lore NI, Cigana C, De Fino I, et al. (2012). Cystic fibrosis-niche adaptation of Pseudomonas aeruginosa reduces virulence in multiple infection hosts. PLoS One 7:e35648
- Luiking YC, Poeze M, Dejong CH, et al. (2004). Sepsis: an arginine deficiency state? Crit Care Med 32:2135–45
- Luzar MA, Montie TC. (1985). Avirulence and altered physiological properties of cystic fibrosis strains of Pseudomonas aeruginosa. Infect Immun 50:572–6
- Luzar MA, Thomassen MJ, Montie TC. (1985). Flagella and motility alterations in Pseudomonas aeruginosa strains from patients with cystic fibrosis: relationship to patient clinical condition. Infect Immun 50:577–82
- Lyczak JB, Cannon CL, Pier GB. (2002). Lung infections associated with cystic fibrosis. Clin Microbiol Rev 15:194–222
- Macia MD, Blanquer D, Togores B, et al. (2005). Hypermutation is a key factor in development of multiple-antimicrobial resistance in Pseudomonas aeruginosa strains causing chronic lung infections. Antimicrob Agents Chemother 49:3382–6
- Mahadeva R, Webb K, Westerbeek RC, et al. (1998). Clinical outcome in relation to care in centres specialising in cystic fibrosis: cross sectional study. BMJ 316:1771–5
- Manos J, Arthur J, Rose B, et al. (2008). Transcriptome analyses and biofilm-forming characteristics of a clonal Pseudomonas aeruginosa from the cystic fibrosis lung. J Med Microbiol 57:1454–65
- Mathee K, Narasimhan G, Valdes C, et al. (2008). Dynamics of Pseudomonas aeruginosa genome evolution. Proc Natl Acad Sci USA 105:3100–5
- Mavrodi DV, Bonsall RF, Delaney SM, et al. (2001). Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol 183:6454–65
- May TB, Shinabarger D, Maharaj R, et al. (1991). Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin Microbiol Rev 4:191–206
- McCutcheon JP, Moran NA. (2012). Extreme genome reduction in symbiotic bacteria. Nat Rev Microbiol 10:13–26
- Mena A, Macia MD, Borrell N, et al. (2007). Inactivation of the mismatch repair system in Pseudomonas aeruginosa attenuates virulence but favors persistence of oropharyngeal colonization in cystic fibrosis mice. J Bacteriol 189:3665–8
- Mena A, Smith EE, Burns JL, et al. (2008). Genetic adaptation of Pseudomonas aeruginosa to the airways of cystic fibrosis patients is catalyzed by hypermutation. J Bacteriol 190:7910–7
- Meyer KF. (1936). Latent infections. J Bacteriol 31:109–35
- Montanari S, Oliver A, Salerno P, et al. (2007). Biological cost of hypermutation in Pseudomonas aeruginosa strains from patients with cystic fibrosis. Microbiology 153:1445–54
- Moran NA, McCutcheon JP, Nakabachi A. (2008). Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42:165–90
- Moran NA. (2006). Symbiosis. Curr Biol 16:R866–871
- Moran NA. (2007). Symbiosis as an adaptive process and source of phenotypic complexity. Proc Natl Acad Sci USA 104:8627–33
- Mowat E, Paterson S, Fothergill JL, et al. (2011). Pseudomonas aeruginosa population diversity and turnover in cystic fibrosis chronic infections. Am J Respir Crit Care Med 183:1674–9
- Munder M. (2009). Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol 158:638–51
- Munson MA, Baumann P, Clark MA, et al. (1991). Evidence for the establishment of aphid-eubacterium endosymbiosis in an ancestor of four aphid families. J Bacteriol 173:6321–4
- Murray TS, Egan M, Kazmierczak BI. (2007). Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr Opin Pediatr 19:83–8
- Nappi AJaEV. (2001). Phylogenetic perspectives on the vertebrate immune system. Vol. 484. Springer. 329–348
- Nixon GM, Armstrong DS, Carzino R, et al. (2001). Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr 138:699–704
- Noah TL, Black HR, Cheng PW, et al. (1997). Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J Infect Dis 175:638–47
- Oberhardt MA, Goldberg JB, Hogardt M, Papin JA. (2010). Metabolic network analysis of Pseudomonas aeruginosa during chronic cystic fibrosis lung infection. J Bacteriol 192:5534–48
- Oliver A. (2010). Mutators in cystic fibrosis chronic lung infection: prevalence, mechanisms, and consequences for antimicrobial therapy. Int J Med Microbiol 300:563–72
- Oliver A, Baquero F, Blazquez J. (2002). The mismatch repair system (mutS, mutL and uvrD genes) in Pseudomonas aeruginosa: molecular characterization of naturally occurring mutants. Mol Microbiol 43:1641–50
- Oliver A, Mena A. (2010). Bacterial hypermutation in cystic fibrosis, not only for antibiotic resistance. Clin Microbiol Infect 16:798–808
- O'Sullivan BP, Freedman SD. (2009). Cystic fibrosis. Lancet 373:1891–904
- Palmer KL, Mashburn LM, Singh PK, Whiteley M. (2005). Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J Bacteriol 187:5267–77
- Palmer KL, Aye LM, Whiteley M. (2007). Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol 189:8079–87
- Palmer JN. (2005). Bacterial biofilms: do they play a role in chronic sinusitis? Otolaryngol Clin North Am 38:1193–201, viii
- Patel HM, Walsh CT. (2001). In vitro reconstitution of the Pseudomonas aeruginosa nonribosomal peptide synthesis of pyochelin: characterization of backbone tailoring thiazoline reductase and N-methyltransferase activities. Biochemistry 40:9023–31
- Payne SH, Loomis WF. (2006). Retention and loss of amino acid biosynthetic pathways based on analysis of whole-genome sequences. Eukaryot Cell 5:272–6
- Piddock LJ. (2006). Multidrug-resistance efflux pumps - not just for resistance. Nat Rev Microbiol 4:629–36
- Pirnay JP, Bilocq F, Pot B, et al. (2009). Pseudomonas aeruginosa population structure revisited. PLoS One 4:e7740
- Pitt TL, Sparrow M, Warner M, Stefanidou M. (2003). Survey of resistance of Pseudomonas aeruginosa from UK patients with cystic fibrosis to six commonly prescribed antimicrobial agents. Thorax 58:794–6
- Pollack M. (2000). Pseudomonas aeruginosa. In: Mandell GL BJ, Dolin R, eds. Principles and practice of infectious diseases, 5th ed. New York, NY: Churchill Livingstone, 2310–27
- Poole K. (2001). Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J Mol Microbiol Biotechnol 3:255–64
- Poulsen M, Erhardt DP, Molinaro DJ, et al. (2007). Antagonistic bacterial interactions help shape host-symbiont dynamics within the fungus-growing ant-microbe mutualism. PLoS One 2:e960
- Proctor RA, von Eiff C, Kahl BC, et al. (2006). Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305
- Qin X, Zerr DM, McNutt MA, et al. (2012). Pseudomonas aeruginosa syntrophy in chronically colonized airways of cystic fibrosis patients. Antimicrob Agents Chemother 56:5971–81
- Racey D, Inglis RF, Harrison F, et al. (2010). The effect of elevated mutation rates on the evolution of cooperation and virulence of Pseudomonas aeruginosa. Evolution 64:515–21
- Ratcliff WC, Denison RF. (2011). Microbiology. Alternative actions for antibiotics. Science 332:547–8
- Rau MH, Hansen SK, Johansen HK, et al. (2010). Early adaptive developments of Pseudomonas aeruginosa after the transition from life in the environment to persistent colonization in the airways of human cystic fibrosis hosts. Environ Microbiol 12:1643–58
- Regnath T, Kreutzberger M, Illing S, et al. (2004). Prevalence of Pseudomonas aeruginosa in households of patients with cystic fibrosis. Int J Hyg Environ Health 207:585–8
- Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. (2004). Nitric oxide in health and disease of the respiratory system. Physiol Rev 84:731–65
- Rosenfeld M, Ramsey BW, Gibson RL. (2003). Pseudomonas acquisition in young patients with cystic fibrosis: pathophysiology, diagnosis, and management. Curr Opin Pulm Med 9:492–7
- Roy PH, Tetu SG, Larouche A, et al. (2010). Complete genome sequence of the multiresistant taxonomic outlier Pseudomonas aeruginosa PA7. PLoS One 5:e8842
- Roy-Burman A, Savel RH, Racine S, et al. (2001). Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis 183:1767–74
- Ruby EG. (2008). Symbiotic conversations are revealed under genetic interrogation. Nat Rev Microbiol 6:752–62
- Sheldon CD, Hodson ME, Carpenter LM, Swerdlow AJ. (1993). A cohort study of cystic fibrosis and malignancy. Br J Cancer 68:1025–8
- Smith EE, Buckley DG, Wu Z, et al. (2006). Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA 103:8487–92
- Son MS, Matthews WJ Jr, Kang Y, et al. (2007). In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect Immun 75:5313–24
- Starkey M, Hickman JH, Ma L, et al. (2009). Pseudomonas aeruginosa rugose small-colony variants have adaptations that likely promote persistence in the cystic fibrosis lung. J Bacteriol 191:3492–503
- Starner TD, McCray PB Jr. (2005). Pathogenesis of early lung disease in cystic fibrosis: a window of opportunity to eradicate bacteria. Ann Intern Med 143:816–22
- Strausbaugh SD, Davis PB. (2007). Cystic fibrosis: a review of epidemiology and pathobiology. Clin Chest Med 28:279–88
- Tangney M, Gahan CG. (2010). Editorial [Hot Topic: Bacterial Vectors for Gene & Cell Therapy]. Curr Gene Ther 10:1–2
- Taylor RF, Hodson ME, Pitt TL. (1992). Auxotrophy of Pseudomonas aeruginosa in cystic fibrosis. FEMS Microbiol Lett 71:243–6
- Thomas SR, Ray A, Hodson ME, Pitt TL. (2000). Increased sputum amino acid concentrations and auxotrophy of Pseudomonas aeruginosa in severe cystic fibrosis lung disease. Thorax 55:795–7
- Valderrey AD, Pozuelo MJ, Jimenez PA, et al. (2010). Chronic colonization by Pseudomonas aeruginosa of patients with obstructive lung diseases: cystic fibrosis, bronchiectasis, and chronic obstructive pulmonary disease. Diagn Microbiol Infect Dis 68:20–7
- Vendeville A, Winzer K, Heurlier K, et al. (2005). Making ‘sense' of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev Microbiol 3:383–96
- von Eiff C, Peters G, Becker K. (2006). The small colony variant (SCV) concept – the role of staphylococcal SCVs in persistent infections. Injury 37:S26–33
- Waters CM, Bassler BL. (2005). Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–46
- Whiteley M, Bangera MG, Bumgarner RE, et al. (2001). Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860–4
- Williams P, Camara M. (2009). Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol 12:182–91
- Williams BJ, Dehnbostel J, Blackwell TS. (2010). Pseudomonas aeruginosa: host defence in lung diseases. Respirology 15:1037–56
- Williams P, Winzer K, Chan WC, Camara M. (2007). Look who's talking: communication and quorum sensing in the bacterial world. Philos Trans R Soc Lond. Ser B, Biol Sci 362:1119–34
- Willcox MD, Zhu H, Conibear TC, et al. (2008). Role of quorum sensing by Pseudomonas aeruginosa in microbial keratitis and cystic fibrosis. Microbiology 154:2184–94
- Winstanley C, Fothergill JL. (2009). The role of quorum sensing in chronic cystic fibrosis Pseudomonas aeruginosa infections. FEMS Microbiol Lett 290:1–9
- Wolter DJ, Emerson JC, McNamara S, et al. (2013). Staphylococcus aureus small-colony variants are independently associated with worse lung disease in children with cystic fibrosis. Clin Infect Dis 57:384–91
- Wu H, Song Z, Givskov M, et al. (2001). Pseudomonas aeruginosa mutations in lasI and rhlI quorum sensing systems result in milder chronic lung infection. Microbiology 147:1105–13
- Xavier JB, Kim W, Foster KR. (2011). A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa. Mol Microbiol 79:166–79
- Yang L, Haagensen JA, Jelsbak L, et al. (2008). In situ growth rates and biofilm development of Pseudomonas aeruginosa populations in chronic lung infections. J Bacteriol 190:2767–76
- Yim G, Wang HH, Davies JFRS. (2007). Antibiotics as signalling molecules. Philos Trans R Soc Lond B Biol Sci 362:1195–200
- Zierdt CH, Schmidt PJ. (1964). Dissociation in Pseudomonas aeruginosa. J Bacteriol 87:1003–10
