Abstract
Three commercial brands of Swedish snus (SWS), an experimental SWS, and the 2S3 reference moist snuff were each tested in four in vitro toxicology assays. These assays were: Salmonella reverse mutation, mouse lymphoma, in vitro micronucleus, and cytotoxicity. Water extractions of each of the 5 products were tested using several different concentrations; the experimental SWS was also extracted using dimethyl sulfoxide (DMSO). Extraction procedures were verified by nicotine determinations. Results for SWS in the mutagenicity assays were broadly negative: there were occasional positive responses, but these were effectively at the highest concentration only (concentrations well above those suggested by regulatory guidelines), and were often associated with cytotoxicity. The 2S3 reference was unequivocally positive in one of the three conditions of the micronucleus assay (MNA), at the highest concentration only. Positive controls produced the expected responses in each assay. The SWS data are contrasted with data reported for combusted tobacco in the form of cigarettes, where strongly positive responses have been routinely reported for mutagenicity and cytotoxicity. These negative findings in a laboratory setting concur with the large amount of epidemiological data from Sweden, data showing that SWS are associated with considerably lower carcinogenic potential when compared with cigarettes.
Table of Contents
Abstract 312
Keywords 312
Introduction 312
Materials and methods 313
Results 316
Discussion 318
Conclusions 319
Acknowledgements 319
Declaration of interest 319
References 319
Introduction
Swedish snus (SWS) are moist to semi-moist, ground, oral tobacco products (CitationRutqvist et al., 2011), which are often placed in “tea-bag” style packaging which consumers usually place behind their upper lip. The products are made from mainly air-cured tobaccos, water, salt, and flavor additives; they are “pasteurized” in a proprietary heat treatment process which satisfies the hygienic requirements of the Swedish Food Act (CitationRutqvist et al., 2011). Data from Swedish and US epidemiology studies (CitationLee and Hamling, 2009; CitationColilla, 2010) have shown that the use of SWS and other “moist snuff” products have effectively no effect on a broad range of cancers and other diseases (CitationLee, 2011), a finding in direct contrast with findings repeatedly reported elsewhere for combusted tobacco products such as cigarettes (CitationIARC, 2004; CitationUS Department of Health and Human Services, 2004).
Epidemiology data have also shown that SWS has been used by many smokers as an aid to quit smoking, with snus use being associated with an increased probability of being a former smoker (CitationLund et al., 2011). The efficacy of SWS in smoking cessation was recently demonstrated in two recent (but quite small) clinical trials (CitationFagerström et al., 2011; CitationJoksic et al., 2011). SWS are very different from smokeless tobacco products (STP) used in regions other than Scandinavia and North America, for example the Sudanese “toombak” (CitationIdris et al., 1998). Therefore, we agree that “oral tobacco products should not be categorized together when considering the public health implications of their use” (CitationStanfill et al., 2011).
Genetic toxicology testing, the study of chemical, physical or biological agents that may interact with DNA resulting in genetic changes, has been a widely used procedure for several decades (CitationClive et al., 1972; CitationAmes et al., 1973), the active principle being that short-term tests involving genetic changes may be indicative of carcinogenic risk in laboratory animals and in humans. The tests are primarily used on individual compounds (e.g. a novel pharmaceutical), largely as an early stage safety evaluation (if the test produces a positive finding, then there probably will be no further work performed on that compound). It is usual to conduct a complementary battery of tests for each compound and to include tests in both bacterial and mammalian cells. A typical approach would be to use the bacterial (usually Salmonella typhimurium) reverse-mutation or “Ames” assay (CitationAmes et al., 1973; CitationAmes et al., 1975; CitationMcCann et al., 1975), with additional tests for chromosome damage and aneuploidy (CitationKirkland et al., 2005; CitationKirkland et al., 2011). Data on these latter endpoints are often obtained using the micronucleus assay (MNA) and the mouse lymphoma assay (MLA); there are recent suggestions that the only tests needed are S. typhimurium and in vitro micronucleus (CitationPfuhler et al., 2007; CitationKirkland et al., 2011). There are well-established international guidelines on how to perform each of these assays (CitationOECD, 1997a,Citationb,Citationc), including the suggestion that “wherever possible, the use of an aqueous solvent/vehicle be considered first.”
A related in vitro test that is commonly used in combination with the above assays is the neutral red uptake (NRU) assay (CitationNemes et al., 1979), a test for cytotoxicity (CitationBorenfreund and Puerner, 1985). Cytotoxicity is a major concern in genotoxicity assays (dead cells cannot mutate) and this factor has the potential to confound the interpretation of negative results. Compared to the assays listed above, only limited guidelines are available on the NRU assay (CitationAndreoli et al., 2003; CitationKing and Jones, 2003).
Combusted tobacco in the form of cigarette smoke condensate (CSC) has been shown to be highly active in a number of in vitro toxicology systems (CitationDeMarini, 2004; CitationDeMarini et al., 2008), particularly in the Salmonella mutagenicity test for the TA98 and TA100 strains in the presence of the Aroclor™ 1254 induced mammalian (rat) liver post-mitochondrial fraction (S9) (CitationRickert et al., 2007; CitationPatskan et al., 2008; CitationGaworski et al., 2010). The MLA has also been used with CSC, and again the mixture has been found to be highly mutagenic (CitationSchramke et al., 2006; CitationDeMarini et al., 2008; CitationWerley et al., 2008). An in vitro MNA for use with Chinese hamster fibroblast cells has been developed to use whole smoke and different components of this complex mixture (CitationOkuwa et al., 2010). Both particulate and vapor phases of smoke from a reference cigarette generated significant responses in this assay. The NRU assay has also been used routinely in the comparative evaluations of both the gas-vapor and particulate phases of cigarette smoke (CitationPatskan et al., 2008; CitationGaworski et al., 2010).
The present study was performed to determine whether SWS are active in the in vitro toxicology assays classically used to predict carcinogenicity in humans. Although there are published reports on the in vitro toxicology of SWS (CitationJansson et al., 1991), the products evaluated and the tests available in a publication from 20 years ago are quite different from those available today. In particular, the MLA does not appear to have been used previously with SWS. Also, concentrations of tobacco-specific nitrosamines, considered by some to be very important in STP (CitationÖsterdahl et al., 2004; CitationIARC, 2007; CitationScientific Committee on Emerging and Newly Identified Health Risks, 2008; CitationWorld Health Organization, 2008; CitationStanfill et al., 2011), are now very much lower in SWS than they were 20 years ago, probably the result of altered manufacturing processes (CitationRutqvist et al., 2011).
Materials and methods
SWS and control samples
The following commercial SWS (manufactured by Swedish Match, Stockholm, Sweden) were tested: General Original Portion Large (hereafter abbreviated as “G”), Catch White Portion Large, Licorice (“CPS”), and Catch Dry White Portion Mini, Licorice (“CDM”). An experimental SWS similar to CDM, but with experimental flavoring agents, was also tested (hereafter abbreviated as “CDM2”). The 2S3 reference moist snuff (CitationNorth Carolina Agricultural Research Service, 2006; CitationBorgerding et al., 2009; CitationJohnson et al., 2009) was also included. provides results of chemical analyses of the 5 different test materials, and compares these with the GothiaTek® standard (CitationRutqvist et al., 2011).
Table 1. Characteristics of the Swedish snus and the reference product, compared with the GothiaTek standard.
Extraction methods
The G, CPS, CDM and 2S3 samples were only tested with extractions using sterile, purified water; the CDM2 samples were tested using both water extractions and extractions with sterile, anhydrous dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Poole, UK). DMSO was use as a second extraction agent for “CDM2”, because of the inclusion in this product of some experimental flavoring agents with limited water solubility.
Extracts were prepared using the following procedure at concentrations of 500 mg of tobacco product per ml, and conducted using sterile containers and solutions, in order to minimise contamination from external sources. Appropriate numbers of sachets of SWS were cut approximately in half, and both the contents and the sachets were weighed and mixed with appropriate volumes of water or DMSO to produce the required w/v concentration. If the tobacco was not finely divided then brief homogenisation was performed. Extractions were performed for 24 h at 37°C, with shaking. At the end of the 24 h extraction period, extracts were centrifuged at approximately 1800g for 30 min, and heavy particulates removed by decanting off the supernatant. The supernatant was then centrifuged at 25,000g for 30 min, and fine particulates removed by decanting off the supernatant. The final supernatant was adjusted to pH 7.4 ± 0.2 with hydrochloric acid or sodium hydroxide (water extracts only). The resulting extracts were filter-sterilized using a 0.2 μm pore size filter (pre-filtering using a larger pore size was performed where required). Where extracts were prepared in two or more separate flasks, extracts were pooled prior to use in the assay. Aliquots of extracts were stored at approximately −80°C, and used within 3 months of extraction. Since extracts of SWS and reference products were each prepared according to the above methodology, with the particulate matter removed prior to assay, stated concentrations for these extracts should be considered to be in terms of equivalent weight of test material, corresponding to the concentration of test material present during the extraction phase.
For the Salmonella assays, treatments were performed using additions of 0.5 ml water extract per plate or 0.1 ml of DMSO extract per plate. For the other assays, treatments were performed using 10% v/v additions of water extract and 1%, 1.5%, or 2% v/v additions of the DMSO extract.
Nicotine contents
Nicotine concentrations were assessed using high-performance liquid chromatography; extraction blanks were used as negative controls in each assay system (CitationTambwekar et al., 2003). Three additional concentrations (200, 300 and 400 mg/ml) were used in addition to that used for the toxicology testing (500 mg/ml).
In vitro assays
The four assays used were the S. typhimurium reverse-mutation assay, the MLA, an in vitro micronucleus assay (MNAvit), and the NRU assay.
Ames assay
Extracts were assessed in a bacterial mutation assay system, using S. typhimurium tester strains TA98 (frameshift), TA100 (base-pair substitution), TA102 (base-pair substitution), TA1535 (base-pair substitution) and TA1537 (frameshift) using pre-incubation methodology. Bacteria were originally obtained from the UK National Centre for Type Cultures and from Glaxo Group Research Ltd. Testing was performed in the absence and presence of S9 (Molecular Toxicology Inc., Boone, NC), as described in OECD guideline 471 (CitationOECD, 1997a). Six concentrations were used for the water extracts: 0.08, 0.40, 2, 10, 50 and 250 mg per plate equivalent (OECD guideline 471 recommends a maximum test concentration for soluble non-cytotoxic substances of 5 mg/plate). Six concentrations were also used for the DMSO extract: 0.016, 0.08, 0.40, 2, 10 and 50 mg per plate equivalent. Three plates were used for each treatment and for the positive controls, with five for the solvent control.
The highest concentrations used were considered to be the highest achievable concentrations in this assay, using the highest extraction concentration that could practically be extracted from the test materials, and the largest volume additions that the assay system could tolerate for each solvent.
The following positive control substances (Sigma-Aldrich) and their concentrations were used for the different tester strains and S9 conditions: 2-nitrofluorene (2NF, TA98, −S9, 5 μg/plate), sodium azide (NaN3, TA100, TA1535, both −S9, 2 μg/plate), 9-aminoacridine (AAC, TA1537, −S9, 50 μg/plate), mitomycin C (MMC, TA102, −S9, 0.2 μg/plate), benzo[a]pyrene (B[a]P, TA98, +S9, 10 μg/plate) and 2-aminoanthracene (AAC, TA100, TA1535, TA1537, and TA102, all +S9, 5 μg/plate except for TA 102, 20 μg/plate).
MLA assay
Extracts were assessed in duplicate cultures of mouse lymphoma L5178Y thymidine kinase (tk+/−) cells (a gift from Dr. D. Clive, Burroughs-Wellcome, NC), as described in OECD Guideline 476 (CitationOECD, 1997c). Treatments were conducted using 3- and 24-h exposures in the absence of S9, and a 3-h treatment in the presence of S9 (CitationClive et al., 1995; CitationMoore et al., 2006), using six concentrations for the water extractions (5, 10, 20, 30, 40 and 50 mg/ml equivalent) and eight concentrations for the DMSO extractions (7.5, 15, 30, 45, 60, 65, 70 and 75 mg/ml equivalent). Treatments were assessed for their effects on relative total growth (RTG) and mutant frequency (MF).
The positive controls (single plate, Sigma-Aldrich) used were 4-nitroquinoline 1-oxide (NQO, −S9 condition) and B[a]P (+S9 condition), at low and high concentrations of 0.05 and 0.1 μg/ml (NQO, 24 h), 0.15 and 0.20 μg/ml (NQO, 3 h), and 2 and 3 μg/ml (B[a]P, both time points), respectively.
MNAvit assay
Extracts were assessed in duplicate cultures of Chinese hamster fibroblast V79 cells (a gift from Dr. E Massey, BAT, UK) in an in vitro MNA, according to OECD guidelines 474 (CitationOECD, 1997b) and 487 (CitationOECD, 2007). Four plates were used for the solvent controls and two plates for the positive controls. The target number of cells to be examined was 1000 for each plate. Treatments were conducted using 3 h (pulse) treatments in the absence and presence of S9, followed by a 17-h recovery period (3 + 17), and 20 h (continuous) treatment in the absence of S9. At least three concentrations were tested in addition to solvent controls, in the range 6.7–50 mg/ml for the water extracts and 0.27–7.5 mg/ml for the DMSO extracts.
The MNA assay detects clastogens and aneugens in cells that have undergone cell division during or after exposure to the test substance (CitationLorge et al., 2006). The assay typically uses protocols with and without the actin polymerization inhibitor cytochalasin B (a mycotoxin); however, we did not perform any testing without cytochalasin B. Addition of this compound prior to the targeted mitosis allows for the identification and selective micronucleus frequency in cells that have completed a single mitosis, because such cells are binucleate. Cytochalasin B (Sigma-Aldrich) inhibits cytokinesis (cell division), but not karyokinesis (nuclear division), resulting in the formation of binucleate cells. Thus, when micronuclei are only counted in binucleate cells, a true measurement of their induction can be obtained. Cytochalasin-B has been shown to have no effect on the overall assay (CitationLorge et al., 2006). Treatments were assessed for resulting counts of micro-nucleated binucleate (MNBN) cells.
The positive controls (Sigma-Aldrich) used were NQO (0.30 μg/ml) and vinblastin (VIN, 0.004 μg/ml), for the −S9 condition, and cyclophosphamide (CPA, 8 μg/ml) for the +S9 condition.
NRU assay
Extracts were assessed in Balb/c 3T3 mouse fibroblast cells (CitationBorenfreund and Puerner, 1985; CitationBabich and Borenfreund, 1990), purchased from the European Cell and Culture Collection. Cytotoxicity was measured by assessment of NRU after 24 h of exposure, expressed as the per cent cell survival. The eight concentrations were 0.39, 0.78, 1.56, 3.13, 6.25, 12.5, 25 and 50 mg/ml for the water extracts and 0.04, 0.08, 0.16, 0.31, 0.63, 1.25, 2.5 and 5 mg/ml for the DMSO extracts. Six replicates were performed for each concentration. The positive control used was sodium dodecyl sulfate (Sigma-Aldrich), used at a single concentration of 100 μg/ml.
Two experiments were performed: one compared samples “G”, “CPS” and “2S3”, and the other compared samples “CDM”, “CDM2” (water extraction), and “CDM2” (DMSO extraction).
Endpoint analyses
For the S. typhimurium analyses, we used pairwise comparisons of mean revertant counts with the solvent control, using Dunnett’s test and a significance level of P < 0.01. Dunnett’s test makes adjustments for multiple corrections: it ensures that the type 1 error rate will be fixed at the desired level by incorporating correction factors into the design of the test table. The significance level used here (<0.01 rather than <0.05 used elsewhere) is well-established and is widely used for bacterial mutation assay data. An estimate was made of dose response, considered to be present if the two highest concentrations both produced significant differences from the solvent controls. We also considered the twofold rule (CitationCariello and Piegorsch, 1996) to infer positive results, modified to a threefold rule for tester strains TA1535 and TA1537 because of their relatively low number of background revertants (c. 10–20 per plate).
For the MLA we used pairwise comparisons of mean MF values with those in the solvent controls, using Dunnett’s test and a significance level of P < 0.05. Linear trends in MF values were evaluated using χ2-tests and a significance level of P < 0.05. We also considered mean MF values that were at least 126 revertants per million cells greater than the mean MF in the solvent controls (termed the global evaluation factor) (CitationMoore et al., 2006).
In the MNAvit study, a binomial dispersion test was used to determine homogeneity between replicates. This was followed by the use of Fisher’s exact test and a significance level of P < 0.05 to compare numbers of MNBN cells in treated groups with those in the concomitant solvent control group. We also considered whether the results obtained for percentages of MNBN cells were higher than those seen in the historical solvent control. These historical values (from 53 prior studies) were 2.9% (3 + 17, −S9), 2.8% (3 + 17, + S9), and 3.0% (0 + 20, −S9).
For the NRU assay, pairwise comparisons of cell survival rates were made at the highest extract concentration, using SigmaPlot version 11.2 (San Jose, CA) and a significance level of P < 0.05.
Results
Nicotine determination
Excellent consistency in nicotine concentrations was obtained between separate water extracts, indicating that the extraction methodology provided consistent levels of extracted material. Estimated nicotine recovery levels (based on information provided on nicotine levels of the different materials used) from 200, 300, 400 and 500 mg/ml equivalent water extracts were 84%, 78%, 76% and 73%, indicating that nicotine content does continue to increase proportionately over these increasing extraction concentrations, with only a relatively small reduction in recovery efficiency.
Ames assay
Results of the S. typhimurium mutagenicity assays for sample “G” are given as , with separate listings for the −S9 and +S9 conditions. Results for the other samples are provided in the supplementary material.
For both conditions, there were several mean revertant counts that reached statistical significance when compared with the solvent control; these counts were often limited to the highest concentration (i.e. there was little or no evidence for a dose-response relationship). There were three cases where the two highest concentrations were both significantly greater than the solvent control: sample “G” for TA102 in the −S9 condition (), sample “CDM” for TA1537 in the −S9 condition, and sample “CDM2” in the water extraction, TA102, −S9 condition. There were only two cases of a doubling (or a tripling) of the mean value for the solvent control, but these were not the same two cases as those described above. The cases here were sample “G”, TA1535, −S9 (mean of 45 revertants compared to 11, a factor of 4.1, ), and sample “CPS”, TA1535, −S9 (mean of 43 revertants compared to 11, a factor of 3.9). The two different assessment methods thus provided different indications of possible mutagenic activity; both of these indications were effectively limited to the highest concentration being tested.
With one exception, mean revertant counts for the positive controls were in the range of 5-to-70-fold significant increases over the mean for the solvent control for the −S9 condition and 4-to-9-fold for the +S9 condition. The exception was for the DMSO extractions of sample “CDM2” with strain TA1537: for the +S9 condition the mean value for the positive control was 2.9 times higher than that for the solvent control.
Table 2. Results of the Salmonella mutagenesis assay with water extracts of Swedish snus sample “G” (−S9 and +9 conditions).
MLA assay
The results of the MLA for sample “G” are given as , with separate listings for the 3-h treatments (both S9 conditions) and the 24-h treatments (−S9 only). Results for the other samples are provided in the supplementary material.
Table 3. Results of the mouse lymphoma assay with water extracts of Swedish snus sample “G” (3-h and 24-h treatment).
There were several mean MFs that reached statistical significance when compared with the solvent control; these differences were sporadic with no apparent dose–response relationship. There were two clear exceptions, both in the 24-h −S9 condition. The first was sample “CDM”, where the two highest treatments both had mean MFs that were significantly greater than the mean for the solvent control. The second was sample “CDM2”, water extraction, where the three highest treatments all had mean MFs that were significantly greater than the mean for the solvent control. Significant linear trends in MFs were noted for several of the 3-h +S9 and 24-h −S9 conditions; none were observed for the 3-h −S9 condition.
There were two cases of mean MFs greater by at least 126 revertants per million cells than the mean for the solvent control, and these were for the same samples as those noted above for conventional statistical significance. The highest treatments for the 24-h−S9 condition with samples “CDM” and “CDM2”, water extraction, had mean MFs of 270 and 327, respectively, compared with the mean value for the solvent control group of 103.
The two positive responses described above were both associated with considerable toxicity: the RTGs for both at the highest concentration were only 8%, less than the suggested minimum of 10% (CitationMoore et al., 2006).
Mean MFs for the positive controls (both doses) were in the range of 2-to-17-fold significant increases over the mean value for the solvent control and were considerably greater than the global evaluation factor.
MNAvit assay
Homogeneity testing did not reveal any major discrepancies between replicates.
The results of the MNAvit assay for sample “G” are given as , with separate listings for the 3 + 17 h treatments (both of the S9 conditions) and the 20 h treatment (the −S9 condition only). Results for the other samples are given in the supplementary material.
Table 4. Results of the in vitro micronucleus assay with water extracts of Swedish snus sample “G”.
There were several incidences of MNBN incidences that reached statistical significance when compared with the concomitant solvent control; in only two cases did the incidences exceed the historical range for the solvent control. The first of these cases was for sample “CDM2”, water extraction, in the 3 + 17 h, −S9 condition. At the highest concentration tested the mean MNBN response was 4.1%, compared with 0.85% for the concomitant solvent controls and with 2.9% for historic solvent controls. However, there was considerable cytotoxicity (70%) associated with this response. The second case was the 2S3 reference product, in the 3 + 17 h, +S9 condition. At the highest concentration tested the mean MNBN response here was 4.08%, compared with 0.89% for the concomitant solvent controls and with 2.8% for historic solvent controls. Cytotoxicity was not excessive in this case (22%), and so the 2S3 response is unequivocally positive.
Positive controls produced responses that were significantly increased when compared with concomitant solvent controls. With one exception, the positive control responses were also significantly increased when compared with historical solvent controls. The exception was NQO in the 3 + 17 h, −S9 condition, where the mean value (2.42%) was less than the historical mean value, even though it was almost threefold higher than the concomitant solvent control (comparable to the positive control for the TA1537 +S9 condition of the Ames assay). When used at the same dose in the 20 + 0 h, −S9 condition, NQO produced an approximately eightfold increase over concomitant solvent controls and an approximately threefold increase over historical solvent controls.
NRU assay
Results of the two experiments are presented as and .
Figure 1. Results of the neutral red uptake assay with samples “G”, “CPS” and “2S3”. Means ± SD (n = 6). *Significantly different (P < 0.05) from the other two means.
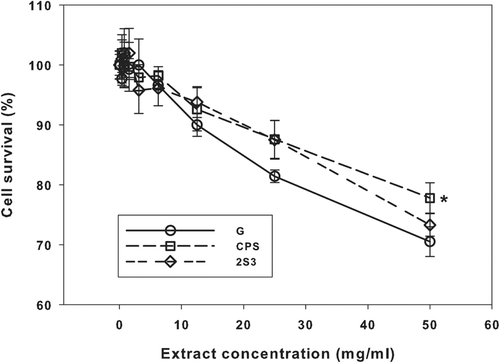
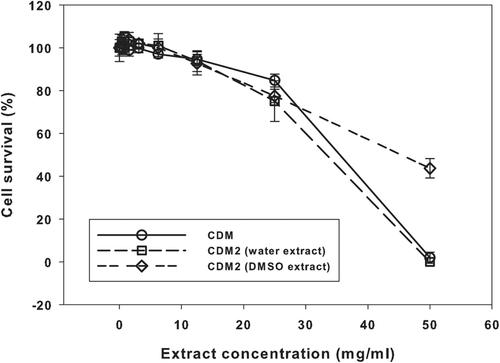
Figure 2. Results of the neutral red uptake assay with samples “CDM” and “CDM2”. Means ± SD (n = 6).
The increasing cytotoxicity (decreasing cell survival) of the extracts from samples “G”, “CPS” and “2S3” were very similar, with the lowest attained survival being approximately 75% (). At the highest extract concentration, the mean cell survival for the “CPS” sample was significantly greater (by about 10%) than those for the “G” and “2S3” samples (Holm-Sidak test, P < 0.05, ).
The decline in cell survival in the “CDM” and “CDM2” (water extraction) samples with increasing extract concentration was effectively identical (), with zero survival at the highest concentration. However, cell survival for the “CDM2” sample (DMSO extraction) at the highest concentration was 44% ().
There was minimal cell survival with the positive control.
Discussion
We showed that various types of in vitro toxicology testing, including those tests classically used to predict carcinogenicity in humans, are feasible for STP such as SWS. Positive control substances demonstrated that each of the tests was working correctly.
We also showed that for SWS in assays such as S. typhimurium, mouse lymphoma and in vitro micronucleus, the results provided no clear biologically-relevant positive responses, as opposed to the statement of “poor performance” made by others (CitationJohnson et al., 2009). In most cases the results for the reference moist snuff were also negative, but there was one unequivocally positive response. Using carefully controlled assays with positive controls, we showed that cytotoxicity can be controlled and indeed (with some exceptions) this was not a problem in the work we describe here. The broadly negative data presented here for SWS contrast strongly with data for cigarettes where the tobacco is combusted; for these products, substantial genotoxicity has been demonstrated repeatedly (CitationDeMarini et al., 2008).
Normally, genetic toxicology data such as those we present here would be the first step in a toxicological evaluation that would end with epidemiology. The reverse is the case here, with extensive epidemiology but minimal genetic toxicology. Our work therefore represents a unique case of being able to “look back” on the toxicological evaluation process; it provides validation of the genetic testing of non-combusted tobacco products. Cigarette smoke on the other hand has shown strong evidence of carcinogenicity by epidemiology and positive results from genetic toxicology tests.
A limitation of the work described here is the fact that a full set of chemical analyses was not made on the actual products tested (nicotine only was analyzed). However, a full set of analyses was made on identical products, and these data are presented in . Another limitation is the lack of agreement in the literature on the statistical criteria to be used to determine a positive response, particularly for the Salmonella mutagenicity assay where the OECD guidelines simply state that “there are several criteria for determining a positive result.” A further limitation is our exclusive use of aqueous extracts, supplemented in one case with DMSO extracts. We are aware of published work with extractions using artificial saliva, and the use of alternative extraction agents may be an avenue for future work.
These broadly negative findings in a controlled laboratory setting add to the large amount of epidemiological data from Scandinavia (CitationColilla, 2010; CitationLee, 2011), data showing that SWS are associated with considerably lower carcinogenic potential when compared with tobacco products involving combustion of the tobacco (CitationUS Department of Health and Human Services, 2004).
Conclusions
For each of the assays, data obtained for negative and positive controls confirmed that the test systems were working correctly.
Ames assay
Extracts of the four SWS and the 2S3 reference did not induce clear increases in MF in five strains of S. typhimurium, both in the absence and in the presence of S9. Two different methods for evaluating the data produced conflicting results. The observed increases in revertant numbers were limited to extreme concentrations of the test material (well in excess of concentrations required by regulatory guidelines, such as OECD 471), and so these findings are considered to be of limited relevance to an overall risk assessment for SWS.
MLA assay
Extracts of the four SWS and the 2S3 reference did not induce clear increases in MF in L5178Y tk+/− cells, both in the absence and in the presence of S9. For the 3-h treatments, no increase in MFs over the global evaluation factor was noted. In two cases with the 24-h treatments, MFs were greater than the global evaluation factor. In each of these cases the RTG values were substantially reduced (indicating considerable cytotoxicity), so these findings are considered to be of limited relevance to an overall risk assessment for SWS.
MNAvit assay
Extracts of the four SWS did not show increased MNBN counts in Chinese hamster fibroblast cells, with an unequivocally positive result for the 2S3 reference at the highest dose in the 3 + 17 h +S9 condition.
NRU assay
No obvious differences were noted between the cytotoxicity results noted in Balb/c 3T3 mouse fibroblast cells for the “G”, “CPS” and “2S3” samples, with the highest extract concentrations only producing approximately 75% cytotoxicity. Complete cytotoxicity was seen with the “CDM” and “CDM2” samples at the highest concentration, with some apparent reduction in cytotoxicity in the “CDM2” sample when DMSO extraction was used.
Declaration of interest
The authors thank the Covance Laboratories (Harrogate, UK) for their skillful contributions to this research. Dr. Coggins is a consultant to Swedish Match and as such he was paid for his contribution to the preparation of this manuscript. Mr. Ballantyne works for Covance Laboratories, who were paid by Swedish Match to perform the work. Drs. Rutqvist and Curvall are employees of Swedish Match.
Supplementary Material
Download PDF (163.5 KB)References
- Ames BN, Durston WE, Yamasaki E, Lee FD. (1973). Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci USA 70:2281–2285.
- Ames BN, Mccann J, Yamasaki E. (1975). Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res 31:347–364.
- Andreoli C, Gigante D, Nunziata A. (2003). A review of in vitro methods to assess the biological activity of tobacco smoke with the aim of reducing the toxicity of smoke. Toxicol In Vitro 17:587–594.
- Babich H, Borenfreund E. (1990). Cytotoxic effects of food additives and pharmaceuticals on cells in culture as determined with the neutral red assay. J Pharm Sci 79:592–594.
- Borenfreund E, Puerner JA. (1985). Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett 24:119–124.
- Borgerding M, Bodnar J, Swauger J. (2009). The chemical composition of smokeless tobacco: a survey of products sold in the United States in 2006 and 2007. Winston-Salem: R.J. Reynolds Tobacco Co.
- Cariello NF, Piegorsch WW. (1996). The Ames test: the two-fold rule revisited. Mutat Res 369:23–31.
- Clive D, Bolcsfoldi G, Clements J, Cole J, Homna M, Majeska J, Moore M, Müller L, Myhr B, Oberly T. (1995). Consensus agreement regarding protocol issues discussed during the mouse lymphoma workshop: Portland, Oregon, May 7, 1994. Environ Mol Mutagen 25:165–168.
- Clive D, Flamm WG, Machesko MR, Bernheim NJ. (1972). A mutational assay system using the thymidine kinase locus in mouse lymphoma cells. Mutat Res 16:77–87.
- Colilla SA. (2010). An epidemiologic review of smokeless tobacco health effects and harm reduction potential. Regul Toxicol Pharmacol 56:197–211.
- DeMarini DM. (2004). Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat Res 567:447–474.
- DeMarini DM, Gudi R, Szkudlinska A, Rao M, Recio L, Kehl M, Kirby PE, Polzin G, Richter PA. (2008). Genotoxicity of 10 cigarette smoke condensates in four test systems: comparisons between assays and condensates. Mutat Res 650:15–29.
- Fagerstrom K, Rutqvist LE, Hughes JR. (2012). Snus as a smoking cessation aid: a randomized placebo-controlled trial. Nicotine Tob Res 14:306–312.
- Gaworski CL, Oldham MJ, Coggins CR. (2010). Toxicological considerations on the use of propylene glycol as a humectant in cigarettes. Toxicology 269:54–66.
- IARC. (2004). Tobacco Smoke and Involuntary Smoking. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon, France: International Agency for Research on Cancer, 1191–1413.
- IARC. (2007). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 89. Smokeless Tobacco and some tobacco-specific N-Nitrosamines. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer.
- Idris AM, Ibrahim SO, Vasstrand EN, Johannessen AC, Lillehaug JR, Magnusson B, Wallström M, Hirsch JM, Nilsen R. (1998). The Swedish snus and the Sudanese toombak: are they different? Oral Oncol 34:558–566.
- Jansson T, Romert L, Magnusson J, Jenssen D. (1991). Genotoxicity testing of extracts of a Swedish moist oral snuff. Mutat Res 261:101–115.
- Johnson MD, Schilz J, Djordjevic MV, Rice JR, Shields PG. (2009). Evaluation of in vitro assays for assessing the toxicity of cigarette smoke and smokeless tobacco. Cancer Epidemiol Biomarkers Prev 18:3263–3304.
- Joksic G, Spasojevic-Tišma V, Antic R, Nilsson R, Rutqvist LE. (2011). Randomized, placebo-controlled, double-blind trial of Swedish snus for smoking reduction and cessation. Harm Reduct J 8:25.
- King AV, Jones PA. (2003). In-house assessment of a modified in vitro cytotoxicity assay for higher throughput estimation of acute toxicity. Toxicol In Vitro 17:717–722.
- Kirkland D, Aardema M, Henderson L, Müller L. (2005). Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens I. Sensitivity, specificity and relative predictivity. Mutat Res 584:1–256.
- Kirkland D, Reeve L, Gatehouse D, Vanparys P. (2011). A core in vitro genotoxicity battery comprising the Ames test plus the in vitro micronucleus test is sufficient to detect rodent carcinogens and in vivo genotoxins. Mutat Res 721:27–73.
- Lee PN. (2011). Summary of the epidemiological evidence relating snus to health. Regul Toxicol Pharmacol 59:197–214.
- Lee PN, Hamling J. (2009). Systematic review of the relation between smokeless tobacco and cancer in Europe and North America. BMC Med 7:36.
- Lorge E, Thybaud V, Aardema MJ, Oliver J, Wakata A, Lorenzon G, Marzin D. (2006). SFTG international collaborative study on in vitro micronucleus test I. General conditions and overall conclusions of the study. Mutat Res 607:13–36.
- Lund KE, Scheffels J, McNeill A. (2011). The association between use of snus and quit rates for smoking: results from seven Norwegian cross-sectional studies. Addiction 106:162–167.
- McCann J, Choi E, Yamasaki E, Ames BN. (1975). Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci USA 72:5135–5139.
- Moore MM, Honma M, Clements J, Bolcsfoldi G, Burlinson B, Cifone M, Clarke J, Delongchamp R, Durward R, Fellows M, Gollapudi B, Hou S, Jenkinson P, Lloyd M, Majeska J, Myhr B, O’Donovan M, Omori T, Riach C, San R, Stankowski LF Jr, Thakur AK, Van Goethem F, Wakuri S, Yoshimura I. (2006). Mouse lymphoma thymidine kinase gene mutation assay: follow-up meeting of the International Workshop on Genotoxicity Testing–Aberdeen, Scotland, 2003–Assay acceptance criteria, positive controls, and data evaluation. Environ Mol Mutagen 47:1–5.
- Nemes Z, Dietz R, Lüth JB, Gomba S, Hackenthal E, Gross F. (1979). The pharmacological relevance of vital staining with neutral red. Experientia 35:1475–1476.
- North Carolina Agricultural Research Service. (2006). Smokeless Tobacco Research Products. Raleigh, NC: North Carolina State University.
- OECD. (1997a). No. 471. Bacterial reverse mutation test. OECD guideline for testing of chemicals. Paris, France.
- OECD. (1997b). No. 474. Mammalian erythrocyte micronucleus test. OECD guideline for testing of chemicals. Paris, France.
- OECD. (1997c). No. 476. In Vitro mammalian cell gene mutation test. OECD guideline for the testing of chemicals. Paris, France.
- OECD. (2007). Draft proposal for a new Guideline 487: In Vitro mammalian cell micronucleus test (MNvit). OECD guideline for the testing of chemicals. Paris, France.
- Okuwa K, Tanaka M, Fukano Y, Nara H, Nishijima Y, Nishino T. (2010). In vitro micronucleus assay for cigarette smoke using a whole smoke exposure system: a comparison of smoking regimens. Exp Toxicol Pathol 62:433–440.
- Osterdahl BG, Jansson C, Paccou A. (2004). Decreased levels of tobacco-specific N-nitrosamines in moist snuff on the Swedish market. J Agric Food Chem 52:5085–5088.
- Patskan GJ, Podraza KF, Meurrens K, Coggins CR, Friedrichs B, Gerstenberg B, Gomm W, Schnell P, Stabbert R, Veltel D, Weber S, Terpstra P. (2008). Toxicological comparisons of three styles of a commercial U.S. cigarette (Marlboro with the 1R4F reference cigarette. Inhal Toxicol 20:695–721.
- Pfuhler S, Albertini S, Fautz R, Herbold B, Madle S, Utesch D, Poth A; Gesellschaft fuer Umwelt-Mutationsforschung. (2007). Genetic toxicity assessment: employing the best science for human safety evaluation part IV: Recommendation of a working group of the Gesellschaft fuer Umwelt-Mutationsforschung (GUM) for a simple and straightforward approach to genotoxicity testing. Toxicol Sci 97:237–240.
- Rickert WS, Wright WG, Trivedi AH, Momin RA, Lauterbach JH. (2007). A comparative study of the mutagenicity of various types of tobacco products. Regul Toxicol Pharmacol 48:320–330.
- Rutqvist LE, Curvall M, Hassler T, Ringberger T, Wahlberg I. (2011). Swedish snus and the GothiaTek® standard. Harm Reduct J 8:11.
- Schramke H, Meisgen TJ, Tewes FJ, Gomm W, Roemer E. (2006). The mouse lymphoma thymidine kinase assay for the assessment and comparison of the mutagenic activity of cigarette mainstream smoke particulate phase. Toxicology 227:193–210.
- Scientific Committee on Emerging and Newly Identified Health Risks. (2008). Health Effects of Smokeless Tobacco Products. Brussels, Belgium: Directorate-General, Health and Consumer Protection, European Commission.
- Stanfill SB, Connolly GN, Zhang L, Jia LT, Henningfield JE, Richter P, Lawler TS, Ayo-Yusuf OA, Ashley DL, Watson CH. (2011). Global surveillance of oral tobacco products: total nicotine, unionised nicotine and tobacco-specific N-nitrosamines. Tob Control 20:e2.
- Tambwekar KR, Kakariya RB, Garg S. (2003). A validated high performance liquid chromatographic method for analysis of nicotine in pure form and from formulations. J Pharm Biomed Anal 32:441–450.
- US Department of Health and Human Services. (2004). The Health Consequences of Smoking. A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Center for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
- Werley MS, Freelin SA, Wrenn SE, Gerstenberg B, Roemer E, Schramke H, Van Miert E, Vanscheeuwijck P, Weber S, Coggins CR. (2008). Smoke chemistry, in vitro and in vivo toxicology evaluations of the electrically heated cigarette smoking system series K. Regul Toxicol Pharmacol 52:122–139.
- World Health Organization. (2008). WHO study group on tobacco product regulation: report on the scientific basis of tobacco product regulation: third report of a WHO study group. WHO technical report series: no. 955. Geneva, Switzerland: World Health Organization.