Abstract
There is controversy on whether occupational exposure to beryllium causes lung cancer. We conducted a systematic review of epidemiologic studies on cancer among workers exposed to beryllium, including a study of seven U.S. production plants which has been recently updated, a study of patients with beryllium disease (largely overlapping with the former study) and several smaller studies. A small excess mortality from lung cancer was detected in the large cohort, which was partially explained by confounding by tobacco smoking and urban residence. Other potential confounders have not been addressed. The excess mortality was mainly among workers employed (often for a short duration) in the early phase of the manufacturing industry. There was no relation with duration of employment or cumulative exposure, whereas average and maximum exposure were associated with lung cancer risk. The use of lagged exposure variables resulted in associations with lung cancer risk; however, these associations were due to confounding by year of birth and year of hire. The studies of beryllium disease patients do not provide independent evidence and the results from other studies do not support the hypothesis of an increased risk of lung cancer or any other cancer. Overall, the available evidence does not support a conclusion that a causal association has been established between occupational exposure to beryllium and the risk of cancer.
Contents
Abstract 107
Introduction 108
Epidemiologic studies of beryllium and cancer 108
Occupational exposure to beryllium 108
Early studies of the Lorain and Reading plants 108
Multi-plant cohort study, including re-analyses 109
Case-control study in reading and subsequent re-analyses 111
US beryllium disease registry studies 112
Other studies of lung cancer among workers exposed to beryllium 113
Study of beryllium disease patients from the United Kingdom 113
Case-control study in Oahu, Hawaii 113
Case-control study in Montreal, Canada 113
Study of plutonium workers in Rocky Flats, Colorado 113
Results on cancers other than lung cancer 113
Discussion 114
Declaration of interest 117
References 117
Introduction
Industrial use of beryllium began in the 1920s and increased substantially during World War II. Until the middle of the 20th century, beryllium was used predominantly in fluorescent lamps, nuclear-weapon components, naval vessels and other defense applications. It is now used in a wide variety of products in several other industries, including aerospace, automotive, biomedical, electronics, energy and electricity, fire protection, instruments and equipment, jewelry, sport equipment, and telecommunications (CitationKreiss et al., 2007). An estimated 134,000 workers are potentially exposed to beryllium in the United States (CitationHenneberger et al., 2004); this number, however, does not include the defense, nuclear and electronic recycling sectors.
It is well established that exposure to beryllium can cause two distinct types of pulmonary disease. Acute beryllium disease is considered a dose-related toxic pneumonitis, although immunological mechanisms have also been proposed; it was first reported in the 1930s, and its incidence greatly decreased after exposure control measures were introduced (CitationVan Ordstrand et al., 1945). Chronic beryllium disease is based on delayed hypersensitivity to beryllium, leading to granulomatous lesions, which occur primarily in the lungs, but can be present also in the skin, liver, and spleen. Beryllium sensitivity precedes the development of the chronic disease and may be detected on the basis of the beryllium lymphocyte proliferation test. The first cases of chronic disease were reported in the mid 1940s in beryllium metal and alloy plants and in fluorescent light bulbs manufacturing (CitationHardy et al., 1946). In the 1940s and early 1950s, cases were also reported among family members of beryllium workers and people living around beryllium plants. Community cases have not been identified among persons first exposed after the early 1950s (CitationMaier et al., 2008) and family member cases became rare following exposure control in the primary industry. The risk of chronic disease in workers exposed during the 1940s and 1950s has been estimated to be 1–10% (CitationEisenbud et al., 1983); the risk increased with the intensity of exposure (CitationMahle et al., 1948). Registries of patients with acute and chronic beryllium disease have been established in the United States CitationEisenbud, 1982), as well as other countries. CitationKreiss and colleagues (2007) recently published a comprehensive review of cross-sectional studies of chronic beryllium disease.
Concern about possible carcinogenicity of beryllium arose when lung cancer was reported in the United States among workers in manufacturing plants and among patients included in the Beryllium Disease Registry since the mid-1960s (CitationHardy et al., 1967; CitationStoeckle et al., 1969; CitationHasan et al., 1974). Since then, several cancer epidemiology studies have been conducted, which have been reviewed in detail in the early 1990s (e.g. CitationIARC, 1993; CitationMacMahon, 1994). Although some recent reviews of beryllium toxicity included a summary of the available results of cancer studies (CitationNRC, 2007; CitationHollins et al., 2009), no comprehensive review of the epidemiological evidence has been reported. We aimed at performing a comprehensive review of epidemiological studies of cancer risk among workers exposed to beryllium, with special emphasis on results reported since the early 1990s. We did not perform a formal meta-analysis of the epidemiologic results because the available literature is mainly based on a small number of non-independent studies: these studies are reviewed in detail. In addition, our review considered other smaller studies of lower relevance though with less emphasis.
Epidemiologic studies of beryllium and cancer
Published reviews of the epidemiology of beryllium formed the basis of our literature search (CitationEPA, 1987; CitationIARC, 1993; CitationMcMahon, 1994; CitationBeryllium Industry Scientific Advisory Committee, 1997; CitationNRC, 2007; CitationHollins et al., 2009). The lists of references of these reviews were then examined to identify relevant papers for primary review. Further, a PubMed search based on the keywords (‘beryllium’ OR ‘berylliosis’) AND (‘cancer’ OR ‘neoplasm’) was conducted to identify additional relevant articles.
Occupational exposure to beryllium
Ample data are available on historical and current workplace beryllium concentrations. In general, the 2 µg/m3 exposure limit introduced in 1949 resulted in a downward worker exposure trend over time (CitationCouch et al., 2011). Reviews of exposure measurements are available (CitationNRC, 2007). In this paper, we focus on available exposure levels in the plants included in the epidemiology studies. In a survey conducted in the Lorain, Ohio plant in 1947–1948 by the Atomic Energy Commission, concentrations of beryllium ranged from 411 µg/m3 in the general air surrounding the mixing operation to 43,300 µg/m3 in the zone of alloy operation (the Lorain plant was closed in 1948). At the Reading, Pennsylvania plant, mean concentrations were estimated to be 1.7–767 μg/m3 in 1935–1960, 1.0–69 μg/m3 in 1961–1970, 0.1–3.1 μg/m3 in 1971–1980, and 0.03–1.4 μg/m3 in 1981–1992 (CitationSanderson et al., 2001c). In the same plant, exposure levels greater than 1000 µg/m3 were reported in 1968–1972 in foundry operations (CitationWagoner et al., 1980). Most measurements made since the 1970s in other production facilities included in the cancer epidemiology studies have resulted in concentrations of beryllium below 2 µg/m3 (CitationKriebel et al., 1988; CitationKreiss et al., 1977; CitationKent et al., 2001; CitationSchuler et al., 2005).
Early studies of the Lorain and Reading plants
Following reports of lung cancer cases among beryllium manufacturing workers (CitationMancuso et al., 1969; CitationMancuso, 1970), Mancuso conducted a cohort study of workers employed for at least 3 months during 1942–1948 in two beryllium plants in Lorain, Ohio (1222 workers), and Reading, Pennsylvania (2044 workers) (CitationMancuso, 1979). Follow-up was through 1975. The standardized mortality ratio (SMR) for lung cancer was 2.00 (95% CI 1.29, 2.95, 25 lung cancer deaths) in the Lorain plant and 1.37 (95% CI 0.98, 1.87, 40 deaths) in the Reading plant. The combined SMR was 1.56 (95% CI 1.20, 1.99). However, in these analyses expected deaths were calculated on the basis of rates for the white male population of the United States for 1942–1967, and rates for 1965–1967 were used for the period 1968–1975, resulting in an underestimate of the number of expected deaths in the order of 10% because lung cancer mortality was increasing among US men in that time period (CitationSaracci, 1985). After correcting for the underestimate of 10% in the expected deaths, the SMR in the Lorain plant fell to 1.8 (95% CI 1.2, 2.7), that in the Reading plant, 1.25 (95% CI 0.9, 1.7), and the combined SMR, 1.42 (95% CI 1.1, 1.8). In both plants, lung cancer mortality was greater among workers with 15 or more years since first employment, and the excess was largely among workers employed for less than 5 years. Analyses by job title or exposure categories were not conducted.
A re-analysis of mortality in the same two plants was conducted by Mancuso (CitationMancuso, 1980). The study population was extended to include workers employed in 1937–1948 (N = 3685), and mortality follow-up was extended to 1976. A cohort of 5929 workers in viscose rayon production who were employed at one of the two companies in 1938–1948 was used for comparison, in addition to the national population. The SMR for lung cancer was 1.58 (95% CI 1.25, 1.97, 80 deaths) based on national rates and 1.40 (95% CI 1.11, 1.74) based on the rates of the viscose rayon workers.
CitationWagoner and colleagues (1980) expanded the cohort study of the Reading plant by including workers employed during 1942–1967 (total N = 3055). Follow-up was to 1975 and expected deaths were calculated based on the national rates in white males. As in the studies by CitationMancuso (1970, Citation1980), 1965–1967 rates were used for the period 1968–1975. The SMR for lung cancer (ignoring the likely underestimate of expected deaths, see above) was 1.37 (95% CI 1.01, 1.82, 47 deaths). The increased mortality was restricted to workers with 25 or more years since first employment (SMR 1.68; 95% CI 1.02, 2.59, 20 deaths); no difference in mortality was apparent by duration of employment. A comparison of the smoking distribution in 1968 among 379 workers in the Reading plant and a 1964–65 national survey of US white males showed a higher proportion of smokers among the workers. The 1968 survey on smoking was conducted by the U.S. Public Health Service (USPHS) and included information from about 10% of the study cohort on current smoking status (nonsmokers, cigarette smokers, ex-smokers, or smokers of pipes or cigars): when they began smoking and how much they smoked per day. It must be noted that very few of the survey participants were employed in the 1940s and 1950s when most of the lung cancer cases and controls began employment (CitationSanderson, 2001a). The estimated confounding odds ratio (i.e. the odds ratio of lung cancer in the cohort because of the different distribution of smoking between the workers and the national population), based on relative risks for tobacco smoking from a large case-control study (CitationHaenszel et al., 1962), was 1.14. Another potential source of bias was due to the use of national vs. local reference rates for mortality. The mortality rates during 1950–1969 were lower in the county where the Reading plant was located as compared to the national rates and, therefore, the expected deaths would have been lower (and the SMR correspondingly higher) had local reference rates been used rather than national rates. The authors estimated the magnitude of this potential bias to be 19%. On the other hand, as discussed in a report by CitationEPA (1987), findings might have been overestimated because (i) as mentioned above, US white male mortality data for 1941–1967 were used to calculate expected values, which resulted in an underestimation in the order of 10% of the number of expected lung cancer deaths as lung cancer death rates in the US were increasing during the period 1968–1975 (CitationSaracci et al., 1985); (ii) the inclusion of one lung cancer death of an individual who was paid for the pre-employment physical but was not hired; (iii) the exclusion of approximately 300 white males employed in jobs similar to those of the workers included in the cohort; (iv) inadequate consideration of the potential confounding effect from lung carcinogens, and (v) failure to consider that many of the employees lived in Reading city, which is an area of elevated lung cancer rates.
Multi-plant cohort study, including re-analyses
The cohorts of workers in the Lorain and Reading plants were included in a study comprising an additional five plants in Ohio and Pennsylvania (CitationWard et al., 1992). The expanded study included 9225 male workers (of whom 320 were non-white) employed for at least 2 days between 1940 and 1969. Follow-up for mortality was through 1988, and both national and local (county) rates were used to calculate expected deaths. A total of 280 lung cancer deaths were observed, resulting in a SMR of 1.26 (95 % CI 1.12–1.42, based on national rates). Use of local (county) rates resulted in a SMR of 1.32 (95% CI, 1.19–1.46). The SMR by plant are reported in . In Lorain and Reading, the excess lung cancer mortality was lower than the one reported by CitationMancuso (1980) and the combined SMR, based on 177 observed deaths was 1.36 (95% CI 1.17, 1.58) vs. 1.58 in the analysis by CitationMancuso (1980). In the remaining four plants (those not included in the previous analyses), a total of 81 lung cancer deaths were observed, resulting in a SMR of 1.06 (95% CI 0.84, 1.32). An additional 22 observed and 14.6 expected deaths occurred among workers who could not be allocated to one specific plant. Mortality from non-neoplastic respiratory diseases was higher in the plants with higher mortality from lung cancer, and vice-versa. The SMRs increased with increasing time since first employment, but there was no relationship with duration of employment. The only significantly increased SMR was for those working less than 1 year (SMR = 1.32; p < 0.01). The variable showing the strongest association with lung cancer mortality was calendar year of first employment where the SMRs were 1.42 (95% CI 1.22, 1.65) for workers first hired before 1950, 1.24 (95% CI 0.99, 1.54) for workers first hired between 1950 and 1959, and 0.62 (95% CI 0.37, 0.98) for workers first hired between 1960 and 1969. Information on smoking status was available for 1466 workers employed in 1968 in four of the plants. The adjustment for smoking was based on a comparison of the smoking distribution of this sample of workers and that of national surveys conducted in 1965 and 1970, using the relative risks for smoking among 50 to 69-year-old men derived from the American Cancer Society Cancer Prevention Study I (CitationHammond, 1966). The adjustment explained more than half of the excess mortality from lung cancer, resulting in an adjusted SMR equal to 1.12 (95% CI 0.99, 1.26).
Table 1. SMR of lung cancer by plant in the multi-plant study of US beryllium manufacture workers: 1988 follow-up (CitationWard et al., 1992), 2005 follow-up (CitationSchubauer-Berigan et al., 2011a) and difference between the two follow-ups.
The data from the multi-plant cohort study were re-analyzed by a different group of researchers (CitationLevy et al., 2002). They introduced a correction factor in the local (county) mortality rates used for the Lorain and Reading plants, since these counties were mainly rural while most cohort members, according to these authors, lived in the two respective cities. They also used alternative relative risks for smoking to calculate adjusted SMRs for beryllium exposure and adopted a meta-analytic approach to combine plant-specific results in order to take into account potential heterogeneity. When the urban-rural difference in county rates was accounted for, the SMR in the Lorain plant based on local rates was 1.14 (95% CI 0.86, 1.48) vs. 1.60 (95% CI 1.21, 2.07) in the original publication (CitationWard et al., 1992). The corresponding SMRs in the Reading plant were 1.07 (95% CI 0.89, 1.28) and 1.42 (95% CI 1.18, 1.70). The smoking-adjusted SMRs reported by CitationLevy and colleagues (2002) were 0.98 (95% CI 0.87, 1.10) when they used the same RR for smoking used by CitationWagoner and coworkers (1980) (see above), and 1.04 (95% CI 0.92, 1.17) when they used the RR for smoking reported in a large U.S. Veterans study (CitationKahn, 1966). The use of a meta-analytic approach did not alter the results.
CitationLevy and coworkers (2009) performed a further re-analysis of the data using multivariate Cox regression to assess the impact of other employment-related factors on the increased SMR by time since first employment observed in the original analysis (CitationWard et al., 1992). The pattern of increasing SMRs with increasing latency was strongly attenuated by inclusion of other variables in the model, including birth cohort and age at first employment.
The mortality follow-up of the cohort from the Ward et al. study has been updated to 2005 (CitationSchubauer-Berigan et al., 2011a). The number of workers employed in the different plants was slightly modified (the overall number of cohort members was 9199 compared to 9225 in the previous analyses); no update of the working history was performed. National reference rates were used. The number of observed deaths from lung cancer was 545 and the SMR was 1.17 (95% CI 1.08, 1.28). The SMR for lung cancer by plant are reported in , together with the difference in results from the previous report of the same cohort (CitationWard et al., 1992). In the update, an additional 265 deaths from lung cancer were identified, compared to 241.7 expected deaths: (SMR = 1.10, 95% CI 0.96, 1.24). The SMR for lung cancer in the plant in Lorain decreased from 1.69 to 1.45, that in the plant in Reading from 1.24 to 1.20, and that in the plant in Hazelton from 1.39 to 1.03 (no significant excess of lung cancer mortality was detected in the remaining plants in either follow-up). Similar to the results presented by CitationWard and colleagues (1992), the excess lung cancer mortality was restricted to workers with less than 1 year of employment, and became apparent after a long latency (more than 35 years in the results reported by CitationSchubauer-Berigan and coworkers (2011a), more than 30 years in the results reported by CitationWard and coworkers (1992)). In internal analyses based on 5436 workers employed at three plants (Elmore, Hazleton, Reading, the latter including 74% of these workers) in which the job-exposure matrix was developed, there was no trend by categories of estimated cumulative exposure (after applying a 10-year lag) Exclusion of workers with exposure to other carcinogens, or exclusion of professional workers did not modify the results; however, exclusion of workers with less than 1 year of employment, who experienced an elevated lung cancer risk and clustered in the categories of lower cumulative exposure, resulted in a statistically significant, positive dose-response relation. The results, according to estimated maximum exposure, showed a higher risk among workers with maximum exposure equal to, or greater than, 10 µg/m3, compared to workers with maximum exposure below 10 µg/m3. There was no trend across categories of increasing maximum exposure.
The potential confounding effect of smoking was assessed by applying an adjustment factor to the risk estimates (in both the external and the internal analyses), based on the difference between the smoking habits of the cohort and that of the United States(or, in the internal analyses, based on differences in smoking habits across categories of the exposure variables). This was similar to the approach taken in the previous follow-up (CitationWard et al., 1992), although there were differences in the assumption on the effect of tobacco smoking on lung cancer risk and in the data on smoking used for the US (see Discussion).
Additional analyses were performed on the 5436 workers with quantitative exposure information based on job-exposure matrices (CitationSchubauer-Berigan et al., 2011b). For each of the 293 cases, 10 age-matched comparison subjects were selected, and different dose-response relations including categorical, power, restricted cubic spline and piecewise log-linear models, were fitted to estimated beryllium exposure indices. The associations between lung cancer mortality and average and maximum estimated exposure, which have been previously reported, were confirmed in these analyses. After adjusting for age, birth cohort, plant, short-term worker status and exposure to asbestos, there was also an association with cumulative beryllium exposure (with 10-year lag). The categorical and the piecewise log-linear models best fitted the data, with the steepest increase in lung cancer risk between 0 and 10 μg/m3 for average and maximum estimated exposure and between 0 and 200 μg/m3-days for cumulative exposure.
Case-control study in reading and subsequent re-analyses
In addition to these cohort analyses, a nested case-control study was conducted at the Reading plant (CitationSanderson et al., 2001b). For the purpose of case ascertainment, the follow-up was updated to 1992. For each deceased lung cancer case, five controls were selected from among cohort members of the same race who survived to at least the age at which the case died. Employment history was updated to 1992, and a job-exposure matrix was developed, including, for each job and department, time-specific estimates of exposure to beryllium fume or dust, various chemical forms of beryllium, and other chemical agents (CitationSanderson et al., 2001c). The linkage of individual employment histories with the job-exposure matrix generated estimates of cumulative, maximum and average exposure to beryllium and other agents. Three sets of results were presented for each exposure index: without lag, with a 10-year lag, and with a 20-year lag.
The SMR for lung cancer was 1.22 (95% CI 1.03, 1.43), similar to that found in the previous follow-up (). A majority of the 142 cases and 710 controls included in the analysis were first employed in the 1940s. The average age at hire was 33 and 37 years for cases and controls, respectively, average duration of employment was 3.7 years for cases and 5.5 years for controls, and the majority of both cases and controls were employed at the plant for less than 1 year. No differences were found according to department of employment, but cases more frequently than controls were employed as general labor and maintenance workers, two jobs which were assigned high beryllium exposure. The results based on categories (quartiles) of different exposure variables are reported in . Odds ratios (OR) for lung cancer significantly decreased with increasing unlagged duration of employment. When this variable was lagged 10 years the decreasing trend in OR disappeared and, when a 20-year lag was applied, a significantly increased OR was observed in the second and third quartiles of the distribution, but not in the top quartile. Similar results were found for cumulative exposure. There was no association between lung cancer risk and unlagged average or maximum intensity of exposure except for a significant excess among those in the third highest quartile for average exposure. When the intensity exposure indices were lagged 10 years, a significantly increased OR was observed in all quartiles compared to the first. When average and maximum intensity of exposure were lagged 20 years, the increase in the fourth quartile was no longer statistically significant. Additional analyses, conducted using exposure indices as continuous variables, showed a statistically significant dose-response relation after log-transformation of the exposure variables and application of a 10- or 20-year lag (the unlagged analysis of log-transformed duration of employment resulted in a statistically significant inverse relationship). Additional analyses excluding professional workers provided results similar to those based on the whole study population. Analyses were also conducted for specific beryllium compounds. Exposure to beryllium oxide and beryllium-copper alloy, the most common types of exposure, explained the results for all forms combined. Exposure to fluorides and copper was highly correlated to beryllium exposure, and the results on lung cancer risk paralleled those for beryllium. An indirect adjustment for smoking was performed using the data from a survey of about 10% of workers who were employed in 1968 (this appears to be the same survey used to adjust for smoking by CitationWard and colleagues (1992). There was no association between indices of beryllium exposure and smoking status in this group of workers.
Figure 1. Odds ratios of lung cancer for quartiles of indices of beryllium exposure (CitationSanderson et al., 2001b). Panel A: Duration of exposure. Panel B: Cumulative exposure. Panel C: Average exposure. Panel D: Maximum exposure. Solid line: no lag; dotted line: 10-year lag; broken line: 20-year lag. Asterisks denote statistically significant odds ratio.
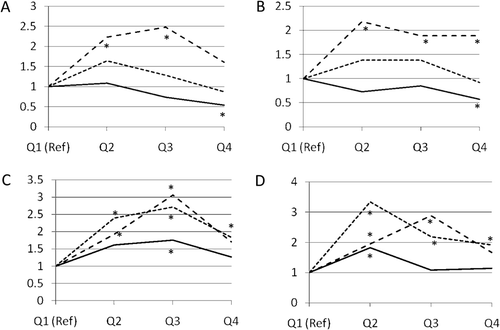
CitationDeubner and colleagues (2001) raised a number of issues on the case-control study at the Reading plant including: (i) the lack of information in the job-exposure matrix on exposure circumstances before 1947 when most cases and controls were employed, (ii) the lack of information on jobs held by these workers outside the beryllium industry (which is particularly relevant because of the short duration of employment in the beryllium industry for most of them), (iii) the fact that the SMR for workers hired in the most recent time period was not reported, despite the fact that a decreased mortality from lung cancer was reported among these workers in the doctoral thesis of the first author of the original paper, (iv) the strategy for matching controls to cases on attained age (i.e. requiring controls to be alive at the age of the death of the cases would preferentially select older controls with a higher age at first employment, resulting in less opportunity to have high lagged exposure), (v) the residual potential confounding by exposure to strong inorganic acids, and (vi) the absence of smoking data from a subsample of cases and controls to estimate an adjusted risk estimate for beryllium exposure. While the authors of the original paper correctly argued that their strategy for selecting and matching controls was methodologically sound (CitationSanderson, 2001a), they were not able to address completely the other criticisms. In particular, the authors of the original paper reported in their response the results for lung cancer among workers first employed after 1960 (SMR 0.23, 95% CI 0.03–0.84) which confirmed the statement by CitationDeubner and colleagues (2001) that no excess mortality was observed among people hired in the recent period. The criticism of a possible bias from the nested case-control design raised by CitationDeubner and colleagues (2001) was addressed in a simulation of the case-control study, based on random allocation of cohort members as cases (CitationDeubner et al., 2007). In that analysis, it was found that, when lag was applied, there was an association between (simulated) case status and average exposure. These arguments were criticized by the authors of the original study (CitationHein et al., 2009) and others (CitationLangholz and Richardson, 2009). The controversy was resolved by CitationRothman and Mosquin (2011), who used a simulation of random assignment of case status to cohort members (an approach expected to result in no association between exposure and case status) to show that confounding by year of birth and year of hire operated under different models of risk-set sampling, when exposures were lagged, while there was no confounding—and no association between exposure and case status—in unlagged analyses.
In a re-analysis of the case-control study at the Reading plant, Levy and colleagues raised the issue that without log-transformation, none of the exposure variables was associated with an increased risk of lung cancer (CitationLevy et al., 2007). Their explanation is that the log-transformation exaggerates the difference between workers with zero exposure (after the application of a lag, that is, workers whose exposure occurred entirely in the period excluded in the lagged analysis) and the workers with non-zero exposure, and the former group of workers includes preferentially controls. The authors of the original case-control study did not provide new evidence to address this criticism (CitationSchubauer-Berigan et al., 2007).
A further re-analysis of the data for Reading from the case-control study was performed by the original authors, with the aim of evaluating the potential confounding effect of age at first employment and cohort of birth (CitationSchubauer-Berigan et al., 2008). The latter variable, which likely reflected differences in smoking pattern across birth cohorts, as well as the fact that workers first employed in the Reading plant during the World War II era tended to be older than other workers, acted as a confounder in the analysis. After adjustment for birth cohort, there was no relation between cumulative beryllium exposure (in both the lagged and the unlagged analysis), and the association with average exposure was weakened and in most cases no longer statistically significant. These results are consistent with those from the simulated analyses by CitationRothman and Mosquin (2011) that provided evidence of confounding by year at first employment and year of birth in lagged analyses.
US beryllium disease registry studies
Following reports of cases of lung cancer among patients with beryllium disease (CitationHardy et al., 1967; CitationStoeckle et al., 1969; CitationHasan and Kazemi, 1974), CitationInfante and colleagues (1980) studied a cohort of 421 white male patients with chronic beryllium disease or acute beryllium-related pneumonitis included in the Beryllium Case Registry during 1952–1975. These patients were mainly employed in beryllium extraction and smelting, metal production and fluorescent tube production. The mortality follow-up was conducted until 1975; seven deaths from lung cancer were observed (SMR = 2.12, 95% CI 0.85, 4.37). As in the early studies in the Lorain and Reading plants, national mortality rates from 1965 to 1967 were used for the period 1968–1975, resulting in a likely underestimate of the expected deaths of about 10%. Six of the lung cancer deaths occurred among patients with beryllium-related acute pneumonitis (SMR 3.14; 95% CI 1.15, 6.84); the remaining lung cancer deaths occurred among patients with chronic beryllium disease (SMR 0.72; 95% CI 0.02, 4.04).
CitationSteenland and Ward (1991) enlarged the study to comprise 689 beryllium disease patients of both genders and all ethnic groups, who were registered between 1952 and 1980, and extended the mortality follow-up to 1988. The SMRs for lung cancer (based on national reference rates) were 2.00 (95% CI 1.33, 2.89, based on 28 deaths); 1.76 (95% CI 1.02, 2.67, based on 22 deaths) for men and 4.04 (95% CI 1.47, 8.81, based on 6 deaths) for women. After stratification by type of beryllium disease, the SMRs were 2.32 (95% CI 1.35, 3.72; 17 deaths) among patients with acute disease and 1.57 (95% CI 0.75, 2.89; 10 deaths) among patients with chronic disease. No trends were observed for time since first exposure to beryllium or duration of exposure. The SMRs were 2.69 (95% CI 1.61, 4.20, 19 deaths) for patients who were employed in beryllium manufacturing and 0.85 (95% CI 0.18, 2.49, 3 deaths) for patients who were employed in the fluorescent light industry (p value of test of heterogeneity 0.05). The SMR for non-malignant respiratory diseases was 34.23 (95% CI 29.1, 40.0, based on 158 deaths), that from poisoning, including beryllium poisoning, was 35.93 (95% CI 19.1, 61.4, 13 deaths). The SMR for non-malignant respiratory diseases were 68.64 (95% CI 57.8, 81.0) among chronic disease patients and 6.55 (95% CI 3.74, 10.6) among acute disease patients. All deaths from poisoning occurred in the chronic disease group. A comparison of smoking habits assessed in 1965 in 141 male and 82 female patients with a national survey conducted in the same year showed a similar proportion of ever smokers in men (59 vs. 61%), and a lower proportion in women (38 vs. 47%). Based on this limited series of patients, tobacco smoking does not appear to act as confounder.
Other studies of lung cancer among workers exposed to beryllium
Study of beryllium disease patients from the United Kingdom
An analysis of the United Kingdom Beryllium Registry identified 30 deaths, of which 25 were from respiratory failure, among 69 patients diagnosed with chronic beryllium disease during 1945–1993 (CitationWilliams, 1996). There was no death from lung cancer. In the US Registry study (CitationSteenland and Ward, 1991), the proportion of lung cancer deaths over total deaths among chronic beryllium disease patients was 10/249, or 4.0%. A similar proportion in the UK study would correspond to 1.2 expected deaths.
Case-control study in Oahu, Hawaii
CitationHinds and colleagues (1985) conducted a study of 261 male cases of lung cancer newly diagnosed during 1979–1982 among residents in Oahu, Hawaii, and 444 age (5-year groups) and sex matched controls selected through random digit dialing. A job-exposure matrix was applied to the primary and secondary occupations of study subjects to assess occupational exposure to seven agents. The smoking-adjusted odds ratio for low-level exposure to beryllium was 1.62 (95% CI 1.04, 2.51), and for high-level exposure was 1.57 (95% CI 0.81, 3.01). No adjustment was made for other occupational exposures to other occupational exposures.
Case-control study in Montreal, Canada
A population-based case-control study with 900 cases and 1000 controls, conducted during 1979–1986 in Montreal, Canada, was designed to assess the effects from exposures to known and suspected occupational carcinogens (CitationSiemiatycki, 1991). Beryllium was one of over 300 agents whose workplace exposure was assessed retrospectively by a team of experts based on detailed questionnaires. The prevalence of beryllium exposure was 0.6%. Results were reported for all associations with p-value smaller than 0.1; results for beryllium exposure were not reported. We conducted a power calculation based on crude odds ratios, which suggested that the minimal OR detectable as significant at α = 0.1 was in the order of 2.
Study of plutonium workers in Rocky Flats, Colorado
Brown and colleagues conducted a study of 180 decedents from lung cancer and 720 controls matched on age, sex and year of birth, nested in a cohort of workers employed at the Rocky Flats Plant in Colorado during 1951–1989 (CitationBrown et al., 2004). Exposure to several agents, including beryllium, was also estimated. No association was found between lung cancer mortality and cumulative exposures to beryllium.
Results on cancers other than lung cancer
Results on all cancer mortality were reported in two studies of manufacturing workers (CitationWagoner et al., 1980; CitationWard et al., 1992) and two studies of Beryllium Registry patients (CitationInfante et al., 1980; CitationSteenland and Ward, 1991). summarizes the results for lung cancer and for all cancer minus lung cancer, obtained by subtracting the number of observed and expected lung cancer deaths from the totals. No excess of cancer other than lung cancer was observed in the studies of manufacturing workers. Patients in the Beryllium Disease Registries experienced a non-significant 30% increase in mortality from cancers other than lung cancer.
Table 2. SMR of all cancers and all cancers minus lung in selected studies of beryllium workers.
In the most recent update of the multi-plant cohort of manufacturing workers, no results for all cancer mortality were reported (CitationSchubauer-Berigan et al., 2011a). Results however were reported for cancers of the nervous system and the urinary tract, the latter based on an analysis of multiple causes of deaths. Although no overall increase in mortality was observed for these two cancers, a significantly increased trend in risk was observed for cumulative beryllium exposure and nervous system cancer and for maximum exposure and urinary tract cancer.
Results on risk of all cancers and of cancers other than lung have been reported among workers at the Oak Ridge National Laboratory, Tennessee. CitationWing and colleagues (1993) studied a cohort of 8318 white male workers employed during 1943–1972 and followed to 1985. A total of 609 workers were included on lists of workers monitored for potential exposure to beryllium. Most of them were non-monthly workers, implying a lower socio-economic status. The relative risk of cancer in this group of workers compared to all other workers in the cohort was 1.38 (95% CI 0.95, 2.00), after adjustment for sociodemographic factors (but not radiation). CitationCarpenter and colleagues (1988) conducted a study of 89 workers in the same facilities who died from cancer of the central nervous system and 356 controls matched on race, sex, facility at which initially employed (cohort), year of birth, and year of hire. Based on job titles and departments, subjects were classified according to exposure to 26 agents, including beryllium. The OR for exposure to beryllium was 1.5 (95% CI 0.6, 3.9). Analyses by probability and duration of exposure yielded inconsistent results.
A case-control study of prostate cancer was conducted in a cohort of workers of the United Kingdom Atomic Energy Authority (CitationRooney et al., 1993). A total of 136 cases and 404 matched controls were included in the study. Exposure to several agents, including beryllium, was assessed based on the occupational history of subjects. Seven cases and 23 controls were classified as exposed to beryllium (OR 0.87; 95% CI 0.30, 2.17).
Discussion
In 1992, IARC classified beryllium as a human carcinogen (CitationIARC, 1993). Subsequent evaluations by EPA (CitationUSEPA, 1998a; CitationUSEPA, 1998b) classified beryllium as a probable human carcinogen and by NTP (CitationUS Dept HHS, 2011) as known to be carcinogenic to humans. Reviews of the epidemiologic literature available at that time of these evaluations concluded that the evidence did not support a causal association between beryllium exposure and cancer (and specifically lung cancer) in humans (CitationNRC,2007; CitationBeryllium Industry Scientific Advisory Committee, 1997).
A unique aspect regarding the evidence of carcinogenicity of beryllium in humans is that most of the available data refer to the population of workers employed in the seven plants in Ohio and Pennsylvania, and in particular, those in Lorain and Reading. This population from the seven plants has formed the basis for repeated cohort and nested case-control analyses, with slightly different selection criteria and approaches for exposure assessment. Although the studies of patients in the U.S. Beryllium Disease Registry (CitationInfante et al., 1980; CitationSteenland and Ward, 1991) have been presented as providing independent evidence, a substantial (albeit undefined) degree of overlap in subjects exists with the studies of manufacturing workers. Consistency of results among different populations, an important component of the guidelines to assess causality in human observational studies (CitationHill, 1965), has not been met in the case of beryllium.
The evidence supporting the hypothesis of carcinogenicity of beryllium comes mainly from the results of the analyses of workers employed in the early phase of the manufacturing industry, essentially before occupational exposure standards were recommended in 1949. These are the workers who suffered most from acute and chronic beryllium disease. In a report of 224 cases of acute disease included during 1952–1981 in the U.S. Beryllium Disease Registry, only 15 (6.7%) were first employed after 1949 (CitationEisenbud and Lisson, 1983). Workers employed in the early technological phase of the industry also experienced an increased risk of lung cancer, as shown in several studies since the late 1970s and early 1980s (CitationWagoner et al., 1980; CitationMancuso, 1979; CitationMancuso, 1980). They were exposed to very high levels of beryllium, and they had a short duration of employment in beryllium manufacturing plants mostly due to adverse reactions from exposure to soluble salts of beryllium. These two factors may explain the results on lung cancer risk by average and maximum estimated beryllium exposure, which were shown in various analyses of beryllium manufacturing workers. However, there are other factors that may be more valid explanations for the increased risk of lung cancer in these workers (CitationMacMahon, 1994; CitationBeryllium Industry Scientific Advisory Committee, 1997). Short-term workers tend to have an increased mortality, even in the absence of exposure to specific hazards. For example, in a study of styrene-exposed workers from seven European countries, workers employed for less than 1 month had a RR for overall mortality of 1.24 (95% CI 1.09, 1.42), compared to workers employed for more than 1 year (CitationBoffetta et al., 1998). This difference might be due to lifestyle factors, including tobacco smoking, but also to employment in other jobs and industries entailing exposure to carcinogens and other hazards. This is particularly of concern because the plants with elevated lung cancer deaths were located in highly industrialized areas. None of these potential sources of confounding has been directly addressed in the studies of workers employed in the early technological phase of the beryllium industry. No information was available on employment in other jobs and industries, and the indirect adjustment for tobacco was based on data collected in the mid- and late-1960s. Although beryllium exposure remains a plausible explanation for the increased lung cancer mortality experienced by these workers, alternative explanations cannot be excluded. As mentioned above, the majority of patients included in the U.S. Beryllium Disease Registry are likely to include these same workers. The fact that no excess risk of lung cancer was reported in Registry patients employed in fluorescent light manufacture support a non-causal interpretation of the association between beryllium exposure and lung cancer risk in similar patients employed in the beryllium industry.
Results for workers employed after 1949 have been reported separately by CitationWard and colleagues (1992), and CitationSanderson and colleagues (2001b), and no significant increase in lung cancer mortality was detected in these workers. Consistent with these results, there was no significantly increased risk among workers employed in the plants other than Reading and Lorain, who did not experience the high pre-1949 exposure levels (only one of the two Cleveland plants operated before 1949). In the restricted cohort of manufacturing workers from the plants in Cleveland, Lucky, Elmore and Hazelton the SMR at the 1988 follow-up was 1.06 (95% CI 0.84, 1.32, based on 81 deaths) (CitationWard et al., 1992); that at the 2005 update was 1.04 (95% CI 0.90, 1.19, based on 196 deaths) (CitationSchubauer-Berigan et al., 2011a).
The results of the recent updated mortality follow-up (CitationSchubauer-Berigan et al., 2011a, CitationSchubauer-Berigan et al., 2011b) add little evidence in favor of, or against, the hypothesis of a carcinogenic risk entailed by exposure to beryllium. The statistical significance of some of the results depends on the increased risk of lung cancer experienced by workers (especially those with short-term employment) employed in the plant in Lorain (which was closed in 1948) and—to a lesser extent—by workers employed in the plants at Reading and Hazelton before 1960. These excesses have been documented in previous reports, and are sufficient to generate a number of the findings including: (i) the apparent trends by time since first employment and maximum exposure, (ii) the fact that results become more ‘positive’ when a lag is applied to the exposure history, and (iii) the lack of a trend according to duration of employment and cumulative employment. In addition, it appears that a large number of analyses were conducted in this study, and it is unclear whether the results reported in the published manuscript were selected a priori or were chosen after having generated a large set of results.
The lack of an association between lung cancer mortality and duration of employment and cumulative beryllium exposure reduces the credibility of a causal relation between beryllium exposure and lung cancer risk. For all known occupational lung carcinogens, such relationships are evident in the primary analyses of the relevant epidemiologic studies, although for some agents further modeling and the application of lags or time windows of exposure reinforce the results. In this respect, it is worth noting the strong dose-response relation observed between unlagged duration of employment and mortality from pneumoconiosis and other respiratory diseases, a category which includes beryllium disease (CitationSchubauer-Berigan et al., 2011a).
Residual confounding by smoking is a central concern. The indirect adjustment performed in the studies of manufacturing workers, including the recent report by CitationSchubauer-Berigan and colleagues (2011b), as well as Beryllium Disease Registry patients (CitationInfante et al., 1980), was based on surveys conducted in the 1960s in a small proportion of workers and on the comparison with national surveys. Arguments against an important role of this type of confounding include the small confounding odds ratio calculated in some of these adjustments and the lack of correlation between smoking status and beryllium exposure indices in the nested case-control study (CitationSanderson et al., 2001b). Counter-arguments include the comparability of smoking data among the different surveys and the low relative risks for smoking used in the adjustment (CitationHaenszel et al., 1962; CitationHammond, 1966; CitationKahn, 1966). In our view, a key issue is the fact that it is unclear whether the available smoking data used for the adjustments were relevant to workers employed before 1949, among whom an excess lung cancer risk has been detected (see below). We conclude therefore that the possibility of residual confounding by smoking in this group of workers has not been adequately addressed. The problem of residual confounding by smoking is particularly acute in the most recent analysis of the multi-plant cohort study (CitationSchubauer-Berigan et al., 2011a) since the assumptions used in that analysis are questionable. The relative risks used for tobacco smoking were lower than those typically found in epidemiological studies conducted in the US (CitationGandini et al., 2008; CitationIARC, 2004). The data on smoking for the US population were derived from the 1966 National Health Interview Survey (CitationNational Center for Health Statistics, 1970): the authors, however, excluded the majority of participants on the basis that their information was provided by proxies and therefore likely biased toward lower smoking rates. The effect of the first choice is an underestimate of the actual confounding effect of smoking; the effect of the second choice is more difficult to evaluate but if indeed non-smokers were selectively excluded from rates used for the US population, the results would again have been toward an underestimate of the confounding effect. The conclusion of the authors was that there was essentially no confounding exerted by smoking (bias factor 0.0997). This conclusion is surprising in light of the previous estimate made by Ward and colleagues (CitationWard et al., 1992) and in general of the results of similar adjustments for smoking in other populations of US blue collar workers employed in the 1950s–1970s (CitationStellman et al., 1988).
Residual confounding by exposure to other occupational agents is an additional concern. As mentioned above, many workers at increased risk of lung cancer had limited occupational skills and a short duration of employment in this industry. This raises the issue of potential employment in other high-risk industries during the remainder of their occupational history. Although adjustment for sociodemographic factors was performed in the nested case-control study in Reading (CitationSanderson et al., 2001b), no direct information on exposure to agents in other jobs and industries is available. The job exposure matrix (JEM) used in the recent analysis of the US multi-plant cohort included information on exposure to other agents in the beryllium industry. In particular, asbestos was reported in 14.8% of JEM entries (no results on mesothelioma risk were reported in any of the publications of this cohort), acid mists in 0.9%, welding fumes in 1.0%, nickel in 4.3%, chromium in 1.0% and silica in 1.7% (CitationCouch et al., 2011). No information is provided however on exposure prevalence for individual cohort members or on the presence or absence of confounding by these agents.
It has been suggested that the secular trend in lung cancer mortality among US men due to the maturation of the smoking epidemics, the use of a lag in a nested case-control study might result in positive confounding for employment-related variables (CitationDeubner and Roth, 2009). This would explain the positive association shown in lagged analyses that did not adjust for date of birth and age at hire (CitationSanderson et al., 2001b), and the lack of such association when date of birth and age at hire were controlled for (CitationLevy and Roth, 2007; CitationSchubauer-Berigan et al., 2008). Indeed, confounding by time-related variables in nested case-control analyses using lagged exposure variables has been demonstrated by CitationRothman and Mosquin (2011).
Although no single mechanism has been identified to support the hypothesis of beryllium carcinogenicity in humans (CitationHollins et al., 2009), the association with beryllium disease suggests a possible mechanistic role of chronic inflammation for interpreting epidemiologic results as supporting the carcinogenicity of beryllium in humans. Chronic inflammation is indeed a mechanism of lung carcinogenesis in humans, and several clinical inflammatory conditions, including chronic obstructive pulmonary disease, tuberculosis, silicosis and asbestosis are associated with increased lung cancer risk (CitationEngels, 2008; CitationBrenner et al., 2011), although mechanisms other than chronic inflammation may also play a role. The association between granuloma-type conditions, such as sarcoidosis, and lung cancer risk, however, is less convincing (CitationLe Jeune et al., 2007). If indeed beryllium exerts a carcinogenic effect by sustained inflammation of the lung, the results of studies of Beryllium Disease Registry patients should show a stronger risk for chronic vs. acute disease, since duration of the disease is a critical factor in other chronic inflammatory conditions, yet the opposite pattern is observed. Similarly, duration of exposure would be an important dimension of exposure in occupational studies; yet, this variable was not associated with risk in most analyses. Furthermore, if inflammation is indeed the mechanism of beryllium carcinogenicity, the use of a time lag in the epidemiologic analysis is not justified, as inflammation acts on late stages of the carcinogenic process, and lagging provides more weight to early exposures, i.e. those relevant for mutagenic agents; yet, results of most analyses become stronger when a lag was applied.
Previous reviews and evaluations of epidemiologic results on beryllium and cancer have focused on the studies among U.S. manufacturing workers and patients in the Beryllium Disease Registry. These studies certainly represent the majority of the evidence. Our review however has identified a few additional investigations which provide relevant information. Despite the limitations due to low statistical power and the rather crude assessment of exposure to beryllium in most of these additional studies, they provide no supportive evidence to the hypothesis of a causal association between beryllium exposure and lung cancer risk in humans. Results for other cancers, albeit sparse, are inconsistent and do not suggest a carcinogenic effect on other organs. Stressing ‘positive’ associations with low prior probability, such as those with cancers of the nervous system and the urinary tract in the most recent update of the multicenter study of manufacturing workers (CitationSchubauer-Berigan et al., 2011a) raises the issues of selective reporting of positive results, which are not likely to be confirmed in subsequent studies (CitationBoffetta et al., 2008).
In conclusion, there is little doubt that excess lung cancer mortality occurred in (mainly short-term) workers employed before 1949 in the Lorain and Reading plants with no excess risk in workers employed after 1949. Such excess mortality contributed to positive associations with estimates of maximum and average beryllium exposure in various studies within this industry and the strengthening of results when a lag period is included in the analysis. It is debatable whether this finding should be interpreted as demonstrating that beryllium is a human carcinogen, since confounding by tobacco smoking and other occupational and lifestyle factors is not adequately excluded. Little supportive evidence comes from other populations. It is unclear whether the results based on patients in the Beryllium Disease Registry provide independent evidence, and the results of studies in other manufacturing plants provide no clear evidence of increased lung cancer risk. Several criticisms raised in previous reviews of this body of evidence are still valid, and probably will never be adequately addressed, because of the difficulty in gathering valid information on exposure circumstances prevalent more than 60 years ago. The overall epidemiologic evidence, therefore, does not support the conclusion that a causal association between exposure to beryllium and cancer in humans has been established. Future research should aim at identifying populations of workers exposed to beryllium which have not been already studied.
Declaration of interest
This study was supported by an unrestricted grant from Materion Brush Inc.
References
- Beryllium Industry Scientific Advisory Committee. (1997). Is beryllium carcinogenic in humans? J Occup Environ Med 39:205–208.
- Boffetta P, Sali D, Kolstad H, Coggon D, Olsen J, Andersen A, Spence A, Pesatori AC, Lynge E, Frentzel-Beyme R, Chang-Claude J, Lundberg I, Biocca M, Gennaro V, Teppo L, Partanen T, Welp E, Saracci R, Kogevinas M. (1998). Mortality of short-term workers in two international cohorts. J Occup Environ Med 40:1120–1126.
- Boffetta P, McLaughlin JK, La Vecchia C, Tarone RE, Lipworth L, Blot WJ. (2008). False-positive results in cancer epidemiology: A plea for epistemological modesty. J Natl Cancer Inst 100:988–995.
- Brenner DR, McLaughlin JR, Hung RJ. (2011). Previous lung diseases and lung cancer risk: A systematic review and meta-analysis. PLoS ONE 6:e17479.
- Brown SC, Schonbeck MF, McClure D, Barón AE, Navidi WC, Byers T, Ruttenber AJ. (2004). Lung cancer and internal lung doses among plutonium workers at the Rocky Flats Plant: A case-control study. Am J Epidemiol 160:163–172.
- Carpenter AV, Flanders WD, Frome EL, Tankersley WG, Fry SA. (1988). Chemical exposures and central nervous system cancers: A case-control study among workers at two nuclear facilities. Am J Ind Med 13:351–362.
- Couch JR, Petersen M, Rice C, Schubauer-Berigan MK. (2011). Development of retrospective quantitative and qualitative job-exposure matrices for exposures at a beryllium processing facility. Occup Environ Med 68:361–365.
- Deubner DC, Lockey JL, Kotin P, Powers MB, Miller F, Rogers AE, Trichopoulos D. (2001). Re: Lung cancer case-control study of beryllium workers. Sanderson WT, Ward EM, Steenland K, Petersen MR. Am. J. Ind. Med. 2001. 39:133–144. Am J Ind Med 40:284–288.
- Deubner DC, Roth HD, Levy PS. (2007). Empirical evaluation of complex epidemiologic study designs: Workplace exposure and cancer. J Occup Environ Med 49:953–959.
- Deubner DC, Roth HD. (2009). Rejoinder: Progress in understanding the relationship between beryllium exposure and lung cancer. Epidemiology 20:341–343.
- Eisenbud M. (1982). Origins of the standards for control of beryllium disease (1947-1949). Environ Res 27:79–88.
- Eisenbud M, Lisson J. (1983). Epidemiological aspects of beryllium-induced nonmalignant lung disease: A 30-year update. J Occup Med 25:196–202.
- Engels EA. (2008). Inflammation in the development of lung cancer: Epidemiological evidence. Expert Rev Anticancer Ther 8:605–615.
- Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, Boyle P. (2008). Tobacco smoking and cancer: A meta-analysis. Int J Cancer 122:155–164.
- Haenszel W, Loveland DB, Sirken MG. (1962). Lung-cancer mortality as related to residence and smoking histories. I. White males. J Natl Cancer Inst 28:947–1001.
- Hammond EC. (1966). Smoking in relation to the death rates of one million men and women. In: Haenszel W. (ed.). Epidemiological Study of Cancer and Other Chronic Diseases. Washington, DC, US Government Printing Office, pp. 127–204.
- Hardy HL, Tabershaw IR. (1946). Delayed chemical pneumonitis occurring in workers exposed to beryllium compounds. J Ind Hyg Toxicol 28:197–211.
- Hardy HL, Rabe EW, Lorch S. (1967). United States, Beryllium Case Registry (1952-1966). Review of its methods and utility. J Occup Med 9:271–276.
- Hasan FM, Kazemi H. (1974). Chronic beryllium disease: A continuing epidemiologic hazard. Chest 65:289–293.
- Hein MJ, Deddens JA, Schubauer-Berigan MK. (2009). Bias from matching on age at death or censor in nested case-control studies. Epidemiology 20:330–338.
- Henneberger PK, Goe SK, Miller WE, Doney B, Groce DW. (2004). Industries in the United States with airborne beryllium exposure and estimates of the number of current workers potentially exposed. J Occup Environ Hyg 1:648–659.
- Hill AB. (1965). The environment and disease: Association or causation? Proc R Soc Med 58:295–300.
- Hinds MW, Kolonel LN, Lee J. (1985). Application of a job-exposure matrix to a case-control study of lung cancer. J Natl Cancer Inst 75:193–197.
- Hollins DM, McKinley MA, Williams C, Wiman A, Fillos D, Chapman PS, Madl AK. (2009). Beryllium and lung cancer: A weight of evidence evaluation of the toxicological and epidemiological literature. Crit Rev Toxicol 39 Suppl 1:1–32.
- Infante PF, Wagoner JK, Sprince NL. (1980). Mortality patterns from lung cancer and nonneoplastic respiratory disease among white males in the beryllium case registry. Environ Res 21:35–43.
- International Agency for Research on Cancer. Beryllium. (1993). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 58. Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry. Lyon, IARC pp. 41–117.
- International Agency for Research on Cancer. (2004). Tobacco smoke. IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans, Vol. 83, Tobacco Smoke and Involuntary Smoking. Lyon, IARC, pp. 51–1187.
- Kahn HA. (1966). The Dorn study of smoking and mortality among U.S. veterans: Report on eight and one-half years of observation. Natl Cancer Inst Monogr 19:1–125.
- Kent MS, Robins TG, Madl AK. (2001). Is total mass or mass of alveolar-deposited airborne particles of beryllium a better predictor of the prevalence of disease? A preliminary study of a beryllium processing facility. Appl Occup Environ Hyg 16:539–558.
- Kreiss K, Mroz MM, Zhen B, Wiedemann H, Barna B. (1997). Risks of beryllium disease related to work processes at a metal, alloy, and oxide production plant. Occup Environ Med 54:605–612.
- Kreiss K, Day GA, Schuler CR. (2007). Beryllium: A modern industrial hazard. Annu Rev Public Health 28:259–277.
- Kriebel D, Sprince NL, Eisen EA, Greaves IA. (1988). Pulmonary function in beryllium workers: Assessment of exposure. Br J Ind Med 45:83–92.
- Langholz B, Richardson D. (2009). Are nested case-control studies biased? Epidemiology 20:321–329.
- Le Jeune I, Gribbin J, West J, Smith C, Cullinan P, Hubbard R. (2007). The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir Med 101:2534–2540.
- Levy PS, Roth HD, Hwang PM, Powers TE. (2002). Beryllium and lung cancer: A reanalysis of a NIOSH cohort mortality study. Inhal Toxicol 14:1003–1015.
- Levy PS, Roth HD, Deubner DC. (2007). Exposure to beryllium and occurrence of lung cancer: A reexamination of findings from a nested case-control study. J Occup Environ Med 49:96–101.
- Levy PS, Roth HD, Deubner DC. (2009). Exposure to beryllium and occurrence of lung cancer: Findings from a cox proportional hazards analysis of data from a retrospective cohort mortality study. J Occup Environ Med 51:480–486.
- Machle W, Beyer E, Gregorious F. (1948). Berylliosis; acute pneumonitis and pulmonary granulomatosis of beryllium workers. Occup Med (Chic Ill) 5:671–683.
- MacMahon B. (1994). The epidemiological evidence on the carcinogenicity of beryllium in humans. J Occup Med 36:15–24; discussion 25.
- Maier LA, Martyny JW, Liang J, Rossman MD. (2008). Recent chronic beryllium disease in residents surrounding a beryllium facility. Am J Respir Crit Care Med 177:1012–1017.
- Mancuso TF, el-Attar AA. (1969). Epidemiological study of the beryllium industry. Cohort methodology and mortality studies. J Occup Med 11:422–434.
- Mancuso TF. (1970). Relation of duration of employment and prior respiratory illness to respiratory cancer among beryllium workers. Environ Res 3:251–275.
- Mancuso TF. (1979). Occupational lung cancer among beryllium workers. In Lemen R, Dement J. (eds.). Dusts and Disease. Park Forest, IL, Pathotox Publishers, pp. 463–471.
- Mancuso TF. (1980). Mortality study of beryllium industry workers’ occupational lung cancer. Environ Res 21:48–55.
- National Center for Health Statistics. (1970). Changes in Cigarette Smoking Habits between 1955 and 1966. Rockville, MD, U.S. Department of Health, Education, and Welfare, pp. 1–33.
- National Research Council, Committee on Toxicology. (2007). Committee on Beryllium Alloy Exposures. Health Effects of Beryllium Exposure: A Literature Review. Washington, DC, National Academy Press, http://www.nap.edu/catalog/12007.html.
- Rooney C, Beral V, Maconochie N, Fraser P, Davies G. (1993). Case-control study of prostatic cancer in employees of the United Kingdom Atomic Energy Authority. BMJ 307:1391–1397.
- Rothman KJ, Mosquin PL. (2011). Confounding after risk-set sampling in the beryllium study of Sanderson et al. Ann Epidemiol 21:773–779.
- Sanderson WT. (2001a) Re: Response to criticisms of “Lung Cancer Case-Control Study of Beryllium Workers.” Am J Ind Med 40:286–288.
- Sanderson WT, Ward EM, Steenland K, Petersen MR. (2001b). Lung cancer case-control study of beryllium workers. Am J Ind Med 39:133–144.
- Sanderson WT, Petersen MR, Ward EM. (2001c). Estimating historical exposures of workers in a beryllium manufacturing plant. Am J Ind Med 39:145–157.
- Saracci R. (1985). Beryllium: Epidemiological evidence. In Wald NJ, Doll R. (eds.). Interpretation of Negative Epidemiological Evidence for Carcinogenicity. (IARC Scientific Publications No. 65), Lyon, IARC, pp. 203–219.
- Schubauer-Berigan MK, Deddens JA, Petersen MR. (2007). Re: Exposure to beryllium and occurrence of lung cancer: A reexamination of findings from a nested case-control study. J Occup Environ Med 49:708–9; author reply 709.
- Schubauer-Berigan MK, Deddens JA, Steenland K, Sanderson WT, Petersen MR. (2008). Adjustment for temporal confounders in a reanalysis of a case-control study of beryllium and lung cancer. Occup Environ Med 65:379–383.
- Schubauer-Berigan MK, Couch JR, Petersen MR, Carreón T, Jin Y, Deddens JA. (2011a). Cohort mortality study of workers at seven beryllium processing plants: Update and associations with cumulative and maximum exposure. Occup Environ Med 68:345–353.
- Schubauer-Berigan MK, Deddens JA, Couch JR, Petersen MR. (2011b). Risk of lung cancer associated with quantitative beryllium exposure metrics within an occupational cohort. Occup Environ Med 68:354–360.
- Schuler CR, Kent MS, Deubner DC, Berakis MT, McCawley M, Henneberger PK, Rossman MD, Kreiss K. (2005). Process-related risk of beryllium sensitization and disease in a copper-beryllium alloy facility. Am J Ind Med 47:195–205.
- Siemiatycki J. (1991). Risk Factors for Cancer in the Workplace. Boca Raton, FL, CRC Press.
- Steenland K, Ward E. (1991). Lung cancer incidence among patients with beryllium disease: A cohort mortality study. J Natl Cancer Inst 83:1380–1385.
- Stellman SD, Boffetta P, Garfinkel L. (1988). Smoking habits of 800,000 American men and women in relation to their occupations. Am J Ind Med 13:43–58.
- Stoeckle JD, Hardy HL, Weber AL. (1969). Chronic beryllium disease. Long-term follow-up of sixty cases and selective review of the literature. Am J Med 46:545–561.
- US Department of Health and Human Service. (2011). National Toxicology Program. Report on Carcinogens. 12th Edition. Research Triangle Park, NC, US Department of Health and Human Service.
- US Environmental Protection Agency. (1987). Health Assessment Document for Beryllium (EPA Report No. 60/8-84-026F). Research Triangle Park, NC, Office of Research and Development.
- US Environmental Protection Agency. (1998a). Beryllium and Compounds (CASRN 7440-41-7). Washington, DC, Integrated Risk Information System, US Environmental Protection Agency, http://www.epa.gov/iris/subst/0012.htm.
- US Environmental Protection Agency. (1998b). Toxicological Review of Beryllium and Compounds (CAS No. 7440-41-7) in Support of Summary Information on the Integrated Risk Information System (IRIS). EPA/635/R-98/008. Washington, DC, US Environmental Protection Agency, http://www.epa.gov/iris/toxreviews/0012-tr.pdf.
- VanOrdstrand HS, Hughes R. (1945). Beryllium poisoning. J Am Med Assoc 129:1084–1090.
- Wagoner JK, Infante PF, Bayliss DL. (1980). Beryllium: An etiologic agent in the induction of lung cancer, nonneoplastic respiratory disease, and heart disease among industrially exposed workers. Environ Res 21:15–34.
- Ward E, Okun A, Ruder A, Fingerhut M, Steenland K. (1992). A mortality study of workers at seven beryllium processing plants. Am J Ind Med 22:885–904.
- Williams WJ. (1996). United Kingdom Beryllium Registry: Mortality and autopsy study. Environ Health Perspect 104 Suppl 5:949–951.
- Wing S, Shy CM, Wood JL, Wolf S, Cragle DL, Tankersley W, Frome EL. (1993). Job factors, radiation and cancer mortality at Oak Ridge National Laboratory: Follow-up through 1984. Am J Ind Med 23:265–279.